Abstract
IMPORTANCE
In animal studies, brain-derived neurotrophic factor (BDNF) has been shown to impact neuronal survival and function and improve synaptic plasticity and long-term memory. Circulating BDNF levels increase with physical activity and caloric restriction, thus BDNF may mediate some of the observed associations between lifestyle and the risk for dementia. Some prior studies showed lower circulating BDNF in persons with Alzheimer disease (AD) compared with control participants; however, it remains uncertain whether reduced levels precede dementia onset.
OBJECTIVE
To examine whether higher serum BDNF levels in cognitively healthy adults protect against the future risk for dementia and AD and to identify potential modifiers of this association.
DESIGN, SETTING, AND PARTICIPANTS
Framingham Study original and offspring participants were followed up from 1992 and 1998, respectively, for up to 10 years. We used Cox models to relate BDNF levels to the risk for dementia and AD and adjusted for potential confounders. We also ran sensitivity analyses stratified by sex, age, and education, as well as related BDNF genetic variants to AD risk. This community-based, prospective cohort study involved 2131 dementia-free participants aged 60 years and older (mean [SD] age, 72 [7] years; 56% women).
MAIN OUTCOMES AND MEASURES
Ten-year incidence of dementia and AD.
RESULTS
During follow-up, 140 participants developed dementia, 117 of whom had AD. Controlling for age and sex, each standard-deviation increment in BDNF was associated with a 33% lower risk for dementia and AD (P = .006 and P = .01, respectively) and these associations persisted after additional adjustments. Compared with the bottom quintile, BDNF levels in the top quintile were associated with less than half the risk for dementia and AD (hazard ratio, 0.49; 95%CI, 0.28–0.85; P = .01; and hazard ratio, 0.46; 95%CI, 0.24–0.86; P = .02, respectively). These associations were apparent only among women, persons aged 80 years and older, and those with college degrees (hazard ratios for AD: 0.65, [95%CI, 0.50–0.85], P = .001; 0.63 [95%CI, 0.47–0.85], P = .002; and 0.27 [95%CI, 0.11–0.65], P = .003, respectively). Brain-derived neurotrophic factor genetic variants were not associated with AD risk.
CONCLUSIONS AND RELEVANCE
Higher serum BDNF levels may protect against future occurrence of dementia and AD. Our findings suggest a role for BDNF in the biology and possibly in the prevention of dementia and AD, especially in select subgroups of women and older and more highly educated persons.
The lifetime risk1 for developing Alzheimer disease (AD) is 1 in 5, and with increasing life expectancy, the number of affected people is projected to increase.2 Despite intensive investigation in recent years, there is still an incomplete understanding of the etiology and pathophysiology of this disabling and costly disease; therefore, the best strategies for prevention and treatment are not known.
Brain-derived neurotrophic factor (BDNF) may explain some of the variation in dementia risk, and because it is inducible by factors such as reduced caloric intake3 and increased physical activity,4,5 it is thought to mediate the association between healthy lifestyle and successful aging. In animal models, BDNF is highly expressed and widely distributed throughout the central nervous system especially in the hippocampus and cerebral cortex6,7 and is important in the survival and function of hippocampal and cortical, as well as cholinergic and dopaminergic, neurons.8–11 In addition, BDNF is critical for synaptic plasticity and memory processing in the adult brain.12,13 Erickson et al14 demonstrated a relationship between reduced serum BDNF and smaller hippocampal volume, as well as poorer memory, in a sample of 142 dementia free individuals aged 59 to 81 years. Similarly, in a Finnish population-based study of 1389 men and women aged 57 to 79 years, plasma BDNF was positively associated with cognitive performance; however, these associations were apparent only in women.15 Reduced levels of BDNF have been observed in the hippocampus and parietal lobe,16,17 as well as in the serum,18–20 of people with mild cognitive impairment and AD compared with cognitively intact individuals or people with vascular dementia.21 Nevertheless, other studies have found no association with AD.22 However, because of the cross-sectional design of these studies, it is not clear whether changes in BDNF levels preceded or followed the cognitive decline and onset of clinical dementia.
In the present study, we examined whether serum BDNF levels were prospectively associated with 10-year risks for incident dementia and AD in a large population-based sample from the Framingham Heart Study (FHS). Because factors such as age, sex, smoking status,23,24 and education25,26 may influence both BDNF levels and dementia risk,27 we explored whether these factors modify the association between BDNF and the risk for incident dementia or AD.
Methods
Study Sample
The FHS is a longitudinal community-based cohort study that was initiated in 1948 with the enrollment of 5209 participants aged 28 to 62 years (original cohort). In 1971, the offspring and their spouses were enrolled as the offspring cohort. Since the study’s inception, participants have had serial examinations including standardized interviews, physician examinations, and laboratory testing. Data were obtained under a protocol approved by the institutional review board of the Boston University Medical Center, and written informed consent was obtained from all participants. More details of the study design have been provided elsewhere.28,29
Of the original cohort, 1026 participants attended examination 23 (mean age, 82 years; 33% males) and from the offspring cohort, 3539 participants attended examination 7 (mean age, 62 years; 46% males). In total, BDNF levels were measured in 3689 participants (669 in the original and 3020 in the offspring cohorts). After exclusion of 1407 offspring participants who were younger than age 60 years, 9 participants with prevalent dementia, and 142 who did not have complete follow-up information, 2131 participants were available for the analyses of association with dementia/AD (655 in the original and 1476 in the offspring cohorts).
Laboratory Measurements of BDNF
Serum BDNF concentrations were measured on previously frozen (stored at −70°C) blood samples drawn in the fasting state from persons who attended the 23rd original cohort examination and the 7th offspring examination. Assays used enzyme-linked immunosorbent assay kits from R&D Systems. The intra-assay and interassay coefficients of variation were 4.8% and 7.6%, respectively.
Ascertainment of Dementia and AD
All FHS participants are under continuous surveillance for impairment in cognitive function. We have previously outlined our screening and surveillance methods for the development of dementia.30 Dementia was diagnosed according to the criteria of the Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition)31 and AD based on the criteria of the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer Disease and Related Disorders Association for definite, probable, or possible AD.32,33
Definition of Covariates
The study covariates included previously described components of the Framingham Stroke Risk Profile34 including age in years, systolic blood pressure in millimeters of mercury, history of diabetes mellitus, cardiovascular disease and atrial fibrillation, and current smoking status. Educational achievement was defined as a 3-class variable (no high-school degree, high-school degree only, or at least a college degree). Total plasma homocysteine (tHcy) was measured using high-performance liquid chromatography with fluorometric detection,35 and depressive symptoms were evaluated with the Center for Epidemiological Studies Depression Scale (CES-D).36 The Physical Activity Index (PAI) was calculated as a composite score based on information collected from a structured questionnaire.37 Total plasma homocysteine, CES-D, and PAI were all log-transformed for analysis. Information on covariates was obtained from the visit in which BDNF was measured except for tHcy, CES-D, and PAI in the original cohort that were obtained from examination 20.
Single-Nucleotide Polymorphism Selection and Genotyping
Genome wide genotyping was available for all participants with good-quality DNA. Genotyping was performed on the Affymetrix GeneChip Human Mapping 500K Array Set and 50K Human Gene Focused Panel through the SHARe Project (SNP-Health Association Resource), and these data were used to impute to the 2.5 million nonmonomorphic, autosomal single-nucleotide polymorphisms (SNPs) described in the HapMap (CEU population). Genotyping, quality control, and imputation methods have been described previously.38 Of 2131 participants available for the analysis of plasma BDNF levels and dementia/AD, 1819 also had adequate genotypic data after quality control, and there were 115 cases of incident AD in this subsample. Mean BDNF levels in this subsample (mean [SD], 23 270 [8144.95] pg/ml) were similar to that of the larger sample studied. We extracted information on 133 SNPs that were within the genomic region up to 60 kb on either side (chr11:27573018–27760181) of the BDNF gene (defined as chr11:27633018–27700181) and related these SNPs to serum BDNF levels and to the risk for AD.
Statistical Analyses
We used multivariable Cox regression analysis to examine the associations of BDNF with incident dementia and AD, modeling BDNF as a continuous variable and also examining the trend across quintiles. In addition, we tested for interaction of BDNF levels with age, sex, smoking status, and education in determining the risk for dementia by including these interaction terms in the Cox regression model. If a significant interaction was found, we ran a stratified model. For age stratification, we chose the cutoff of 80 years. All primary analyses were first adjusted for age, sex, and cohort (original or offspring). In model B, we additionally adjusted for education and for the following vascular covariates previously associated in FHS with stroke and brain injury: systolic blood pressure, prevalent cardiovascular disease, atrial fibrillation and diabetes mellitus, current smoking status, and apolipoprotein ε4 genotype. In model C, we additionally adjusted for other metabolic covariates that have been related to cognitive outcomes outside FHS: total cholesterol, body mass index (calculated as weight in kilograms divided by height in meters squared), and use of statins. In model D, we also included lifestyle and biomarker measures of CES-D and PAI scores and tHcy, in addition to the covariates in model C. The additional covariates adjusted for in model D were available for all offspring participants but only for participants in the original cohort who attended examination 20. The associations of SNPs contained within the BDNF region with serum BNDF levels were examined using linear regression and their association with the risk for AD was examined using Cox models. Both analyses used additive genetic models and adjusted for age, sex, and occult population stratification and familial relationships within the Framingham cohorts. We additionally examined the association with AD within sex-specific strata (using models adjusted only for age and family relationships).
Results
At baseline, the mean (SD) serum BDNF of the sample was 23 043 (8274) pg/mL, the mean (SD) age was 72 (7) years, and 932 (44%) were men. The baseline characteristics according to BDNF levels are presented in Table 1. Participants with BDNF levels at the bottom 2 quintiles were significantly older, and they were more likely to be males, to have a college degree or higher, and to have a history of atrial fibrillation compared with those with higher BDNF levels. Moreover, they were less likely to be current smokers and their total cholesterol was lower.
Table 1.
Baseline Characteristics
| Characteristic | No. (%) |
P Value |
|
|---|---|---|---|
| Quintiles 1–2 |
Quintiles 3–5 |
||
| Total No. | 852 | 1279 | |
| BDNF, mean (SD), ng/mL | 15 242 (4033) | 28 240 (5972) | |
| Age, mean (SD), y | 72.8 (7.6) | 72.1 (7.5) | .03 |
| Women | 242 (50) | 775 (61) | <.001 |
| College degree | 231 (28) | 296 (24) | .04 |
| Systolic blood pressure, mean (SD), mm Hg | 135 (20) | 136 (20) | .56 |
| Antihypertensive medication | 389 (45.8) | 584 (45.8) | .99 |
| Current smoker | 52 (6) | 115 (9) | .02 |
| Diabetes mellitus | 145 (17) | 185 (15) | .11 |
| Prevalent cardiovascular disease | 192 (23) | 255 (20) | .15 |
| Atrial fibrillation | 77 (9) | 66 (5) | <.001 |
| Total cholesterol, mean (SD), mg/dL | 195 (36) | 204 (37) | <.001 |
| Total homocysteine, median (IQR), µmol/L | 9.0 (4.3) | 9.0 (3.9) | .09 |
| Depression (CES-D score), median (IQR) | 3.0 (6.0) | 3.0 (6.0) | .23 |
| Physical Activity Index, median (IQR) | 35.7 (8.0) | 35.8 (8.2) | .18 |
Abbreviations: BDNF, brain-derived neurotrophic factor; CES-D, Center for Epidemiological Studies Depression Scale; IQR, interquartile range.
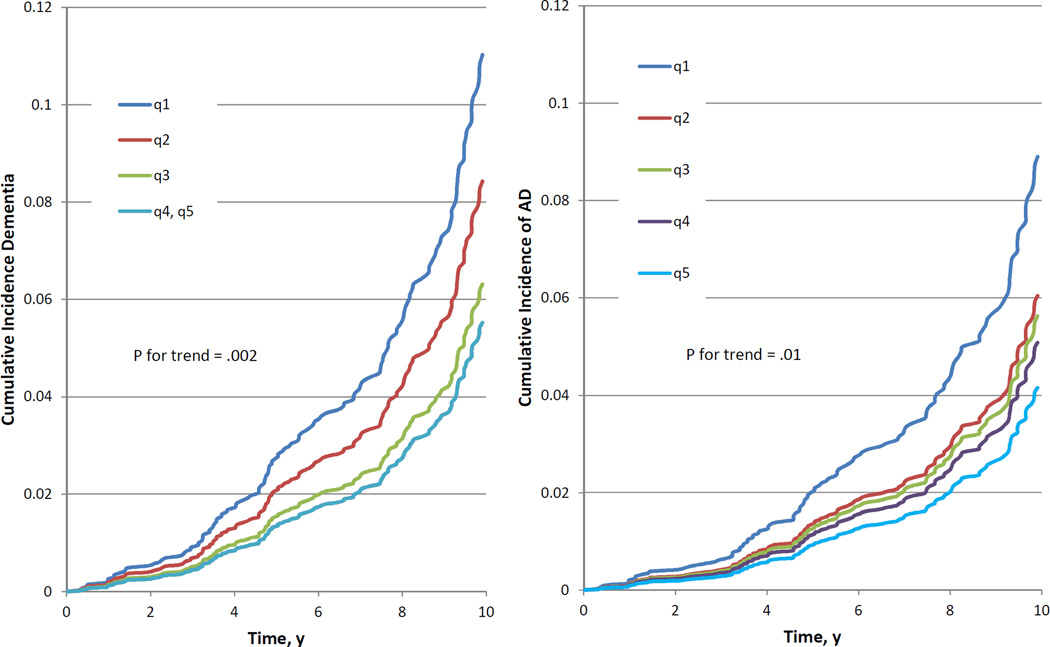
During a median of 10 years of follow-up, 140 participants developed dementia, 117 of whom had AD. After controlling for age, sex, and cohort, each 1-SD increment of serum BDNF level was associated with a 23% lower risk for future dementia (P = .006) and AD (P = .01) (Table 2). There was a significant trend toward a lower risk for dementia and AD with increasing quintiles of BDNF levels (P for trend = .002 and .01 for dementia and AD, respectively), and compared with the lowest quintile, the top quintile of BDNF level was associated with less than half the risk for developing dementia (hazard ratio [HR], 0.49; 95%CI, 0.28–0.85; P = .01) and AD (HR, 0.46; 95% CI, 0.24–0.86; P = .02) (Table 2 and Figure). The results remained significant after further adjustment for education, systolic blood pressure, history of cardiovascular disease, atrial fibrillation, diabetes mellitus, smoking status, and apolipoprotein ε4, as well as for total cholesterol, body mass index, and statin use (Table 3). Additional adjustment for tHcy, CES-D, and PAI resulted in a HR of 0.80 (95% CI, 0.64–0.99; P = .04) for dementia and 0.80 (95%CI, 0.63–1.01; P = .05) for AD (data not tabulated). As previously mentioned, these variables were available only for a subset of participants from the original cohort who attended examination 20 in addition to examination 23, and thus they may be subject to bias.
Table 2.
Association of Serum Brain-Derived Neurotrophic Factor Levels and Risk for Incident Dementia and Alzheimer Diseasea
| Dementia 140/2131 |
Alzheimer Disease 117/2131 |
|||
|---|---|---|---|---|
| Group | Hazard Ratio (95% CI) |
P Value |
Hazard Ratio (95% CI) |
P Value |
| Per SD | 0.77 (0.64–0.93) | .006 | 0.77 (0.63–0.95) | .01 |
| Quintile 1 | 1 [Reference] | 1 [Reference] | ||
| Quintile 2 | 0.75 (0.47–1.20) | .24 | 0.67 (0.39–1.14) | .14 |
| Quintile 3 | 0.56 (0.34–0.92) | .02 | 0.62 (0.37–1.06) | .08 |
| Quintile 4 | 0.49 (0.29–0.81) | .006 | 0.56 (0.33–0.96) | .04 |
| Quintile 5 | 0.49 (0.28–0.85) | .01 | 0.46 (0.24–0.86) | .02 |
| P value for trend | .002 | .01 | ||
Model A: Adjusted for age, sex, and cohort.
Figure.
Cumulative Incidence of Alzheimer Disease by Quintiles of Serum Brain-Derived Neurotrophic Factor Levels
Table 3.
Association Between Serum Brain-Derived Neurotrophic Factor Levels (Per SD) and Risk for Incident Dementia and Alzheimer Disease
| Dementia | Alzheimer Disease | |||
|---|---|---|---|---|
| Model | Hazard Ratio (95% CI) |
P Value |
Hazard Ratio (95% CI) |
P Value |
| Ba | 0.77 (0.63–0.93) | .006 | 0.78 (0.63–0.95) | .02 |
| Cb | 0.77 (0.63–0.94) | .008 | 0.77 (0.63–0.95) | .02 |
Model B: Adjusted for age; sex; cohort; education; systolic blood pressure; history of cardiovascular disease, atrial fibrillation, and diabetes mellitus; smoking status; and apolipoprotein ε4.
Model C: Model B + total cholesterol, body mass index, and statin use.
We found significant interactions of age and sex with BDNF in determining the risk for AD (P = .03 and P = .048 for age and sex, respectively) and for education (college degree or higher vs others) with BDNF in determining both the risks for dementia (P = .003) and AD (P = .01). The associations between BDNF levels and the risk for incident dementia and AD were significant only in women (HR, 0.70; 95%CI, 0.55–0.89; P = .003, and HR, 0.65; 95%CI, 0.50–0.85; P = .001, for dementia and AD, respectively), in people aged 80 years or older (HR, 0.66; 95% CI, 0.50–0.87; P = .003, and HR, 0.63; 95% CI, 0.47–0.85; P = .002, for dementia and AD, respectively), and in participants with a college degree or higher (HR, 0.31; 95% CI, 0.16–0.60; P < .001, and HR, 0.27; 95%CI, 0.11–0.65; P = .003, for dementia and AD, respectively (Table 4, model A). We did not find any interaction between BDNF and smoking status in determining the risk for AD or dementia. These stratum-specific associations remained unchanged after additional adjustment for vascular risk factors, apolipoprotein ε4, and education, where appropriate (Table 4, model B).
Table 4.
Association Between Serum Brain-Derived Neurotrophic Factor Levels and Risk for Incident Dementia and Alzheimer Disease Stratified by Sex, Age, and Educationa,b
| Dementia | Alzheimer Disease | |||
|---|---|---|---|---|
| Variable | Hazard Ratio (95% CI) |
P Value |
Hazard Ratio (95% CI) |
P Value |
| Women | ||||
| Total No. | 86/1199 | 76/1199 | ||
| Model A | 0.70 (0.55–0.89) | .003 | 0.65 (0.50–0.85) | .001 |
| Model B | 0.69 (0.54–0.88) | .003 | 0.65 (0.50–0.84) | .001 |
| Men | ||||
| Total No. | 54/932 | 41/932 | ||
| Model A | 0.88 (0.66–1.18) | .39 | 0.99 (0.72–1.38) | .97 |
| Model B | 0.88 (0.64–1.20) | .42 | 1.03 (0.73–1.47) | .85 |
| <80 y | ||||
| Total No. | 67/1792 | 55/1792 | ||
| Model A | 0.92 (0.70–1.19) | .51 | 0.98 (0.73–1.31) | .88 |
| Model B | 0.92 (0.71–1.19) | .52 | 0.94 (0.71–1.24) | .65 |
| ≥80 y | ||||
| Total No. | 73/339 | 62/339 | ||
| Model A | 0.66 (0.50–0.87) | .003 | 0.63 (0.47–0.85) | .002 |
| Model B | 0.64 (0.49–0.85) | .002 | 0.65 (0.48–0.88) | .005 |
| No college degree | ||||
| Total No. | 120/1554 | 106/1554 | ||
| Model A | 0.83 (0.68–1.01) | .07 | 0.83 (0.67–1.02) | .08 |
| Model B | 0.84 (0.68–1.02) | .08 | 0.83 (0.67–1.03) | .08 |
| College degree | ||||
| Total No. | 18/527 | 11/527 | ||
| Model A | 0.31 (0.16–0.60) | <.001 | 0.27 (0.11–0.65) | .003 |
| Model B | 0.27 (0.13–0.57) | <.001 | 0.26 (0.10–0.69) | .007 |
Model A: Adjusted for age, sex, and cohort.
Model B: Adjusted for age (except for the age stratification); sex (except for the sex stratification); education (except for the education stratification); cohort; systolic blood pressure; history of cardiovascular disease, atrial fibrillation, and diabetes mellitus; smoking status; and apolipoprotein ε4.
There were 133 SNPs contained within the BDNF region and none of them were significantly associated with AD risk, both overall and separately, in men and women (eTable and eFigure 1 in the Supplement) after correction for multiple testing. Moreover, BDNF SNPs explained only a small proportion of the variability in serum BDNF levels (eFigure 2 in the Supplement).
Discussion
In our community-based cohort, dementia-free individuals with higher BDNF levels were less likely to develop dementia and AD, independent of other risk factors. The association between BDNF and the risk for incident dementia and AD was apparent only in women, older persons, and those with at least a college degree.
Our findings add to the previous literature by demonstrating that BDNF is not only reduced in persons with AD,18–20 but may also be reduced in healthy people who are destined to develop dementia or AD. The prodromal phase of AD is long and characterized by cognitive deficits39,40 and structural brain magnetic resonance image changes.41–43 Furthermore, findings of associations between some risk factors for dementia, such as hypertension and obesity, could only be demonstrated in prospective studies where these risk factors were measured at mid-life rather than in cross-sectional studies or when the risk factor was assessed closer to the time when the disease became apparent.44,45 Hence, it is to be expected that if BDNF has an active pathophysiologic role in mediating the association of these risk factors with AD, reduced levels will be already apparent at these early stages. This finding is in accordance with a previous study that observed lower BDNF levels in mild cognitive impairment,46 a condition recognized as a prodromal stage of AD. The fact that BDNF levels predicted dementia and AD independently of putative risk factors further suggests that it may be an active participant in the mechanism underlying these conditions rather than an incidental risk marker.
Little data exists on factors that may modify the association between BDNF levels and the risk for dementia. Sex, age, and smoking status may be associated with BDNF levels,23,24 as well as dementia risk, and thus we examined whether they also modify the association between BDNF levels and the risk for incident dementia/AD. Our finding that high BDNF levels predict lower risk for dementia/AD only in women is consistent with previous studies. In a Japanese sample, polymorphism in Val66Met, a BDNF gene, was associated with AD only in women.47 Other data suggest that plasma BDNF is a biomarker of impaired memory and general cognitive function in aging women but not men.15 These findings may support the hypothesis that BDNF interaction with estrogen,48 other sex hormones,49 or another sex-specific mechanism may underlie the development of AD. The finding of an association only in older individuals may be explained by the decline in BDNF levels with age.23 Because younger individuals have relatively high levels of BDNF, it is possible that their dementia/AD events are due to other processes. Because BDNF plays a role in synaptic plasticity and functional efficiency, it is postulated that it mediates the influence of educational attainment on cognitive reserve. Persons sensitive to the effects of BDNF may achieve greater education but also be more susceptible to the adverse impact of a decline in BDNF with increasing age. Our finding that the risk for dementia and AD is related to BDNF levels only in highly educated people needs to be tested in subsequent studies. If this and the former interactions are confirmed, they may provide insight into some of the complex mechanisms underlying dementia and AD and may set the road for future observational and experimental studies focusing on specific subpopulations. They may also explain inconsistencies between prior reports owing to different distributions of these variables in different study samples and pooled analyses that might obscure real associations in subsamples.
The absence of a significant association between BDNF SNPs and the risk for AD is not inconsistent with the association we show between serum BDNF levels and the risk for AD in women since genetic variation does not appear to explain a substantial proportion of the variation in BDNF levels. Rather, environmental factors, such as mood, diet, and physical activity,3,4 likely alter BDNF levels and BDNF may be a biological intermediate between these lifestyle factors and their impact of AD pathology and risk, a hypothesis that needs further exploration in additional studies.
The strengths of this study were its community-based prospective design, the large dementia-free sample, and the careful surveillance for end points. We were able to adjust for multiple potential confounders; however, we could not exclude the possibility that some other unknown factors may have affected the results. In addition to this limitation, the number of people in some stratified categories was small and the over-whelmingly European origin of the study sample limits the generalizability of our results.
Conclusions
We suggest that serum BDNF may play a role in the development of AD, especially in older women, the group at highest risk for AD. This is of particular interest because serum BDNF levels can be elevated through simple lifestyle measures such as increased physical activity. Brain-derived neurotrophic factor may also serve as a novel predictor of dementia and AD in healthy adults and as a biomarker of dementia risk and prognosis. Finally, BDNF might also have a potential therapeutic effect in AD. This effect could be reached by noninvasive means, such as physical activity and caloric-restricted diet, or by the administration of exogenous BDNF and/or by stimulating its receptor expression.50 Lee et al51 recently showed that inhibition of specific microRNA enhances BDNF protein levels, synaptogenesis, and neurogenesis, as well as improves memory function in mice. They concluded that this inhibitor may be therapeutically effective for AD. Future clinical trials and observational studies should consider the possibilities of a compensation effect in early disease and of an effect modification by various demographic and lifestyle factors.
Supplementary Material
Acknowledgments
Funding/Support: This work received support from the National Heart, Lung and Blood Institute’s Framingham Heart Study (contract no. N01-HC-25195) and grants from the National Institute of Neurological Disorders and Stroke (NS17950); the National Heart, Lung and Blood Association (HL93029, U01HL 096917); and the National Institute of Aging (AG08122, AG16495, AG033193, AG031287, and P30AG013846).
Role of the Sponsor: The funding agencies had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Additional Contributions: This work was supported by the dedication of the Framingham Heart Study participants.
Footnotes
Author Contributions: Drs Beiser and Seshadri had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Weinstein, Beiser, Wolf, DeStefano, Vasan, Seshadri.
Acquisition of data: Weinstein, Beiser, Chen, Vorgias, Au, Pikula, Wolf, Seshadri.
Analysis and interpretation of data: Weinstein, Beiser, Choi, Preis, Chen, Seshadri.
Drafting of the manuscript: Weinstein, Seshadri.
Critical revision of the manuscript for important intellectual content: All authors.
Statistical analysis: Beiser, Choi, Preis, DeStefano.
Obtained funding: Wolf, Seshadri.
Administrative, technical, or material support: Chen, Vorgias, Vasan.
Study supervision: Chen, Seshadri.
Conflict of Interest Disclosures: None reported.
Publisher's Disclaimer: Disclaimer: The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Neurological Disorders and Stroke; the National Heart, Lung and Blood Institute; the National Institute of Aging; or the National Institutes of Health.
REFERENCES
- 1.Seshadri S, Wolf PA. Lifetime risk of stroke and dementia: current concepts, and estimates from the Framingham Study. Lancet Neurol. 2007;6(12):1106–1114. doi: 10.1016/S1474-4422(07)70291-0. [DOI] [PubMed] [Google Scholar]
- 2.Ferri CP, Prince M, Brayne C, et al. Alzheimer’s Disease International. Global prevalence of dementia: a Delphi Consensus Study. Lancet. 2005;366(9503):2112–2117. doi: 10.1016/S0140-6736(05)67889-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee J, Seroogy KB, Mattson MP. Dietary restriction enhances neurotrophin expression and neurogenesis in the hippocampus of adult mice. J Neurochem. 2002;80(3):539–547. doi: 10.1046/j.0022-3042.2001.00747.x. [DOI] [PubMed] [Google Scholar]
- 4.Neeper SA, Gómez-Pinilla F, Choi J, Cotman C. Exercise and brain neurotrophins. Nature. 1995;373(6510):109. doi: 10.1038/373109a0. [DOI] [PubMed] [Google Scholar]
- 5.Vaynman S, Ying Z, Gomez-Pinilla F. Interplay between brain-derived neurotrophic factor and signal transduction modulators in the regulation of the effects of exercise on synaptic-plasticity. Neuroscience. 2003;122(3):647–657. doi: 10.1016/j.neuroscience.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 6.Phillips HS, Hains JM, Laramee GR, Rosenthal A, Winslow JW. Widespread expression of BDNF but not NT3 by target areas of basal forebrain cholinergic neurons. Science. 1990;250(4978):290–294. doi: 10.1126/science.1688328. [DOI] [PubMed] [Google Scholar]
- 7.Wetmore C, Ernfors P, Persson H, Olson L. Localization of brain-derived neurotrophic factor mRNA to neurons in the brain by in situ hybridization. Exp Neurol. 1990;109(2):141–152. doi: 10.1016/0014-4886(90)90068-4. [DOI] [PubMed] [Google Scholar]
- 8.Ghosh A, Carnahan J, Greenberg ME. Requirement for BDNF in activity-dependent survival of cortical neurons. Science. 1994;263(5153):1618–1623. doi: 10.1126/science.7907431. [DOI] [PubMed] [Google Scholar]
- 9.Lindholm D, Carroll P, Tzimagiogis G, Thoenen H. Autocrine-paracrine regulation of hippocampal neuron survival by IGF-1 and the neurotrophins BDNF, NT-3 and NT-4. Eur J Neurosci. 1996;8(7):1452–1460. doi: 10.1111/j.1460-9568.1996.tb01607.x. [DOI] [PubMed] [Google Scholar]
- 10.Alderson RF, Alterman AL, Barde YA, Lindsay RM. Brain-derived neurotrophic factor increases survival and differentiated functions of rat septal cholinergic neurons in culture. Neuron. 1990;5(3):297–306. doi: 10.1016/0896-6273(90)90166-d. [DOI] [PubMed] [Google Scholar]
- 11.Hyman C, Hofer M, Barde YA, et al. BDNF is a neurotrophic factor for dopaminergic neurons of the substantia nigra. Nature. 1991;350(6315):230–232. doi: 10.1038/350230a0. [DOI] [PubMed] [Google Scholar]
- 12.Alonso M, Vianna MR, Depino AM, et al. BDNF-triggered events in the rat hippocampus are required for both short- and long-term memory formation. Hippocampus. 2002;12(4):551–560. doi: 10.1002/hipo.10035. [DOI] [PubMed] [Google Scholar]
- 13.Bekinschtein P, Cammarota M, Izquierdo I, Medina JH. BDNF and memory formation and storage. Neuroscientist. 2008;14(2):147–156. doi: 10.1177/1073858407305850. [DOI] [PubMed] [Google Scholar]
- 14.Erickson KI, Prakash RS, Voss MW, et al. Brain-derived neurotrophic factor is associated with age-related decline in hippocampal volume. J Neurosci. 2010;30(15):5368–5375. doi: 10.1523/JNEUROSCI.6251-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Komulainen P, Pedersen M, Hänninen T, et al. BDNF is a novel marker of cognitive function in ageing women: the DR’s EXTRA Study. Neurobiol Learn Mem. 2008;90(4):596–603. doi: 10.1016/j.nlm.2008.07.014. [DOI] [PubMed] [Google Scholar]
- 16.Peng S, Wuu J, Mufson EJ, Fahnestock M. Precursor form of brain-derived neurotrophic factor and mature brain-derived neurotrophic factor are decreased in the pre-clinical stages of Alzheimer’s disease. J Neurochem. 2005;93(6):1412–1421. doi: 10.1111/j.1471-4159.2005.03135.x. [DOI] [PubMed] [Google Scholar]
- 17.Durany N, Michel T, Kurt J, Cruz-Sanchez FF, Cervas-Navarro J, Riederer P. Brain-derived neurotrophic factor and neurotrophin-3 levels in Alzheimer's disease brains. Int J Dev Neurosci. 2000;18(8):807–813. [PubMed] [Google Scholar]
- 18.Lee JG, Shin BS, You YS, et al. Decreased serum brain-derived neurotrophic factor levels in elderly korean with dementia. Psychiatry Investig. 2009;6(4):299–305. doi: 10.4306/pi.2009.6.4.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Laske C, Stransky E, Leyhe T, et al. BDNF serum and CSF concentrations in Alzheimer’s disease, normal pressure hydrocephalus and healthy controls. J Psychiatr Res. 2007;41(5):387–394. doi: 10.1016/j.jpsychires.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 20.Forlenza OV, Diniz BS, Teixeira AL, et al. Effect of brain-derived neurotrophic factor Val66Met polymorphism and serum levels on the progression of mild cognitive impairment. World J Biol Psychiatry. 2010;11(6):774–780. doi: 10.3109/15622971003797241. [DOI] [PubMed] [Google Scholar]
- 21.Yasutake C, Kuroda K, Yanagawa T, Okamura T, Yoneda H. Serum BDNF, TNF-alpha and IL-1beta levels in dementia patients: comparison between Alzheimer’s disease and vascular dementia. Eur Arch Psychiatry Clin Neurosci. 2006;256(7):402–406. doi: 10.1007/s00406-006-0652-8. [DOI] [PubMed] [Google Scholar]
- 22.O’Bryant SE, Hobson VL, Hall JR, et al. Texas Alzheimer’s Research Consortium. Serum brain-derived neurotrophic factor levels are specifically associated with memory performance among Alzheimer’s disease cases. Dement Geriatr Cogn Disord. 2011;31(1):31–36. doi: 10.1159/000321980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bus BA, Molendijk ML, Penninx BJ, et al. Determinants of serum brain-derived neurotrophic factor. Psychoneuroendocrinology. 2011;36(2):228–239. doi: 10.1016/j.psyneuen.2010.07.013. [DOI] [PubMed] [Google Scholar]
- 24.Bus BA, Tendolkar I, Franke B, et al. Serum brain-derived neurotrophic factor: determinants and relationship with depressive symptoms in a community population of middle-aged and elderly people. World J Biol Psychiatry. 2012;13(1):39–47. doi: 10.3109/15622975.2010.545187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Richards M, Sacker A. Lifetime antecedents of cognitive reserve. J Clin Exp Neuropsychol. 2003;25(5):614–624. doi: 10.1076/jcen.25.5.614.14581. [DOI] [PubMed] [Google Scholar]
- 26.Andel R, Vigen C, Mack WJ, Clark LJ, Gatz M. The effect of education and occupational complexity on rate of cognitive decline in Alzheimer’s patients. J Int Neuropsychol Soc. 2006;12(1):147–152. doi: 10.1017/S1355617706060206. [DOI] [PubMed] [Google Scholar]
- 27.Qiu C, Kivipelto M, von Strauss E. Epidemiology of Alzheimer’s disease: occurrence, determinants, and strategies toward intervention. Dialogues Clin Neurosci. 2009;11(2):111–128. doi: 10.31887/DCNS.2009.11.2/cqiu. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dawber TR, Meadors GF, Moore FE., Jr Epidemiological approaches to heart disease: the Framingham Study. Am J Public Health Nations Health. 1951;41(3):279–281. doi: 10.2105/ajph.41.3.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Feinleib M, Kannel WB, Garrison RJ, McNamara PM, Castelli WP. The Framingham Offspring Study: design and preliminary data. Prev Med. 1975;4(4):518–525. doi: 10.1016/0091-7435(75)90037-7. [DOI] [PubMed] [Google Scholar]
- 30.Seshadri S, Wolf PA, Beiser A, et al. Lifetime risk of dementia and Alzheimer’s disease: the impact of mortality on risk estimates in the Framingham Study. Neurology. 1997;49(6):1498–1504. doi: 10.1212/wnl.49.6.1498. [DOI] [PubMed] [Google Scholar]
- 31.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 32.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34(7):939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 33.Berg L. Clinical Dementia Rating (CDR) Psychopharmacol Bull. 1988;24(4):637–639. [PubMed] [Google Scholar]
- 34.Wolf PA, D’Agostino RB, Belanger AJ, Kannel WB. Probability of stroke: a risk profile from the Framingham Study. Stroke. 1991;22(3):312–318. doi: 10.1161/01.str.22.3.312. [DOI] [PubMed] [Google Scholar]
- 35.Seshadri S, Wolf PA, Beiser AS, et al. Association of plasma total homocysteine levels with subclinical brain injury: cerebral volumes, white matter hyperintensity, and silent brain infarcts at volumetric magnetic resonance imaging in the Framingham Offspring Study. Arch Neurol. 2008;65(5):642–649. doi: 10.1001/archneur.65.5.642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Beekman AT, Deeg DJ, Van Limbeek J, Braam AW, De Vries MZ, Van Tilburg W. Criterion validity of the Center for Epidemiologic Studies Depression scale (CES-D): results from a community-based sample of older subjects in the Netherlands. Psychol Med. 1997;27(1):231–235. doi: 10.1017/s0033291796003510. [DOI] [PubMed] [Google Scholar]
- 37.Kannel WB, Sorlie P. Some health benefits of physical activity: the Framingham Study. Arch Intern Med. 1979;139(8):857–861. [PubMed] [Google Scholar]
- 38.Seshadri S, Fitzpatrick AL, Ikram MA, et al. CHARGE Consortium; GERAD1 Consortium; EADI1 Consortium. Genome-wide analysis of genetic loci associated with Alzheimer disease. JAMA. 2010;303(18):1832–1840. doi: 10.1001/jama.2010.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bäckman L, Jones S, Berger AK, Laukka EJ, Small BJ. Multiple cognitive deficits during the transition to Alzheimer’s disease. J Intern Med. 2004;256(3):195–204. doi: 10.1111/j.1365-2796.2004.01386.x. [DOI] [PubMed] [Google Scholar]
- 40.Elias MF, Beiser A, Wolf PA, Au R, White RF, D’Agostino RB. The preclinical phase of Alzheimer disease: a 22-year prospective study of the Framingham Cohort. Arch Neurol. 2000;57(6):808–813. doi: 10.1001/archneur.57.6.808. [DOI] [PubMed] [Google Scholar]
- 41.Debette S, Beiser A, DeCarli C, et al. Association of MRI markers of vascular brain injury with incident stroke, mild cognitive impairment, dementia, and mortality: the Framingham Offspring Study. Stroke. 2010;41(4):600–606. doi: 10.1161/STROKEAHA.109.570044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tondelli M, Wilcock GK, Nichelli P, De Jager CA, Jenkinson M, Zamboni G. Structural MRI changes detectable up to ten years before clinical Alzheimer's disease. Neurobiol Aging. 2012;33(4):825.e825–825.e836. doi: 10.1016/j.neurobiolaging.2011.05.018. [DOI] [PubMed] [Google Scholar]
- 43.Kaye JA, Swihart T, Howieson D, et al. Volume loss of the hippocampus and temporal lobe in healthy elderly persons destined to develop dementia. Neurology. 1997;48(5):1297–1304. doi: 10.1212/wnl.48.5.1297. [DOI] [PubMed] [Google Scholar]
- 44.Whitmer RA, Sidney S, Selby J, Johnston SC, Yaffe K. Midlife cardiovascular risk factors and risk of dementia in late life. Neurology. 2005;64(2):277–281. doi: 10.1212/01.WNL.0000149519.47454.F2. [DOI] [PubMed] [Google Scholar]
- 45.Kivipelto M, Helkala EL, Laakso MP, et al. Midlife vascular risk factors and Alzheimer’s disease in later life: longitudinal, population based study. BMJ. 2001;322(7300):1447–1451. doi: 10.1136/bmj.322.7300.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sperling RA, Aisen PS, Beckett LA, et al. Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):280–292. doi: 10.1016/j.jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fukumoto N, Fujii T, Combarros O, et al. Sexually dimorphic effect of the Val66Met polymorphism of BDNF on susceptibility to Alzheimer’s disease: new data and meta-analysis. Am J Med Genet B Neuropsychiatr Genet. 2010;153B(1):235–242. doi: 10.1002/ajmg.b.30986. [DOI] [PubMed] [Google Scholar]
- 48.Scharfman HE, MacLusky NJ. Estrogen and brain-derived neurotrophic factor (BDNF) in hippocampus: complexity of steroid hormone-growth factor interactions in the adult CNS. Front Neuroendocrinol. 2006;27(4):415–435. doi: 10.1016/j.yfrne.2006.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kaur P, Jodhka PK, Underwood WA, et al. Progesterone increases brain-derived neurotrophic factor expression and protects against glutamate toxicity in a mitogen-activated protein kinase- and phosphoinositide-3 kinase-dependent manner in cerebral cortical explants. J Neurosci Res. 2007;85(11):2441–2449. doi: 10.1002/jnr.21370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tapia-Arancibia L, Aliaga E, Silhol M, Arancibia S. New insights into brain BDNF function in normal aging and Alzheimer disease. Brain Res Rev. 2008;59(1):201–220. doi: 10.1016/j.brainresrev.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 51.Lee ST, Chu K, Jung KH, et al. miR-206 regulates brain-derived neurotrophic factor in Alzheimer disease model. Ann Neurol. 2012;72(2):269–277. doi: 10.1002/ana.23588. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.