Abstract
Objectives. We used dynamical systems modeling to describe how a prenatal behavioral intervention that adapts to the needs of each pregnant woman may help manage gestational weight gain and alter the obesogenic intrauterine environment to regulate infant birth weight.
Methods. This approach relies on integrating mechanistic energy balance, theory of planned behavior, and self-regulation models to describe how internal processes can be impacted by intervention dosages, and reinforce positive outcomes (e.g., healthy eating and physical activity) to moderate gestational weight gain and affect birth weight.
Results. A simulated hypothetical case study from MATLAB with Simulink showed how, in response to our adaptive intervention, self-regulation helps adjust perceived behavioral control. This, in turn, changes the woman’s intention and behavior with respect to healthy eating and physical activity during pregnancy, affecting gestational weight gain and infant birth weight.
Conclusions. This article demonstrates the potential for real-world applications of an adaptive intervention to manage gestational weight gain and moderate infant birth weight. This model could be expanded to examine the long-term sustainable impacts of an intervention that varies according to the participant’s needs on maternal postpartum weight retention and child postnatal eating behavior.
Public health agencies1,2 advocate preventive interventions among pregnant women, particularly overweight and obese pregnant women (OW/OBPW), to assist women in meeting the Institute of Medicine (IOM) gestational weight gain (GWG) guidelines in an effort to make a long-term impact on the obesity epidemic. Despite this recommendation, nearly 60% of OW/OBPW exceed GWG guidelines,2 which has been shown to independently predict the onset of obesity,2–4 type 2 diabetes,5,6 and cardiovascular diseases7 among women and their offspring. Thus, the prenatal period may be an opportune time to intervene and break the intergenerational cycle of obesity by reducing fetus exposure to an “obesogenic” intrauterine environment8,9 through promoting maternal energy balance (EB). Although the underlying mechanism for how maternal prenatal obesity “programs” fetal development, related metabolic disorders,10,11 and later obesity during childhood and adulthood12–14 remains unclear, it is common to use high birth weight as a surrogate marker for intrauterine growth and as an indicator of the conditions experienced in utero.15
Despite focused prevention efforts, behavioral intervention studies show little to no evidence for preventing excessive GWG among OW/OBPW.16,17 Even more importantly, few, if any, existing GWG interventions have had an impact on rates of high infant birth weight. Thus, there is a critical need to develop effective and efficient interventions to prevent excessive maternal GWG and high infant birth weight. One potential reason for why GWG interventions have had some success among normal weight but not overweight women is that OW/OBPW may have unique barriers that require a higher intervention (i.e., more intensive) dosage to managing GWG than the single dose selected in interventions that rely on a “one size fits all” approach (i.e., fixed, time-invariant intervention). Another reason is that many factors influence GWG including behavioral (EB: energy intake [EI] and physical activity [PA]), psychological (attitude, perceived control, intention), sociodemographic (age, parity), and physical (body mass index [BMI], defined as weight in kilograms divided by the square of height in meters [kg/m2]; fat mass),2 and thus, interventions are needed that consider how changes in these factors influence changes in GWG.
A time-varying (i.e., “just-in-time”), individually tailored intervention that provides each woman, especially OW/OBPW, with the support needed to manage GWG and adapts to her unique needs over time across the pregnancy may be a promising approach to manage GWG and prevent high birth weight. This approach enhances potency and conserves resources (i.e., cost savings associated with delivering only necessary dosages to participants), and thus, it has the potential to increase compliance and improve effectiveness of treatment compared with fixed interventions that may or may not work for individuals depending on their needs.18 We have developed the conceptual framework for such an intervention. Furthermore, we have used control systems engineering principles (in general) and dynamical modeling approaches (in particular) to inform our individually tailored, time-varying GWG intervention that uses decision algorithms (i.e., controllers that will assign the optimized intervention dosage) to increase intervention effectiveness and improve participant outcomes.19,20 However, little is known about how our intervention (or any existing GWG intervention) affects infant birth weight.
The goal of this study was to build on our existing research to discuss how our prenatal intervention not only helps women to manage their gestational weight gain, but also might alter the obesogenic fetus environment to regulate infant birth weight. A simulated hypothetical case study will be presented illustrating the basic workings of this model and demonstrating proof of concept for how self-regulation and adaptive interventions with decision rules influence GWG during pregnancy and, in turn, has an impact on infant birth weight. Exploratory simulations of our adaptive GWG intervention21 will be generated from data based on an intergenerational fetal EB model22 and artificial parameters to examine the effects of creating a healthy maternal–fetus eating and PA environment on infant birth weight.
METHODS
Our behavioral intervention includes several components informed by past research and pilot data23–30 (i.e., education, goal-setting, self-monitoring, and healthy eating [HE] and PA active learning) and intervenes on mechanisms of EB and dynamical models of planned behavior and self-regulation to influence prenatal GWG. Specifically, experimental31 and intervention studies21,26,27,29 show that when people are taught how to set appropriate goals; problem-solve to overcome barriers; self-monitor GWG, HE, and PA; and manage their time, they are more likely to achieve their goals and see positive behavioral outcomes (e.g., engage in HE and PA, manage weight). Furthermore, active participation in HE strategies to reduce dietary energy density and increase fruit and vegetable consumption (e.g., portion size or control, reading food labels, weighing food, meal preparation and planning demonstrations, and meal replacements)27,32–34 and face-to-face onsite PA training26,29 are effective for lowering energy intake, increasing PA, decreasing body weight, and increasing weight loss—all of which have been integrated into the active learning component.
Different dosages of these intervention components are assigned across time in response to the needs of each woman much like clinical practice.18,35 Decision rules are used to define changes in the intervention (i.e., when and how much to adapt intervention dosages). This dosage level is based on variables that are expected to have an impact on the effect of the “tailoring variable,” in this case, GWG, and the level of intervention that is required to address the needs of the individual. In this intervention, the tailoring variable (GWG) is frequently assessed (daily) so the intervention can be adjusted on an ongoing basis (every 4 weeks) to determine if a woman is within her GWG goal. These decision rules are informed by IOM guidelines2 and clinical insight.
Dynamical Systems Model
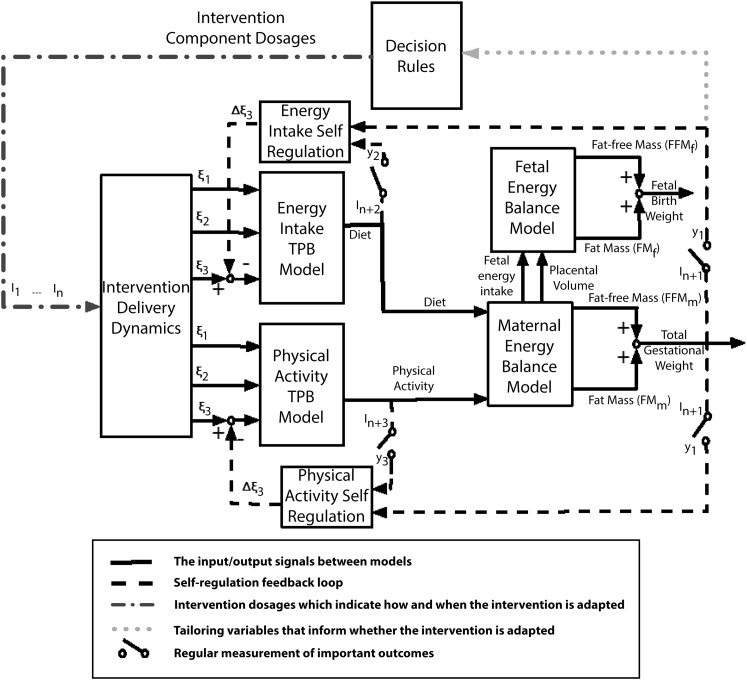
To improve the understanding of the dynamic response associated with our behavioral intervention (i.e., how changes in GWG and fetal growth respond to changes in EI and physical activity levels [PAL] in a system of EB and behavioral models),21,22,36 we will apply principles and methods from systems science and engineering37 including dynamical modeling, simulation, and controller design. The structure of the dynamical systems model is described and shown in Figure 1. The model is implemented by using the MATLAB with Simulink software package version R2012a (The MathWorks Inc, Natick, MA).
FIGURE 1—
Schematic representation for an adaptive fetal birth weight and gestational weight gain intervention.
Note. EI = energy intake; FFMf = fetal fat-free mass; FFMm = mother’s fat-free mass; FMf = fetal fat mass; FMm = mother’s fat mass; GWG = gestational weight gain; PA = physical activity; TPB = theory of planned behavior. Solid lines are the input or output signals between models. The dashed lines represent the signals influencing self-regulation loop. The dotted line represents the tailoring variable, which is used by decision rules to inform whether the intervention is adapted or not. The alternating dots and dashes line stands for the dosages of intervention components that are generated by decision rules based on the participant’s past performance and measurement of tailoring variables. The outputs of intervention delivery dynamics serve as the inputs to EI–TPB and PA–TPB models, which indirectly influence GWG and fetal birth weight through energy balance models, and decision rules dictate if the intervention is adapted or stays in course according to the tailoring variable (GWG).
The model presented here is an extension of the adaptive GWG intervention developed in Dong et al.21 This expanded model includes the fetal energy model,22 which is based on the first law of thermodynamics and incorporated maternal dietary intake and PAL to capture how they will affect fetal birth weight. This model is divided into 5 main segments:
1. maternal and fetal EB models predicting changes in body mass as a result of EI and PA,
2. 2 theory of planned behavior (TPB) models describing how maternal EI and PA are affected by behavioral variables,
3. 2 self-regulation modules outlining how success expectances during the intervention influence a participant’s motivation to achieve a goal,
4. an intervention delivery module that relates the magnitude or duration of intervention components to the inputs of the TPB models, and
5. decision rules outlining when or how to adapt intervention dosages for each OW/OBPW and enabling tailoring of the intervention to the specific needs of each woman.
Dynamical models corresponding to the behavioral theories are based on the concept of fluid analogies.38 Insight gained from simulation include a time course of the intervention and an evaluation of the decision rules as reflected by multiple “just in time” augmentations (i.e., active learning, PA, and self-monitoring adaptions occurring at different points throughout the intervention in response to decision rules) that are calculated in a systematic, operationalized manner. This dynamical systems model can be further utilized as the basis for designing and achieving a fully optimized behavioral intervention for managing GWG relying on a modern control engineering paradigm known as model predictive control20; accomplishing this represents one of the ultimate goals of this research.
Our intervention is conceptually based on multiple adaptations (i.e., “step-up” in dosages); however, for ease of presentation, we summarize 3 possible adaptations in Table 1. The baseline intervention is standard of care plus daily GWG, HE, and PA self-monitoring, plus 1 face-to-face session per week with an instructor who will provide goal-setting guidance, deliver education, and use weekly HE and PA plans to provide tailored meals to meet calorie goals and safe PA to meet PA guidelines. Women will be given a Wi-Fi scale for wireless uploading of daily weight, a scale for measuring food, an activity monitor to assess movement and sleep, and will be asked to record foods and beverages consumed (and amounts) by using the online Super Tracker System. The adaptations used to increase the frequency and dosage of the intervention over the course of pregnancy include, for example, HE meal preparation, portion size, and menu planning demonstrations; meal replacements; guided PA sessions with an instructor; electronic instructor feedback and support; and e-health booster messages (video, text, e-mail).
TABLE 1—
Summary of Dosage Augmentations per the If–Then Decision Rule on Gestational Weight Gain
| Options | Adaptation |
| Baseline intervention | NA |
| Step up 1 | Baseline + hands-on healthy eating demonstration guided by an instructor |
| Step up 2 | Baseline + 1 + 30 min of moderate physical activity guided by an instructor |
| Step up 3 | Baseline + 1 + 2 + daily electronic (e.g., texting or social media) feedback for motivational support |
Note. NA = not applicable. Baseline intervention includes self-monitoring, education, and guidance.
Maternal and Fetal Energy Balance Model
The maternal and fetal EB model is based on the 2-compartment model.36 It relies on the conservation of energy principle in
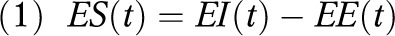 |
where ES(t) is the energy stored, EI(t) is the energy intake, and EE(t) is the energy expenditure at time t, measured daily. The ES(t) term can be expanded into the sum of the instantaneous change of the 2 compartments: fat-free mass (FFM) and fat mass (FM), multiplied by their respective energy densities (λFFMm and λFMm). Both mother’s body mass and fetal birth weight correspond to the sum of its 2 compartments, respectively. The maternal EB model can be described in
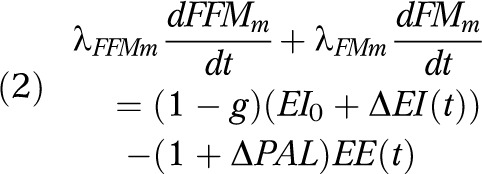 |
where λFFMm = 771 kilocalories per kilogram and λFMm = 9500 kilocalories per kilogram, g = 0.03, which is the nutrient partitioning constant; ΔEI(t) and ΔPAL(t) represent the change of EI and PAL, respectively.
The fetal EB model22 can be expressed in a similar manner:
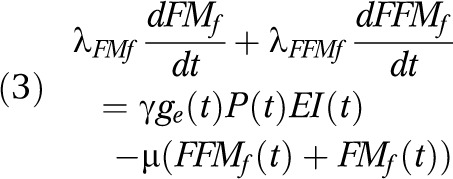 |
where λFFMf = 670 kilocalories per kilogram and λFMf = 9500 kilocalories per kilogram, the first term on the right side is the formulation of fetal energy intake, EIf(t), as a product of the total maternal calories consumed, EI(t), per day, percentage factor of daily glycemic index of maternal diet ge(t) and placental volume P(t). In this equation 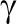 = 0.000234, which is introduced as a conversion constant measured in 1 mL−1. Both ge(t) and P(t) are functions of mother’s PAL. The second term on the right side in equation 3 is fetal energy expenditure, EEf(t), which is modeled with μ as the proportion that fetal body mass contributes to energy expenditure (μ = 32 kcal/kg/d). The change of mother’s EI and PAL during the intervention will influence fetal EI, placental volume, and ultimately fetal FM and birth weight.
= 0.000234, which is introduced as a conversion constant measured in 1 mL−1. Both ge(t) and P(t) are functions of mother’s PAL. The second term on the right side in equation 3 is fetal energy expenditure, EEf(t), which is modeled with μ as the proportion that fetal body mass contributes to energy expenditure (μ = 32 kcal/kg/d). The change of mother’s EI and PAL during the intervention will influence fetal EI, placental volume, and ultimately fetal FM and birth weight.
Theory of Planned Behavior
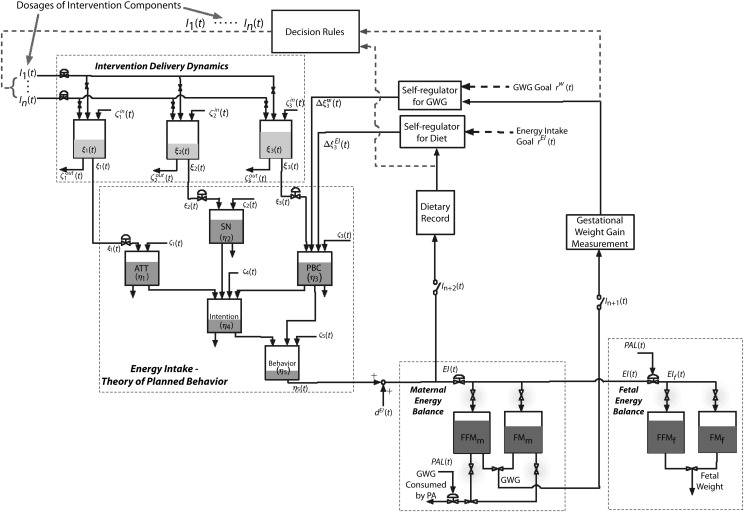
The TPB39 is a social–cognitive theory that describes the behavioral component of our intervention. It assumes that one’s positive or negative evaluations of a behavior (attitude η1), perceived pressures from others to perform a behavior (subjective norm η2), and personal resources or ability (perceived behavioral control [PBC] η3) influences the intention or motivation, η4, of an individual. Intention and PBC, in turn, influence behavior, η5.38 A dynamic TPB model can be postulated as 5 inventories, with each medium gray tank representing 1 component of TPB depicted in Figure 2. How full or empty the tank is indicates the value of each component for that particular individual (e.g., social norms, PBC). Figure 2 shows the comprehensive fluid analogy and interrelationship between systems for the EI loop (see EI–TPB box), with inflows corresponding to exogenous variables ξ1, ξ2, and ξ3 (i.e., dosage of each intervention component influencing EI and PA).40,41 The dynamical model of the intention inventory, for example, can be generated by applying the principle of conservation of mass:
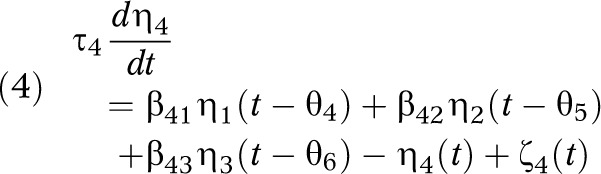 |
FIGURE 2—
The adaptive fetal birth weight and gestational weight gain intervention for the energy intake loop, illustrated as a fluid analogy by using the concept of a network of production inventory systems, very much akin to systems found in process control.
Note. ATT = attitude; EI = energy intake; FFM = fat-free mass; FM = fat mass; PAL = physical activity level; PBC = perceived behavioral control; SN = subjective norm; TPB = theory of planned behavior. These types of systems usually use “tanks” to depict the process.40,41 The level of each tank (i.e., how full or empty each tank is) is the indicator of the value for that variable. In this figure, the dark gray tanks are maternal and fetal energy balance models, the light gray tanks are intervention delivery dynamics, and the middle gray tanks represent EI–TPB. In EI–TPB model, how full the tank is indicates the value of each component in TPB for that particular individual.
where βij represent gains of the system, τ4 and θi are time constants and delay, and ζ4 is disturbance.38 The left side of equation 4 is the accumulation of the intention inventory; the first 3 terms on the right side are the inflows, the fourth term is the outflow, and the last term is the disturbance. The dynamical model (i.e., attitude, subjective norm, PBC, and behavior) can be obtained similarly for the other inventories.
Self-Regulation Theory
Self-regulation theory42 assumes that human behavior is goal-directed and regulated by feedback control processes. Individuals tend to engage in activities in which they believe they can succeed; this confidence in performance success will influence the PBC inflow, which reflects the individual’s perception of her ability to perform a given behavior. As a result, self-monitoring and goal setting are core strategies of behavior modification (i.e., used to increase healthy eating and PA to promote weight management).
Thus, in our adapted GWG and fetal birth weight intervention, self-regulation is implemented as a controller20,21 that adjusts the PBC inflows to the TPB models on the basis of the discrepancies between the reference values and tailoring variable (i.e., measured GWG), which is shown by the 2 loops on the right side of Figure 2. The tuning parameters in the self-regulatory controller allow flexibility to describe different responses of participant’s self-regulation.20,21
The intervention delivery module that is depicted in Figure 2 relates the magnitude and duration of the intervention components to the inflows of the TPB models and considers that we treat the dosage of each intervention component as contributing to the inflows to EI–TPB and PA–TPB models. The effects of the intervention on these behavioral variables that constitute the inflows to TPB models accumulate and, hence, integration is required.
Decision Rules
In an adaptive, time-varying intervention, decision rules operationalize the changes to the frequency and intensity of the intervention dosage by eliminating, adding, or altering the dosage of existing components (e.g., in our example, increasing the number of PA sessions to manage GWG) based on the changing needs of the participants during the intervention.18 In this article, if–then decision rules acting on values of tailoring variables obtained from a dynamical systems model illustrate how the adaptive intervention works for managing GWG and regulating infant birth weight throughout pregnancy.
The decision rules (Table 1) that were developed by our research team in a National Institutes of Health–funded study will be used to evaluate GWG in 4-week cycles. If a woman is within her GWG goal, the intervention dosage will be sustained. If she is exceeding her GWG goal, a more intensive intervention is needed to manage her GWG and, therefore, the intervention dosage is adapted (i.e., “stepped up”) to increase potency (Table 1).
Simulations
The simulations in this section are based on a hypothetical 25-year-old female with pregravid body mass 75 kilograms (165 pounds), 160 centimeters in height, which classifies her as overweight (BMI = 29.3). We selected maternal age by using 2010 data from the Centers for Disease Control and Prevention illustrating mean age of mother at first birth is 25.4 years.43 In both the intervention and nonintervention treatment, we assumed age of gestation at time of delivery to be 40 weeks. We posited that, with no intervention, this woman will increase her EI starting from day 35, with the rate of EI increase slowed in the second and third trimester but still above her estimated energy requirements throughout pregnancy (i.e., she will gradually consume slightly more calories throughout her second and third trimesters). The participant is sedentary at the time of conception (PAL = 1.65) and potentially engaged in less PA from the second to third trimester as she gains weight. The intervention can help improve her PAL during pregnancy. The model parameters in the behavioral models are summarized in the material available as a supplement to the online version of this article at http://www.ajph.org.
The proposed hypothetical intervention aims to help the participant manage her GWG within the IOM guidelines and prevent high infant birth weight. The case study assumes the participant enters the intervention with the baseline program at gestational week 14 (day 98) and she starts engaging in self-regulatory behaviors (e.g., weighing herself to monitor GWG, monitoring EI and PAL). The dosage of the intervention components is adapted every 4 weeks on the basis of decision rules of whether she is meeting or not meeting her GWG goal until week 37 (day 260). In the example here, as shown in Table 1, we focused on the augmentation of 2 of the intervention components previously described in Dong et al.,21 engaging in HE (step up 1) and PA behaviors (step up 2).
RESULTS
Figure 3 shows 2 hypothetical simulation scenarios for a 25-year-old overweight woman with predicted maternal weight gain, EI, PAL, and fetal weight gain for the case study described previously when she (1) receives our adaptive intervention and (2) does not receive our intervention. In both simulations, during the first trimester, we assumed this overweight woman will increase her EI as she is aware of her pregnancy and she will remain sedentary with little or no activity (PAL is 1.65) throughout the first trimester.
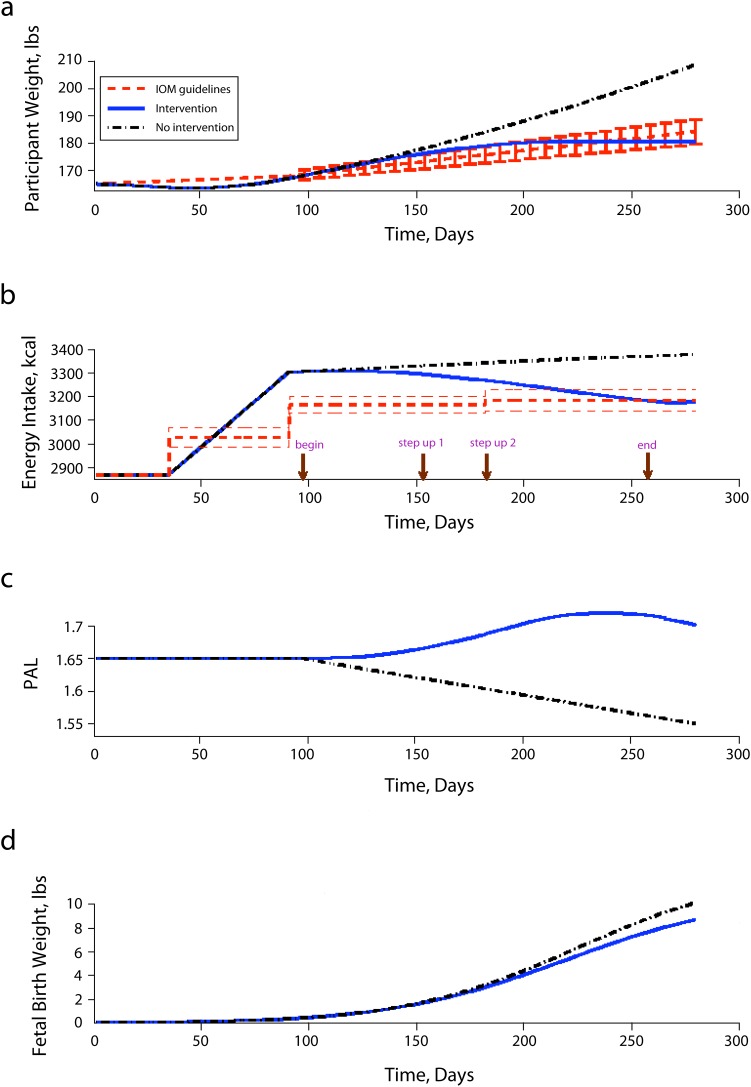
FIGURE 3—
Simulation responses for (a) maternal body mass, (b) energy intake, (c) physical activity level, (d) and fetal birth weight.
Note. IOM = Institute of Medicine; PAL = physical activity level. Red lines represent the 2009 Institute of Medicine guidelines applied on a daily basis, the blue solid lines represent the case with intervention and self-regulation, and the black dashed lines represent the case with no intervention.
In the first scenario, the intervention starts around day 100 with the participant receiving the baseline intervention. At this dose of intervention, the participant still has high EI leading to GWG above the IOM guidelines at the time of her second assessment cycle. Thus, her intervention dosage is increased. Once the intervention is adapted, she gradually lowers her EI. Meanwhile, the intervention also forces her to be involved in PA via face-to-face PA sessions with an instructor, leading to the increase of her PAL from sedentary (PAL = 1.65) at the start of the intervention to moderately active (PAL = 1.70) around day 196 (i.e., the start of her third trimester). The highest PAL that she achieves is 1.721 around day 230 (week 32), maintaining this level of activity until day 245 (week 35). Around this time, she starts to reduce her PAL slightly to 1.702 as most women (even those participating in interventions) tend to decrease their PA in the third trimester as they approach delivery. However this final value of PAL is not only still above the initial PAL 1.65, but is also in the moderately active PAL range.
Because this intervention is adapted to this woman’s specific needs, her EI decreases and PA increases during the intervention, and her rate or speed of weight gain (pounds) slows in the second trimester. As a result, with participation in the individually tailored, adaptive intervention, she meets her GWG goal at day 180, and keeps her EI within the IOM guidelines at day 210. At time of delivery, she meets the IOM guidelines for GWG based on her prepregnancy classification of overweight, gaining a total of 16 pounds.2 Furthermore, by modifying the intrauterine dietary intake and PA environment, her infant is born at 40 weeks gestation at 8.718 pounds, which is within the range of normal and healthy birth weight.
The simulation result for the no-intervention case shows that, without intervention, this same woman gradually increases her EI from the start of her second trimester (3300 kilocalories) to the end of her third trimester (3380 kilocalories). Without receiving an intervention, she increases her EI by about 500 kilocalories during pregnancy, which is about 150 kilocalories higher than recommended during the third trimester for an overweight (almost obese) woman. Also, from the beginning of the second to end of her third trimester, her PAL decreases from 1.65 to 1.55, making her even more sedentary. Without intervention, she gains 43 pounds and exceeds the GWG guidelines of the IOM. Furthermore, because of excessive GWG (high EI and low PA), this mother delivers an infant weighing 10.18 pounds. This infant would be considered macrosomic (birth weight ≥ 90% for gestational age), which has negative health outcomes for both mother and infant, especially for those born weighing more than 9 pounds 15 ounces as in this case.
DISCUSSION
The incorporation of intervention dosage adaptations via decision rules with our comprehensive dynamical model to simulate an intergenerational GWG intervention that adapts to the needs of each OW/OBPW could have substantial effects on maternal–fetus eating and PA environment, GWG, and infant birth weight. The results from our case study simulations showed how, in response to our intervention, self-regulation helps adjust PBC, which consequently changes the woman’s intention and ultimately behavior with respect to HE and PA during pregnancy, thereby having an impact on both GWG and infant birth weight. From these hypothetical simulations, we can better understand that OW/OBPW may need adaptive interventions that tailor to their specific needs to create a healthy intrauterine environment in an effort to meet GWG goals, which, in turn, moderates infant birth weight. Specifically in our simulation, after receiving 2 of the 3 potential adaptations, this woman meets her GWG goal, delivering a healthy-sized baby whereas the woman who did not receive our intervention gains excessive weight and delivers a macrosomic (large for gestational age) baby.
Based on the simulation, in addition to influencing GWG and infant birth weight, a strength of our individually tailored, adaptive, behavioral intervention is that the lifestyle changes made during the prenatal period may be sustainable postpartum and, thus, potentially influence the environment the child will enter after birth. As a result, our prenatal intervention may have long-term impacts on maternal postpartum weight retention as well as child food acceptance, intake, and weight status. Extensive evidence suggests that maternal prenatal and postnatal food choices provide the fetus and infant with very early experiences with food flavors via both amniotic fluid and breast milk.24,44–47 These early experiences provide a “flavor bridge” that can begin to familiarize the fetus and infant with flavors of the maternal diet, depending on the variety of the mother’s diet, thereby promoting later infant acceptance of those foods during the transition to solids and later in life.24,44–47 On the basis of these findings, we hypothesized that our proposed behavioral intervention, which is designed to create a healthy intrauterine food environment, may also have sustainable effects on the postnatal food environment and ultimately infant food acceptance. As a result, our behavioral intervention may influence infant intake of commonly rejected foods, namely vegetables, thereby further reducing risk for controlling feeding practices that have been associated with childhood obesity. We aim to test this assumption in future research.
This article represents exploratory simulation work in which we have generated results from data based on the EB model described in Thomas et al.22 and artificial parameters. Thus, an observational trial or experiment is needed to fully validate this model and allow it to achieve its full usefulness (e.g., the fact that this model can enable intervention optimization through control system design techniques from engineering). What we have illustrated in this article points to an important application of the dynamical model. Collecting intensive observational trial data will allow us to validate our proof of concept; evaluate decision rules that enable time-varying, adaptive interventions; and ultimately lead to the development of hybrid model predictive control algorithm for this problem to act as optimal decision policies to achieve the automated dosage as described in Dong et al.20
In conclusion, this study demonstrates the potential for real-world applications of an adaptive intervention to manage GWG in OW/OBPW and moderate infant birth weight and provides a plausible “proof of concept” of our approach. The ultimate goal is to validate this simulation by examining the effectiveness of a real-life implementation of our intervention on both GWG and infant birth weight (i.e., short-term effects). This would also allow us to get parameter estimates for our model and use control systems engineering approaches to optimize our adaptive time-varying interventions by using a predictive controller to assign dosages of each intervention component. Lastly, our model could be expanded to examine the long-term sustainable effects of individually tailored, adaptive interventions on the eating and PA environments of mothers and their offspring. Specifically, future extensions of our model might include the impact of changes in percentage breastfeeding and use of controlling feeding practices on postpartum weight retention and child weight.
Acknowledgments
Support for this work has been provided by the Office of Behavioral and Social Sciences Research of the National Institutes of Health and the National Institute on Drug Abuse through grants R21 DA024266, K25 DA021173, and P50 DA010075-14. In addition, support for this work was provided by the National Heart, Lung, and Blood Institute of the National Institutes of Health through grant R01 HL119245-01.
We appreciate the important contributions of Diana M. Thomas for the development of the maternal and fetal energy balance equations predicting gestational weight gain and birth weight, and Linda M. Collins for reviewing and providing valuable input when we were preparing this article.
Human Participant Protection
A simulated hypothetical case study is presented; thus, institutional review board approval was not required.
References
- 1.Wojcicki JM, Heyman MB. Let’s Move—childhood obesity prevention from pregnancy and infancy onward. N Engl J Med. 2010;362(16):1457–1459. doi: 10.1056/NEJMp1001857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Institute of Medicine and National Research Committee to Reexamne IOM Pregnancy Guidelines. Weight Gain During Pregnancy: Reexamining the Guidelines. Washington, DC: National Academic Press; 2009. [Google Scholar]
- 3.Gunderson EP, Abrams B. Epidemiology of gestational weight gain and body weight changes after pregnancy. Epidemiol Rev. 1999;21(2):261–275. doi: 10.1093/oxfordjournals.epirev.a018001. [DOI] [PubMed] [Google Scholar]
- 4.Amorim AR, Rossner S, Neovius M, Lourenco PM, Linne Y. Does excess pregnancy weight gain constitute a major risk for increasing long-term BMI? Obesity (Silver Spring) 2007;15(5):1278–1286. doi: 10.1038/oby.2007.149. [DOI] [PubMed] [Google Scholar]
- 5.Siega-Riz AM, Viswanathan M, Moos MKet al. A systematic review of outcomes of maternal weight gain according to the Institute of Medicine recommendations: birthweight, fetal growth, and postpartum weight retention Am J Obstet Gynecol 20092014339e1–e14 [DOI] [PubMed] [Google Scholar]
- 6.Rooney BL, Schauberger CW, Mathiason MA. Impact of perinatal weight change on long-term obesity and obesity-related illnesses. Obstet Gynecol. 2005;106(6):1349–1356. doi: 10.1097/01.AOG.0000185480.09068.4a. [DOI] [PubMed] [Google Scholar]
- 7.Rasmussen KM, Abrams B. Gestational weight gain and later maternal health: are they related? Am J Clin Nutr. 2011;93(6):1186–1187. doi: 10.3945/ajcn.111.016758. [DOI] [PubMed] [Google Scholar]
- 8.Patti ME. Intergenerational programming of metabolic disease: evidence from human populations and experimental animal models. Cell Mol Life Sci. 2013;70(9):1597–1608. doi: 10.1007/s00018-013-1298-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Adamo KB, Ferraro ZM, Brett KE. Can we modify the intrauterine environment to halt the intergenerational cycle of obesity? Int J Environ Res Public Health. 2012;9(4):1263–1307. doi: 10.3390/ijerph9041263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Catalano PM, Ehrenberg HM. The short- and long-term implications of maternal obesity on the mother and her offspring. BJOG. 2006;113(10):1126–1133. doi: 10.1111/j.1471-0528.2006.00989.x. [DOI] [PubMed] [Google Scholar]
- 11.Chandler-Laney P, Bush N. Maternal obesity, maternal health, and prenatal programming of offspring obesity. Open Obesity J. 2011;3:42–50. [Google Scholar]
- 12.Reilly JJ, Armstrong J, Dorosty AR et al. Early life risk factors for obesity in childhood: cohort study. BMJ. 2005;330(7504):1357. doi: 10.1136/bmj.38470.670903.E0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martin JA, Hamilton BE, Ventura SJ et al. Births: final data for 2009. Natl Vital Stat Rep. 2011;60(1):1–70. [PubMed] [Google Scholar]
- 14.Nohr EA, Vaeth M, Baker JL, Sorensen TI, Olsen J, Rasmussen KM. Pregnancy outcomes related to gestational weight gain in women defined by their body mass index, parity, height, and smoking status. Am J Clin Nutr. 2009;90(5):1288–1294. doi: 10.3945/ajcn.2009.27919. [DOI] [PubMed] [Google Scholar]
- 15.Fallucca S, Vasta M, Sciullo E, Balducci S, Fallucca F. Birth weight: genetic and intrauterine environment in normal pregnancy. Diabetes Care. 2009;32(12):e149. doi: 10.2337/dc09-1489. [DOI] [PubMed] [Google Scholar]
- 16.Thangaratinam S, Rogozinska E, Jolly K et al. Effects of interventions in pregnancy on maternal weight and obstetric outcomes: meta-analysis of randomised evidence. BMJ. 2012;344:e2088. doi: 10.1136/bmj.e2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Muktabhant B, Lumbiganon P, Ngamjarus C, Dowswell T. Interventions for preventing excessive weight gain during pregnancy. Cochrane Database Syst Rev. 2012;4:CD007145. doi: 10.1002/14651858.CD007145.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Collins LM, Murphy SA, Bierman KL. A conceptual framework for adaptive preventive interventions. Prev Sci. 2004;5(3):185–196. doi: 10.1023/b:prev.0000037641.26017.00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rivera DE, Pew MD, Collins LM. Using engineering control principles to inform the design of adaptive interventions: a conceptual introduction. Drug Alcohol Depend. 2007;88(suppl 2):S31–S40. doi: 10.1016/j.drugalcdep.2006.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dong Y, Rivera DE, Downs DS, Savage JS, Thomas DM, Collins LM. Hybrid model predictive control for optimizing gestational weight gain behavioral interventions. Proc Am Control Conf. 2013:1970–1975. doi: 10.1109/acc.2013.6580124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dong Y, Rivera DE, Thomas DM et al. A dynamic systems model for improving gestational weight gain behavioral interventions. Proc Am Control Conf. 2012:4059–4064. doi: 10.1109/acc.2012.6315424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thomas DM, Clapp JF, Shernce S. A foetal energy balance equation based on maternal exercise and diet. J R Soc Interface. 2008;5(21):449–455. doi: 10.1098/rsif.2007.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heerwagen MJ, Miller MR, Barbour LA, Friedman JE. Maternal obesity and fetal metabolic programming: a fertile epigenetic soil. Am J Physiol Regul Integr Comp Physiol. 2010;299(3):R711–R722. doi: 10.1152/ajpregu.00310.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Knowler WC, Fowler SE, Hamman RF et al. The Diabetes Prevention Program Research Group. 10-year follow-up of diabetes and weight loss in the Diabetes Prevention Program Outcomes Study. Lancet. 2009;374(9702):1677–1686. doi: 10.1016/S0140-6736(09)61457-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bray G, Gregg E, Haffner S et al. Baseline characteristics of the randomised cohort from the Look AHEAD (Action for Health in Diabetes) study. Diab Vasc Dis Res. 2006;3(3):202–215. doi: 10.3132/dvdr.2006.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Downs DS. Determinants and outcomes of physical activity in pregnancy: findings from Active Moms, a randomized physical activity intervention for pregnant women. Ann Behav Med. 2011;41:S135. [Google Scholar]
- 27.Ello-Martin JA, Roe LS, Ledikwe JH, Beach AM, Rolls BJ. Dietary energy density in the treatment of obesity: a year-long trial comparing 2 weight-loss diets. Am J Clin Nutr. 2007;85(6):1465–1477. doi: 10.1093/ajcn/85.6.1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Savage JS, Marini M, Birch LL. Dietary energy density predicts women’s weight change over 6 y. Am J Clin Nutr. 2008;88(3):677–684. doi: 10.1093/ajcn/88.3.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Downs DS, DiNallo JM, Rauff EL, Ulbrecht JS, Birch LL, Paul IM. Pregnant women’s exercise motivation and behavior: preliminary findings from a randomized physical activity intervention. J Sport Exerc Psychol. 2010;32:S157–S158. [Google Scholar]
- 30.Phelan S, Jankovitz K, Hagobian T, Abrams B. Reducing excessive gestational weight gain: lessons from the weight control literature and avenues for future research. Womens Health (Lond Engl) 2011;7(6):641–661. doi: 10.2217/whe.11.70. [DOI] [PubMed] [Google Scholar]
- 31.Downs DS, Singer RN. Goal setting and implementation intentions: preliminary support for increasing exercise behavior. J Hum Mov Stud. 2003;45(5):419–432. [Google Scholar]
- 32.Ledikwe JH, Rolls BJ, Smiciklas-Wright H et al. Reductions in dietary energy density are associated with weight loss in the PREMIER trial. Am J Clin Nutr. 2007;85(5):1212–1221. doi: 10.1093/ajcn/85.5.1212. [DOI] [PubMed] [Google Scholar]
- 33.Flechtner-Mors M, Ditschuneit HH, Johnson TD, Suchard MA, Adler G. Metabolic and weight loss effects of long-term dietary intervention in obese patients: four-year results. Obes Res. 2000;8(5):399–402. doi: 10.1038/oby.2000.48. [DOI] [PubMed] [Google Scholar]
- 34.Ditschuneit HH, Flechtner-Mors M, Johnson TD, Adler G. Metabolic and weight-loss effects of a long-term dietary intervention in obese patients. Am J Clin Nutr. 1999;69(2):198–204. doi: 10.1093/ajcn/69.2.198. [DOI] [PubMed] [Google Scholar]
- 35.Kumar S, Nilsen W, Pavel M, Srivastava M. Mobile health: revolutionizing healthcare through transdisciplinary research. Computer. 2013;46(1):28–35. [Google Scholar]
- 36.Thomas DM, Navarro-Barrientos JE, Rivera DE et al. Dynamic energy-balance model predicting gestational weight gain. Am J Clin Nutr. 2012;95(1):115–122. doi: 10.3945/ajcn.111.024307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Collins LM, Murphy SA, Strecher V. The multiphase optimization strategy (MOST) and the sequential multiple assignment randomized trial (SMART): new methods for more potent eHealth interventions. Am J Prev Med. 2007;32(5, suppl):S112–S118. doi: 10.1016/j.amepre.2007.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Navarro-Barrientos JE, Rivera DE, Collins LM. A dynamical model for describing behavioural interventions for weight loss and body composition change. Math Comput Model Dyn Syst. 2011;17(2):183–203. doi: 10.1080/13873954.2010.520409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ajzen I. Attitude, Personality, and Behavior. Berkshire, England: Open University Press; 2005. [Google Scholar]
- 40.Schwartz JD, Rivera DE. A process control approach to tactical inventory management in production-inventory systems. Int J Prod Econ. 2010;125(1):111–124. [Google Scholar]
- 41.Schwartz JD, Wang W, Rivera DE. Optimal tuning of process control-based decision policies for inventory management in supply chains. Automatica. 2006;42:1311–1320. [Google Scholar]
- 42.Carver CS, Scheier MF. On the Self-Regulation of Behavior. Cambridge, England: University of Cambridge; 1998. [Google Scholar]
- 43.Martin JA, Hamilton BE, Ventura MA, Osterman MJ, Wilson EC, Mathews TJ. Hyattsville, MD: US Department of Health and Human Services, Centers for Disease Control and Prevention; 2012. Births: final data from 2010. [PubMed] [Google Scholar]
- 44.Schaal B, Marlier L, Soussignan R. Human foetuses learn odours from their pregnant mother’s diet. Chem Senses. 2000;25(6):729–737. doi: 10.1093/chemse/25.6.729. [DOI] [PubMed] [Google Scholar]
- 45.Kanjilal S, Gregg EW, Cheng YJ et al. Socioeconomic status and trends in disparities in 4 major risk factors for cardiovascular disease among US adults, 1971–2002. Arch Intern Med. 2006;166(21):2348–2355. doi: 10.1001/archinte.166.21.2348. [DOI] [PubMed] [Google Scholar]
- 46.Imperatore G, Cheng YJ, Williams DE, Fulton J, Gregg EW. Physical activity, cardiovascular fitness, and insulin sensitivity among US adolescents: the National Health and Nutrition Examination Survey, 1999–2002. Diabetes Care. 2006;29(7):1567–1572. doi: 10.2337/dc06-0426. [DOI] [PubMed] [Google Scholar]
- 47.Rauff EL, Downs DS. Mediating effects of body image satisfaction on exercise behavior, depressive symptoms, and gestational weight gain in pregnancy. Ann Behav Med. 2011;42(3):381–390. doi: 10.1007/s12160-011-9300-2. [DOI] [PubMed] [Google Scholar]