Abstract
Objectives. We aimed to (1) evaluate the relation between home age and concentrations of multiple chemical contaminants in settled dust and (2) discuss the feasibility of using lead hazard controls to reduce children’s exposure to persistent organic pollutants.
Methods. As part of the California Childhood Leukemia Study, from 2001 to 2007, we used a high-volume small surface sampler and household vacuum cleaners to collect dust samples from 583 homes and analyzed the samples for 94 chemicals with gas chromatography–mass spectrometry and inductively coupled plasma mass spectrometry. We evaluated relations between chemical concentrations in dust and home age with Spearman rank correlation coefficients.
Results. Dust concentrations of lead, polychlorinated biphenyls, organochlorine insecticides, and polycyclic aromatic hydrocarbons were correlated with home age (ρ > 0.2; P < .001), whereas concentrations of pyrethroid insecticides and polybrominated diphenyl ethers were not.
Conclusions. Dust in older homes contains higher levels of multiple, persistent chemicals than does dust in newer homes. Further development of strategies to reduce chemical exposures for children living in older homes is warranted.
Settled dust found indoors is a mixture of biologically derived materials, particles deposited from indoor aerosols, particles deposited from building materials (e.g., deteriorated paint), and soil particles that infiltrate from outdoors (e.g., soil tracked indoors on shoes).1 Because typical cleaning removes only a portion of dust from indoor environments (e.g., when vacuuming a carpet), indoor dust acts as a reservoir for chemical contamination.2 Settled dust can be an important source of chemical exposures, especially for young children, who have frequent hand-to-mouth contact.3 As early as 1904, Gibson suggested the importance of dust as a route of exposure to lead, hypothesizing that “painted surfaces . . . become a dry easily detachable powder . . . carried to the mouths of children . . . who bite their nails, suck their fingers or eat with unwashed hands.”4(p302)
More than a century after dust was first implicated in childhood lead poisoning, children’s exposure to lead-contaminated dust remains a major public health issue, especially for children living in older homes.5,6 Lead-based paint, the major source of lead in the residential environment,7 was banned in the United States in 1978. Thus, homes that predate the ban are more likely to contain lead-based paint, and these older homes have greater lead contamination than do newer homes.8 Numerous investigators have reported a relation between home age and lead levels in dust (Table A, available as a supplement to the online version of this article at http://www.ajph.org). In addition to lead, several organic chemicals that were used extensively worldwide have been banned, including polychlorinated biphenyls (PCBs) and organochlorine insecticides; and these contaminants likewise have been found at higher levels in dust from older homes compared with newer homes (Table B, available as a supplement to the online version of this article at http://www.ajph.org).
As part of the California Childhood Leukemia Study (CCLS), we collected dust samples from 583 homes. We showed in previous multivariable models that home age was positively associated with levels of PCBs,9 polycyclic aromatic hydrocarbons (PAHs),10,11 and the organochlorine insecticides chlordane and dichlorodiphenyltrichloroethane (DDT)12 in dust samples from CCLS homes. We present the first systematic evaluation of the relation between home age and concentrations of a broad suite of chemical contaminants in dust samples from CCLS homes. Because no established protocol exists to control persistent organic pollutants in homes, we describe mitigation strategies that have been used successfully for lead hazard control and discuss their potential utility in reducing children’s exposure to organic chemicals.
METHODS
The CCLS is a population-based case–control study that seeks to identify genetic and environmental risk factors for childhood leukemia. We collected dust samples from homes of case participants and control participants younger than 8 years at diagnosis (similar reference date for control participants) who were enrolled in the study from December 1999 to November 2007 and who were still living at the diagnosis home. Sampled homes were composed of a wide range of construction dates (pre-1900 to post-2000), types (single-family homes to multifamily apartments), sizes (< 1000 sq ft to > 5000 sq ft), and income levels (annual income: < $15 000 to > $75 000).
Dust Sampling
We collected dust samples from 583 CCLS homes with the high-volume small surface sampler (n = 415) and household vacuum cleaners (n = 499) from 2001 to 2007, as previously described.13 Initially, dust was collected with both sampling methods, and samples that were collected with the standardized high-volume small surface sampler protocol14 were used in chemical analyses. However, because chemical concentrations in dust samples collected by high-volume small surface sampler and household vacuum from the same homes were found to be comparable,13 starting in 2006, household vacuum sampling was used in place of the more labor-intensive high-volume small surface sampler protocol. Dust samples were collected by high-volume small surface sampler from a carpet or rug in the room where the child spent the most time while awake (commonly the family room). Vacuum dust samples were collected by removing the used bag or by emptying the loose dust from the household vacuum cleaner into a sealable polyethylene bag. Dust samples were stored in the dark at 4°C for up to 10 years prior to chemical analysis. When dust was collected with the high-volume small surface sampler, the sampling area was measured; thus, chemical concentrations (mass of chemical per mass of dust) and chemical loadings (mass of chemical per area sampled) were calculated. In contrast, when dust was collected with a household vacuum cleaner, the sampling area was unknown, and only chemical concentrations were calculated.
Chemical Analysis
We used a multiresidue analysis scheme to analyze 74 chemicals from several compound classes (organochlorine insecticides, organophosphate insecticides, carbamate insecticides, pyrethroid insecticides, phenoxy herbicides, amide herbicides, dinitroaniline herbicides, PAHs, PCBs, metals, and tobacco markers) at Battelle Memorial Institute (Columbus, OH), as previously described.13 A complete list of analytes is available in Table C (available as a supplement to the online version of this article at http://www.ajph.org). To accommodate the diverse group of organic analytes, 3 different extraction methods were used (hexane:acetone, dichloromethane, and acetonitrile:sodium acid phosphate) in combination with gas chromatography–mass spectrometry in the multiple ion detection mode. In addition, metals were analyzed with microwave-assisted acid digestion in combination with inductively coupled plasma mass spectrometry. These initial chemical analyses used 168 dust samples (29%) collected from household vacuum cleaners and 415 dust samples (71%) collected via high-volume small surface sampler. Most of the 415 homes sampled with the high-volume small surface sampler also provided a household vacuum sample that was not initially analyzed.
Subsequently, 292 of the available dust samples collected from household vacuum cleaners were analyzed for 20 polybrominated diphenyl ethers at the California Department of Toxic Substances Control (Berkeley), as previously described.15 Of the 292 homes with a vacuum dust sample analyzed for polybrominated diphenyl ethers, 106 homes had a sample from the same vacuum bag that was previously analyzed for the other 74 chemicals, whereas 186 homes had a dust sample collected by high-volume small surface sampler that was previously analyzed for the other 74 chemicals. Briefly, dust samples were extracted with a 95:5 hexane:methylene chloride mixture and analyzed for polybrominated diphenyl ethers with high-resolution gas chromatography–high-resolution mass spectrometry in the multiple ion detection mode.
Questionnaire Responses
At the time of dust collection, participants were asked to identify their residence’s date of construction (pre-1940, 1940–1949, 1950–1959, 1960–1969, 1970–1979, 1980–1984, 1985–1989, or post-1989) and type, their child’s race/ethnicity, and their household’s annual income. Of 583 respondents, 513 were able to estimate their residence’s construction date; 70 respondents did not know the age of their home and were therefore excluded from statistical analyses that used home age. In the 415 homes that were sampled with the high-volume small surface sampler, participants were also asked to identify the age (in months) of the rug or carpet that was sampled (413 participants responded; carpet ages ranged from < 1 month to 30 years).
Statistical Analysis
We used nonparametric Spearman rank correlation coefficients to identify relations between chemicals, relations between chemical levels and home age (an ordinal variable), and relations between chemical levels and carpet age. Our primary statistical analysis included dust samples collected by both high-volume small surface sampler and household vacuum cleaners; thus, chemical levels were characterized by concentration. We conducted secondary statistical analyses of both chemical concentrations and loadings for dust samples collected by high-volume small surface sampler. We also conducted analyses stratified by household annual income, child’s race/ethnicity, and residence type. We set all values that were below the analytical limit of detection to a value of zero (equivalent to the lowest rank when estimating Spearman rank correlation).
RESULTS
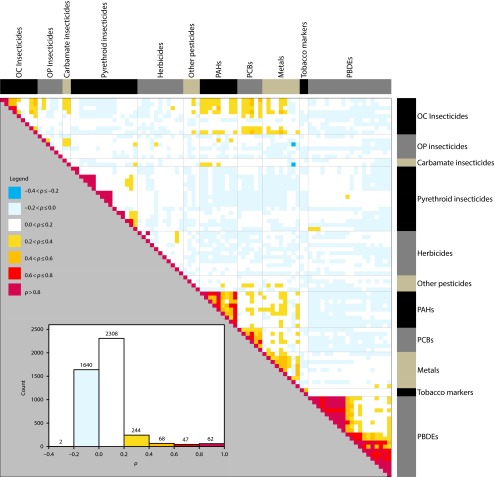
Figure 1 shows a matrix of Spearman rank correlation coefficients between concentrations of 94 chemicals measured in 583 dust samples collected from CCLS homes. As expected, the chemicals with the strongest correlation were found within chemical classes—these relations are characterized as areas of red near the diagonal line in Figure 1. For example, several of the PAHs were highly correlated (ρ > 0.6) with each other (e.g., ρ = 0.90 for benzo[a]anthracene, chrysene), as were some of the organochlorine insecticides, pyrethroid insecticides, PCBs, and polybrominated diphenyl ethers. More interestingly, there was also moderate correlation (ρ = 0.2–0.6) between individual chemicals across some of the chemical classes (e.g., between some organochlorine insecticides, PAHs, PCBs, and metals)—these relations are shown in orange. For example, lead is correlated with DDT, chrysene, and PCB-180 (ρ = 0.40, 0.37, 0.36, respectively; P < .001 for each).
FIGURE 1—
Spearman rank correlation coefficients between concentrations of 94 chemicals measured in 583 dust samples collected from California Childhood Leukemia Study homes: 2001–2007.
Note. OC = organochlorine; OP = organophosphate; PAHs = polycyclic aromatic hydrocarbons; PBDEs = polybrominated diphenyl ethers; PCBs = polychlorinated biphenyls. Chemicals are grouped by class, and groups are shown along the top and right borders (in black, gray, and gold). Inset is a histogram of correlation coefficients for the 94 chemicals.
Table C shows the detection frequency, median, and interquartile range of each of 94 chemicals measured in 513 dust samples collected from CCLS homes, stratified by the residence construction date. Of the 94 chemicals analyzed, 21 chemicals from various classes were detected in fewer than 20% of the participating homes. We generally did not observe a significant correlation between home age and concentrations of these 21 infrequently detected chemicals.
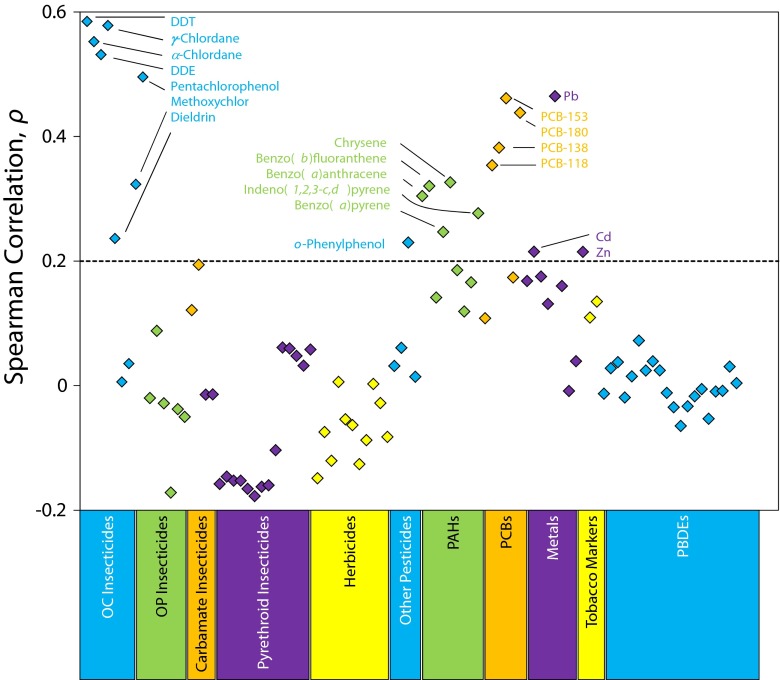
Figure 2 shows Spearman rank correlation coefficients between home age and chemical concentrations for each of 94 chemicals measured in 513 dust samples collected from CCLS homes. Of the 94 chemicals analyzed, dust concentrations of DDT (and its metabolite dichlorodiphenyldichloroethylene) and chlordane (both the α and the γ isomers) were most positively correlated with home age (ρ > 0.5; P < .001), indicating that these chemicals were found at higher concentrations in dust from older homes than in dust from newer homes. Likewise, dust concentrations of 3 other organochlorine insecticides (dieldrin, methoxychlor, and pentachlorophenol), 4 PCBs, and 5 PAHs were positively correlated with home age (ρ > 0.2; P < .001). We also observed significant positive correlations between home age and concentrations of lead (ρ > 0.46; P < .001) and other metals (cadmium and zinc; ρ > 0.2; P < .001). By contrast, concentrations of chemicals that were first produced at high volumes within the past 30 years, such as polybrominated diphenyl ethers and pyrethroid insecticides, were either unrelated to, or negatively correlated with, home age.
FIGURE 2—
Spearman rank correlation coefficients between home age and chemical levels for each of 94 chemicals measured in 513 dust samples collected from California Childhood Leukemia Study homes: 2001–2007.
Note. DDE = dichlorodiphenyldichloroethylene; DDT = dichlorodiphenyltrichloroethane; OC = organochlorine; OP = organophosphate; PAHs = polycyclic aromatic hydrocarbons; PBDEs = polybrominated diphenyl ethers; PCBs = polychlorinated biphenyls. Chemicals are grouped by class and groups are shown by color along the bottom border (blue, green, orange, purple, yellow). Chemicals highly correlated with home age are labeled (if ρ > 0.2)
Compared with the relations observed in the complete data set (Figure 2), the relations between home age and chemical concentrations were similar in the subset of 415 homes sampled with the high-volume small surface sampler (Table D, available as a supplement to the online version of this article at http://www.ajph.org). For example, for the 20 chemicals that were most positively correlated with home age (those with ρ > 0.2, highlighted in Figure 2), the correlation coefficients were generally at least as large in the subset of samples collected via high-volume small surface sampler. Likewise, in the subset of 415 homes that were sampled with the high-volume small surface sampler, most of the relations between home age and chemical loadings were similar to relations between home age and chemical concentrations (Table D). One exception was lead, for which concentrations were more strongly correlated with home age than were loadings (ρ = 0.44 and 0.29, respectively). In stratified analyses, the positive correlations between home age and chemical concentrations were observed in homes from all strata of income, race/ethnicity, and residence type (Table E, available as a supplement to the online version of this article at http://www.ajph.org).
For most chemicals, the age of the carpet or rug sampled was less strongly correlated with the chemical concentrations and loadings than was home age. For the 20 chemicals that were most positively correlated with home age, none were as strongly correlated with carpet age (ρ < 0.2 for each chemical; Table D).
DISCUSSION
We observed moderate correlations between contaminants with disparate sources, such as lead, PCBs, organochlorine insecticides, and PAHs. Likewise, we observed relations between concentrations of these contaminants in dust and home age. On the basis of these observations, we suggest that older homes are likely to contain dust contaminated by multiple persistent chemicals from various classes.
In the United States, the production and distribution of PCBs were halted in 1979. Thus, PCB-contaminated construction materials, such as ceiling tiles, insulation, building joint caulk, floor finish, paint, and roofing material, are more likely to be present in homes built before 1980 than in more recently constructed residences.9 Moreover, homes built before 1980 may have contained PCB-contaminated consumer products, such as fluorescent light ballasts, refrigerators, televisions, carpet pads, and air conditioners, which may have contaminated household dust or surfaces.9 Several investigators have reported a relation between home age and PCB levels in dust (Table B). Like PCBs, many of the insecticides that were positively associated with home age in our analysis (DDT, chlordane, dieldrin, methoxychlor, and pentachlorophenol also have been restricted or banned in the United States. In support of our findings, multiple investigators have reported a relation between home age and levels of DDT or chlordane in dust (Table B).
Importantly, in addition to confirming previous reports that banned organic contaminants are found at higher levels in dust from older homes than in dust from newer homes, we discovered that PAHs—chemicals that are still being generated—are also found at higher levels in older homes. As evidenced by the decreasing trends in ambient air PAH concentrations in California,16 many PAH sources that contributed to dust-PAH contamination in CCLS homes, including emissions from vehicular traffic, cigarettes, and fireplaces, likely diminished over time. We hypothesize that older homes were initially contaminated by these PAH sources in the past and that long-lasting PAH residues contribute to current PAH concentrations in dust.
Our systematic evaluation of the relation between home age and a broad suite of chemical contaminants complements prior findings from the CCLS, which suggested that home age was predictive of levels of PCBs,9 chlordane, DDT,12 and PAHs10,11 in dust samples.
Given that dust samples from older homes contain higher levels of lead, PCBs, restricted-use pesticides, and PAHs than dust samples from newer homes, we suggest that residents living in older homes may be more highly exposed to these chemicals. Accidental ingestion of settled dust is an important source of chemical exposures for young children,3 and numerous investigators have determined that dust-lead loadings are good indicators of blood-lead levels in children.17 Although the link between dust levels and body burdens is less well established for organic contaminants, it is reasonable to suspect that settled dust is also an important source of exposure to these chemicals,18,19 especially for children.20–22
The potential detrimental effect of ingesting dust contaminated with multiple persistent chemicals has not been evaluated. One outcome of concern for young children living in older homes may be adverse neurodevelopment (e.g., IQ deficits). Investigators have shown that exposures to lead,23 PCBs,24,25 DDT,26–29 and PAHs30–32 are associated with adverse neurodevelopment in young children, but the joint effect of coexposure to multiple chemicals is not known. Perera et al.32 reported that for 122 5-year-old Chinese children, in utero exposure to PAH (as measured by benzo[a]pyrene-DNA adducts in umbilical cord blood) was associated with more severe IQ deficits in the presence of coexposure to secondhand smoke. Likewise, Boucher et al.33 showed that for 196 Inuit children, the association between in utero lead exposure (as measured by lead levels in umbilical cord blood) and response-inhibition deficits was intensified by coexposures to PCBs or methylmercury. Similarly, the risk of adverse neurodevelopment or other adverse health outcomes may be increased for young children exposed to multiple persistent chemicals through the ingestion of settled dust.
The US Department of Housing and Urban Development (HUD) has an established method for controlling lead hazards in homes built before 1978 that targets primary sources of lead (e.g., via encapsulation, enclosure, or paint stabilization of building components with lead-based paint) as well as lead-contaminated dust and lead-contaminated bare soil.34 The HUD protocol has achieved sustainable reductions in dust-lead loadings and children’s blood-lead levels in intervention studies.35–39 For example, in 1034 homes from 11 states, Dixon et al.37 reported that in combination with professional cleaning, the encapsulation or removal of lead-based paint resulted in 95% lower dust-lead loadings on floors. Generally, blood-lead levels in American children continue to decrease40 partially because of HUD’s aggressive program to mitigate lead exposure in older US homes.8
In contrast to lead, relatively little guidance is available for homeowners who want to avoid exposure to persistent organic pollutants, such as PCBs, pesticides, or PAHs. Moreover, there is little empirical evidence to support the use of any particular organic pollutant control program. In theory, an effective strategy could be modeled after the lead hazard control program: address primary chemical sources and remove contaminated dust. However, unlike lead, the list of potential primary sources of organic chemicals inside a home would be extensive and could include building materials and ongoing human activities (e.g., cigarette smoking or pesticide application). Moreover, testing household surfaces or dust for the presence of organic chemicals would be more analytically intensive and expensive than testing for lead. For both of these reasons, controlling sources of organic pollutants in a contaminated home would be more complicated than controlling lead-based paint.
A more limited mitigation strategy that focuses solely on dust removal also has been considered for lead hazard control. Rhoads et al.41 reported that professional cleaning (cleaning carpets with a vacuum cleaner equipped with a high-efficiency particulate air filter and cleaning uncarpeted surfaces with detergent solution) reduced children’s blood-lead levels, especially when homes were cleaned frequently. Tohn et al.42 showed that professional cleaning initially reduced dust-lead loadings, but those reductions did not persist 1 year later. Both studies suggested that successful dust reduction strategies require continuous cleaning. Likewise, we hypothesize that repeated cleaning designed to remove contaminated dust may limit children’s exposure to organic pollutants in older homes, and we suggest that this hypothesis be tested in controlled intervention studies.
Currently, only limited evidence indicates that specific cleaning practices can reduce levels of organic pollutants. McCaule et al.43 showed that wiping windowsills and steam cleaning carpets reduced organophosphorus pesticide residues in some homes from an agricultural community in Oregon. The National Institute for Occupational Safety and Health (NIOSH) also reported the effectiveness of steam cleaning in reducing vacuum-dust levels of chlorinated hydrocarbons in highly contaminated homes.44 Additionally, NIOSH found that wiping surfaces with a specially formulated alkaline detergent was effective in reducing PCB loadings in a Minnesota high school.45
However, the utility of a dust-control strategy for reducing children’s exposures to organic pollutants is potentially limited by several factors. First, vacuuming performed without proper equipment or training can expose children to airborne dust particles contaminated with organic pollutants.44 Moreover, semivolatile organic pollutants can move from their sources and deposit on various household surfaces, including countertops or children’s toys46; they can be absorbed into wood, drywall, and plaster47; and they can persist in hard-to-clean reservoirs, such as carpet pads.2,48 Dust removal may result in only limited reductions in levels of organic pollutants, because newly generated dust may be quickly contaminated by residual organic pollutant reservoirs. Our results provide circumstantial support for this hypothesis. For example, we suggest that the lack of correlation between carpet age and carpet-dust DDT levels is evidence of partitioning of DDT between contaminated household surfaces such as countertops and drywall and newly replaced carpets.
We emphasize the need for further research to identify safe and cost-effective mitigation strategies for older homes contaminated with organic pollutants. Specifically, we suggest that future investigators test the efficacy of repeated professional cleaning of carpets and other surfaces in reducing children’s exposure to organic pollutants.
One limitation of our analysis was the use of questionnaire responses to characterize home and carpet age. Because we did not verify public records or sales receipts, ages of some participants’ homes and carpets may have been misclassified. Underlying factors not included in this analysis, such as the presence of indoor and outdoor chemical sources and the condition of the home, also may explain the observed relation between home age and chemical levels in dust. Another limitation of our analysis was that some of the chemicals we measured were infrequently detected in dust samples from participating homes, which reduced our power to identify relations between home age and levels of these chemicals.
To maximize the size of our primary statistical analysis, we included dust samples collected with both the high-volume small surface sampler and household vacuum cleaners. The major drawback of using dust samples collected from household vacuum cleaners is the absence of a consistent sampling protocol across study homes. Each model of vacuum cleaner has a unique combination of flow rate, sampling efficiency, and range of particle sizes collected. Likewise, each vacuum cleaner may have been used in a different combination of rooms and at different proximity to chemical sources. It was reassuring that our results were similar whether we included or excluded dust samples collected from household vacuum cleaners in our statistical analysis.
Because the area sampled by a household vacuum cleaner is not well defined, chemical loadings could not be calculated for vacuum samples, and, as a result, our primary statistical analysis used chemical concentrations. It is not known whether dust loadings or dust concentrations are more representative of children’s exposure to organic pollutants. It has been reported that blood-lead levels are more highly correlated with dust-lead loadings than with dust-lead concentrations, suggesting that dust-lead loadings are more useful markers of childhood lead exposures.49 In the subset of samples we collected with the high-volume small surface sampler, our results were similar for chemical concentrations and loadings.
In summary, we showed that lead, PCBs, organochlorine pesticides, and PAHs are found at higher levels in dust collected from older homes than in dust collected from newer homes. We conclude that residents living in older homes potentially experience coexposures to many of these persistent chemicals, which may result in adverse health effects, especially for children. Whether strategies that have been successful for controlling lead hazards in older homes will be helpful in controlling organic pollutants is uncertain. Mitigation strategies for older homes with dust contaminated by organic pollutants should be developed.
Acknowledgments
This work was supported in part by the National Institute of Environmental Health Sciences (grants R01ES009137, R01ES015899, P42ES0470518, P01ES018172); by the Intramural Research Program of the National Cancer Institute (NCI), National Institutes of Health (subcontracts 7590-S-04, 7590-S-01); by the NCI (contract N02-CP-11015); and by the Environmental Protection Agency (grant RD83451101).
Patricia A. Buffler, PhD, died September 26, 2013, at age 75. We dedicate this article to her memory; she was a brilliant researcher who inspired us all.
We thank the families for their participation. We thank our collaborators at the Battelle Memorial Institute (Marcia Nishioka) and the California Department of Toxic Substances Control (F. Reber Brown, PhD, June-Soo Park, PhD, and Myrto Petreas, PhD) for conducting chemical analyses. We also thank the clinical investigators at the following collaborating hospitals for help in recruiting patients: University of California Davis Medical Center (Jonathan Ducore, MD), University of California San Francisco (Mignon Loh, MD, and Katherine Matthay, MD), Children’s Hospital of Central California (Vonda Crouse, MD), Lucile Packard Children’s Hospital (Gary Dahl, MD), Children’s Hospital Oakland (James Feusner, MD), Kaiser Permanente Oakland (Daniel Kronish, MD, and Stacy Month, MD), Kaiser Permanente Roseville (Kent Jolly, MD, and Vincent Kiley, MD), Kaiser Permanente Santa Clara (Carolyn Russo, MD, Denah Taggart, MD, and Alan Wong, MD), and Kaiser Permanente San Francisco (Kenneth Leung, MD). Finally, we acknowledge the entire California Childhood Leukemia Study staff for their effort and dedication.
Note. P. A. Buffler served on the Board of Directors for FMC Agricultural Products from 1999 to 2011. All other authors declare that they have no competing financial interests.
Human Participant Protection
We obtained written informed consent from the children’s parents, and study protocols involving research with human participants were approved by the institutional review boards at the University of California, Berkeley, and all other participating institutions.
References
- 1.Butte W, Heinzow B. Pollutants in house dust as indicators of indoor contamination. Rev Environ Contam Toxicol. 2002;175:1–46. [PubMed] [Google Scholar]
- 2.Roberts JW, Wallace LA, Camann DE et al. Monitoring and reducing exposure of infants to pollutants in house dust. Rev Environ Contam Toxicol. 2009;201:1–39. doi: 10.1007/978-1-4419-0032-6_1. [DOI] [PubMed] [Google Scholar]
- 3.Cohen Hubal EA, Sheldon LS, Burke JM et al. Children’s exposure assessment: a review of factors influencing children’s exposure, and the data available to characterize and assess that exposure. Environ Health Perspect. 2000;108(6):475–486. doi: 10.1289/ehp.108-1638158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gibson JL. A plea for painted railings and painted walls of rooms as the source of lead poisoning amongst Queensland children. 1904. Public Health Rep. 2005;120(3):301–304. doi: 10.1177/003335490512000314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pirkle JL, Kaufmann RB, Brody DJ, Hickman T, Gunter EW, Paschal DC. Exposure of the US population to lead, 1991-1994. Environ Health Perspect. 1998;106(11):745–750. doi: 10.1289/ehp.98106745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dixon SL, Gaitens JM, Jacobs DE et al. Exposure of US children to residential dust lead, 1999-2004, II: the contribution of lead-contaminated dust to children’s blood lead levels. Environ Health Perspect. 2009;117(3):468–474. doi: 10.1289/ehp.11918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lanphear BP, Roghmann KJ. Pathways of lead exposure in urban children. Environ Res. 1997;74(1):67–73. doi: 10.1006/enrs.1997.3726. [DOI] [PubMed] [Google Scholar]
- 8.Jacobs DE, Clickner RP, Zhou JY et al. The prevalence of lead-based paint hazards in US housing. Environ Health Perspect. 2002;110(10):A599–A606. doi: 10.1289/ehp.021100599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Whitehead TP, Ward MH, Colt JS et al. Determinants of polychlorinated biphenyls in dust from homes in California, USA. Environ Sci Processes Impacts. 2013;15(2):339–346. doi: 10.1039/c2em30721a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Whitehead T, Metayer C, Gunier RB et al. Determinants of polycyclic aromatic hydrocarbon levels in house dust. J Expo Sci Environ Epidemiol. 2011;21(2):123–132. doi: 10.1038/jes.2009.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Whitehead TP, Metayer C, Petreas M, Does M, Buffler PA, Rappaport SM. Polycyclic aromatic hydrocarbons in residential dust: sources of variability. Environ Health Perspect. 2013;121(5):543–550. doi: 10.1289/ehp.1205821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gunier RB, Ward MH, Whitehead TP, et al. Time trends of insecticide concentrations in house dust from northern and central California residences 2001–2006. Presented at: International Society of Exposure Science 22nd Annual Meeting; October 28–November 1, 2012; Seattle, WA.
- 13.Colt JS, Gunier RB, Metayer C et al. Household vacuum cleaners vs the high-volume surface sampler for collection of carpet dust samples in epidemiologic studies of children. Environ Health. 2008;7:6. doi: 10.1186/1476-069X-7-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.ASTM Standard D5438, 1993. Standard Practice for Collection of Floor Dust for Chemical Analysis. West Conshohocken, PA: ASTM International; 2005. [Google Scholar]
- 15.Whitehead TP, Brown FR, Metayer C et al. Polybrominated diphenyl ethers in residential dust: sources of variability. Environ Int. 2013;57-58:11–24. doi: 10.1016/j.envint.2013.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.California Environmental Protection Agency, California Air Resources Board. Statewide ambient toxics data summaries. 2013. Available at: http://www.arb.ca.gov/adam/toxics/statesubstance.html. Accessed May 24, 2013.
- 17.Lanphear BP, Matte TD, Rogers J et al. The contribution of lead-contaminated house dust and residential soil to children’s blood lead levels: a pooled analysis of 12 epidemiologic studies. Environ Res. 1998;79(1):51–68. doi: 10.1006/enrs.1998.3859. [DOI] [PubMed] [Google Scholar]
- 18.Rudel RA, Seryak LM, Brody JG. PCB-containing wood floor finish is a likely source of elevated PCBs in residents’ blood, household air and dust: a case study of exposure. Environ Health. 2008;7:2. doi: 10.1186/1476-069X-7-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Knobeloch L, Turyk M, Imm P, Anderson H. Polychlorinated biphenyls in vacuum dust and blood of residents in 20 Wisconsin households. Chemosphere. 2012;86(7):735–740. doi: 10.1016/j.chemosphere.2011.10.048. [DOI] [PubMed] [Google Scholar]
- 20.Chuang JC, Callahan PJ, Lyu CW, Wilson NK. Polycyclic aromatic hydrocarbon exposures of children in low-income families. J Expo Anal Environ Epidemiol. 1999;9(2):85–98. doi: 10.1038/sj.jea.7500003. [DOI] [PubMed] [Google Scholar]
- 21.Gevao B, Al-Bahloul M, Zafar J, Al-Matrouk K, Helaleh M. Polycyclic aromatic hydrocarbons in indoor air and dust in Kuwait: implications for sources and nondietary human exposure. Arch Environ Contam Toxicol. 2007;53(4):503–512. doi: 10.1007/s00244-006-0261-6. [DOI] [PubMed] [Google Scholar]
- 22.Harrad S, Ibarra C, Robson M et al. Polychlorinated biphenyls in domestic dust from Canada, New Zealand, United Kingdom and United States: implications for human exposure. Chemosphere. 2009;76(2):232–238. doi: 10.1016/j.chemosphere.2009.03.020. [DOI] [PubMed] [Google Scholar]
- 23.Lanphear BP, Hornung R, Khoury J et al. Low-level environmental lead exposure and children’s intellectual function: an international pooled analysis. Environ Health Perspect. 2005;113(7):894–899. doi: 10.1289/ehp.7688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jacobson JL, Jacobson SW. Intellectual impairment in children exposed to polychlorinated biphenyls in utero. N Engl J Med. 1996;335(11):783–789. doi: 10.1056/NEJM199609123351104. [DOI] [PubMed] [Google Scholar]
- 25.Stewart PW, Lonky E, Reihman J, Pagano J, Gump BB, Darvill T. The relationship between prenatal PCB exposure and intelligence (IQ) in 9-year-old children. Environ Health Perspect. 2008;116(10):1416–1422. doi: 10.1289/ehp.11058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ribas-Fitó N, Cardo E, Sala M et al. Breastfeeding, exposure to organochlorine compounds, and neurodevelopment in infants. Pediatrics. 2003;111(5):e580–e585. doi: 10.1542/peds.111.5.e580. [DOI] [PubMed] [Google Scholar]
- 27.Eskenazi B, Marks AR, Bradman A et al. In utero exposure to dichlorodiphenyltrichloroethane (DDT) and dichlorodiphenyldichloroethylene (DDE) and neurodevelopment among young Mexican American children. Pediatrics. 2006;118(1):233–241. doi: 10.1542/peds.2005-3117. [DOI] [PubMed] [Google Scholar]
- 28.Ribas-Fitó N, Torrent M, Carrizo D et al. In utero exposure to background concentrations of DDT and cognitive functioning among preschoolers. Am J Epidemiol. 2006;164(10):955–962. doi: 10.1093/aje/kwj299. [DOI] [PubMed] [Google Scholar]
- 29.Torres-Sánchez L, Schnaas L, Rothenberg SJ et al. Prenatal p,p-DDE exposure and neurodevelopment among children 3.5-5 years of age. Environ Health Perspect. 2013;121(2):263–268. doi: 10.1289/ehp.1205034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Perera FP, Li Z, Whyatt R et al. Prenatal airborne polycyclic aromatic hydrocarbon exposure and child IQ at age 5 years. Pediatrics. 2009;124(2):e195–e202. doi: 10.1542/peds.2008-3506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Edwards SC, Jedrychowski W, Butscher M et al. Prenatal exposure to airborne polycyclic aromatic hydrocarbons and children’s intelligence at 5 years of age in a prospective cohort study in Poland. Environ Health Perspect. 2010;118(9):1326–1331. doi: 10.1289/ehp.0901070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Perera F, Li TY, Lin C, Tang D. Effects of prenatal polycyclic aromatic hydrocarbon exposure and environmental tobacco smoke on child IQ in a Chinese cohort. Environ Res. 2012;114:40–46. doi: 10.1016/j.envres.2011.12.011. [DOI] [PubMed] [Google Scholar]
- 33.Boucher O, Burden MJ, Muckle G et al. Response inhibition and error monitoring during a visual go/no-go task in Inuit children exposed to lead, polychlorinated biphenyls, and methylmercury. Environ Health Perspect. 2012;120(4):608–615. doi: 10.1289/ehp.1103828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guidelines for the Evaluation and Control of Lead-Based Paint Hazards in Housing. 2nd ed. Washington, DC: US Department of Housing and Urban Development; 2012. [Google Scholar]
- 35.Galke W, Clark S, Wilson J et al. Evaluation of the HUD lead hazard control grant program: early overall findings. Environ Res. 2001;86(2):149–156. doi: 10.1006/enrs.2001.4259. [DOI] [PubMed] [Google Scholar]
- 36.Clark S, Grote J, Wilson J et al. Occurrence and determinants of increases in blood lead levels in children shortly after lead hazard control activities. Environ Res. 2004;96(2):196–205. doi: 10.1016/j.envres.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 37.Dixon SL, Wilson JW, Scott Clark C, Galke WA, Succop PA, Chen M. Effectiveness of lead-hazard control interventions on dust lead loadings: findings from the evaluation of the HUD Lead-Based Paint Hazard Control Grant Program. Environ Res. 2005;98(3):303–314. doi: 10.1016/j.envres.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 38.Wilson J, Pivetz T, Ashley P et al. Evaluation of HUD-funded lead hazard control treatments at 6 years post-intervention. Environ Res. 2006;102(2):237–248. doi: 10.1016/j.envres.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 39.Clark S, Galke W, Succop P et al. Effects of HUD-supported lead hazard control interventions in housing on children’s blood lead. Environ Res. 2011;111(2):301–311. doi: 10.1016/j.envres.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 40.Centers for Disease Control and Prevention. Lead—CDC’s National Surveillance Data (1997-2012) 2013. Available at: http://www.cdc.gov/nceh/lead/data/national.htm. Accessed April 24, 2013.
- 41.Rhoads GG, Ettinger AS, Weisel CP et al. The effect of dust lead control on blood lead in toddlers: a randomized trial. Pediatrics. 1999;103(3):551–555. doi: 10.1542/peds.103.3.551. [DOI] [PubMed] [Google Scholar]
- 42.Tohn ER, Dixon SL, Wilson JW, Galke WA, Clark CS. An evaluation of one-time professional cleaning in homes with lead-based paint hazards. Appl Occup Environ Hyg. 2003;18(2):138–143. doi: 10.1080/10473220301437. [DOI] [PubMed] [Google Scholar]
- 43.McCaule LA, Travers R, Lasarev M, Muniz J, Nailon R. Effectiveness of cleaning practices in removing pesticides from home environments. J Agromed. 2006;11(2):81–88. doi: 10.1300/J096v11n02_11. [DOI] [PubMed] [Google Scholar]
- 44.National Institute for Occupational Safety and Health. Report to Congress on Workers’ Home Contamination Study Conducted Under the Workers’ Family Protection Act (29 U.S.C. 671a) Cincinnati, OH: National Institute for Occupational Safety and Health; 1995. pp. 95–123. Available at: http://www.cdc.gov/niosh/docs/95-123. Accessed March 28, 2014. [Google Scholar]
- 45.Orris P, Kominsky JR. Health Hazard Evaluation Report: Hill-Murray High School, Maplewood, Minnesota. Cincinnati, OH: National Institute for Occupational Safety and Health; 1984. HETA 82-310-1475. [Google Scholar]
- 46.Lewis RG, Fortune CR, Blanchard FT, Camann DE. Movement and deposition of two organophosphorus pesticides within a residence after interior and exterior applications. J Air Waste Manag Assoc. 2001;51(3):339–351. doi: 10.1080/10473289.2001.10464281. [DOI] [PubMed] [Google Scholar]
- 47.Clark JM, Bing-Canar J, Renninger S et al. Methyl parathion in residential properties: relocation and decontamination methodology. Environ Health Perspect. 2002;110(suppl 6):1061–1070. doi: 10.1289/ehp.02110s61061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shin HM, McKone TE, Tulve NS, Clifton MS, Bennett DH. Indoor residence times of semivolatile organic compounds: model estimation and field evaluation. Environ Sci Technol. 2013;47(2):859–867. doi: 10.1021/es303316d. [DOI] [PubMed] [Google Scholar]
- 49.Lanphear BP, Emond M, Jacobs DE et al. A side-by-side comparison of dust collection methods for sampling lead-contaminated house dust. Environ Res. 1995;68(2):114–123. doi: 10.1006/enrs.1995.1015. [DOI] [PubMed] [Google Scholar]