Abstract
Objectives. The objective of this study was to determine the role international travel plays in US Campylobacter epidemiology and antimicrobial resistance.
Methods. In this study, epidemiological and antimicrobial resistance data, encompassing the years 2005 to 2011, from 10 sites participating in the Foodborne Diseases Active Surveillance Network were linked. The 10 sites are represented by 7 states that conducted surveillance on a statewide level, and 3 states which conducted county-level surveillance. Cases of Campylobacter among persons with history of international travel in the week prior to illness were compared with cases among individuals with no international travel.
Results. Approximately 18% of Campylobacter infections were estimated to be associated with international travel, and 60% of international travel-associated infections had a quinolone-resistant Campylobacter isolate.
Conclusions. We confirm that international travel plays a significant role in campylobacteriosis diagnosed in the United States. Recognizing this is important to both medical management decisions and understanding burden and attribution estimates of US campylobacteriosis and antibiotic-resistant campylobacteriosis.
In the United States, Campylobacter is estimated to affect more than 1.3 million people annually, with more than 14.3 laboratory-confirmed cases per 100 000 people.1,2 Many of these infections are attributed to high levels (reported at 47% in the United States in 2011) of bacterial contamination in US poultry3 and to other domestic US exposures. However, there are an estimated 400 to 500 million cases of campylobacteriosis occurring annually worldwide,4 and recent findings suggest a large proportion of US Campylobacter infections might actually be acquired internationally and diagnosed upon return to the United States.5 Of particular concern is that there appears to be a high prevalence of antibiotic resistance in Campylobacter outside of the United States, primarily in developing countries.6,7
Understanding the epidemiology of travel-associated Campylobacter infections is important for increasing awareness of this infection in international travelers and among providers that care for these patients. It is also important for assessing the burden of domestically acquired Campylobacter illnesses in the United States and attributing infections to certain food commodities. Lastly, it should be considered in determining effectiveness of US interventions, including efforts of the US Department of Agriculture (USDA) and the US Food and Drug Administration (FDA), to address foodborne bacterial infections in general and antimicrobial resistance in particular.
We used data collected by the Foodborne Diseases Active Surveillance Network (FoodNet) from 2005 to 2011 to describe Campylobacter-infected persons, including their demographics, travel history, and outcomes such as hospitalization and death. In addition, we linked antimicrobial susceptibility testing results from select isolates as part of the National Antimicrobial Resistance Monitoring System (NARMS) to epidemiological surveillance data to help determine the contribution of international travel to antimicrobial resistance detected in the United States.
METHODS
We obtained information about campylobacteriosis cases via FoodNet, an active, population-based surveillance network established by the US Centers for Disease Control and Prevention (CDC) in 1996 as part of the Emerging Infections Program.8 FoodNet is a collaborative program of the CDC, 10 state health departments, academic institutions, USDA’s Food Safety and Inspection Service, and FDA. The sites in this network routinely identify all laboratory-confirmed cases of 9 foodborne pathogens: Campylobacter, Cryptosporidium, Cyclospora, Listeria, Salmonella, Shiga-toxin producing Escherichia coli, Shigella, Vibrio, and Yersinia. The populations under surveillance at the 10 FoodNet sites contain 47.5 million persons, representing roughly 15% of the US population. Since 2004, Connecticut, Georgia, Maryland, Minnesota, New Mexico, Oregon, and Tennessee conduct statewide surveillance; California, Colorado, and New York monitor select counties within the state. The proportion of campylobacteriosis cases differs by state, with New Mexico contributing the fewest (an average of 6%), and California contributing the highest proportion (16% on average). These 10 catchment areas have remained constant since 2004, and the proportion of cases identified by each site does not vary greatly over time. For some patients, sites obtain demographic and international travel information prior to illness onset, including destination and travel dates.9
NARMS is a national collaboration that tracks antimicrobial resistance of enteric bacterial pathogens.10 Viable Campylobacter isolates received at the site-specific public health laboratories are routinely sent to the CDC NARMS laboratory for antimicrobial susceptibility and other testing. Depending on the burden of Campylobacter infections and expected annual number of isolates received at each site, a scheme was developed by the CDC to obtain and test a sample of isolates.10 From 2005 through 2009, every second isolate received by California, Colorado, Connecticut, and New York, every fifth isolate received by Minnesota, and all isolates received by Georgia, Maryland, New Mexico, Oregon, and Tennessee were submitted to NARMS. Beginning in 2010, the scheme was changed to every other isolate from Georgia and Maryland, and every third from New Mexico; the other sites were unchanged. This was done to avoid oversampling from the 3 sites, which had increased submissions over time, relative to the other sites. This difference in sampling schemes causes variation in the proportion of FoodNet cases linked to NARMS data by site each year.
NARMS routinely tests Campylobacter for resistance to a number of antibiotics, including those in the following classes: aminoglycosides, ketolides, lincosamides, macrolides, quinolones, phenicols, and tetracyclines.10 For this study, 2 quinolones, ciprofloxacin and nalidixic acid, as well as 2 macrolides, azithromycin and erythromycin, were chosen for analysis because of their clinical relevance for Campylobacter treatment.11 We defined resistance, intermediate susceptibility (when applicable), and susceptibility to these 4 antimicrobials using criteria from the Clinical and Laboratory Standards Institute and NARMS, and we removed the few isolates with intermediate susceptibility from the analysis to avoid skewing of results because of misclassification.10 FoodNet patient data and NARMS resistance data were linked using a common state lab ID. The link was verified using age, sex, race, county of residence, specimen collection date, and specimen source.
Definitions and Categorizations
A confirmed case of campylobacteriosis was defined as any culture-confirmed Campylobacter infection. We reviewed cases reported by FoodNet sites for the 7-year period 2005 through 2011. For this study, we considered a case to be international travel-associated if associated with international travel during the week prior to onset of illness. In this article, “non-travel” refers to patients without international travel, and patients reporting domestic travel fall into this group.
International destinations were categorized into 10 regions and 29 subregions, based on the United Nations Statistical Division Country and Area classifications12 and proportion of cases traveling to each destination. North America included Bermuda and Canada. Puerto Rico and the US Virgin Islands were not considered international destinations. Patients who had visited multiple destinations in different UN area classifications or whose destinations were unknown were not included in destination-related analyses. For parts of the analysis, Mexico, China, and Peru were considered individual destinations owing to disproportionately large numbers of travelers and patients with antimicrobial resistance.
Study Population
Collection of travel status varied by site; Colorado, Maryland, Minnesota, New York, New Mexico, and Tennessee consistently reported greater than 50% known travel status, while California, Connecticut, Georgia, and Oregon all had lower percentages of travel status determination over all 7 years. However, travel status determination improved for all sites over the period 2005 through 2011. To determine whether the nonuniformity of data collection across sites and years dramatically impacted the population characteristics, we compared demographic information such as age, sex, race, and ethnicity across sites for cases with and without known travel status. Cases with known and unknown travel status were similar in age and gender distribution. Not surprisingly, race and ethnicity were not known for a higher proportion of cases that also had unknown travel status compared with cases in which travel status was known; however we determined that the cases with known travel status were still generally representative of FoodNet campylobacteriosis cases overall and that missing information about race, ethnicity, or travel status did not affect the overall analyses sufficiently to warrant excluding any of the sites from the analysis.
Data Analysis
Case-patient demographics, outcomes, and the proportion of resistant organisms were compared between cases with different travel status. In addition, the prevalence of resistant organisms by destination was determined. The χ2 test, the t-test, and logistic regression were used to evaluate demographic differences between groups. We used multivariable logistic regression to calculate odds ratios (ORs) for hospitalization and death comparing international travel-associated cases to non–travel-associated cases, as well as to evaluate antimicrobial resistance. We also used a quasipoisson regression model to evaluate differences in hospital duration. Variables with a P value less than .1 in univariate analysis and stepwise forward selection were included in the models. Spearman’s rank order correlation coefficient was used to assess correlation between antimicrobial susceptibility results for antibiotics of the same class. P values of .05 or less were considered significant. Statistical analyses were completed using R (R Foundation for Statistical Computing, Vienna, Austria, version 2.15.2—“Trick or Treat,” 2012).
RESULTS
From 2005 through 2011 there were 42 402 laboratory confirmed cases of Campylobacter among all FoodNet sites. Travel status was known for 24 433 cases (58%), and of these, 4389 (18%) were associated with international travel (Figure 1). The proportion of international travel-associated cases decreased significantly from a high of 21% in 2005 to a low of 16% in 2011. Of the 4389 cases involving international travel, 3554 (81%) involved travel to a single region (Figure 1). Of those, Asia (excluding China) and Mexico were the destinations for the greatest proportion of cases, accounting for 21% and 20% respectively, followed by Western Europe (17%), South America (14%), Central America excluding Mexico (12%), Africa (7.4%), China (2.5%), Eastern Europe (2.2%), North America excluding the United States (1.7%), and Australia/New Zealand (1.1%).
FIGURE 1—
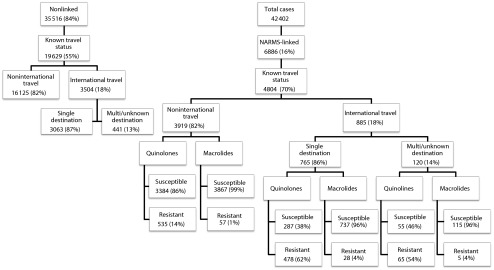
Flowchart diagramming number of cases analyzed in each study population: United States, 2005–2011.
Note. NARMS = National Antimicrobial Resistance Monitoring System. Of 42 402 FoodNet Campylobacter cases reported between 2005–2011, < 8000 should have been submitted to the NARMS, bringing the percentage of linkage among available isolates to 88%.
Demographics between international travel-associated cases and non–travel-associated cases varied for some characteristics. Age, sex, and ethnicity were similar between groups, although cases associated with international travel were slightly older and more likely to be female than were non–travel-associated cases (Table 1). For cases in which race was known, the proportion of international travel-associated cases reported as Black was lower than the proportion of Black non–travel-associated cases when compared with White cases. By constrast, the proportion of international travel-reported cases as Asian was higher than the proportion of non–travel-associated cases.
TABLE 1—
Demographics and Outcomes of FoodNet Campylobacter Cases With Known Travel Status: United States, 2005–2011
| Characteristic | No international Travel, No. (%) or Mean (Range) | International Travel, No. (%) or Mean (Range) | OR (95% CI) | P |
| Age, y | 36.19 (0-101.4) | 37.13 (0-90.1) | 1.00 (1.000, 1.003) | .014 |
| Sex | ||||
| Male | 10 999 (54.91) | 2326 (53.06) | 1.00 (Ref) | |
| Female | 9033 (45.09) | 2058 (46.94) | 1.08 (1.01, 1.15) | .026 |
| Race | ||||
| White | 15 735 (78.50) | 3211 (73.16) | 1.00 (Ref) | |
| Black | 864 (4.31) | 135 (3.08) | 0.76 (0.63, 0.92) | .006 |
| Asian | 408 (2.04) | 189 (4.31) | 2.27 (1.90, 2.70) | < .001 |
| Other | 807 (4.03) | 153 (3.49) | 0.93 (0.78, 1.10) | .415 |
| Ethnicity | ||||
| Non-Hispanic | 15 320 (88.92) | 3269 (88.90) | 1.00 (Ref) | |
| Hispanic | 1909 (11.08) | 408 (11.10) | 1.00 (0.89, 1.12) | .978 |
| Hospitalized | ||||
| No | 15 881 (80.58) | 3987 (93.44) | 1.00 (Ref) | |
| Yes | 3827 (19.42) | 280 (6.56) | 0.29 (0.23, 0.31) | < .001 |
| Deaths | ||||
| No | 19 635 (99.92) | 4256 (99.98) | 1.00 (Ref) | |
| Yes | 15 (0.08) | 1 (0.02) | 0.31 (0.02, 1.92) | .349 |
| Total | 20 044 | 4389 |
Note. CI = confidence interval; OR = odds ratio. Sites include the following states: CA, CO, CT, GA, MD, MN, NM, NY, OR, and TN.
Cases associated with international travel were significantly less likely to require hospitalization compared with non–travel-associated cases, (OR = 0.26; 95% confidence interval [CI] = 0.22, 0.30; P < .001); however, duration of hospital stay was not significantly different between international travel- and non–travel-associated cases that required hospitalization (coefficient = 0.93; 95% CI = 0.78, 1.10; P = .395). Very few deaths were reported overall among cases with known travel status (16 total), and only 1 death was reported in the international travel group, so differences in case fatality ratios could not be assessed (Table 1).
NARMS antimicrobial resistance data were available for roughly 16% of all FoodNet campylobacteriosis cases reported between 2005 and 2011. Antimicrobial resistance data were available for 4804 (20%) of the campylobacteriosis cases with known travel status (Figure 1). Demographics among the subset of 4804 NARMS-linked cases with known travel status were not substantially different from the total group of 24 433 cases with known travel history.
Overall, 23% of all NARMS-linked cases in this study were quinolone-resistant, and 2.0% were macrolide-resistant. Of the 1078 quinolone-resistant NARMS-linked cases with known travel status, 543 (50%) were associated with international travel and 535 (50%) were associated with no international travel in the 7 days prior to illness onset. The relative proportion of quinolone-resistant cases associated with international travel decreased significantly over the 7-year period from 69% to 44%. The 543 international travel-associated quinolone-resistant cases represent 61% of all travel-associated campylobacteriosis cases from all years. By contrast, the 535 non–travel-associated quinolone-resistant cases represent only 14% of non–travel-associated cases. The overall proportion of quinolone-sensitive cases with known travel status associated with international travel was only 9.2% (342 cases), again with a significant decrease over time (12% to 7.7%).
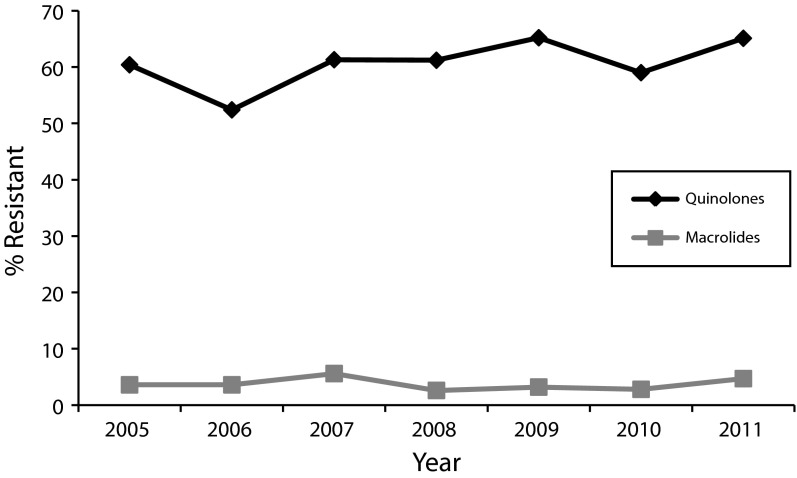
Although the overall proportion of cases with macrolide resistance was relatively low, cases with macrolide-resistant Campylobacter were associated with international travel 37% of the time (33 cases), compared with only 18% (852 cases) of cases with macrolide-susceptible isolates. This proportion decreased significantly among macrolide-susceptible isolates, from 23% to 16%, although there was no significant change among macrolide-resistant isolates overall during that time period. Similar to the findings with quinolones, 3.7% of international travel-associated Campylobacter isolates cases were macrolide-resistant, yet only 1.4% of non–travel-associated isolates had macrolide resistance. For both antibiotic classes, the differences in proportion resistant between international and non–travel-associated cases were significant (ciprofloxacin OR = 9.96; 95% CI = 8.31, 11.96; P < .001; azithromycin OR = 2.76; 95% CI = 1.71, 4.38; P < .001). Over the period 2005 through 2011, there was no significant increase in quinolone resistance among international travel-associated cases (60% to 65%; χ2 = 5.17; df = 6; P = .522; Figure 2). There was no significant change in resistance to macrolides over the same period (Figure 2).
FIGURE 2—
Difference in resistance between quinolones and macrolides among international travel-associated Campylobacter cases reported to FoodNet: 2005–2011.
Among international travel-associated cases, the proportion with quinolone-resistant Campylobacter isolates that required hospitalization was 5.8. The proportion requiring hospitalization with quinolone-susceptible Campylobacter was 6.8%. Among non–travel-associated cases, these proportions were 20% and 80% for resistant Campylobacter, and 18% and 82% for susceptible isolates, respectively. In multivariable analysis with travel as an additional covariate, antibiotic resistance did not appear to be significantly associated with odds of requiring hospitalization (ciprofloxacin OR = 0.96; 95% CI = 0.75, 1.21; P = .728; azithromycin OR = 1.22; 95% CI = 0.62, 2.21; P = .536); nor did it appear to be associated with duration of hospital stay among hospitalized cases (ciprofloxacin OR = 1.07; 95% CI = 0.90, 1.29; P = .427; azithromycin OR = 0.82; 95% CI = 0.46, 1.35; P = .475). However, when we stratified cases by travel status, having macrolide-resistant Campylobacter increased the odds of hospitalization among international travel-associated cases more than 3 times that of those with a susceptible organism (OR = 3.63; 95% CI = 1.13, 9.83, P = .017). This was not the case for non–travel-associated cases (OR = 0.82; 95% CI = 0.35, 1.25, P = .19).
The largest proportion of cases with known international travel to a single destination were associated with travel to locations in Asia, Mexico, and South America. Among cases associated with travel to these locations, a high proportion had Campylobacter isolates resistant to the 2 quinolones (78%, 62%, and 83%, respectively). Far fewer cases were associated with Eastern Europe as the destination, but almost 80% of isolates from travelers to this region were resistant. Only 2.5% of cases were associated with China as their sole destination, but of isolates received from these 18 travelers, 100% had quinolone-resistant Campylobacter. Peru also had a large proportion of resistant Campylobacter; roughly 6.4% of cases were associated with this as their sole destination, and 93% of those had a quinolone-resistant organism. Africa, Australia/New Zealand, and North America had the lowest proportions of resistant isolates (Table 2). There was very little resistance to the macrolides azithromycin and erythromycin. The proportion of macrolide-resistant Campylobacter isolates was highest in cases associated with travel to China (11%), Asia excluding China (5.8%), and Eastern Europe (5.3%). The proportion of macrolide resistance among cases associated with travel to other international destinations was less than 5% (Table 2).
TABLE 2—
Proportion of FoodNet Campylobacter Cases and Antimicrobial Resistant Isolates by Region: 2005–2011
| Destination | Travelers to Single Destination,a No. (%) | Total Isolates Submitted, No. | Quinolone Resistant Isolates, No. (%) | Macrolide Resistant Isolates, No. (%) |
| Africa | 295 (7.42) | 74 | 28 (37.34) | 1 (1.35) |
| Asiab | 835 (20.99) | 154 | 120 (77.92) | 9 (5.84) |
| China | 101 (2.54) | 18 | 18 (100.00) | 2 (11.11) |
| Australia/New Zealand | 44 (1.11) | 10 | 0 (0.00) | 0 (0.00) |
| Central Americab | 490 (12.32) | 104c | 53 (50.96) | 3 (2.86) |
| South America | 801 (20.13) | 115 | 95 (82.61) | 5 (4.35) |
| Mexico | 559 (14.05) | 145 | 90 (62.07) | 6 (4.14) |
| Eastern Europe | 69 (1.73) | 19 | 15 (78.95) | 1 (5.26) |
| Western Europe | 697 (17.52) | 139c | 69 (49.64) | 2 (1.45) |
| North America | 87 (2.19) | 17 | 2 (11.76) | 0 (0.00) |
| Total | 3554 | 795 | 490 | 29 |
Of 4141 cases with known international travel destination, 277 reported multiple destinations.
Numbers for Asia exclude China. Numbers for Central America exclude Mexico.
There were 105 isolates from cases reporting Central America as their destination and 138 isolates from cases reporting Western Europe submitted for azithromycin resistance testing.
DISCUSSION
This study demonstrates the important contribution of international travel to Campylobacter disease burden in the United States, as well as to the acquisition of antibiotic resistant infections. We estimate that during 2005 through 2011, roughly 18% of US Campylobacter cases were associated with international travel in the week before illness onset. This is comparable to proportions described in other studies5 and higher than what has been reported for many other enteric bacterial infections.8 Differences in the characteristics of international travel-associated cases and non–travel-associated cases were observed. Travel-associated cases were slightly older on average than non–travel-associated cases, possibly because there were fewer infants and young children traveling than adults. According to the US Travel Association, travelers with young children constitute only 30% of US adults traveling for leisure, and the highest proportion of travelers (31%) were born between 1965 and 1980.13 There were also subtle differences in travel-associated campylobacteriosis by race. Destination also varied slightly with race, with more international travel-associated cases among Asians reporting travel to Asia and more international travel-associated cases among Blacks reporting travel to Africa. The cause could be case-patients having emigrated from these countries or having friends and family residing there, and other research has demonstrated that these travelers are more likely than first-time visitors to contract an illness.14 However, our study could not assess country of origin for cases or the nature of the travel.
The odds of hospitalization were markedly decreased for international travel-associated cases compared to those without international travel. This may be attributed to the “healthy traveler effect.” This effect has been observed in many other studies, including those demonstrating a reduced risk of heart disease and death after vacation,15 as well as long-term stress reduction attributed to “leisure coping.”16 Additionally, previous research has observed both decreased duration of hospital stay and decreased mortality among international travelers with a diarrheal infection.17,18 Although our study was not able to fully assess infection severity, the reduced risk of hospitalization among travelers coupled with these previous research findings supports the idea that individuals who travel internationally are healthier and therefore have better outcomes than case-patients without international travel.
Among all cases with a quinolone-resistant Campylobacter infection in which travel status was known, half of them were associated with international travel. This proportion declined slightly from 2005 through 2011; however, this decline occurred in the setting of an overall increase in the actual number of resistant cases of both international travel-associated cases and those not associated with international travel. And importantly, the number of US residents traveling internationally decreased dramatically over the last few years, from a peak of 4.08 million overseas air travelers in 2007 to a low of 3.61 million in 2011.19 Should overall international travel increase again, the proportion of antimicrobial-resistant cases associated with international travel could increase as well.
Among international travel-associated cases, 60% of the Campylobacter isolates were resistant to quinolones compared with 13% of the non–travel-associated cases. This differs from a 2004 study which reported a different trend; from 1998 to 1999 more quinolone-resistant Campylobacter infections were acquired domestically than internationally, although the risk of acquiring quinolone-resistant Campylobacter was still more than 7 times higher among international travel-associated cases than those not associated with international travel.6 This higher proportion among domestic cases in the late 1990s was speculated to be related to the introduction of fluoroquinolones for use in US poultry flocks in 1995 (sarafloxacin) and 1996 (enrofloxacin).6,20 Macrolide resistance was less common and did not increase substantially over the period 2004 through 2010; however, as with quinolone resistance, macrolide resistance was identified in a higher proportion of international travel-associated cases than those reporting no international travel. Our findings suggest that a substantial proportion of antimicrobial-resistant, particularly quinolone-resistant, Campylobacter isolated in the United States in recent years is acquired outside of the United States. Resistance to quinolones is high and increasing in certain countries, and therefore the increase in quinolone resistance seen in the United States might be ascribed in part to travel to these locations.7,21,22 Additionally, the increase seen in international travel-associated cases over domestic cases might be attributed in part to the less restricted use of quinolones in some parts of the world. In 1998, the FDA conducted an assessment of human health risk associated with the use of quinolones in poultry and the development of resistance in Campylobacter.23 Combined with additional information from the FDA and NARMS implicating quinolones in selection of resistant Campylobacter,24 this assessment resulted in a suspension of quinolone use by the US poultry industry (sarafloxacin in 2001 and enrofloxacin in 2005); however, because of a fitness advantage of quinolone-resistant Campylobacter, resistant strains have been observed to persist in food animal communities years after the cessation of antibiotic use.22,25,26
This study had several limitations. Acquisition of epidemiological data varied by site as well as over time, and travel status was not known for a substantial proportion of FoodNet campylobacteriosis cases. We did not identify any major differences in most demographic and outcome information between cases with known travel status and those in which travel status was not determined. However, if there are substantial differences in international travel between cases with unknown and known travel status, our estimates could either be too high or too low. In addition, no systematic attempt was made to collect detailed exposure information, including information about antibiotic use among either travel-associated or non–travel-associated cases. In recognition of the importance of this information, all FoodNet sites are now attempting to obtain travel status and related information for a higher proportion of reported cases. In particular, better characterization of antibiotic use among travel-associated cases could improve understanding of travel-associated resistance.
Secondly, travel destination was not obtained for all cases with known international travel, and destination-related results were presented only for cases associated with travel to a single destination. Furthermore, because we were not able to identify denominator data to determine the numbers of travelers to various destinations by state of residence for all FoodNet sites and all destinations, we were unable to calculate rates of infection among travelers or determine risks associated with travel.
Another limitation is that the submission scheme for sending Campylobacter isolates to NARMS varied for some sites over the study period, which affected the number of cases with antimicrobial data to link to epidemiological data. This makes it possible that resistance among international travel-associated and non–travel-associated campylobacteriosis cases differs from our report. Additionally, the level of resistance to certain antimicrobials is known to differ by species of Campylobacter. Because of the low number of nonjejuni isolates, we were unable to accurately assess any differences in resistance by species. Finally, we did not detect major changes in the proportion of travel-associated infections from 2005 through 2011, nor did we detect substantial changes in the proportions of antibiotic-resistant isolates associated with international travel during that period; however, it is possible that this 7-year period was too short to detect important changes in these factors.
This and other studies clearly document that a substantial proportion of campylobacteriosis cases identified in the United States are associated with international travel. US providers should be aware of the high prevalence of resistance among travelers, and consider culture and antibiotic resistance testing for patients with international travel. Furthermore, strategies aimed at reducing antibiotic resistance clearly must be a multinational effort. Additionally important is the considerable percentage of antibiotic-resistant Campylobacter, particularly quinolone-resistant Campylobacter found in US cases associated with international travel. This has great relevance for estimating the burden of Campylobacter cases, both resistant and susceptible, associated with exposures in the United States, for attributing infections to potential exposures, including specific foods and food commodities, and for assessing the effects of US efforts aimed at reducing these domestically acquired infections. Continued surveillance for campylobacteriosis cases through FoodNet and for antimicrobial resistance among cases through NARMS will be essential to monitoring these effects and continued efforts over time.
Acknowledgments
This work was supported by Cooperative Agreement Number 5U50CK000203 from the Centers for Disease Control and Prevention.
We would like to thank Brenna Hogan and Jordan Cahoon at the Maryland Health Department for help with initial data cleaning and editing. Additional thanks go to FoodNet and NARMS staff, including Jennifer Huang, Kevin Joyce, Barbara Mahon, Felicita Medalla, and Jared Reynolds at the Centers for Disease Control and Prevention for collecting and providing data, as well as assisting on early analysis.
Note. The opinions expressed by authors contributing to this journal do not necessarily reflect the opinions of the Centers for Disease Control and Prevention or the instutions with which the authors are affiliated.
Human Participant Protection
Information about human participants was collected as part of public health surveillance and de-identified prior to analysis.
References
- 1.Scallan E, Hoekstra RM, Angulo FJ et al. Foodborne illness acquired in the United States—major pathogens. Emerg Infect Dis. 2011;17(1):7–15. doi: 10.3201/eid1701.P11101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. Incidence and trends of infection with pathogens transmitted commonly through food—Foodborne Diseases Active Surveillance Network, 10 US sites, 1996–2012. MMWR Morb Mortal Wkly Rep. 2013;62(15):283–287. [PMC free article] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. CDC—Campylobacter, General Information. 2013. Available at: http://www.cdc.gov/nczved/divisions/dfbmd/diseases/campylobacter. Accessed December 23, 2013.
- 4.Ruiz-Palacios GM. The health burden of Campylobacter infection and the impact of antimicrobial resistance: playing chicken. Clin Infect Dis. 2007;44(5):701–703. doi: 10.1086/509936. [DOI] [PubMed] [Google Scholar]
- 5.Kendall ME, Crim S, Fullerton K et al. Travel-associated enteric infections diagnosed after return to the United States, Foodborne Diseases Active Surveillance Network (FoodNet), 2004–2009. Clin Infect Dis. 2012;54(suppl 5):S480–S487. doi: 10.1093/cid/cis052. [DOI] [PubMed] [Google Scholar]
- 6.Kassenborg HD, Smith KE, Vugia DJ et al. Fluoroquinolone-resistant Campylobacter infections: eating poultry outside of the home and foreign travel are risk factors. Clin Infect Dis. 2004;38(suppl 3):S279–S284. doi: 10.1086/381597. [DOI] [PubMed] [Google Scholar]
- 7.Pollett S, Rocha C, Zerpa R et al. Campylobacter antimicrobial resistance in Peru: a ten-year observational study. BMC Infect Dis. 2012;12:193–200. doi: 10.1186/1471-2334-12-193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention. CDC—Emerging Infections Program (EIP)–DPEI–NCEZID. 2011. Available at: http://www.cdc.gov/ncezid/dpei/eip. Accessed November 7, 2012.
- 9.Centers for Disease Control and Prevention. Foodborne Diseases Active Surveillance Network (FoodNet): FoodNet Surveillance Report for 2011 (Final Report) Atlanta, GA: 2012. pp. 1–52. [Google Scholar]
- 10.Centers for Disease Control and Prevention. National Antimicrobial Resistance Monitoring System for Enteric Bacteria (NARMS): Human Isolates Final Report, 2010. Atlanta, GA: 2012. pp. 1–75. [Google Scholar]
- 11.de Saussure PP. Management of the returning traveler with diarrhea. Therap Adv Gastroenterol. 2009;2(6):367–375. doi: 10.1177/1756283X09346668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.UNdata. United Nations Statistical Division Country and Area classifications—composition of Regions. 2012. Available at: http://millenniumindicators.un.org/unsd/methods/m49/m49regin.htm. Accessed November 7, 2012.
- 13.United States Travel Association. Travel facts and statistics. 2011. Available at: http://www.ustravel.org/news/press-kit/travel-facts-and-statistics. Accessed October 17, 2012.
- 14.Leder K, Tong S, Weld L et al. Illness in travelers visiting friends and relatives: a review of the GeoSentinel Surveillance Network. Clin Infect Dis. 2006;43(9):1185–93. doi: 10.1086/507893. [DOI] [PubMed] [Google Scholar]
- 15.Gump BB, Matthews K. Are vacations good for your health? The 9-year mortality experience after the Multiple Risk Factor Intervention Trial. Psychosom Med. 2000;62:608–612. doi: 10.1097/00006842-200009000-00003. [DOI] [PubMed] [Google Scholar]
- 16.Iwasaki Y. Counteracting stress through leisure coping: a prospective health study. Psychol Health Med. 2006;11(2):209–220. doi: 10.1080/13548500500155941. [DOI] [PubMed] [Google Scholar]
- 17.Koch K, Kristensen B, Holt HM, Ethelberg S, Mølbak K, Schønheyder HC. International travel and the risk of hospitalization with non-typhoidal Salmonella bacteremia. A Danish population-based cohort study, 1999–2008. BMC Infect Dis. 2011;11(1):277–285. doi: 10.1186/1471-2334-11-277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ternhag A, Törner A, Svensson A, Giesecke J, Ekdahl K. Mortality following Campylobacter infection: a registry-based linkage study. BMC Infect Dis. 2005;5:70–74. doi: 10.1186/1471-2334-5-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Program IATS. (I-92 data) Monthly tourism statistics. Available at: http://tinet.ita.doc.gov/research/monthly/departures/index.html. Accessed May 2, 2013.
- 20.Smith KE, Besser JM, Hedberg C et al. Quinolone-resistant Campylobacter jejuni infections in Minnesota, 1992–1998. N Engl J Med. 1999;340(20):1525–1532. doi: 10.1056/NEJM199905203402001. [DOI] [PubMed] [Google Scholar]
- 21.Moore JE, Barton MD, Blair IS et al. The epidemiology of antibiotic resistance in Campylobacter. Microbes Infect. 2006;8(7):1955–1966. doi: 10.1016/j.micinf.2005.12.030. [DOI] [PubMed] [Google Scholar]
- 22.Luangtongkum T, Jeon B, Han J, Plummer P, Logue CM, Zhang Q. Antibiotic resistance in Campylobacter: emergence, transmission and persistence. Future Microbiol. 2009;2:189–200. doi: 10.2217/17460913.4.2.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Food and Drug Administration. The Human Health Impact of Fluoroquinolone Resistant Campylobacter Attributed to the Consumption of Chicken Food and Drug Administration Center for Veterinary Medicine Document. Atlanta, GA: 2000. pp. 1–113. [Google Scholar]
- 24.Nelson JM, Chiller TM, Powers JH, Angulo FJ. Fluoroquinolone-resistant Campylobacter species and the withdrawal of fluoroquinolones from use in poultry: a public health success story. Clin Infect Dis. 2007;44(7):977–980. doi: 10.1086/512369. [DOI] [PubMed] [Google Scholar]
- 25.Price LB, Lackey LG, Vailes R, Silbergeld E. The persistence of fluoroquinolone-resistant Campylobacter in poultry production. Environ Health Perspect. 2007;115(7):1035–1039. doi: 10.1289/ehp.10050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Food and Drug Administration. FDA CVM proposes to withdraw poultry fluoroquinolones approval. 2000. Available at: http://www.fda.gov/AnimalVeterinary/NewsEvents/CVMUpdates/ucm133743.htm. Accessed December 18, 2012.