Abstract
Objectives. Our objective was to create a system dynamics model specific to weight gain and obesity in women of reproductive age that could inform future health policies and have the potential for use in preconception interventions targeting obese women.
Methods. We used our system dynamics model of obesity in women to test various strategies for family building, including ovulation induction versus weight loss to improve ovulation. Outcomes included relative fecundability, postpartum body mass index, and mortality.
Results. Our system dynamics model demonstrated that obese women who become pregnant exhibit increasing obesity levels over time with elevated morbidity and mortality. Alternatively, obese women who lose weight prior to pregnancy have improved reproductive outcomes but may risk an age-related decline in fertility, which can affect overall family size.
Conclusions. Our model highlights important public health issues regarding obesity in women of reproductive age. The model may be useful in preconception counseling of obese women who are attempting to balance the competing risks associated with age-related declines in fertility and clinically meaningful weight loss.
More than half of women of reproductive age in the United States are overweight or obese.1 This situation raises a number of health concerns, but most proximate for many of these young women is the fact that obesity is related to subfertility, often as a result of anovulation associated with polycystic ovary syndrome (PCOS).2 Also of concern, obesity is associated with serious obstetrical risks, including preeclampsia, gestational diabetes, and stillbirth.2 Perhaps most concerning for society is that maternal obesity increases the offspring’s risk for future obesity3–5 as well as obesity-related conditions such as diabetes.6,7
Because of the known risks associated with maternal obesity, clinicians often encourage obese women with subfertility to lose weight before they initiate fertility treatment. However, deferring fertility treatment involving weight loss can be prohibitive for some women because the time required to lose weight may also reduce their chances of conception, particularly if they are older than 35 years.8 Furthermore, published outcome data on weight loss in this population are scarce. Thus, obese women with subfertility may accept obesity-related risks and proceed with fertility treatment.
Given the prevalence of obesity among women of reproductive age, studies of prepregnancy weight loss aimed at improving fertility and maternal and infant outcomes are important. Unfortunately, such studies in real-life situations are difficult for several reasons, including poor compliance with weight loss strategies, limitations in resources, and the time required to achieve clinically meaningful weight loss. Innovations offered through system dynamics may help, as system dynamics allows for simulated yet clinically informative research on complex, real-world problems.9,10 The growing body of knowledge regarding weight loss and reproductive outcomes among obese women can be assimilated into a model incorporating system dynamics techniques to determine how to optimize outcomes for obese women with infertility.
Current dynamic models of weight change consider 2 major body components of nonpregnant humans: fat mass and fat-free mass.11–25 Although helpful, these models do not account for pregnancy, an important, gender-specific event associated with weight change over time. Previous conceptual models have assessed weight change specific to pregnancy,26–30 but only Thomas et al.25 modeled gestational weight gain mathematically. Their model, based on longitudinal study data on 63 pregnant women who took part in a study conducted by Butte et al.,31 focused on regression equations relating fat-free mass to fat mass. Other models investigating pregnancy-specific concerns include those of Osgood et al.,32 who studied gestational diabetes at the population level, and Thomas et al.,24 who developed a fetal energy balance model to capture fetal growth.
Although these pregnancy models are helpful, such individual-level models do not consider prepregnancy obesity, nor do they consider weight gain in women across the life span. This latter point is particularly important given that many younger women who are obese have few health care needs outside of those related to reproductive health. However, as these women age, they are at increased risk for conditions associated with obesity such as diabetes and cardiovascular disease. Pregnancy and its associated weight changes may affect these risks.
Understanding complex interactions over time between pregnancy weight gain and postpartum weight retention is critical in identifying opportunities for interventions so that effective and comprehensive public health prevention efforts for obese women and their offspring can be designed and implemented. Here we propose a system dynamics model of the feedback mechanisms driving gestational weight gain and offer a simulation of fecundity, pregnancy weight gain, and mortality in the clinical case of an obese woman with PCOS and associated anovulatory subfertility.
METHODS
We adopted a system dynamics approach to study obesity in the following phases of the female reproductive period: prepregnancy, gestational, and postpartum. In each phase, different factors interact and intervene to influence weight change. The resulting causal map is shown in Figure 1, incorporating the conventions described by Sterman.33 In this model, causal links with a positive polarity indicate that if the cause increases in magnitude, the effect will also increase, all else being equal.
FIGURE 1—
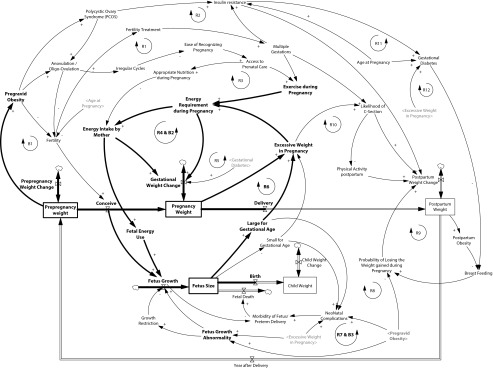
Causal map outlining basic elements that may affect projected changes in body weight over time among obese women of reproductive age.
Note. B = balancing feedback loop; R = reinforcing feedback loop. The causal structure demonstrates complex interactions between sociological and physiological factors contributing to obesity and reflects how these elements may contribute to transgenerational obesity. We simulated the prepregnancy and pregnancy phases shown by the highlighted links and variables.
For example, when “pregravid obesity” increases, “fetus growth abnormality” also increases, and thus the causal link from pregravid obesity to fetus growth abnormality is positive. However, the link from “pregravid obesity” to “fertility” has a negative polarity, meaning that the greater the extent to which the woman is obese, the lower her likelihood of becoming pregnant, all else being equal. If these causal connections form a closed chain, the result is a feedback loop that is either reinforcing (“R” in Figure 1) or balancing (“B”). Reinforcing feedback loops can lead to virtuous or vicious cycles, whereas balancing loops are goal-seeking system structures.33
Our model considered distinct sets of stock variables related to weight change in the different reproductive phases and separate stock variables related to fetal growth and child weight (after birth). Relationships between factors influencing weight change are demonstrated by the reinforcing loops in Figure 1 and include physical activity, insulin resistance, gestational diabetes, PCOS, multiple gestations, assisted reproductive technologies, and contraception.
Prepregnancy obesity is associated with subfertility and increased use of fertility treatment, including assisted reproductive technologies.34 Fertility treatments are associated with an increase in the risk of multiple gestations, which in turn lead to increased gestational weight gain as a result of reduced exercise during pregnancy as well as a higher likelihood of a caesarean section, contributing to maternal obesity (loop R1 in Figure 1). If subfertility caused by obesity goes untreated, there are fewer pregnancies and less chance for gestational weight gain (loop B1).
The reinforcing loop R3 emphasizes how prepregnancy obesity would delay pregnancy recognition, thereby delaying access to prenatal care and elevating gestational weight gain. If prenatal care were available earlier in a woman’s pregnancy, it would lead to appropriate nutrition and physical activity and thereby reduce maternal obesity.
The R2 loop demonstrates how prepregnancy obesity elevates the risk of PCOS and consequently leads to an increase in insulin resistance, weight gain after pregnancy, and maternal obesity. Insulin resistance also causes gestational diabetes, which may contribute to excessive weight gain in pregnancy (loop R5). Furthermore, excessive weight during pregnancy leads to an elevated risk of gestational diabetes and, thus, higher gestational weight gain (loop R12). Obesity in pregnancy also increases the likelihood of a caesarean section, frequently reducing the mother’s physical activity after delivery. This can lead to higher postpartum weight gain and further aggravation of maternal obesity (loop R10).
Reinforcing loops R6 and R7 demonstrate associations between prepregnancy obesity and increased fetal macrosomia, increased gestational weight gain, and increased maternal obesity. A macrosomic fetus would also be at an increased risk for elevated weight in infancy and childhood obesity. In addition, maternal obesity is associated with fetal growth restriction, another fetal growth abnormality, and could lead to a small-for-gestational-age fetus (loop B3), also associated with obesity later in life. It is important to consider as well that an obese mother has higher energy requirements during pregnancy, and therefore she must increase her intake of calories. Without sufficient caloric intake, she will experience weight loss during pregnancy (loop B2).
Obese mothers are more susceptible to diabetes than nonobese mothers, particularly if they developed gestational diabetes during their pregnancy. This decreases their chances of breastfeeding after delivery35 and thus increases their obesity risk (loop R12). Furthermore, when the mother is obese after delivery, she is less likely to breastfeed; in addition to elevating the risk of childhood obesity, this also tends to lead to higher postpartum weight retention and a higher risk of diabetes (loop R9).
Case Scenario
As our system dynamics model illustrates, obesity, weight loss, and fertility represent a complex system composed of diverse factors that may interact with trade-offs that vary over time.10 To simplify our model and demonstrate a potential clinical application, we applied the model to a commonly encountered clinical case scenario that highlights salient decisions faced by many women of reproductive age who are affected by obesity.
The patient in our case study is a 34-year-old nulliparous White woman presenting for a preconceptional consultation. She is healthy and has no known medical problems. She stopped her oral contraceptive pills 6 months ago and has had 1 period since. The only significant factor in her physical examination is her weight of 253 pounds (114.8 kg). At 5 feet, 3 inches (160 cm) tall, her body mass index (BMI, defined as weight in kilometers divided by the square of height in meters) is 44.8.
The reproductive, intergenerational, and long-term health risks associated with obesity are discussed with the patient, and her clinician raises several points for her to consider. For example, her lack of menses indicates that she may have PCOS, one of the most common endocrine disorders encountered by women; there is an increased incidence of PCOS among obese women associated with subfertility secondary to oligo-ovulation (i.e., infrequent or irregular ovulation).36 Accordingly, she will likely require an intervention to achieve ovulation. Several medications can be given to induce ovulation, but most are associated with an increased risk of multiple gestations.
Alternatively, overweight women with PCOS often ovulate spontaneously after weight loss. The patient is advised that a BMI below 30 would be ideal for minimizing her obstetrical risks. By losing 91 pounds, she would achieve a BMI of 29.2 and likely ovulate spontaneously. The drawback is that losing 91 pounds requires a substantial amount of time. Given that she is 34 years old, her age alone has an impact on fertility, and waiting 6 months to lose weight can have a negative impact on oocyte quality.8 By contrast, losing 5% of body weight (13 pounds for this woman) can restore ovulation in women with PCOS; however, if the woman in our scenario chooses not to lose a significant amount of weight, she faces increasing obesity and associated risks over time.
This woman faces competing risks, including the time required for losing weight versus age-associated declines in fertility. Our simulated systems model may help such women balance these risks and make appropriate decisions.
The Simulation
As mentioned, our patient has important decisions to make. She can take medication to become pregnant or lose a prespecified amount of weight to achieve ovulation and spontaneous conception. Because there are lasting risks and benefits related to each scenario, a simulation of our system dynamics model might be helpful in evaluating these options. For this simulation, we focus on the prepregnancy and pregnancy phases of our model (shown by the highlighted links and variables in Figure 1).
In simulating our model, we incorporated elements from currently available models of weight gain. The Hall et al.18 model, an energy balance model across the life span, was chosen to characterize the prepregnancy phase. Because the Hall et al. model lacks input regarding pregnancy, we used the Thomas et al.25 model, the only computational model of gestational weight currently available, to inform our modeling of the pregnancy phase.
The Thomas et al.25 pregnancy energy balance model has limitations that required developing a new model. Their model does not account for extreme obesity (BMI of ≥ 40) because it is heavily dependent on a data set collected from 63 women of whom only 8 were classified as overweight (BMI of 25–29.9) and 4 as obese (BMI of ≥ 30).31 Therefore, the model cannot be reliably applied to a wider variety of individuals that includes those who are obese and extremely obese.
Furthermore, the Thomas et al.25 model estimates total energy expenditure (TEE) as a monolithic quantity comprising basal metabolic rate (BMR), activity energy expenditure, and the thermic effect of food.31 In other words, the model does not compute different elements of BMR, physical activity, and the thermic effect of food separately. Thus, TEE values cannot be adjusted if an individual has higher or lower levels of physical activity, different BMR levels, or adaptive thermogenesis. As a result, the model does not allow for individual adjustments in important contributors to weight gain such as physical activity level and BMR.
Moreover, the TEE values computed in the Thomas et al.25 model are larger than the corresponding estimates in the Hall et al.18 model. For example, the prepregnancy TEE for a woman who weighs 75 kilograms is computed as approximately 2900 kilocalories per day in the Thomas et al. model, as opposed to 1989 kilocalories per day for a nonexercising woman in the Hall et al. model. Because the Thomas et al. model also estimates base energy intake (EI) values according to TEE values, the model is incapable of accurately estimating EI values, although it can predict gestational weight gain on the basis of changes in caloric intake values during pregnancy.
Also, the Thomas et al.25 model does not consider fetal growth and weight gain and maternal weight gain separately despite the fact that doing so would be helpful in modeling important conditions such as fetal growth restriction. In addition, in estimating growth of fat-free mass, the model considers the aggregate growth of placental tissues, uterus expansion, increases in the size of mammary glands, and increases in blood flow and extracellular fluid. However, the model results do not distinguish which components exhibit larger or smaller growth, which would be helpful in identifying signs of abnormality in a pregnancy.37,38
We suggest using a modified version of the Hall et al. model to predict maternal weight gain and the Thomas et al.25 model to predict fetal growth. In the Hall et al.18 model, the gap between energy intake and energy expenditure (Energy Stored = EI − TEE) is reserved in fat mass and fat-free mass. EI is equal to the caloric intake of food and beverages. TEE is computed from adding terms such as BMR, physical activity, thermic effect of food, adaptive thermogenesis, and energy required for the synthesis of fat mass and fat-free mass, which are computed separately in the model. Because the model does not make assumptions regarding initial weight, it can be applied to any range of weight categories, including obese and extremely obese.
To adjust the Hall et al.18 model to capture gestational weight gain, one needs to consider additional components that start to grow after conception and during pregnancy. During pregnancy, extra dietary energy is required to support the weight gain (values in parentheses are averages) resulting from the growth of the placenta (0.7 kg) and fetus (3.2–3.6 kg), increases in amniotic fluid (0.9 kg) and maternal tissues including the uterus (0.9 kg) and breasts (0.45–1.4 kg), and increases in amounts of blood (1.4–1.8 kg), extracellular water (0.9–1.4), and maternal fat stores (2.7–3.6 kg), as well as their associated maintenance energy consumption.39,40 To model pregnancy weight dynamics, we needed to model how energy is deposited in maternal and fetal tissues. Thus, we needed to model the growth rate for each of these components and their required energy expenditure (for further details on the assumptions made in these areas, see Appendix A, available as a supplement to the online version of this article at http://www.ajph.org).
RESULTS
We simulated the 4 different scenarios shown in Figure 2 and Table 1. In the fertility treatment scenario, if the patient considered decides not to lose weight but takes medication to induce ovulation, she can expect to become pregnant at the age of 34 years but will conceive with a BMI of 45. Considering her age and BMI, her hazard ratio (relative to the BMI reference category of 22.5–24.9) for all-cause mortality is 3.7. After delivery, her BMI will likely be higher at 47. As a result of the medication, she may have multiple gestations and increased pregnancy complications, including gestational diabetes and its associated risks. Because she is likely to be extremely obese after delivery, she is at high risk of encountering difficulty in breastfeeding and will not accrue the associated benefits for herself and her child.
FIGURE 2—
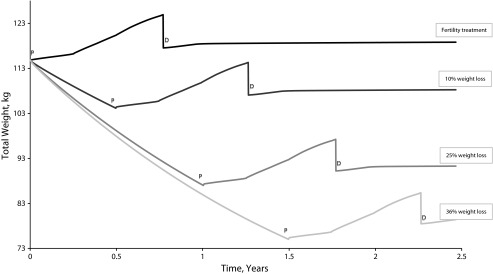
Prepregnancy weight loss and pregnancy weight gain with additional energy intake of 117, 360, and 475 kcal per day in the first, second, and third trimesters, respectively.
Note. D = weight after delivery; P = pregravid weight.
TABLE 1—
Analysis of the 4 Different Simulation Scenarios
| Scenario |
||||
| Outcome | Fertility Treatmenta | 10% Weight Loss | 25% Weight Loss | 36% Weight Loss |
| Lifestyle change | ||||
| Calorie reduction, kcal/d | . . . | 681 | 805 | 854 |
| Change in physical activity (min/frequency per wk) | . . . | Walking lightly (20/3) | Walking lightly (20/5) and running at a medium level (15/3) | Walking lightly (30/5) and running lightly (25/3) |
| Duration, mo | . . . | 6 | 12 | 18 |
| Resulting body weight loss, % (amount, kg) | . . . | 10 (11) | 25 (28) | 36 (40) |
| Simulation model outcomes | ||||
| Age at pregnancy, y | 34 | 34 | 35 | 36 |
| Pregravid | ||||
| Weight, kg | 114.8 | 104.1 | 87 | 75 |
| BMI, kg/m2 | 44.8 | 40.7 | 34.0 | 29.3 |
| 40 wk of pregnancy | ||||
| Maternal weight, kg | 121.2 | 110.8 | 94.0 | 82.4 |
| Fetal weight, kg | 3.7 | 3.5 | 3.2 | 2.9 |
| Postpartum | ||||
| Weight, kg | 119.8 | 109.4 | 92.6 | 80.9 |
| BMI, kg/m2 | 46.8 | 42.7 | 36.2 | 31.6 |
| Outcomes from the literature | ||||
| Fecundability ratio | ||||
| Weight basedb | . . .c | 0.52 | 0.53 | 0.80 |
| Age basedd | . . .c | 0.74 | 0.55 | 0.55 |
| Mortality hazard ratio (95% CI)e | 3.70 (3.03, 4.50) | 3.70 (3.03, 4.50) | 1.79 (1.61, 1.99) | 1.44 (1.29, 1.61) |
| Gestational diabetes risk, ORf | 5.55 | 5.55 | 3.01 | 1.97 |
| Risk of adverse fetal outcomes | ||||
| Breastfeeding cessation, OR (95% CI)g | 2.54 (1.70, 3.79) | 2.54 (1.70, 3.79) | 2.54 (1.70, 3.79) | 1.52 (1.02, 2.28) |
| Large for gestational age, OR (95% CI)h | 2.08 (1.95, 2.23) | 2.08 (1.95, 2.23) | 2.08 (1.95, 2.23) | 1.53 (1.44, 1.63) |
| Fetal insulin resistancei | 0.31* | 0.31* | 0.31* | 0.31* |
Note. BMI = body mass index; CI = confidence interval; OR = odds ratio.
Woman opts for no lifestyle change and instead pursues ovulation induction.
Fecundability ratio (relative to normal-weight women) for women aged 30 years or older.41
The conception rate among women with polycystic ovary syndrome who are treated with clomiphene citrate is 39.5%,42 and the rate is similar to that among women receiving letrozole.
Fecundability ratio relative to the reference group of women 19–26 years of age.43
Based on BMI and age group.44
Based on prepregnancy BMI (relative to normal-weight women).45
Odds of cessation by 1 week (relative to normal-weight women).46
Relative to normal-weight women.47
Correlation coefficient relative to maternal pregravid weight.48
*P = .007.
The alternative scenarios tested preconceptional weight loss ranging between 11 and 41 kilograms. Although losing weight will increase the fecundability ratio, it will require between 6 and 18 months depending on how much weight is lost. Increasing age negatively influences women’s chances of becoming pregnant, but a lower BMI will reduce mortality and improve their pregnancy outcomes. For example, in our third scenario, our patient can lose 25% of her current weight by consuming 805 fewer kilocalories per day than her usual intake of 2545 kilocalories per day, walking lightly for 20 minutes 5 times a week, and running for 15 minutes 3 times a week. Over the course of a year, she can expect to reach a BMI of 34 and to have a lower mortality hazard ratio of about 1.79. The chances of the woman in this scenario becoming pregnant are lower than those in the other scenarios given that she is aging and is still obese before her pregnancy. However, she will experience a far healthier pregnancy and delivery and be at lower risk of adverse fetal outcomes.
Furthermore, Figure 2 shows that after her delivery, her weight reaches an unstable equilibrium that is path dependent on her pregravid weight. The phenomenon of path dependence is common in natural and human systems. In this pattern of behavior, small random events early in the history of a system determine the ultimate end state, even when all end states are equally likely at the beginning.33 Hammond10 describes this pattern as characteristic of complex adaptive systems whose subsequent direction is strongly affected by the particular order in which events occur.
During her pregnancy, our patient’s energy intake is higher owing to her additional energy requirements. After delivery, if she still consumes additional food following the pattern of pregnancy, the positive feedback loop of energy balance will lead to even further weight gain. This is also an outcome of path dependency; that is, once a dominant behavior has been established, “the costs of switching become prohibitive, so the equilibrium is self-enforcing: the system has locked in.”33(p406) The lock-in phenomenon persists “until an architectural shift or external shock renders the dominant design obsolete.”33(p406) Our patient will continue to gain weight unless she makes a substantial effort to change her diet and activity level.
DISCUSSION
Our novel system dynamics model demonstrates how the risks faced by obese women who are considering pregnancy can be quantified through incorporation of important physiological, sociological, and gender-specific factors affecting weight gain and retention. In addition to providing a framework for a future decision tool that can be used to help women make important preconceptional decisions, our system dynamics approach illustrates a number of novel concepts that may be important with respect to the obesity epidemic. Our simulation model can easily be used by clinical practitioners, researchers, or patients, and it can be applied to any individual case by initializing it on the basis of a woman’s weight, height, age, and ethnicity. Users can test different weight-loss scenarios to assess the impact on maternal and fetal weight and the subsequent risks and benefits. The results of such experiments with individuals of different ages and ethnicities can provide quantitative evidence to inform public health policies and promote obesity prevention among women of reproductive age.
Simulation modeling related to obesity has aspired to an integrative perspective that captures diverse health effects outside the purview of traditional models. However, the large majority of simulation models have not included characterizations of dynamic processes involving weight patterns related to women’s reproductive health and childbearing. Thus, previous models have limited their scope to effects that are common between men and women. This omission is important not only in light of the impact of obesity on fertility, but also because of the recognized association between the prenatal and perinatal periods and adverse health outcomes and health status across the life course.6,7,49–51 Consideration must be given as well to the substantial evidence suggesting that pregnancy experiences may play a key driving role in shaping the risk of chronic diseases in mothers, children, and future generations.6,32,50
The goal of the model described here is to deepen and operationalize initial insights from the few previous simulation studies that have assessed weight in the context of pregnancy.32 This model can also serve as an important step toward providing an actionable tool that helps women, clinicians, and those involved in the field of public health understand how best to manage the tangled web of causation associated with weight dynamics and reproductive health and outcomes.
Acknowledgments
Financial support was provided by the National Cancer Institute (Washington University Translational Research on Energetics and Cancer Project 4; contract U54 CA155496), the National Institutes of Health Office of Behavioral and Social Sciences Research (contract NNSN276200900017C/HHSN276201200027C), and the Women’s Reproductive Health Research Scholar Program (grant K12 HD063086).
Human Participant Protection
No protocol approval was necessary because no human participants were involved.
References
- 1.Dalenius K, Brindley P, Smith B, Reinold C, Grummer-Strawn L. Pregnancy Nutrition Surveillance System 2010 Report. Atlanta, GA: Centers for Disease Control and Prevention; 2012. [Google Scholar]
- 2.Jungheim ES, Moley KH. Current knowledge of obesity’s effects in the pre- and periconceptional periods and avenues for future research. Am J Obstet Gynecol. 2010;203(6):525–530. doi: 10.1016/j.ajog.2010.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brisbois TD, Farmer AP, McCargar LJ. Early markers of adult obesity: a review. Obes Rev. 2012;13(4):347–367. doi: 10.1111/j.1467-789X.2011.00965.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dietz WH. Critical periods in childhood for the development of obesity. Am J Clin Nutr. 1994;59(5):955–959. doi: 10.1093/ajcn/59.5.955. [DOI] [PubMed] [Google Scholar]
- 5.Gunderson EP. Childbearing and obesity in women: weight before, during, and after pregnancy. Obstet Gynecol Clin North Am. 2009;36(2):317–332. doi: 10.1016/j.ogc.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dabelea D, Knowler WC, Pettitt DJ. Effect of diabetes in pregnancy on offspring: follow-up research in the Pima Indians. J Matern Fetal Med. 2000;9(1):83–88. doi: 10.1002/(SICI)1520-6661(200001/02)9:1<83::AID-MFM17>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 7.Dyck RF, Klomp H, Tan L. From “thrifty genotype” to “hefty fetal phenotype”: the relationship between high birthweight and diabetes in Saskatchewan Registered Indians. Can J Public Health. 2001;92(5):340–344. doi: 10.1007/BF03404975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.American College of Obstetricians and Gynecologists. ACOG committee opinion: age-related fertility decline. Obstet Gynecol. 2008;112(2):409–411. doi: 10.1097/AOG.0b013e318183fbe6. [DOI] [PubMed] [Google Scholar]
- 9.Ip EH, Rahmandad H, Shoham DA et al. Reconciling statistical and systems science approaches to public health. Health Educ Behav. 2013;40(suppl 1):123S–131S. doi: 10.1177/1090198113493911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hammond RA. Complex systems modeling for obesity research. Prev Chronic Dis. 2009;6(3):A97. [PMC free article] [PubMed] [Google Scholar]
- 11.Abdel-Hamid TK. Modeling the dynamics of human energy regulation and its implications for obesity treatment. Syst Dyn Rev. 2002;18(4):431–471. [Google Scholar]
- 12.Butte NF, Christiansen E, Sorensen TI. Energy imbalance underlying the development of childhood obesity. Obesity (Silver Spring) 2007;15(12):3056–3066. doi: 10.1038/oby.2007.364. [DOI] [PubMed] [Google Scholar]
- 13.Chow CC, Hall KD. The dynamics of human body weight change. PLOS Comput Biol. 2008;4(3):e1000045. doi: 10.1371/journal.pcbi.1000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Christiansen E, Garby L. Prediction of body weight changes caused by changes in energy balance. Eur J Clin Invest. 2002;32(11):826–830. doi: 10.1046/j.1365-2362.2002.01036.x. [DOI] [PubMed] [Google Scholar]
- 15.Christiansen E, Garby L, Sorensen TI. Quantitative analysis of the energy requirements for development of obesity. J Theor Biol. 2005;234(1):99–106. doi: 10.1016/j.jtbi.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 16.Flatt JP. Carbohydrate-fat interactions and obesity examined by a two-compartment computer model. Obes Res. 2004;12(12):2013–2022. doi: 10.1038/oby.2004.252. [DOI] [PubMed] [Google Scholar]
- 17.Hall KD. Mechanisms of metabolic fuel selection: modeling human metabolism and body-weight change. IEEE Eng Med Biol Mag. 2010;29(1):36–41. doi: 10.1109/MEMB.2009.935465. [DOI] [PubMed] [Google Scholar]
- 18.Hall KD, Sacks G, Chandramohan D et al. Quantification of the effect of energy imbalance on bodyweight. Lancet. 2011;378(9793):826–837. doi: 10.1016/S0140-6736(11)60812-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kozusko FP. Body weight setpoint, metabolic adaption and human starvation. Bull Math Biol. 2001;63(2):393–403. doi: 10.1006/bulm.2001.0229. [DOI] [PubMed] [Google Scholar]
- 20.Kozusko FP. The effects of body composition on setpoint based weight loss. Math Comput Model. 2002;35(9–10):973–982. [Google Scholar]
- 21.Navarro-Barrientos JE, Rivera DE, Collins LM. A dynamical model for describing behavioural interventions for weight loss and body composition change. Math Comput Model Dyn Syst. 2011;17(2):183–203. doi: 10.1080/13873954.2010.520409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Song B, Thomas DM. Dynamics of starvation in humans. J Math Biol. 2007;54(1):27–43. doi: 10.1007/s00285-006-0037-7. [DOI] [PubMed] [Google Scholar]
- 23.Thomas DM, Ciesla A, Levine JA, Stevens JG, Martin CK. A mathematical model of weight change with adaptation. Math Biosci Eng. 2009;6(4):873–887. doi: 10.3934/mbe.2009.6.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thomas DM, Clapp JF, Shernce S. A foetal energy balance equation based on maternal exercise and diet. J R Soc Interface. 2008;5(21):449–455. doi: 10.1098/rsif.2007.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thomas DM, Navarro-Barrientos JE, Rivera DE et al. Dynamic energy-balance model predicting gestational weight gain. Am J Clin Nutr. 2012;95(1):115–122. doi: 10.3945/ajcn.111.024307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Davis EM, Stange KC, Horwitz RI. Childbearing, stress and obesity disparities in women: a public health perspective. Matern Child Health J. 2012;16(1):109–118. doi: 10.1007/s10995-010-0712-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hill B, Skouteris H, McCabe M et al. A conceptual model of psychosocial risk and protective factors for excessive gestational weight gain. Midwifery. 2013;29(2):110–114. doi: 10.1016/j.midw.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 28.Huang TT, Brownson PR, Esposito L, Green L, Homer C. Next steps in obesity prevention: applying the systems approach. Child Obes. 2013;9(1):11–14. doi: 10.1089/chi.2013.9102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nader PR, Huang TT, Gahagan S, Kumanyika S, Hammond RA, Christoffel KK. Next steps in obesity prevention: altering early life systems to support healthy parents, infants, and toddlers. Child Obes. 2012;8(3):195–204. doi: 10.1089/chi.2012.0004. [DOI] [PubMed] [Google Scholar]
- 30.Phillips J, King R, Skouteris H. A conceptual model of psychological predictors of postpartum weight retention. J Reprod Infant Psychol. 2012;30(3):278–288. [Google Scholar]
- 31.Butte NF, Wong WW, Treuth MS, Ellis KJ, O’Brian Smith E. Energy requirements during pregnancy based on total energy expenditure and energy deposition. Am J Clin Nutr. 2004;79(6):1078–1087. doi: 10.1093/ajcn/79.6.1078. [DOI] [PubMed] [Google Scholar]
- 32.Osgood ND, Dyck RF, Grassmann WK. The inter- and intragenerational impact of gestational diabetes on the epidemic of type 2 diabetes. Am J Public Health. 2011;101(1):173–179. doi: 10.2105/AJPH.2009.186890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sterman J. Business Dynamics: Systems Thinking and Modeling for a Complex World. Boston, MA: Irwin/McGraw-Hill; 2000. [Google Scholar]
- 34.Vahratian A, Smith YR. Should access to fertility-related services be conditional on body mass index? Hum Reprod. 2009;24(7):1532–1537. doi: 10.1093/humrep/dep057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gunderson EP. Breastfeeding after gestational diabetes pregnancy: subsequent obesity and type 2 diabetes in women and their offspring. Diabetes Care. 2007;30(suppl 2):S161–S168. doi: 10.2337/dc07-s210. [DOI] [PubMed] [Google Scholar]
- 36.Fauser BC, Tarlatzis BC, Rebar RW et al. Consensus on women’s health aspects of polycystic ovary syndrome (PCOS): the Amsterdam ESHRE/ASRM-Sponsored 3rd PCOS Consensus Workshop Group. Fertil Steril. 2012;97(1):28–38. doi: 10.1016/j.fertnstert.2011.09.024. [DOI] [PubMed] [Google Scholar]
- 37.Fowden AL, Moore T. Maternal-fetal resource allocation: co-operation and conflict. Placenta. 2012;33(suppl 2):e11–e15. doi: 10.1016/j.placenta.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 38.Oyelese Y. Placenta, umbilical cord and amniotic fluid: the not-less-important accessories. Clin Obstet Gynecol. 2012;55(1):307–323. doi: 10.1097/GRF.0b013e318248818e. [DOI] [PubMed] [Google Scholar]
- 39.Cunningham FG, Gant NF, Leveno KJ . Williams Obstetrics Textbook and Study Guide. 21st ed. New York, NY: McGraw-Hill Professional Publishing; 2001. [Google Scholar]
- 40.Hytten FE, Chamberlain G. Clinical Physiology in Obstetrics. St. Louis, MO: Mosby; 1991. [Google Scholar]
- 41.Wise LA, Rothman KJ, Mikkelsen EM, Sorensen HT, Riis A, Hatch EE. An Internet-based prospective study of body size and time-to-pregnancy. Hum Reprod. 2010;25(1):253–264. doi: 10.1093/humrep/dep360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Legro RS, Barnhart HX, Schlaff WD et al. Clomiphene, metformin, or both for infertility in the polycystic ovary syndrome. N Engl J Med. 2007;356(6):551–566. doi: 10.1056/NEJMoa063971. [DOI] [PubMed] [Google Scholar]
- 43.Dunson DB, Colombo B, Baird DD. Changes with age in the level and duration of fertility in the menstrual cycle. Hum Reprod. 2002;17(5):1399–1403. doi: 10.1093/humrep/17.5.1399. [DOI] [PubMed] [Google Scholar]
- 44.Berrington de Gonzalez A, Hartge P, Cerhan JR et al. Body-mass index and mortality among 1.46 million white adults. N Engl J Med. 2010;363(23):2211–2219. doi: 10.1056/NEJMoa1000367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Torloni MR, Betrán AP, Horta BL et al. Prepregnancy BMI and the risk of gestational diabetes: a systematic review of the literature with meta-analysis. Obes Rev. 2009;10(2):194–203. doi: 10.1111/j.1467-789X.2008.00541.x. [DOI] [PubMed] [Google Scholar]
- 46.Donath SM, Amir LH. Maternal obesity and initiation and duration of breastfeeding: data from the Longitudinal Study of Australian Children. Matern Child Nutr. 2008;4(3):163–170. doi: 10.1111/j.1740-8709.2008.00134.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yu Z, Han S, Zhu J, Sun X, Ji C, Guo X. Pre-pregnancy body mass index in relation to infant birth weight and offspring overweight/obesity: a systematic review and meta-analysis. PLoS One. 2013;8(4):e61627. doi: 10.1371/journal.pone.0061627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Catalano PM, Presley L, Minium J, Hauguel-de Mouzon S. Fetuses of obese mothers develop insulin resistance in utero. Diabetes Care. 2009;32(6):1076–1080. doi: 10.2337/dc08-2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Boney CM, Verma A, Tucker R, Vohr BR. Metabolic syndrome in childhood: association with birth weight, maternal obesity, and gestational diabetes mellitus. Pediatrics. 2005;115(3):e290–e296. doi: 10.1542/peds.2004-1808. [DOI] [PubMed] [Google Scholar]
- 50.Dabelea D, Hanson RL, Lindsay RS et al. Intrauterine exposure to diabetes conveys risks for type 2 diabetes and obesity: a study of discordant sibships. Diabetes. 2000;49(12):2208–2211. doi: 10.2337/diabetes.49.12.2208. [DOI] [PubMed] [Google Scholar]
- 51.Whincup PH, Kaye SJ, Owen CG et al. Birth weight and risk of type 2 diabetes: a systematic review. JAMA. 2008;300(24):2886–2897. doi: 10.1001/jama.2008.886. [DOI] [PubMed] [Google Scholar]


