Abstract
Disulfiram has shown promise as a pharmacotherapy for cocaine dependence in clinical settings, although it has many targets, and the behavioral and molecular mechanisms underlying its efficacy are unclear. One of many biochemical actions of disulfiram is inhibition of dopamine β-hydroxylase (DBH), the enzyme that converts dopamine (DA) to norepinephrine (NE) in noradrenergic neurons. Thus, disulfiram simultaneously reduces NE and elevates DA tissue levels in the brain. In rats, both disulfiram and the selective DBH inhibitor nepicastat block cocaine-primed reinstatement, a paradigm which is thought to model some aspects of drug relapse. This is consistent with some clinical results and supports the use of DBH inhibitors for the treatment of cocaine dependence. The present study was conducted to confirm and extend these results in nonhuman primates. Squirrel monkeys trained to self-administer cocaine were pretreated with disulfiram or nepicastat prior to cocaine-induced reinstatement sessions. Neither DBH inhibitor altered cocaine-induced reinstatement. Unexpectedly, nepicastat administered alone induced a modest reinstatement effect in squirrel monkeys, but not in rats. To investigate the neurochemical mechanisms underlying the behavioral results, the effects of DBH inhibition on extracellular DA were analyzed in the nucleus accumbens (NAc) using in vivo microdialysis in squirrel monkeys. Both DBH inhibitors attenuated cocaine-induced DA overflow in the NAc. Hence, the attenuation of cocaine-induced changes in accumbal DA neurochemistry was not associated with altered cocaine-seeking behavior. Overall, the reported behavioral effects of DBH inhibition in rodent models of relapse did not extend to nonhuman primates under the conditions used in the current studies.
Introduction
Cocaine abuse is a major public health concern, yet there is currently no Food and Drug Administration–approved effective pharmacotherapy for cocaine addiction. Because cocaine addiction is often characterized by recurring relapse to drug taking, relapse prevention is critical for effective treatment strategies. Norepinephrine (NE) has been shown to play an important role in reinstatement of drug self-administration, an animal model of relapse (Weinshenker and Schroeder, 2007; Gaval-Cruz and Weinshenker, 2009). Specifically, attenuating NE production and/or transmission can attenuate drug-induced (Zhang and Kosten, 2005), stress-induced (Erb et al., 2000; Leri et al., 2002), and cue-induced reinstatement (Smith and Aston-Jones, 2011; Schroeder et al., 2013) in rats and drug-induced reinstatement in squirrel monkeys (Lee et al., 2004; Platt et al., 2007). The major metabolite of disulfiram, diethyldithiocarbamate, inhibits dopamine β-hydroxylase (DBH), the enzyme that converts dopamine (DA) to NE (Goldstein et al., 1964; Musacchio et al., 1966). Disulfiram-induced inhibition of DBH leads to decreased tissue NE levels and increased tissue DA levels (Goldstein et al., 1964; Musacchio et al., 1966; Bourdelat-Parks et al., 2005; Schroeder et al., 2010). Consistent with an intervention that lowers brain NE levels, disulfiram blocks cocaine-induced reinstatement of cocaine-seeking in rats (Schroeder et al., 2010). Furthermore, the selective DBH inhibitor nepicastat blocks cocaine-, footshock-, and cue-induced reinstatement in rats (Schroeder et al., 2010, 2013), supporting the potential use of DBH inhibitors as cocaine pharmacotherapies.
A decrease in DA levels in the prefrontal cortex (PFC) (McFarland and Kalivas, 2001) or nucleus accumbens (NAc) (Anderson et al., 2006) also reduces reinstatement of cocaine-seeking in rats. Given that NE facilitates DA neuronal firing and DA release, DBH inhibition should attenuate excitatory drive onto mesolimbic DA neurons (Gaval-Cruz and Weinshenker, 2009). Therefore, even though brain tissue DA levels increase with DBH inhibition, extracellular DA levels should actually decrease. Consistent with this prediction, mice treated with the DBH inhibitor fusaric acid (Weinshenker et al., 2008) and mice genetically lacking DBH (Schank et al., 2006) have decreased basal extracellular DA. However, Devoto and colleagues found that neither disulfiram (Devoto et al., 2012) nor nepicastat (Devoto et al., 2013) has an effect on cocaine-induced DA overflow in the nucleus accumbens, yet both markedly increase cocaine-induced DA overflow in the medial prefrontal cortex of rats. Accordingly, the neurochemical mechanisms underlying the effects of DBH inhibition on cocaine-induced reinstatement require further elucidation.
The effects of DBH inhibition on cocaine-induced changes in behavior and neurochemistry have not been characterized previously in nonhuman primates. Given the importance of establishing nonhuman primate models for the translation of medications development to effective treatments in humans, studies evaluating interactions between DBH inhibitors and cocaine in nonhuman primates are clearly warranted. The goal of the present study was to determine whether DBH inhibition reduces drug-induced reinstatement in squirrel monkeys, as previously reported in rats, and to assess whether inhibition of NE synthesis via DBH inhibition affects cocaine-induced DA overflow in the ventral striatum. We hypothesized that DBH inhibition would attenuate both cocaine-induced reinstatement and cocaine-induced increases in extracellular DA in squirrel monkeys.
Materials and Methods
Nonhuman Primate Studies
Subjects.
A total of 10 male squirrel monkeys (Saimiri sciureus) weighing between 850 and 1100 g served as subjects. Animal assignments to specific experimental protocols are identified in Tables 1 and 2. Subjects were individually housed, fed twice daily with ad libitum access to water, and were provided with daily enrichment. All subjects previously served in behavioral studies that involved administration of compounds acting on monoaminergic and/or glutamatergic systems (Kimmel et al., 2007; Bauzo et al., 2009, 2012; Fantegrossi et al., 2009; Manvich et al., 2012a,b). Additionally, all subjects in reinstatement experiments had previously served in behavioral studies in which reinstatement to cocaine-seeking was either attenuated or enhanced (Manvich et al., 2012a,b). All studies were conducted in strict accordance with the National Institutes of Health’s Guide for Care and Use of Laboratory Animals, the American Association for Accreditation of Laboratory Animal Care, and were approved by the Institutional Animal Care and Use Committee of Emory University.
TABLE 1.
Squirrel monkey assignments: reinstatement
Assignments of the 6 monkeys used in the behavioral experiments.
| Dose | Reinstatement |
Ptx Time | s175 | s191 | s197 | s204 | s203 | s209 | |
|---|---|---|---|---|---|---|---|---|---|
| Ptx | Prime | ||||||||
| 10 Dis | Coc | 2 h | X | X | |||||
| 10 Nep | Coc | 2 h | X | X | X | ||||
| 30 Nep | Coc | 2 h | X | X | X | ||||
| 10 Nep | Coc | 30 min | X | X | X | ||||
| 30 Nep | Coc | 4 h | X | X | X | ||||
| 30 Nep | Coc | 24 h | X | X | X | ||||
| 30 Nep | Coc | 5 day | X | X | X | ||||
| 10 Nep | Yoh | 30 min | X | X | |||||
| 10 Nep | — | 30 min | X | X | |||||
| 10 Nep | — | 2 h | X | X | |||||
| Maint EDmax Coc dose (mg/kg/inf) | 0.3 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | |||
| Rein EDPeak Coc dose (mg/kg) | 1.0 | 1.0 | 1.0 | 0.3 | |||||
Coc, cocaine; Dis, disulfiram; EDmax, maximally effective unit dose of cocaine (self-administration); inf, infusion; Maint, maintenance; Nep, nepicastat; Ptx, pretreatment; Rein, reinstatement; Yoh, yohimbine.
TABLE 2.
Squirrel monkey assignments: in vivo microdialysis
Assignments of the 4 monkeys used in the neurochemistry experiments.
| Ptx | Challenge | s183 | s184 | s192 | s195 |
|---|---|---|---|---|---|
| 10 Dis | 1.0 Coc | X | X | X | |
| 10 Nep | 1.0 Coc | X | X | X | X |
Coc, cocaine; Dis, disulfiram; Nep, nepicastat; Ptx, pretreatment.
Apparatus.
Experimental sessions were conducted in a ventilated, sound-attenuated chamber in which each subject was seated comfortably in a commercially available primate chair (Modular Primate Chair; Med Associates Inc., St. Albans, VT). The chair was equipped with an operant panel consisting of a series of red and white lights, a response lever, and a white noise amplifier which remained activated for the duration of all behavioral sessions to lessen the influence of ambient noise. Med-PC IV software (Med Associates Inc.) was interfaced with each chamber to allow for automated output control and recording of lever presses. A motor-driven syringe pump (for behavioral studies: model PHD2000; for in vivo microdialysis: model 11Plus Dual-Syringe; Harvard Apparatus, Holliston, MA) was mounted on top of the operant chamber for automated delivery of solutions.
Surgery.
All surgeries were conducted under aseptic conditions. Animals were initially anesthetized with Telazol (tiletamine HCl and zolazepam HCl, 2.0 mg i.m.) and ketamine HCl (20 mg i.m.), and anesthesia was maintained throughout the procedure with inhaled isoflurane (0.5–1.5%). Subjects in behavioral experiments were prepared with a chronic indwelling venous catheter in the femoral or jugular vein as previously described (Kimmel et al., 2007; Bauzo et al., 2009). Subjects were fitted with a custom-made nylon mesh jacket (Lomir Biomedical Inc., Malone, NY) to protect the outer portion of the catheter. To maintain catheter patency, catheters were flushed daily with 0.2 ml of saline and, when not in use, filled with heparinized saline (100 units/ml). For in vivo microdialysis experiments, subjects were implanted with bilateral guide cannulae (CMA/11; CMA Microdialysis, Acton, MA) using stereotaxic techniques as described previously (Czoty et al., 2000). Guide cannulae targeted the nucleus accumbens based on the following coordinates from the ear bar: anterior/posterior + 15.0, medial/lateral ± 3.0. When subjects were not actively participating in microdialysis experiments, stainless-steel stylets were situated within the cannulae to protect the surgical preparation. For all surgical procedures, preoperative antibiotics (ceftriaxone) and postoperative analgesics (meloxicam) were administered by veterinary staff, and subjects were closely monitored after surgery.
Cocaine Self-Administration and Reinstatement.
Subjects were trained to self-administer cocaine under a second-order schedule of reinforcement, as previously described (Manvich et al., 2012a,b). Each session began with the illumination of a pair of red lights. During a 600-second fixed interval (FI), a fixed-ratio 20 (FR20) schedule was in effect such that every 20th lever press extinguished the red lights and briefly illuminated a white light for 2 seconds, followed immediately by reillumination of the red lights. Once the FI elapsed, the schedule progressed into a 200-second limited hold. The first FR20 completed during the limited hold extinguished the red lights and resulted in an intravenous bolus infusion of cocaine (0.03–0.3 mg/kg/infusion in 0.5 ml; 25 ml/min flow rate) paired with a 15-second white light, followed by a 60-second time-out during which all lights were extinguished and responses had no programmed consequences. If the subject failed to complete a FR20 during the limited hold, the red lights were extinguished and the schedule moved directly into the time-out. Each daily session consisted of five FI components, and sessions were conducted 5–6 days per week. Response rates were calculated for each individual component and then averaged across the session. The maximally effective unit dose of cocaine (i.e., the unit dose of cocaine that maintained the highest rates of responding) was identified for each individual subject and used as the maintenance dose between reinstatement sessions.
Once response rates were stable, subjects progressed to the extinction phase, during which saline infusions were substituted for cocaine and the white stimulus light was withheld. Extinction criteria were met when the overall response rate within a single session was ≤20% of the mean response rate of the three previous maintenance sessions. Reinstatement tests occurred on the day immediately following extinction of responding. During reinstatement sessions, the white stimulus light was reintroduced but saline continued to be substituted for cocaine infusions. For cocaine-induced reinstatement sessions, subjects were administered a noncontingent, intravenous bolus infusion of cocaine [vehicle (veh), 0.03–1.0 mg/kg] 5 minutes prior to the onset of the session. For each subject, the dose of cocaine that induced maximal rates of responding was determined and deemed the EDPeak. Reinstatement sessions were preceded by a drug pretreatment of either disulfiram [(veh, 10 mg/kg i.m.) given acutely 2 hours prior to the cocaine prime] or nepicastat [(veh, 10 and 30 mg/kg i.m.) given acutely 30 minutes to 24 hours prior to the cocaine prime or subchronically for 5 consecutive days prior to the session]. For nepicastat-induced reinstatement, nepicastat (veh, 10 mg/kg i.m.) was administered either 30 or 120 minutes prior to the onset of the session. For yohimbine-primed reinstatement, yohimbine (veh, 0.3 mg/kg i.m.) was administered 5 minutes prior to the onset of the session. For drug interaction studies, a nepicastat pretreatment (veh, 10 mg/kg i.m.) was administered 120 minutes prior to yohimbine (0.3 mg/kg i.m.) administration. Reinstatement tests for each drug dose or combination were separated by the re-establishment of maintenance cocaine self-administration and subsequent extinction.
In Vivo Microdialysis.
The microdialysis protocols used in the present study were similar to those described previously (Czoty et al., 2000; Kimmel et al., 2005, 2007; Bauzo et al., 2009; Manvich et al., 2012a,b). On test days, a CMA/11 dialysis probe (CMA Microdialysis, North Chelmsford, MA) with a shaft length of 20 mm and active dialysis membrane measuring 2 × 0.24 mm was inserted into the guide cannula and perfused with artificial cerebrospinal fluid (1.0 mM Na2HPO4, 150 mM NaCl, 3 mM KCl, 1.3 mM CaCl2, 1.0 mM MgSO4, and 0.15 mM ascorbic acid, pH 7.4–7.56) at a rate of 2.0 μl/min. After a 60-minute equilibration sample was collected, three baseline samples were collected at 10-minute intervals for determination of basal DA concentrations. Following baseline sample collection, microdialysis proceeded with the following drug administration conditions: disulfiram (10 mg/kg i.m.) administered 30 minutes prior to cocaine (1.0 mg/kg i.m.), and nepicastat (veh, 10 mg/kg i.m.) administered 30 minutes prior to cocaine (1.0 mg/kg i.m.). Following drug administration, additional 10-minute samples were collected for a total session duration of 4–5 hours. For each subject, all drug combinations within a given experiment were acquired from the ipsilateral hemisphere. Times of access at each brain site were separated by at least 2 weeks. The order of drug dose combinations was randomized within subjects.
All samples were refrigerated or frozen until immediately prior to analysis. Probes were tested in vitro both prior to and immediately after each session to determine probe viability and percentage of recovery. To confirm site integrity, following experimental sample collection, the KCl concentration within the perfused artificial cerebrospinal fluid was increased to 100 mM, and a final 10-minute sample was collected. A robust increase in extracellular DA levels confirmed site viability. Samples were analyzed and DA concentrations were determined using high-performance liquid chromatography with electrochemical detection as previously described (Kimmel et al., 2007; Bauzo et al., 2009, 2012).
Drugs.
Cocaine HCl (National Institute on Drug Abuse, Research Technology Branch, Research Triangle Park, NC) was dissolved in 0.9% sterile saline. Yohimbine HCl (Sigma-Aldrich, St. Louis, MO) was sonicated and dissolved in 0.9% sterile saline. Nepicastat (Synosia Therapeutics, South San Francisco, CA) was sonicated and dissolved at a concentration of 30 mg/ml in a 20:20:60 mixture of 95% ethanol, Tween 80 (Sigma-Aldrich), and 0.9% sterile saline for the low dose. The high dose of nepicastat was sonicated and dissolved at a concentration of 80 mg/ml in a 25:25:50 mixture. Disulfiram (Sigma-Aldrich) was sonicated in sterile water and injected as a suspension. Doses were calculated from the salt weights.
Rodent Studies
Subjects.
Subjects were 7 male Sprague-Dawley rats, weighing 200–225 g upon arrival (Charles River, Wilmington, MA). All subjects were individually housed under a reverse light/dark cycle (lights on from 8:00 PM to 8:00 AM) and given ad libitum access to standard rodent chow and water, except during behavioral sessions. All experiments were conducted in strict accordance with the National Institutes of Health’s Guide for Care and Use of Laboratory Animals and were approved by the Emory University Institutional Animal Care and Use Committee.
Surgery.
Subjects were anesthetized with isoflurane and implanted with chronic indwelling jugular catheters using standard methods as described previously (Schroeder et al., 2010). In brief, catheters were inserted into the right jugular vein then threaded subcutaneously through the skin between the shoulder blades and anchored with suture material. Catheters were flushed daily with 0.05 ml of gentamicin (4 mg/ml) and 0.1 ml of heparinized saline (300 U/ml) throughout the duration of experiments.
Food Training.
Prior to drug self-administration training, all rats were first trained to lever press for food reinforcement in standard rat operant chambers (Med Associates Inc.) equipped with a house light, two levers (active and inactive), stimulus lights above each lever, and a food-pellet receptacle located between the two operant levers. Responses on the active lever resulted in delivery of a single 45-mg food pellet (F0165; Bio-Serv, Frenchtown, NJ) according to a FR1 schedule of reinforcement, whereas responses on the inactive lever had no programmed consequences. Daily 6-hour sessions continued until the subject met training criteria defined as at least 70% selection of the active lever and at least 100 reinforcers earned, typically within one to three sessions.
Cocaine Self-Administration and Reinstatement.
Daily 2-hour cocaine self-administration sessions were conducted using an FR1 schedule of reinforcement as described previously (Schroeder et al., 2010, 2013). Sessions began with the extension of both the active and inactive levers and illumination of the house light. Responses on the active lever resulted in delivery of an intravenous infusion of cocaine (0.5 mg/kg in a volume of 0.167 ml/kg) and the initiation of a 20-second time-out period, during which the house light was extinguished, the cue light above the active lever was illuminated, and responses on both levers were recorded but had no programmed consequences. Sessions were terminated once 2 hours had elapsed or 40 reinforcers were earned, whichever occurred first. Once rats achieved maintenance criteria (<20% variance on the active lever and at least 75% preference for the active lever for 3 consecutive days, with a minimum of five total sessions), lever pressing was extinguished such that responses on the previously active lever had no programmed consequences. Extinction criteria were achieved when active lever presses over 3 consecutive days were <25% of the average number of active lever presses during the last 3 days of maintenance. For reinstatement sessions, subjects were administered nepicastat (50 mg/kg i.p.) either 30 or 120 minutes prior to being placed in the operant chambers under extinction conditions. Following this first reinstatement session, extinction criteria were re-established. A second nepicastat reinstatement test was then conducted using the opposite pretreatment time in a counterbalanced fashion. To ensure that the rats were capable of exhibiting reinstatement of cocaine-seeking behavior, they were again extinguished prior to a third reinstatement test, in which subjects were primed with cocaine (10 mg/kg i.p.) immediately prior to the reinstatement session.
Drugs.
Nepicastat (Synosia Therapeutics) was sonicated in saline containing 1.5% dimethylsulfoxide and 1.5% Cremophor EL (Sigma-Aldrich) by volume, and injected as a suspension. Cocaine HCl (National Institute on Drug Abuse) was dissolved in sterile saline. Intraperitoneal drug administration was administered in a volume of 1.0 ml/kg. Drug doses were calculated as the salt weight.
Data Analysis
For nonhuman primate reinstatement experiments, response rates across sessions were normalized to the percentage of average responding during the last three maintenance sessions of cocaine self-administration. For rat experiments, the dependent measure was the number of responses on both active and inactive levers. Data were analyzed using repeated-measures analyses of variance (ANOVAs) with post-hoc Bonferroni tests, or paired t tests, as specified.
For in vivo microdialysis studies, only samples collected within the first 60 minutes following a cocaine challenge were analyzed because the effects of cocaine typically return to near-baseline levels within 60 minutes postcocaine administration. For each subject, DA levels were normalized as the percentage of the mean of the three baseline values. Data were analyzed using a two-way repeated-measures ANOVA with post-hoc Bonferroni test.
Data were graphically plotted and analyzed using GraphPad version 5.01 (GraphPad Software Inc., La Jolla, CA). For all statistical analyses, significance was accepted at the 95% level of confidence (α = 0.05).
Results
Cocaine-Primed Reinstatement in Squirrel Monkeys
Disulfiram Pretreatment.
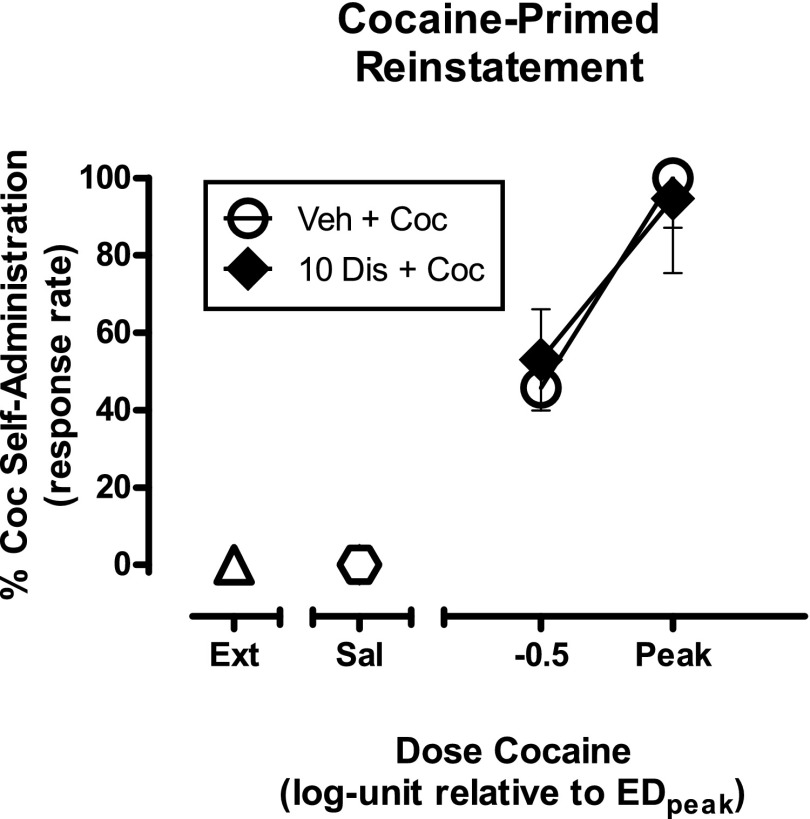
The effects of a 2-hour pretreatment of disulfiram (N = 2) are shown in Fig. 1. Mean rates of responding during maintenance of cocaine self-administration were 1.32 ± 0.21 responses/second (resp/s). The EDPeak priming dose of cocaine increased responding to 100% of levels maintained during cocaine self-administration. Priming with a cocaine dose one-half log unit less than the EDPeak increased responding to nearly 50% of maintenance levels. Disulfiram pretreatment (10 mg/kg) did not affect cocaine-induced reinstatement at either dose of cocaine. Two-way repeated-measures ANOVA indicated a main effect of cocaine dose (F1,3 = 15.57, P = 0.029), but no significant main effect of disulfiram pretreatment (F1,3 = 0.01, P = 0.939) or interaction (F1,3 = 0.27, P = 0.641).
Fig. 1.
Effects of a 2-hour pretreatment with disulfiram (10 mg/kg) on cocaine-induced reinstatement in squirrel monkeys (N = 2). Data (mean ± S.E.M.) are expressed as the percentage of responding maintained during cocaine self-administration sessions. Priming with an EDPeak dose of cocaine reinstated responding to levels near those maintained by cocaine self-administration, whereas priming with a cocaine dose a half log unit less than the EDPeak reinstated responding to levels approximately 50% of those maintained by cocaine self-administration. Disulfiram did not alter the reinstatement effects of either priming dose of cocaine. Coc, cocaine; Dis, disulfiram; Ext, extinction; Sal, saline; Veh, vehicle.
Nepicastat Pretreatment.
The effects of a 2-hour pretreatment with nepicastat (N = 3) are shown in Fig. 2. Mean rates of responding during maintenance of cocaine self-administration were 1.56 ± 0.1 resp/s. The EDPeak priming dose of cocaine increased responding to approximately 60–80% of levels maintained during cocaine self-administration. Nepicastat (10 mg/kg) given 2 hours before the start of the reinstatement session did not affect cocaine-induced reinstatement [paired t(2) = 3.8, P = 0.068] (Fig. 2A). Similarly, a 2-hour pretreatment of a higher dose of nepicastat (30 mg/kg) did not affect cocaine-induced reinstatement [paired t(2) = 0.75, P = 0.534] (Fig. 2B).
Fig. 2.
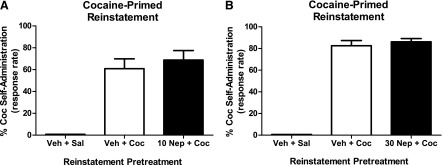
Effects of nepicastat (10 and 30 mg/kg) pretreatment on cocaine-induced reinstatement in squirrel monkeys (N = 3). Data (mean ± S.E.M.) are expressed as the percentage of responding maintained during cocaine self-administration sessions. Priming with a maximally EDPeak dose of cocaine reinstated responding to levels approximately 60–80% of those maintained by cocaine self-administration. A 2-hour pretreatment with 10 mg/kg (A) or 30 mg/kg (B) of nepicastat did not alter the reinstatement effects of cocaine. Coc, cocaine; Nep, nepicastat; Sal, saline; Veh, vehicle.
Given the absence of significant drug interactions in the previous experiments, subsequent experiments systematically manipulated nepicastat pretreatment time. A shorter (30-minute) pretreatment time was evaluated in combination with the EDPeak of cocaine as well as a priming dose one-half log unit less than the EDPeak. Nepicastat (10 mg/kg) pretreatment (N = 3) did not affect cocaine-induced reinstatement at either dose of cocaine (data not shown). Two-way repeated-measures ANOVA indicated a significant main effect for cocaine dose (F1,4 = 8.29, P = 0.045) but no significant main effect for nepicastat pretreatment (F1,4 = 0.69, P = 0.453) or interaction (F1,4 = 2.20, P = 0.212). Similarly, a 24-hour pretreatment time was evaluated in combination with two priming doses of cocaine, and nepicastat (30 mg/kg) pretreatment (N = 3) did not affect cocaine-induced reinstatement at either dose of cocaine (data not shown). Additionally, neither a 4-hour pretreatment with 10 mg/kg nepicastat (N = 3) nor a subchronic, 5-day pretreatment with 30 mg/kg nepicastat (N = 3) affected cocaine-induced reinstatement (data not shown).
Yohimbine-Primed Reinstatement
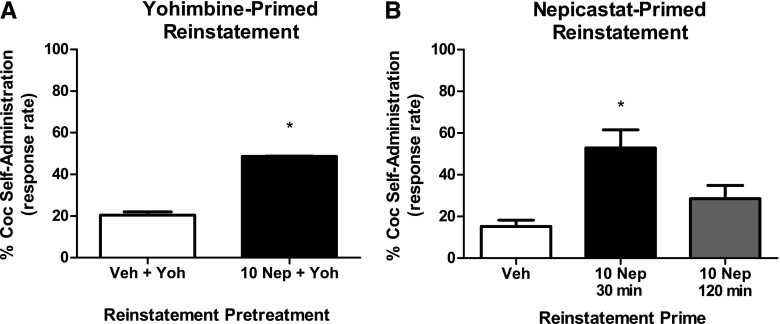
Yohimbine has been shown previously to induce reinstatement to cocaine seeking in squirrel monkeys, ostensibly by blocking the α2-adrenergic (AR) inhibitory autoreceptor, thereby increasing NE release (Lee et al., 2004). Accordingly, yohimbine-primed reinstatement was used as a positive control to evaluate the role of NE in reinstatement (N = 2). Mean rates of responding during maintenance of cocaine self-administration were 1.79 ± 0.08 resp/s. Yohimbine (0.3 mg/kg) alone did not induce responding above extinction criteria (Fig. 3A). Interestingly, a nepicastat pretreatment (10 mg/kg), given 120 minutes prior to the yohimbine prime, significantly increased response rates to ∼50% of maintenance response rates [t(2) = 18.18, P = 0.003].
Fig. 3.
(A) Effects of a 2-hour pretreatment with nepicastat (10 mg/kg) on yohimbine-induced reinstatement (0.3 mg/kg) in squirrel monkeys (N = 2). Data (mean ± S.E.M.) are expressed as the percentage of responding maintained during cocaine self-administration sessions. Yohimbine had a modest effect on responding during reinstatement, whereas a nepicastat pretreatment in combination with yohimbine enhanced responding to approximately 50% of levels maintained by cocaine self-administration. *P < 0.005, compared with vehicle control. (B) Reinstatement effects of nepicastat (10 mg/kg) administered alone as a 30- or 120-minute pretreatment in squirrel monkeys (N = 2). Data (mean ± S.E.M.) are expressed as the percentage of responding maintained during cocaine self-administration sessions. Nepicastat significantly increased response rates when administered 30 minutes before the start of the session. *P < 0.01, compared with vehicle control at the same pretreatment time. Coc, cocaine; Nep, nepicastat; Veh, vehicle; Yoh, yohimbine.
Nepicastat-Primed Reinstatement
Given the unexpected interactions observed between yohimbine and nepicastat, subsequent experiments evaluated whether nepicastat alone would induce reinstatement to cocaine seeking (N = 2). Mean rates of responding during maintenance of cocaine self-administration were 1.61 ± 0.08 resp/s. Priming injections of nepicastat (10 mg/kg) were given 30 or 120 minutes before the start of reinstatement sessions (Fig. 3B). When given 30 minutes prior to the start of the session, nepicastat induced a significant increase in responding to greater than 50% of maintenance response rates. When nepicastat was administered 120 minutes prior to the session, there was no significant effect. Two-way repeated-measures ANOVA indicated a main effect of nepicastat treatment (F1,7 = 19.65, P = 0.003), but no significant main effect of time (F1,7 = 4.51, P = 0.071) or interaction (F1,7 = 4.44, P = 0.073). Post-hoc analyses indicated nepicastat treatment was significantly different from vehicle treatment (P < 0.01).
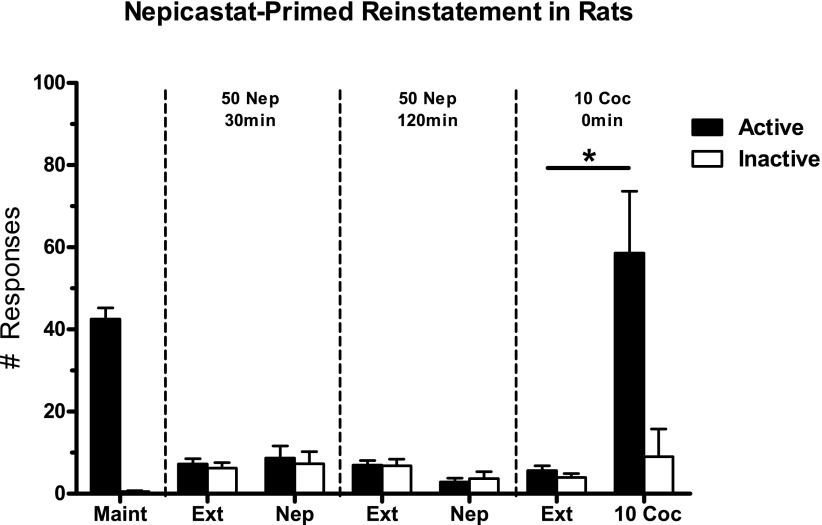
In contrast to the current results observed in squirrel monkeys, nepicastat has been reported to attenuate cocaine-primed reinstatement in rats (Schroeder et al., 2010). However, nepicastat-induced reinstatement in rats has not been evaluated previously. Accordingly, experiments were conducted to evaluate nepicastat-induced reinstatement in rats (N = 7). Following cocaine self-administration and extinction, rats were primed with nepicastat (50 mg/kg, 30 or 120 minutes prior to session) or cocaine (10 mg/kg immediately prior to session) (Fig. 4). Two-way repeated-measures ANOVA revealed significant main effects of active/inactive lever (F1,6 = 53.58, P = 0.0003), treatment (F6,36 = 8.252, P < 0.0001), and a lever × treatment interaction (F6,36 = 20.96, P < 0.0001). Post-hoc analyses indicated that nepicastat did not induce responding that was significantly different from extinction conditions. In contrast, cocaine induced a robust reinstatement effect in the same rats (P < 0.001 compared with extinction).
Fig. 4.
Reinstatement effects of nepicastat (50 mg/kg) administered alone as a 30- or 120-minute pretreatment in rats (N = 7). Maintenance values indicate the average of the final 3 days of maintenance sessions. Extinction values indicate the average of the 3 extinction days immediately prior to the subsequent reinstatement session. Shown are the mean ± S.E.M. active and inactive lever responses. *P < 0.001 active lever responses between extinction and the cocaine (10 mg/kg) prime. Coc, cocaine; Ext, extinction; Maint, maintenance; Nep, nepicastat.
In Vivo Microdialysis
The effects of DBH inhibition on cocaine-induced DA overflow in the NAc were evaluated in unanesthetized squirrel monkeys (N = 4). Mean basal levels of DA unadjusted for probe recovery were 3.30 ± 1.09 nM. Cocaine administration following a vehicle pretreatment increased extracellular DA in the NAc to 200–240% of basal DA levels within 20 minutes, and DA returned to near-baseline levels within 60 minutes postdrug injection. However, there was no significant increase in DA concentration following the combined administration of disulfiram (10 mg/kg) and cocaine (1.0 mg/kg) (Fig. 5A; N = 3) or nepicastat (10 mg/kg) and cocaine (1.0 mg/kg) (Fig. 5B; N = 4). Two-way repeated-measures ANOVA indicated a significant main effect of disulfiram dose (F1,20 = 63.40, P = 0.0013), but not time (F5,20 = 1.80, P < 0.1594) or interaction (F5,20 = 2.29, P = 0.0844). Two-way repeated-measures ANOVA indicated a significant main effect of nepicastat dose (F1,48 = 6.08, P = 0.0487), time (F8,48 = 4.51, P = 0.0004), and interaction (F8,48 = 2.56, P = 0.0208). Subsequent post-hoc analyses indicated that both disulfiram and nepicastat significantly attenuated the peak increase in DA levels (at 20 minutes) following cocaine administration compared with vehicle pretreatment (P < 0.01 for disulfiram, P < 0.001 for nepicastat).
Fig. 5.
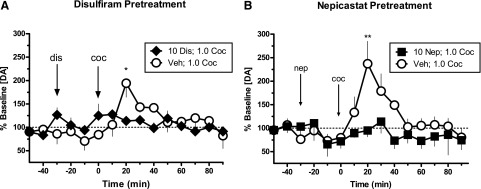
Effects of DBH inhibition on extracellular DA levels in the NAc of unanesthetized squirrel monkeys administered disulfiram (10 mg/kg) 30 minutes prior to cocaine (1.0 mg/kg) (N = 3) (A) or nepicastat (10 mg/kg) 30 minutes prior to cocaine (1.0 mg/kg) (N = 4) (B). Data points (mean ± S.E.M.) are expressed as the percentage of baseline DA levels before drug administration. *P < 0.01; **P < 0.001, compared with vehicle control. Coc, cocaine; Dis, disulfiram; Nep, nepicastat; Veh, vehicle.
Discussion
The purpose of the present study was to determine whether DBH inhibition reduces cocaine-induced reinstatement in nonhuman primates, as previously reported in rats (Schroeder et al., 2010), and to assess whether inhibition of NE synthesis via DBH inhibition affects cocaine-induced DA overflow in the ventral striatum. We hypothesized that DBH inhibition would attenuate both cocaine-induced reinstatement and cocaine-induced increases in extracellular DA in squirrel monkeys. However, neither disulfiram nor nepicastat attenuated cocaine-induced reinstatement in squirrel monkeys in the present study. Although studies have shown that changes in noradrenergic signaling can modulate DA transmission within the mesocorticolimbic DA system (Grenhoff et al., 1993; Grenhoff and Svensson, 1993; Sommermeyer et al., 1995; Darracq et al., 1998; Weinshenker and Schroeder, 2007; Mitrano et al., 2012), the effects of DBH inhibition on stimulant-induced increases in DA levels in the NAc of rodents have produced conflicting results with either a decrease found in mice (Schank et al., 2006; Weinshenker et al., 2008) or no change detected in rats despite decreases in basal NE overflow (Devoto et al., 2012, 2013). In the present study, both disulfiram and nepicastat attenuated cocaine-induced DA overflow in the NAc of squirrel monkeys. Despite the disparate results obtained in nonhuman primates and rodents, it is clearly evident in both species that attenuation of cocaine-induced reinstatement by DBH inhibitors is not directly linked to DA overflow in the NAc. These finding are supported by a recent study conducted in rhesus monkeys showing that attenuation of cocaine-induced reinstatement by the 5-hydroxytryptamine 2A antagonist M100907 [(R)-(+)-a-(2,3-dimethoxyphenyl)-1-[2-(4-fluorophenyl)ethyl]-4-pipidinemethanol] was not associated with drug-induced changes in extracellular DA levels in the NAc (Murnane et al., 2013).
There are several procedural differences to consider when comparing the outcome of behavioral and neurochemical experiments in the present study. Different groups of subjects were used for reinstatement and dialysis, so recent drug history could represent a potential confound. All subjects had extensive drug histories to consider. The cocaine prime was administered intravenously for reinstatement and intramuscularly for microdialysis because subjects assigned to the latter experiments were not prepared with indwelling venous catheters. However, cocaine-induced increases in DA were rapid following intramuscular administration and followed a time course that closely resembles the intravenous route of administration. The pretreatment time for disulfiram was 2 hours for reinstatement and 30 minutes for microdialysis. However, pilot experiments were conducted with various pretreatment times for disulfiram, including 30 minutes, and under no condition did it attenuate cocaine-induced reinstatement. Note that nepicastat was also ineffective in attenuating cocaine-induced reinstatement even when given at the same 30-minute pretreatment time used in dialysis experiments. In fact, extensive manipulation of nepicastat pretreatment time and dose failed to reveal any changes in cocaine-induced reinstatement.
Some of the differences observed in the effects of DBH inhibitors between squirrel monkeys and rats may be attributed to inherent species differences in noradrenergic function. Our prediction that rodents and nonhuman primates would exhibit similar behavioral responses assumed that their noradrenergic systems would respond similarly in a cocaine-induced reinstatement paradigm. Unfortunately, few studies have investigated the influence of the noradrenergic system on cocaine self-administration in nonhuman primates (Woolverton, 1987; Macey et al., 2003; Beveridge et al., 2005; Wee et al., 2006; Negus et al., 2007), and even fewer have specifically studied its influence on reinstatement (Lee et al., 2004; Platt et al., 2007; Valdez et al., 2007). Additionally, there are existing examples of divergent effects of noradrenergic treatments on cocaine-induced behavioral responses between rodents and nonhuman primates. For example, the effects of a pretreatment with the α1-AR antagonist prazosin differed between rats and squirrel monkeys. Zhang and Kosten (2005) found that prazosin dose-dependently attenuated cocaine-primed reinstatement in rats, whereas prazosin had no effect on cocaine-induced reinstatement in squirrel monkeys (Platt et al., 2007). Furthermore, although the α2-AR antagonist yohimbine was able to induce reinstatement in both rats and squirrel monkeys, the effect of a pretreatment with the α2-AR agonist clonidine differed between the species. In rats, clonidine had no effect on yohimbine-induced reinstatement (Brown et al., 2009), whereas clonidine dose-dependently attenuated yohimbine-induced reinstatement in squirrel monkeys (Lee et al., 2004). The current study presents another example of differing effects of a noradrenergic manipulation on the behavioral pharmacology of cocaine in nonhuman primates and rodents.
Notable methodological differences between the reinstatement paradigms used to test the effects of DBH inhibition in rats and squirrel monkeys should also be considered. In the Schroeder et al. (2010) study conducted in rats, the cue lights that functioned as the conditioned reinforcer for the rats were removed during extinction sessions and remained absent during reinstatement tests. Conversely, in the present study, the conditioned reinforcer cue lights were removed during extinction sessions but restored during the reinstatement sessions. Thus, the squirrel monkeys were effectively experiencing a combined cocaine + cue-induced reinstatement session. Cue-induced reinstatement is much less sensitive to NE blockade than cocaine- or stress-induced reinstatement (Smith and Aston-Jones, 2011; Schroeder et al., 2013). Additional important methodological differences include the schedules of reinforcement used and the drug history of the experimental subjects. Hence, it is premature to conclude that the different effects observed for DBH inhibition on cocaine-induced reinstatement depend largely on species differences.
Last, nepicastat administered alone partially reinstated cocaine seeking in squirrel monkeys, an unexpected outcome that was not observed in rats. However, a recent study in rats by Manvich et al. (2013) reported that disulfiram and nepicastat failed to substitute for cocaine in a drug discrimination paradigm but enhanced the discriminative-stimulus effects of cocaine as evidenced by a significant leftward shift of the cocaine dose-response function. Disulfiram and nepicastat pretreatment also conferred cocaine-like discriminative-stimulus effects to the selective norepinephrine uptake inhibitor reboxetine. Hence, the latter results are not inconsistent with the drug interactions between nepicastat and yohimbine observed in squirrel monkeys in the present study. Both disulfiram and nepicastat increase basal and cocaine-induced DA overflow in the PFC of rats (Devoto et al., 2012, 2013). Accordingly, Manvich et al. (2013) speculated that the augmentation of cocaine’s discriminative stimulus effects by DBH inhibitors may be linked to dopaminergic effects in the PFC. In the present study, we were unable to develop a paradigm to target the PFC of squirrel monkeys for in vivo microdialysis. We were also unable to confidently measure NE levels in the accumbal dialysate samples. These technical limitations restricted the present study to analysis of DA in the NAc, but future experiments should assess the effects of catecholamine neurochemistry following DBH inhibition in the PFC of nonhuman primates.
In summary, the present study demonstrated that DBH inhibition in squirrel monkeys attenuated cocaine-induced DA overflow in the NAc but was ineffective in suppressing cocaine-induced reinstatement. The latter results observed in nonhuman primates contrast with those reported recently in rodents. The relevance of these findings to DBH inhibition as a potential pharmacotherapy for cocaine relapse prevention remains to be determined. Although several clinical trials with disulfiram showed a positive result for treatment of cocaine use (Carroll et al., 1998, 2004; George et al., 2000; Petrakis et al., 2000; Grassi et al., 2007), some subsequent studies have been less encouraging. For example, in one clinical trial, low doses of disulfiram treatment resulted in worse retention and increased cocaine-positive urine samples (Oliveto et al., 2011). In a follow-up study by Carroll et al. (2012), the disulfiram treatment group with no 12-step program support had the highest rate of cocaine use. The results of all of these trials must be interpreted with caution because the latest reports indicate that the ability of disulfiram to reduce cocaine use is dependent on both dose and pharmacogenetic considerations (Haile et al., 2012; Kosten et al., 2013; Shorter et al., 2013). Thus, despite these conflicting outcomes, there does appear to be sufficient evidence to continue the evaluation of DBH inhibitors as cocaine pharmacotherapies.
Acknowledgments
The authors thank Mi Zhou, Juliet Brown, and Lisa Neidert for expert technical assistance. They also thank the Emory University Division of Animal Care and the animal care, veterinary care, and facilities staff at Yerkes National Primate Research Center for their exceptional services.
Abbreviations
- ANOVA
analysis of variance
- AR
adrenergic
- DA
dopamine
- DBH
dopamine β-hydroxylase
- EDPeak
maximally effective dose of presession drug prime (reinstatement)
- FI
fixed interval
- FR
fixed ratio
- M100907
(R)-(+)-a-(2,3-dimethoxyphenyl)-1-[2-(4-fluorophenyl)ethyl]-4-pipidinemethanol
- NAc
nucleus accumbens
- NE
norepinephrine
- PFC
prefrontal cortex
- resp
responses
Authorship Contributions
Participated in research design: Cooper, Kimmel, Manvich, Schmidt, Weinshenker, Howell.
Conducted experiments: Cooper, Kimmel, Manvich, Schmidt.
Performed data analysis: Cooper, Manvich.
Wrote or contributed to the writing of the manuscript: Cooper, Kimmel, Manvich, Schmidt, Weinshenker, Howell.
Footnotes
This work was supported by the National Institutes of Health National Institute on Drug Abuse [Grants R01-DA012514, K05-DA031246, R01-DA027535, R03-DA034867, R01-DA017963, and T32-DA015040]; the National Institutes of Health National Institute of General Medical Sciences [Grants P51-RR00165 and UL1-RR025008]; the Office of Research Infrastructure Programs [Grant ODP51OD11132]; and the Howard Hughes Medical Institute [Grant 56006762].
This article was prepared while H.L.K. was employed at the National Institutes of Health. The opinions expressed in this article are the author's own and do not reflect the view of the National Institutes of Health, the Department of Health and Human Services, or the United States government.
With the exception of income received from their primary employers, D.A.C., H.L.K., D.F.M., K.T.S., and L.L.H. have received no financial support or compensation from any individual or company entity over the past 3 years for research or professional service, and there are no personal financial holdings that could be perceived as constituting a conflict of interest. D.W. is coinventor on a patent concerning the use of selective dopamine β-hydroxylase inhibitors for the treatment of cocaine dependence [Weinshenker D and Malison RT (2012) inventors, Yale University and Emory University, assignee. Methods and compositions for treatment of drug addiction. US patent US-2013-0274303-A1. 2012 Oct 2].
Parts of this study represent partial fulfillment dissertation: Cooper DA (2014) Pharmacologic Dopamine β-Hydroxylase Inhibition: Effect On Cocaine-Induced Behavior and Neurochemistry. Doctoral dissertation, Emory University, Atlanta, GA.
Preliminary findings from these experiments were previously presented as follows: Cooper DA, Kimmel HK, and Howell LL (2012) Effects of dopamine beta-hydroxylase (DBH) inhibition on cocaine seeking behavior in squirrel monkeys. Society for Neuroscience Meeting; 2012 October 13–17; New Orleans, LA.
References
- Anderson SM, Schmidt HD, Pierce RC. (2006) Administration of the D2 dopamine receptor antagonist sulpiride into the shell, but not the core, of the nucleus accumbens attenuates cocaine priming-induced reinstatement of drug seeking. Neuropsychopharmacology 31:1452–1461 [DOI] [PubMed] [Google Scholar]
- Bauzo RM, Kimmel HL, Howell LL. (2009) Interactions between the mGluR2/3 agonist, LY379268, and cocaine on in vivo neurochemistry and behavior in squirrel monkeys. Pharmacol Biochem Behav 94:204–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauzo RM, Kimmel HL, Howell LL. (2012) The cystine-glutamate transporter enhancer N-acetyl-L-cysteine attenuates cocaine-induced changes in striatal dopamine but not self-administration in squirrel monkeys. Pharmacol Biochem Behav 101:288–296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beveridge TJ, Smith HR, Nader MA, Porrino LJ. (2005) Effects of chronic cocaine self-administration on norepinephrine transporters in the nonhuman primate brain. Psychopharmacology (Berl) 180:781–788 [DOI] [PubMed] [Google Scholar]
- Bourdélat-Parks BN, Anderson GM, Donaldson ZR, Weiss JM, Bonsall RW, Emery MS, Liles LC, Weinshenker D. (2005) Effects of dopamine beta-hydroxylase genotype and disulfiram inhibition on catecholamine homeostasis in mice. Psychopharmacology (Berl) 183:72–80 [DOI] [PubMed] [Google Scholar]
- Brown ZJ, Tribe E, D’souza NA, Erb S. (2009) Interaction between noradrenaline and corticotrophin-releasing factor in the reinstatement of cocaine seeking in the rat. Psychopharmacology (Berl) 203:121–130 [DOI] [PubMed] [Google Scholar]
- Carroll KM, Fenton LR, Ball SA, Nich C, Frankforter TL, Shi J, Rounsaville BJ. (2004) Efficacy of disulfiram and cognitive behavior therapy in cocaine-dependent outpatients: a randomized placebo-controlled trial. Arch Gen Psychiatry 61:264–272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll KM, Nich C, Ball SA, McCance E, Rounsavile BJ. (1998) Treatment of cocaine and alcohol dependence with psychotherapy and disulfiram. Addiction 93:713–727 [DOI] [PubMed] [Google Scholar]
- Carroll KM, Nich C, Shi JM, Eagan D, Ball SA. (2012) Efficacy of disulfiram and Twelve Step Facilitation in cocaine-dependent individuals maintained on methadone: a randomized placebo-controlled trial. Drug Alcohol Depend 126:224–231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czoty PW, Justice JB, Jr, Howell LL. (2000) Cocaine-induced changes in extracellular dopamine determined by microdialysis in awake squirrel monkeys. Psychopharmacology (Berl) 148:299–306 [DOI] [PubMed] [Google Scholar]
- Darracq L, Blanc G, Glowinski J, Tassin JP. (1998) Importance of the noradrenaline-dopamine coupling in the locomotor activating effects of D-amphetamine. J Neurosci 18:2729–2739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devoto P, Flore G, Saba P, Bini V, Gessa GL. (2013) The dopamine beta-hydroxylase inhibitor nepicastat increases dopamine release and potentiates psychostimulant-induced dopamine release in the prefrontal cortex. Addict Biol DOI: 10.1111/adb.12026. [DOI] [PubMed] [Google Scholar]
- Devoto P, Flore G, Saba P, Cadeddu R, Gessa GL. (2012) Disulfiram stimulates dopamine release from noradrenergic terminals and potentiates cocaine-induced dopamine release in the prefrontal cortex. Psychopharmacology (Berl) 219:1153–1164 [DOI] [PubMed] [Google Scholar]
- Erb S, Hitchcott PK, Rajabi H, Mueller D, Shaham Y, Stewart J. (2000) Alpha-2 adrenergic receptor agonists block stress-induced reinstatement of cocaine seeking. Neuropsychopharmacology 23:138–150 [DOI] [PubMed] [Google Scholar]
- Fantegrossi WE, Bauzo RM, Manvich DM, Morales JC, Votaw JR, Goodman MM, Howell LL. (2009) Role of dopamine transporters in the behavioral effects of 3,4-methylenedioxymethamphetamine (MDMA) in nonhuman primates. Psychopharmacology (Berl) 205:337–347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaval-Cruz M, Weinshenker D. (2009) mechanisms of disulfiram-induced cocaine abstinence: antabuse and cocaine relapse. Mol Interv 9:175–187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- George TP, Chawarski MC, Pakes J, Carroll KM, Kosten TR, Schottenfeld RS. (2000) Disulfiram versus placebo for cocaine dependence in buprenorphine-maintained subjects: a preliminary trial. Biol Psychiatry 47:1080–1086 [DOI] [PubMed] [Google Scholar]
- Goldstein M, Anagnoste B, Lauber E, McKeregham MR. (1964) Inhibition of Dopamine-Beta-Hydroxylase by Disulfiram. Life Sci 3:763–767 [DOI] [PubMed] [Google Scholar]
- Grassi MC, Cioce AM, Giudici FD, Antonilli L, Nencini P. (2007) Short-term efficacy of Disulfiram or Naltrexone in reducing positive urinalysis for both cocaine and cocaethylene in cocaine abusers: a pilot study. Pharmacol Res 55:117–121 [DOI] [PubMed] [Google Scholar]
- Grenhoff J, Nisell M, Ferré S, Aston-Jones G, Svensson TH. (1993) Noradrenergic modulation of midbrain dopamine cell firing elicited by stimulation of the locus coeruleus in the rat. J Neural Transm 93:11–25 [DOI] [PubMed] [Google Scholar]
- Grenhoff J, Svensson TH. (1993) Prazosin modulates the firing pattern of dopamine neurons in rat ventral tegmental area. Eur J Pharmacol 233:79–84 [DOI] [PubMed] [Google Scholar]
- Haile CN, Mahoney JJ, 3rd, Newton TF, De La Garza R., 2nd (2012) Pharmacotherapeutics directed at deficiencies associated with cocaine dependence: focus on dopamine, norepinephrine and glutamate. Pharmacol Ther 134:260–277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimmel HL, Ginsburg BC, Howell LL. (2005) Changes in extracellular dopamine during cocaine self-administration in squirrel monkeys. Synapse 56:129–134 [DOI] [PubMed] [Google Scholar]
- Kimmel HL, O’Connor JA, Carroll FI, Howell LL. (2007) Faster onset and dopamine transporter selectivity predict stimulant and reinforcing effects of cocaine analogs in squirrel monkeys. Pharmacol Biochem Behav 86:45–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosten TR, Wu G, Huang W, Harding MJ, Hamon SC, Lappalainen J, Nielsen DA. (2013) Pharmacogenetic randomized trial for cocaine abuse: disulfiram and dopamine β-hydroxylase. Biol Psychiatry 73:219–224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee B, Tiefenbacher S, Platt DM, Spealman RD. (2004) Pharmacological blockade of alpha2-adrenoceptors induces reinstatement of cocaine-seeking behavior in squirrel monkeys. Neuropsychopharmacology 29:686–693 [DOI] [PubMed] [Google Scholar]
- Leri F, Flores J, Rodaros D, Stewart J. (2002) Blockade of stress-induced but not cocaine-induced reinstatement by infusion of noradrenergic antagonists into the bed nucleus of the stria terminalis or the central nucleus of the amygdala. J Neurosci 22:5713–5718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macey DJ, Smith HR, Nader MA, Porrino LJ. (2003) Chronic cocaine self-administration upregulates the norepinephrine transporter and alters functional activity in the bed nucleus of the stria terminalis of the rhesus monkey. J Neurosci 23:12–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manvich DF, DePoy LM, Weinshenker D. (2013) Dopamine β-hydroxylase inhibitors enhance the discriminative stimulus effects of cocaine in rats. J Pharmacol Exp Ther 347:564–573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manvich DF, Kimmel HL, Cooper DA, Howell LL. (2012a) The serotonin 2C receptor antagonist SB 242084 exhibits abuse-related effects typical of stimulants in squirrel monkeys. J Pharmacol Exp Ther 342:761–769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manvich DF, Kimmel HL, Howell LL. (2012b) Effects of serotonin 2C receptor agonists on the behavioral and neurochemical effects of cocaine in squirrel monkeys. J Pharmacol Exp Ther 341:424–434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland K, Kalivas PW. (2001) The circuitry mediating cocaine-induced reinstatement of drug-seeking behavior. J Neurosci 21:8655–8663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitrano DA, Schroeder JP, Smith Y, Cortright JJ, Bubula N, Vezina P, Weinshenker D. (2012) α-1 Adrenergic receptors are localized on presynaptic elements in the nucleus accumbens and regulate mesolimbic dopamine transmission. Neuropsychopharmacology 37:2161–2172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murnane KS, Winschel J, Schmidt KT, Stewart LM, Rose SJ, Cheng K, Rice KC, Howell LL. (2013) Serotonin 2A receptors differentially contribute to abuse-related effects of cocaine and cocaine-induced nigrostriatal and mesolimbic dopamine overflow in nonhuman primates. J Neurosci 33:13367–13374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musacchio JM, Goldstein M, Anagnoste B, Poch G, Kopin IJ. (1966) Inhibition of dopamine-beta-hydroxylase by disulfiram in vivo. J Pharmacol Exp Ther 152:56–61 [PubMed] [Google Scholar]
- Negus SS, Mello NK, Blough BE, Baumann MH, Rothman RB. (2007) Monoamine releasers with varying selectivity for dopamine/norepinephrine versus serotonin release as candidate “agonist” medications for cocaine dependence: studies in assays of cocaine discrimination and cocaine self-administration in rhesus monkeys. J Pharmacol Exp Ther 320:627–636 [DOI] [PubMed] [Google Scholar]
- Oliveto A, Poling J, Mancino MJ, Feldman Z, Cubells JF, Pruzinsky R, Gonsai K, Cargile C, Sofuoglu M, Chopra MP, et al. (2011) Randomized, double blind, placebo-controlled trial of disulfiram for the treatment of cocaine dependence in methadone-stabilized patients. Drug Alcohol Depend 113:184–191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrakis IL, Carroll KM, Nich C, Gordon LT, McCance-Katz EF, Frankforter T, Rounsaville BJ. (2000) Disulfiram treatment for cocaine dependence in methadone-maintained opioid addicts. Addiction 95:219–228 [DOI] [PubMed] [Google Scholar]
- Platt DM, Rowlett JK, Spealman RD. (2007) Noradrenergic mechanisms in cocaine-induced reinstatement of drug seeking in squirrel monkeys. J Pharmacol Exp Ther 322:894–902 [DOI] [PubMed] [Google Scholar]
- Schank JR, Ventura R, Puglisi-Allegra S, Alcaro A, Cole CD, Liles LC, Seeman P, Weinshenker D. (2006) Dopamine beta-hydroxylase knockout mice have alterations in dopamine signaling and are hypersensitive to cocaine. Neuropsychopharmacology 31:2221–2230 [DOI] [PubMed] [Google Scholar]
- Schroeder JP, Cooper DA, Schank JR, Lyle MA, Gaval-Cruz M, Ogbonmwan YE, Pozdeyev N, Freeman KG, Iuvone PM, Edwards GL, et al. (2010) Disulfiram attenuates drug-primed reinstatement of cocaine seeking via inhibition of dopamine β-hydroxylase. Neuropsychopharmacology 35:2440–2449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder JP, Epps SA, Grice TW, Weinshenker D. (2013) The selective dopamine β-hydroxylase inhibitor nepicastat attenuates multiple aspects of cocaine-seeking behavior. Neuropsychopharmacology 38:1032–1038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shorter D, Nielsen DA, Huang W, Harding MJ, Hamon SC, Kosten TR. (2013) Pharmacogenetic randomized trial for cocaine abuse: disulfiram and α1A-adrenoceptor gene variation. Eur Neuropsychopharmacol 23:1401–1407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RJ, Aston-Jones G. (2011) α(2) Adrenergic and imidazoline receptor agonists prevent cue-induced cocaine seeking. Biol Psychiatry 70:712–719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommermeyer H, Frielingsdorf J, Knorr A. (1995) Effects of prazosin on the dopaminergic neurotransmission in rat brain. Eur J Pharmacol 276:267–270 [DOI] [PubMed] [Google Scholar]
- Valdez GR, Platt DM, Rowlett JK, Rüedi-Bettschen D, Spealman RD. (2007) Kappa agonist-induced reinstatement of cocaine seeking in squirrel monkeys: a role for opioid and stress-related mechanisms. J Pharmacol Exp Ther 323:525–533 [DOI] [PubMed] [Google Scholar]
- Wee S, Wang Z, He R, Zhou J, Kozikowski AP, Woolverton WL. (2006) Role of the increased noradrenergic neurotransmission in drug self-administration. Drug Alcohol Depend 82:151–157 [DOI] [PubMed] [Google Scholar]
- Weinshenker D, Ferrucci M, Busceti CL, Biagioni F, Lazzeri G, Liles LC, Lenzi P, Pasquali L, Murri L, Paparelli A, et al. (2008) Genetic or pharmacological blockade of noradrenaline synthesis enhances the neurochemical, behavioral, and neurotoxic effects of methamphetamine. J Neurochem 105:471–483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinshenker D, Schroeder JP. (2007) There and back again: a tale of norepinephrine and drug addiction. Neuropsychopharmacology 32:1433–1451 [DOI] [PubMed] [Google Scholar]
- Woolverton WL. (1987) Evaluation of the role of norepinephrine in the reinforcing effects of psychomotor stimulants in rhesus monkeys. Pharmacol Biochem Behav 26:835–839 [DOI] [PubMed] [Google Scholar]
- Zhang XY, Kosten TA. (2005) Prazosin, an alpha-1 adrenergic antagonist, reduces cocaine-induced reinstatement of drug-seeking. Biol Psychiatry 57:1202–1204 [DOI] [PubMed] [Google Scholar]