Abstract
A substituted aryl amide derivative of 6-naltrexamine—17-cyclopropylmethyl-3,14β-dihydroxy-4,5α-epoxy-6β-[(4′-trimethylfluoro)benzamido]morphinan-hydrochloride—(compound 5), previously shown to be a potent κ-opioid receptor antagonist, was used to characterize the physicochemical properties and efficacy to decrease alcohol self-administration in alcohol-preferring rats (P-rats) and binge-like P-rats. Previous studies showed that compounds closely related to compound 5 possessed favorable properties regarding penetration of the blood-brain barrier. Pharmacokinetic studies showed that compound 5 had acceptable bioavailability. In contrast to other κ-receptor antagonists, in particular norbinaltorphimine, compound 5 showed favorable drug-like properties. Based on these findings, further studies were done. Safety studies showed that compound 5 was not hepatotoxic at doses 200-fold greater than an efficacious dose. The effects of compound 5 or naltrexone on the hepatotoxicity of thiobenzamide were investigated. In contrast to naltrexone, which exacerbated thiobenzamide-mediated hepatotoxicity, compound 5 was observed to be hepatoprotective. Based on the physicochemical properties of compound 5, the compound was examined in rat animal models of alcohol self-administration. The inhibition of ethanol self-administration by compound 5 in alcohol-dependent and alcohol-nondependent P-rats trained to self-administer a 10% (w/v) ethanol solution, using operant techniques, showed very potent efficacy (i.e., estimated ED50 values of 4–5 μg/kg). In a binge-like P-rat animal model, inhibition of alcohol self-administration by compound 5 had an estimated ED50 value of 8 μg/kg. The results suggest that compound 5 is a potent drug-like κ-opioid receptor antagonist of utility in alcohol cessation medications development.
Introduction
Ethanol overuse is a serious public health disorder with significant social and economic consequences. In 1994, naltrexone (compound 1; Scheme 1), a pure opioid μ-receptor antagonist with relatively low affinity for δ- and κ-opioid receptors and no abuse potential (Tabakoff and Hoffman, 1983), was approved by the US Food and Drug Administration for treatment of alcoholism. A number of studies suggest that alcohol interacts with endogenous opioid systems (Grisel et al., 1995; Gianoulakis et al., 1996). Antagonizing opioid receptors decreases the effects of alcohol-mediated pleasure-inducing endogenous opioids. By attenuating the positive reinforcing effects of alcohol consumption, opioid receptor antagonists directly affect alcohol-seeking behavior (Pastor and Aragon, 2006). A decrease in alcohol consumption by antagonism of opioid receptors suggests direct effects of this reinforcement system, and animal studies have shown that µ-, δ-, and κ-opioid receptors contribute to alcohol-induced reinforcement (Ulm et al., 1995; Herz, 1997).
Scheme 1.
Chemical structures of compounds 1–5.
Based on a number of clinical studies, naltrexone is effective in decreasing alcohol consumption in heavy drinkers (Pettinati et al., 2006) and in treating alcoholism (Anton et al., 1999; Bouza et al., 2004). However, naltrexone is not successful in treating all alcoholics, and adverse effects, including intolerable nausea (Croop et al., 1997) and hepatotoxicity (Mitchell et al., 1987; Mason et al., 1999), confound treatment of patients with liver disease. However, most reports (Sax et al., 1994; Brewer and Wong, 2004; Yen et al., 2006) suggest that naltrexone itself does not cause clinically significant hepatotoxicity. Relatively low bioavailability of naltrexone (Anton et al., 1999) and possibly genetic variability of the opioid receptors (Oslin et al., 2006) may explain the less than consistent efficacy of naltrexone (Roozen et al., 2006).
Thiobenzamide is a well characterized hepatotoxin that causes centrilobular necrosis (Hanzlik et al., 1978, 1980) and requires S-oxidative metabolic bioactivation for full expression of its hepatotoxicity (Cashman and Hanzlik, 1981; Hanzlik and Cashman, 1983). Hepatotoxicity of toxic doses of thiobenzamide is maximal 24 hours after administration and thus can provide an excellent acute model system to examine the effect of 17-cyclopropylmethyl-3,14β-dihydroxy-4,5α-epoxy-6β-[(4′-trimethylfluoro)benzamido]morphinan-hydrochloride (compound 5) or naltrexone on the exacerbation or protection of hepatotoxicity.
In contrast to naltrexone, a more selective κ-opioid receptor antagonist is norbinaltorphimine (nor-BNI). Nor-BNI is effective at decreasing alcohol self-administration in small animals (Walker and Koob, 2008; Walker et al., 2011). Despite its promise, nor-BNI possesses very long-lasting effects (Horan et al., 1992) and is possibly unstable to oxidation (Osa et al., 2007). Similar to other long-acting κ-opioid antagonists, such as 5′-guanidinonaltrindole (GNTI) and (3R)-7-hydroxy-N-[(2S)-1-[(3R,4R)-4-(3-hydroxyphenyl)-3,4-dimethylpiperidin-1-yl]-3-methylbutan-2-yl]-1,2,3,4-tetrahydroisoquinoline-3-carboxamide (JDTic), nor-BNI has a very long time course of κ-opioid receptor antagonism (Munro et al., 2012). Thus, there is a need for a relatively fast-acting drug-like κ-opioid receptor antagonist that possesses appropriate pharmacokinetic and biodistribution properties consistent with a reversible drug.
Studies using rodent animal models have shown that naltrexone decreases alcohol self-administration (Benjamin et al., 1993; Stromberg et al., 2001), suggesting that these types of agents may prevent the reinforcing effects of alcohol consumption (Bouza et al., 2004). The alcohol-preferring rat (P-rat) has been effectively used as a small animal model to study binge drinking (Li et al., 1987). In the P-rat, naltrexone (Biggs and Myers, 1998; Gilpin et al., 2008; Ji et al., 2008) and other opioids (Weiss et al., 1990) have been shown to be effective in decreasing alcohol self-administration. Nalmefene (Scheme 1), the 6-methylene analog of naltrexone, is a more potent κ-opioid antagonist than naltrexone and is an effective antagonist of alcohol self-administration in outbred and P-rats (June et al., 1998, 2004).
Herein, we report on the evaluation of a potent κ-opioid antagonist as an alcohol self-administration cessation agent. The κ-opioid antagonists are anticipated to show a dual action by inhibiting alcohol reinforcement and stimulating dopamine release to decrease craving. Compound 5 (Scheme 1) has been previously reported to decrease alcohol self-administration in Wistar rats. In this study, we extend the analysis to alcohol-preferring and binge-like P-rats. The results show that compound 5 is a very potent, relatively short-acting agent that decreases alcohol self-administration in P-rats and binge-like P-rats. Compound 5 possesses good physicochemical properties and is very drug-like, and in contrast to naltrexone, protects from the hepatotoxicity of a potent hepatotoxin in rats. The rationale for our work was to develop a relatively short-acting drug-like κ-opioid antagonist by replacing the metabolically labile 6-keto moiety of naltrexone with an amide moiety, thus leading to an agent with potent pharmacological activity and potentially less hepatotoxicity.
Materials and Methods
Chemicals
Naltrexone and nalmefene hydrochloride (compounds 1 and 2, respectively) were obtained from Tyco Mallincrodt (St. Louis, MO). We synthesized 17-cyclopropylmethyl-3,14β-dihydroxy-4,5α-epoxy-6β-[(4′-bromo)benzamido]morphinan-hydrochloride (compound 3) and compound 5 as previously described (Ghirmai et al., 2009) (Scheme 1).
Diisopropylethylamine (DIPEA), (dimethylamino) phosphonium hexa-fluorophosphate (BOP), HBF4, Pd(OAc)2, tetrabutylammonium hydroxide, thiobenzamide, heparin, and Supersac were obtained from Sigma-Aldrich (St. Louis, MO) and were used as received. All of the solvents and buffers used were obtained in the highest grade commercially available from VWR (San Diego, CA).
General Procedures
Synthetic chemical reactions were run under a positive pressure of nitrogen with magnetic stirring at ambient temperature using oven-dried glassware unless otherwise indicated. Silica gel (230–400 mesh) was used for column chromatography. Dichloromethane (DCM) was dried by filtration through a column of neutral alumina and stored over activated 4 Å molecular sieves under nitrogen prior to use. All other solvents and reagents were used as received. 1H-NMR spectra were recorded at 300.0 MHz on a Varian Mercury 300 instrument (Palo Alto, CA). Chemical shifts were reported in ppm (δ) relative to CDCl3 at 7.26 ppm. NMR spectra were recorded in CDCl3. Mass spectra were obtained with a Hitachi spectrometer (Dallas, TX) operating in the electrospray ionization mode. Analytical purities were determined by reverse-phase high-performance liquid chromatography (HPLC) using a Hitachi D2500 Hitachi Chromato-integrator, an L-6000 Hitachi pump, and an L-4200 UV-visible Hitachi detector (285 nm) using a reverse phase system (5 µm × 4.6 mm × 250 mm). The mobile phase was 20% 0.05 M tetrabutylammonium hydroxide and 80% methanol using isocratic elution at a flow rate of 1 ml/min. Analytical work for the pharmacokinetic studies was done at Microconstants, Inc. (San Diego, CA).
Animals.
Animal work was conducted in accordance with the Guide for the Care and Use of Laboratory Animals as adopted by the National Institutes of Health. Formal approval to conduct the experiments was obtained from the Institutional Animal Care and Use Committees of the Human BioMolecular Research Institute and Behavioral Pharma, Inc. Animals were assigned randomly to experimental groups, allowed to acclimatize to the facilities for 1 week, and given commercial rat chow and sterile distilled water ad libitum. For the studies with thiobenzamide, male Sprague-Dawley rats weighing 300–400 g from Harlan (San Jose, CA) were used. For pharmacokinetic studies, cannulated male Sprague-Dawley rats (Harlan) weighing 250–300 g at the time of the experiment were housed individually and maintained in a temperature-controlled environment on a 12-hour light/dark cycle (off 7:30 AM; on 7:30 PM). Except during testing, animals were given free access to food and water. Animals administered compounds via the oral route were deprived of food 10 hours before the experiment. For toxicology studies, compound 5 was administered to male Sprague-Dawley rats weighing 300–350 g (Harlan). Twenty-four hours after the last dose of compound 5, animals were killed, blood was obtained and centrifuged, and serum was separated and frozen for analysis of serum clinical chemistry at IDEXX Laboratories (Sacramento, CA). For alcohol self-administration studies, male alcohol-preferring Wistar rats (225–249 g) were obtained from the University of Indiana (Indianapolis, IN) and were housed in groups of two or three and maintained in a temperature-controlled environment on a 12-hour light/dark cycle (off 7:30 AM; on 7:30 PM). Except during behavioral testing, animals were given free access to food and water.
Chemical Synthesis
6-β-(4′-Trifluoromethyl-2′,3′,5′,6′-Tetradeutrio)Benzamido-14-Hydroxy-17-(Cyclopropylmethyl)Nordesmorphine.
6-β-(4′-Trifluoromethyl-2′,3′,5′,6′-tetradeutrio)benzamido-14-hydroxy-17-(cyclopropylmethyl)nordesmorphine (compound 4; Scheme 1) was synthesized by combining β-naltrexamine and 4-CF3-benzoic acid-d4 with BOP dissolved in anhydrous DCM, followed by addition of DIPEA. After removal of the ester at the 3-position by treatment with potassium carbonate, compound 4 was obtained in quantitative yield and converted to its hydrochloride salt. The requisite 4-CF3-benzoic acid-d4 was obtained by following a literature procedure for a nondeuterated analog (Watson et al., 2008). A mixture of K2CO3 (624 mg, 4.5 mmol, 1.5 equiv.), DCPP × HBF4 (73.2 mg, 0.12 mmol, 4% mol), and Pd(OAc)2 (13.5 mg, 0.06 mmol, 2% mol) was placed in an 8-dram vial. The vial was sealed with a septum and purged with Ar. A solution of 4-chloro-trifluoromethyl-toluene-d4 (554 mg, 3 mmol) in dimethylsulfoxide (3 ml) and H2O (108 μl, 108 mg, 6 mmol, 2 equiv.) was added via syringe, and an atmosphere of CO was added to the vial and purged three times and run for 15 hours at 100°C under a CO atmosphere. The mixture was cooled and diluted with 0.25 M NaOH aq. (65 ml) and extracted with DCM (2 × 25 ml). The aqueous layer was neutralized with 3 M HCl (10 ml) and extracted with Et2O (3 × 50 ml). The combined organic material was dried over Na2SO4, filtered, and evaporated to give 4-CF3-benzoic acid-d4 as a white solid, 550 mg, in 94% yield.
Compound 4-d4 was obtained by following a previously reported procedure (Ghirmai et al., 2008). β-Naltrexamine (100 mg, 0.29 mmol), 4-CF3-benzoic acid-d4 (113.3 mg, 0.584 mmol, 2 equiv.), and BOP (258 mg, 0.584 mmol, 2 equiv.) were placed in anhydrous DCM (4 ml) and DIPEA (152 µl, 0.876 mmol, 3 equiv.) was added and the reaction was stirred overnight at room temperature to afford the ester-amide. After purification by flash chromatography (100% EtOAc) the ester-amide was dissolved in methanol and potassium carbonate was added. The mixture was stirred at room temperature for 3 hours, potassium carbonate was removed by filtration, and the product was purified by preparative thin layer chromatography (CHCl3/MeOH) 20/1 to obtain in quantitative yield the desired product. The purity was >98% on the basis of HPLC and liquid chromatography–mass spectrometry (LC-MS).
1H-NMR (CDCl3) δ 0.13–0.18 (m, 2H), 0.53–0.59 (m, 2H), 0.81–0.92 (m, 1H), 1.39–1.62 (m, 3H), 1.66–1.74 (m, 1H), 1.89–2.0 (m, 1H), 2.17–2.26 (m, 2H), 2.39 (d, J = 6.3 Hz, 2H), 2.65 (d, J = 9.9 Hz, 2H), 3.04 (d, J = 13.4 Hz, 1H), 3.16 (d, J = 5.2 Hz, 1H), 3.64 (d, J = 11.0 Hz, 1H), 4.08–4.18 (m, 2H), 4.63 (d, J = 5.24 Hz, 1H), 6.52 (d, J = 8.0 Hz, 1H), 6.67 (d, J = 8.0 Hz, 1H), 7.79 (d, J = 9.0 Hz, 1H). Electrospray ionization/MS m/z = 518.95 [M+H].
Pharmacokinetic Studies
The night before the oral pharmacokinetic study, the animals were fasted. Groups of two jugular cannulated rats were administered compound 5 hydrochloride by the intravenous route of administration (20 or 50 µg/kg, 1 ml/kg) or the oral route of administration (200 µg/kg, 2 ml/kg) in isotonic saline. For the intravenous study of compound 5, blood was taken at 5, 15, 30, 60, 120, 240 minutes and 6 and 10 hours. For the oral study, blood was taken at 15, 30, 60, 120, and 240 minutes and 6 and 10 hours. Blood was combined with 2 IUs of heparin and immediately cooled to 4°C. Separated plasma was brought to a pH of 10 with ammonium hydroxide, and 400 pg/ml compound 4 was added as an internal standard and extracted with hexane/methyl-tert-butyl ether (3:1, v:v). After centrifugation at 13,000g for 5 minutes, the organic fraction was collected and the solvent was removed with a stream of argon. The residue was reconstituted in water:acetonitrile:formic acid (80:20:0.1, v:v) and run isocratically in 0.1% formic acid in water, 0.1% formic acid in acetonitrile (60:40) using a Waters Acquity instrument and Waters XEVO tandem quadrupole detector (Waters, Milford, MA). An aliquot was analyzed by reverse-phase HPLC using a Synergi Polar RP column (2.1 × 150 mm, 4 µm; Phenomenex, Torrance, CA) maintained at 45°C. The mobile phase was nebulized using heated nitrogen in a Z-spray source/interface set to electrospray positive mode. The ionized compounds were detected using tandem mass spectrometry, and both compounds 4 and 5 had retention times of 2.7 minutes in the LC-MS experiment. The standard curve was run between 20–20,000 pg/ml. The calibration curves were obtained by fitting the in-transformed peak height ratios of compound 4/5, and their log-transformed standard concentrations to an appropriate regression equation using MassLynx software (Waters). Pharmacokinetic data were determined using an in-house Excel Macro program for pharmacokinetic parameters.
In Vivo Hepatotoxicology Studies
Thiobenzamide was administered intraperitoneally as a very fine suspension in corn oil (2 mmol/kg, 274 mg/kg, 4 ml/kg). Naltrexone hydrochloride (500 µg/kg, 1 ml/kg i.p.) was administered in sterile saline. Compound 5 hydrochloride (20 µg/kg, 1 ml/kg i.p.) was administered in sterile saline. On the day of the experiment, groups of six animals each were administered thiobenzamide or vehicle as a challenge dose. Twenty-four hours after the challenge dose, treatments were administered. The compound treatments were as follows: vehicle, naltrexone (1.3 µmol/kg or 500 µg/kg), or compound 5 (0.036 µmol/kg or 20 µg/kg). Forty-eight hours after administration of thiobenzamide or vehicle, the animals were killed and blood was collected in heparin-treated syringes and centrifuged; serum was immediately frozen. Serum was sent to IDEXX Laboratories, and serum clinical values were obtained. The mean and standard deviations of the values were calculated and are summarized in Table 2.
TABLE 2.
Effect of κ antagonism on the hepatotoxicity of thiobenzamide
| Condition | Alkaline Phosphatasea | SGPT (ALT) | SGOT (AST) | Albumin | BUN |
|---|---|---|---|---|---|
| Control | 227.3 ± 13.8 | 44.7 ± 8.7 | 82.3 ± 27.6 | 2.9 ± 0.1 | 23.3 ± 3.2 |
| Thiobenzamide alone | 150.5 ± 55.6* | 798 ± 447.1* | 1021 ± 775.8* | 2.6 ± 0.3 | 66.2 ± 34.9* |
| Thiobenzamide + compound 5 | 122.5 ± 18.8 | 613.7 ± 349.2** | 993 ± 172.2 | 2.8 ± 0.4 | 43.2 ± 7.4 |
| Thiobenzamide + naltrexone | 169 ± 84.5 | 1749.8 ± 245.1*** | 1461.8 ± 312.3 | 2.5 ± 0.2 | 57.8 ± 23.9 |
ALT, alanine aminotransferase; AST, aspartate aminotransferase; BUN, blood urea nitrogen.
Mean ± S.D. of values from six animals.
P < 0.05 for control versus thiobenzamide (274 mg/kg) alone.
P < 0.05 for thiobenzamide (274 mg/kg) alone versus thiobenzamide + naltrexone (500 µg/kg).
P < 0.05 for thiobenzamide (274 mg/kg) + compound 5 (20 µg/kg) versus thiobenzamide (274 mg/kg) + naltrexone (500 µg/kg).
Operant Procedure for Oral EtOH and Supersaccharin Self-Administration Training
Ethanol or Supersac self-administration training was conducted in standard alcohol vapor chambers (La Jolla Alcohol Research, La Jolla, CA) located in sound-attenuated, ventilated cubicles. Two 35-ml syringes dispensed either EtOH, water, or Supersac through plastic tubing into two stainless steel drinking cups mounted 4 cm above the grid floor and centered on the front panel of each chamber. Each drinking cup held two reinforcer deliveries (0.1 ml of fluid/reinforcer). Two retractable levers were located 4.5 cm to either side of the drinking cups. Fluid delivery and recording of operant responses were controlled by a microcomputer.
In brief, animals were trained to voluntarily self-administer 10% (w/v) EtOH (n = 11) or Supersac (n = 11) by the oral route using the saccharin fadeout method (Rassnick et al., 1993) and were tested for their response for EtOH or Supersac solution in a two-lever free-choice situation. Once baseline EtOH and Supersac intakes were achieved (i.e., when responding across 3 consecutive days varied less than 20% and response rates corresponded to pharmacologically relevant blood alcohol levels [BALs]), dose-response testing for compound 5 commenced. BALs were measured once per week but never immediately before or after testing, as this was deemed too stressful to the animal. Typically, BALs were obtained 2–3 days prior to testing. BAL levels during these experiments were maintained at 150–200 mg%. To allow for complete dissipation of any carryover effects, a 1-week washout period, in which rats were rebaselined during daily 30-minute operant sessions, occurred between testing of different doses.
Ethanol Self-Administration Studies
P-rats were divided into alcohol binge drinkers (n = 11) and Supersac controls (n = 11). Prior to two-bottle choice training, all rats were given an initial 2-hour training session during which they were allowed to drink Supersac in a single-bottle situation. Rats were allowed 30-minute drinking sessions for 9–14 consecutive days before pharmacologic manipulation occurred. After baseline training, rats were injected subcutaneously with one of several doses of compound 5, (0.00312, 0.00625, and 0.0125 mg/kg, 1 ml/kg) 30 minutes before two-bottle choice test sessions in a within-subjects Latin square design. Rats were allowed to self-administer every day during this time, but were injected with test compound every other day. Rats were tested during their active cycle.
Data Analysis
Statistics, regression analyses, and determination of ED50 values were done using GraphPad Prism (version 4.02; GraphPad, San Diego, CA). Data on the effect of opioids on thiobenzamide hepatotoxicity were expressed as mean ± S.D. and analyzed with the Student’s t test for the difference between two means with a Welch correction. Multiple means were analyzed by a randomized one-way analysis of variance. When the analysis indicated that a significant difference existed, the means of each group were compared by the Student-Newman-Keuls test. In the analyses, the level of significance chosen was P < 0.05. For alcohol and Supersac self-administration studies, data were simultaneously collected online from multiple operant chambers. Results of the operant procedure were reported as mean cumulative number of bar presses ± S.E.M. for ethanol or Supersac and normalized for body weight (i.e., gram of ethanol per kilogram body weight; milliliter of Supersac per kilogram body weight). The effects of compound 5 on alcohol (gram per kilogram) intake and Supersac (milliliter per kilogram) intake were analyzed by one-way repeated measures analysis of variance, with the dose of compound 5 as a within-subjects factor. In general, tests for homogeneity of variance were first conducted on the data. If the scores did not violate the assumption of homogeneity of variance, appropriate analyses of variance were conducted. Data were analyzed using the StatView statistical package on a PC-compatible computer. Mixed-design analyses of variance were used with test compound treatments as a within-subjects factor (i.e., repeated measures design for test compound treatment). A priori analysis examining individual test compound doses to vehicle control dose was conducted using paired t tests. Significant test compound effects were defined as having P < 0.05 compared with vehicle-treated rats.
Results
The chemical synthesis of 17-cyclopropylmethyl-3,14β-dihydroxy-4,5α-epoxy-6β-[(4′-trimethylfluoro)benzamido]morphinan (Scheme 1) was efficiently accomplished as described previously (Ghirmai et al., 2009). As a standard for pharmacokinetic studies, a deuterated analog, compound 4, was efficiently synthesized (Scheme 1). Thus, deuterated compound 4 was synthesized by combining β-naltrexamine, 4-CF3-benzoic acid-d4, and BOP dissolved in anhydrous DCM followed by addition of DIPEA. After removal of the ester by treatment with potassium carbonate, compound 4 was obtained in quantitative yield.
As previously reported, compound 5 was evaluated in the presence of opioid receptors using a 5-O-(3-[35S]thio)triphosphate ([35S]GTPγS) assay (Traynor and Nahorski, 1995). The [35S]GTPγS binding data showed that compound 5 was a partial agonist at the μ-opioid receptor and was an antagonist of δ- and κ-opioid receptors (Ghirmai et al., 2009). In the presence of the nociceptin opioid (NOP) receptor, compound 5 had very low affinity and did not stimulate agonist-induced GTPγS binding. Compound 5 was found to potently decrease basal binding at NOP. Compound 5 was a high-affinity compound that showed low or partial agonist activity in the GTPγS binding experiment and was tested for inhibition of agonist-induced GTPγS binding at each opioid receptor. Compound 5 produced potent inhibition at both κ- and NOP-receptors and modest inhibition at the δ-receptor but not at the μ-receptor. Compound 5 was shown to possess potent antagonism for the κ-opioid and NOP-receptors, and it was taken forward for in vivo studies. As described below, further kinetic analysis was done to characterize the pharmaceutical properties of compound 5.
Metabolic Stability and Pharmacokinetics.
As reported previously, the metabolic stability of compound 5 was examined in the presence of rat, mouse, and human liver preparations plus the appropriate NADPH-generating system (Ghirmai et al., 2009). Compared with nalmefene, compound 5 was quite metabolically stable. In the presence of mouse or human liver microsomes, compound 5 possessed half-life values in excess of 112 minutes and was judged to be quite metabolically stable. In the presence of rat liver microsomes, overall compound 5 was somewhat less metabolically stable, but the half-life values observed did not preclude evaluation of the compounds in vivo.
Evaluation of the inhibition of selective functional activity of cytochrome P450 (P450) was done as previously reported (Ghirmai et al., 2009) for compound 5 as a control on the apparent metabolic stability. The P450 enzyme assays were done using standard conditions as previously described (Denton et al., 2004). Compared with nalmefene, compound 5 possessed less inhibitory potency against the P450s studied (i.e., CYP3A4, -2B6, -2C9, -2C19, and -2D6). A possible exception was CYP2C19, which appeared to be more sensitive than nalmefene to inhibition by compound 5. Because no significant inhibition of P450 was observed, and based on the low plasma concentration of compound 5 observed (i.e., 2–8 ng/ml; Table 1) it is unlikely that compound 5 inhibits P450 and alcohol metabolism in vivo at the doses used in this study. This is based on the well recognized relationship (i.e., I/Ki) that predicts the potential for in vivo interactions (Wienkers and Heath, 2005). If I/Ki is greater than 1, then a significant interaction is predicted. In the case herein, the I/Ki ratio is 0.0003, assuming a Ki of 10 µM. Therefore, no significant interaction is predicted. At the concentrations that are effective at decreasing alcohol self-administration (i.e., 5–10 µg/kg), there is virtually no effect of compound 5 on P450-mediated alcohol metabolism. Accordingly, compound 5 was advanced to pharmacokinetic studies.
TABLE 1.
Pharmacokinetic parameters for lead compound 5
| Route |
Dose |
Cmax
|
Tmax
|
Area under the Curve |
CL/F |
t1/2
|
|---|---|---|---|---|---|---|
| µg/kg | pg/ml | hr | pg × h/ml | l/h/kg | h | |
| i.v. | 20 | 2230 | 0.08 | 1704 | 11.73 | 1.9 |
| i.v. | 50 | 7790 | 0.08 | 3559 | 14.05 | 1.5 |
| Oral | 200 | 89 | 2 | 578 | 346 | 3.4 |
CL, clearance; F, bioavailability.
In Vivo Studies with Compound 5.
The pharmacokinetics (PK) of compound 5 were examined in male Sprague-Dawley rats by the intravenous (two doses, 20 and 50 µg/kg) and oral (one dose, 200 µg/kg) routes of administration. The doses were chosen to mimic the situation in efficacy studies and still be above the lowest limit of detection (20 pg/ml in plasma) by liquid chromatography–tandem mass spectrometry (LC-MS/MS). Serum was extracted and analytes were determined by LC-MS/MS. Table 1 shows the PK parameters for compound 5. The preliminary PK studies of the para-bromophenyl analog of compound 5 (i.e., compound 3; Scheme 1) have been previously reported (Ghirmai et al., 2009) and are in general agreement with the results described below for compound 5.
The hydrochloride salt of compound 5 was administered to two groups of three rats via the oral (200 µg/kg) or intravenous (20 µg/kg) routes of administration. After oral administration of compound 5, the time to achieve maximum concentration (Tmax) was 120 minutes, and the apparent half-life (t1/2) was 3.4 hour. After intravenous administration of compound 5, the Tmax was 5 minutes and the t1/2 was 114 minutes. A summary of the pharmacokinetic parameters is listed in Table 1. The bioavailability was calculated at 11%. Previously, reported data showed that the brain tissue/plasma ratio of the closely related para-bromophenyl analog compound 3 (i.e., a ratio of 2.3:1) was adequate to proceed with in vivo studies (Ghirmai et al., 2009).
Before extensive efficacy studies were conducted, preliminary toxicology studies were undertaken to help establish the safety of compound 5. Range-finding toxicology studies were done in male Sprague-Dawley rats. Compound 5 was very well tolerated in rats. Doses as great as 4 mg/kg (oral) of compound 5 did not show any adverse effects and clinical chemistry analysis of plasma revealed no liver or kidney toxicity. A dose of 4 mg/kg compound 5 is a dose that is 200-fold greater than an estimated efficacious dose. Long-term dosing of compound 5 for 7 days at a dose of 2 mg/kg (i.e., a dose that is 100-fold greater than an estimated efficacious dose) showed no signs of clinical toxicity on the basis of analysis of plasma clinical chemistry. Compared with rats treated with vehicle alone, 7-day dosing of compound 5 at 2 mg/kg caused no apparent liver or kidney toxicity.
Effect of Compound 5 or Naltrexone on an Animal Model of Acute Hepatotoxicity.
The effect of compound 5 or naltrexone on the relative hepatotoxicity of coadministered thiobenzamide to rats was determined. As shown in Table 2, thiobenzamide (2 mmol/kg i.p.) produced significant hepatotoxicity at 48 hours postadministration compared with vehicle (i.e., 17.8- and 12.4-fold increases in hepatotoxicity, respectively) on the basis of serum glutamic-pyruvic transaminase (SGPT) and serum glutamic oxaloacetic transaminase (SGOT) values. Administration of compound 5 (20 µg/kg i.p.) 24 hours after thiobenzamide (2 mmol/kg i.p. in corn oil) showed decreases in SGPT and SGOT values (i.e., nearly 4-fold and 0.4-fold, respectively, decreases in hepatotoxicity compared with thiobenzamide alone). In contrast, administration of naltrexone (500 µg/kg i.p.) 24 hours after thiobenzamide exacerbated the hepatotoxicity of thiobenzamide. Compared with thiobenzamide alone, administration of thiobenzamide and then naltrexone increased SGPT and SGOT levels over 21- and 17.8-fold, respectively. Compared with administration of naltrexone, administration of compound 5 24 hours after thiobenzamide significantly decreased hepatotoxicity of thiobenzamide (P = 0.0034). The hepatoprotective effect of compound 5 on thiobenzamide hepatotoxicity was statistically significant compared with the lack of any hepatoprotective effect of naltrexone on thiobenzamide hepatotoxicity (P = 0.0005). The hepatoprotective effect of compound 5 on thiobenzamide hepatotoxicity as judged by SGOT values was nearly statistically significant compared with the lack of any hepatoprotective effect of naltrexone on thiobenzamide hepatotoxicity (P = 0.055). There was no statistically significant difference of treatment by compound 5 or naltrexone on the toxicity of thiobenzamide on the basis of serum albumin or blood urea nitrogen values.
In Vivo Alcohol Self-Administration Studies.
Previously, we showed that compound 5 possessed potent effects on ethanol intake in nondependent Wistar rats trained to self-administer a 10% (w/v) ethanol solution, utilizing operant techniques (Ghirmai et al., 2009). As a positive control, nalmefene hydrochloride was also examined. Previous studies showed that compound 5, naltrexone, and nalmefene inhibited alcohol self-administration, with ED50 values of 0.019, 0.5, and 0.040 mg/kg, respectively, in the Wistar rat model. Because compound 5 showed considerable potency at inhibition of alcohol self-administration it was studied further in alcohol-preferring rats (i.e., P-rats). We based the dose selection of compound 5 in P-rats on the outcome of the testing of compound 5 in nondependent normal Wistar rats.
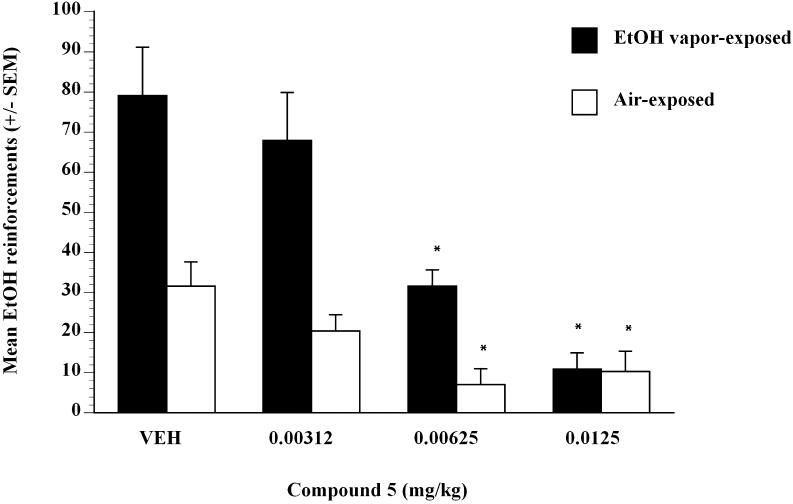
Results showed that P-rats voluntarily and orally self-administered amounts of alcohol to produce blood alcohol levels on average of 0.071 g% following 30-minute self-administration sessions. The average sweetened alcohol solution intake in P-rat vehicle controls during drug testing was 9.0 ml (1.5 g/kg) in the absence of food or water deprivation. Compound 5 was administered subcutaneously in a Latin square design dose-range study and showed significant efficacy. A detailed study using compound 5 from 0.003125 to 0.0125 mg/kg showed that the compound was efficacious at inhibiting sweetened alcohol self-administration in nondependent (air-exposed) and EtOH-dependent (EtOH vapor–exposed) P-rats (Fig. 1). Compound 5 pretreatment dose-dependently decreased intake of sweetened alcohol solution by P-rats (Fig. 1). Analysis revealed that compound 5 at 0.00312, 0.00625, and 0.0125 mg/kg doses significantly suppressed alcohol intake in alcohol-dependent P-rats (P < 0.05). Analysis revealed that compound 5 at 0.00625 and 0.0125 mg/kg doses significantly suppressed alcohol intake in alcohol-nondependent P-rats (P < 0.05) (Fig. 1).
Fig. 1.
Operant lever presses for ethanol by alcohol-dependent (black bars) and alcohol-nondependent (white bars) P-rats after injection of compound 5 doses (0, 0.00312, 0.00625, 0.0125 mg/kg). Operant tests occurred 6 hours after termination of vapor exposure (i.e., 6-hour withdrawal). *P < 0.05 significant difference from vehicle condition in alcohol-dependent or alcohol-nondependent control P-rats.
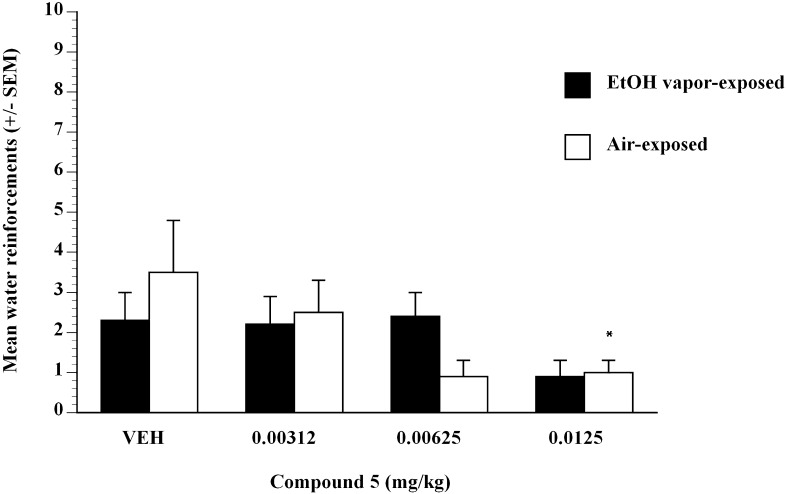
To test whether the effect of compound 5 was selective for sweetened ethanol, the effect of compound 5 on self-administration of water (Fig. 2) was examined. Treatment with compound 5 did not have an overall effect on the self-administration of water compared with vehicle. In control alcohol-dependent P-rats that consumed water, analysis did not reveal any significant effect of compound 5 dose on water intake (Fig. 2). In control alcohol-nondependent P-rats that consumed water, analysis did not reveal any significant effect of compound 5 dose on water intake except at the 0.0125 mg/kg dose (Fig. 2). Data represented mean responses for EtOH after compound 5 (0.0–0.0125 mg/kg) administration in nondependent controls (air-exposed, n = 8) and ethanol-dependent (EtOH vapor–exposed, n = 10) P-rats after 6-hour withdrawal. Compound 5 produced decreases in EtOH self-administration at 0.00625 and 0.0125 mg/kg compared with air (white bars) and EtOH vapor–exposed (black bars) vehicle controls (P < 0.05) (Fig. 1). The ED50 for compound 5 in EtOH-dependent (black bars) P-rats was estimated to be 0.0044 mg/kg, and in nondependent rats (white bars) it was estimated to be 0.005 mg/kg, using linear regression methods.
Fig. 2.
Operant lever presses for water by alcohol-dependent (black bars) and alcohol-nondependent (white bars) P-rats after injection of compound 5 (0, 0.00312, 0.00625, 0.0125 mg/kg). Operant tests occurred 6 hours after termination of vapor exposure (i.e., 6-hour withdrawal). *P < 0.05 significant difference from vehicle condition in alcohol-dependent or alcohol-nondependent control P-rats.
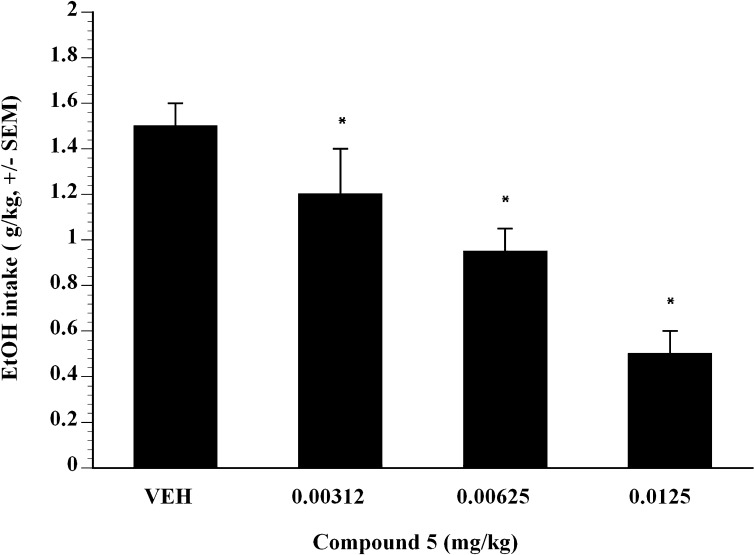
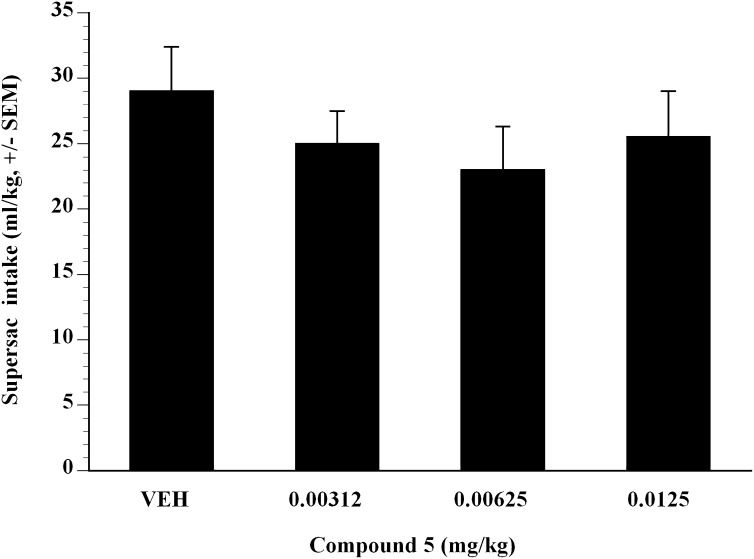
To further examine the effect of compound 5 on alcohol self-administration, compound 5 was examined on alcohol self-administration in binge-like P-rats. The term binge-like P-rats was used because the animals did not quite achieve BALs that are normally associated with binge-drinking P-rats (i.e., binge-like P-rats attained 1.2–1.4 g/kg EtOH in a 30-minute session, whereas binge-like P-rats generally achieve 1.5 g/kg EtOH in a 30 minute session). Compound 5 was administered subcutaneously in a Latin square design dose-range study and showed significant efficacy. Doses of compound 5 from 0.00312 to 0.0125 mg/kg showed that compound 5 inhibited Supersac-sweetened alcohol self-administration in binge-like P-rats (Fig. 3). Compared with vehicle, analysis showed that at all doses examined, compound 5 significantly suppressed binge-like alcohol intake in P-rats (P < 0.05). The ED50 was estimated to be 0.008 mg/kg in binge-like P-rats (Fig. 3). To test whether the effect of compound 5 was selective for Supersac-sweetened ethanol, the effect of compound 5 on self-administration of Supersac (Fig. 4) was examined. In control animals that only consumed Supersac, analysis did not reveal any significant effect of compound 5 for the doses examined on Supersac intake (Fig. 4).
Fig. 3.
Mean ± S.E.M. intake (gram per kilogram) of Supersac sweetened (3% glucose + 0.125% saccharin) 10% (w/v) alcohol solution by P-rats in the alcohol binge-like group (n = 12) after pretreatment with one of four doses of compound 5 (0, 0.00312, 0.00625, 0.0125 mg/kg). P < 0.05, significant difference from vehicle condition.
Fig. 4.
Mean ± S.E.M. Supersac intake (milliliter per kilogram) by Supersac control P-rats (n = 12) in the following pretreatment with one of four doses of compound 5 (0, 0.00312, 0.00625, 0.0125 mg/kg). Data revealed no nonspecific effect on fluid intake after pretreatment with compound 5.
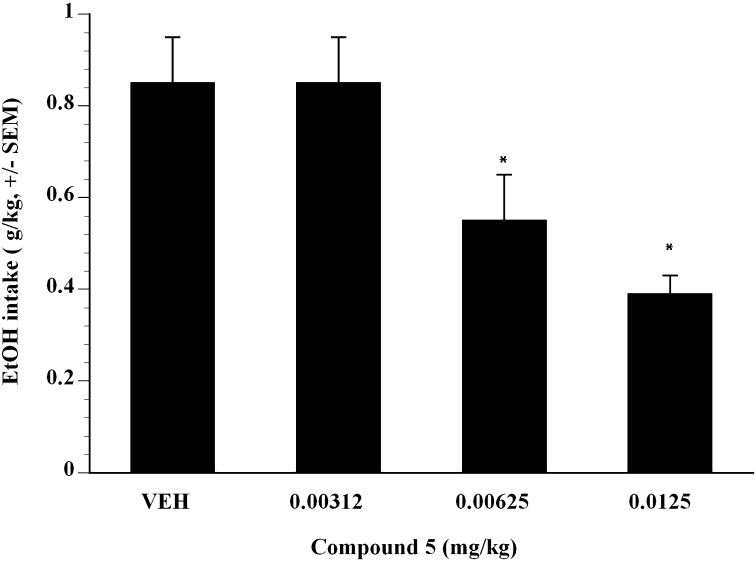
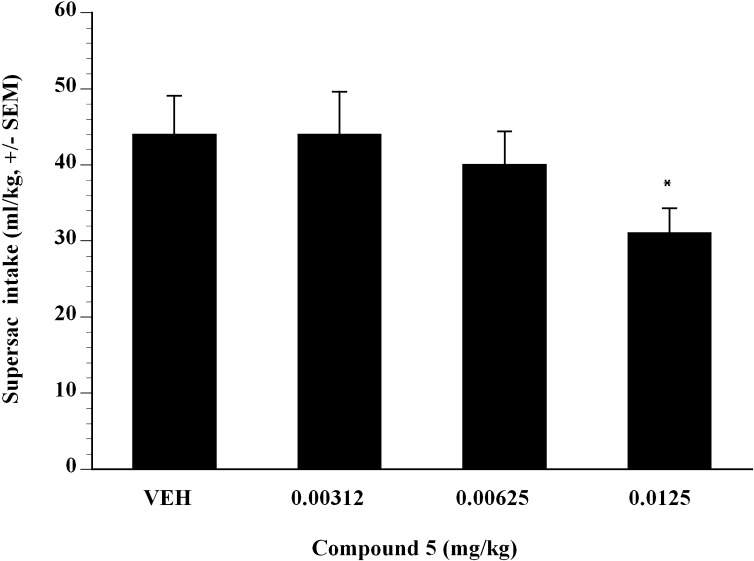
Next, the effect of compound 5 on alcohol self-administration in binge-like Wistar rats was examined. Compound 5 was administered subcutaneously in a Latin square design dose-range study and showed significant efficacy. Doses of compound 5 from 0.00312 to 0.0125 mg/kg showed that compound 5 inhibited Supersac-sweetened alcohol self-administration in binge-like Wistar rats (Fig. 5). Compared with vehicle, analysis showed that 0.00625 and 0.0125 mg/kg compound 5 significantly suppressed binge-like alcohol intake in Wistar rats (P < 0.05). The ED50 was estimated to be 0.012 mg/kg in binge-like Wistar rats (Fig. 5). To test whether the effect of compound 5 was selective for Supersac-sweetened ethanol, the effect of compound 5 on self-administration of Supersac was examined (Fig. 6). In control animals that only consumed Supersac, analysis did not reveal any significant effect of compound 5 for the doses examined on Supersac intake except 0.0125 mg/kg (Fig. 6).
Fig. 5.
Mean ± S.E.M. intake (gram per kilogram) of Supersac-sweetened (3% glucose + 0.125% saccharin) 10% (w/v) alcohol solution by Wistar rats in the alcohol binge-like group (n = 12) after pretreatment with one of four doses of compound 5 (0, 0.00312, 0.00625, 0.0125 mg/kg). *P < 0.05, significant difference from vehicle condition.
Fig. 6.
Mean ± S.E.M. Supersac (3% glucose + 0.125% saccharin) intake (milliliter per kilogram) by Supersac control Wistar rats (n = 12) after pretreatment with one of four doses of compound 5 (0, 0.00312, 0.00625, 0.0125 mg/kg). *P < 0.05, significant difference from vehicle condition.
Discussion
Replacement of the C-6 ketone group of naltrexone with an aryl amide substituent as in compound 5 afforded a compound that inhibited the self-administration of alcohol in P-rats and in binge-like P rats. Compound 5 is a reversible, relatively short-acting κ-opioid receptor antagonist. It is much more drug-like and much shorter-acting than nor-BNI. Compound 5 is lipophilic (i.e., log P = 3.73), and based on its pharmacokinetics rapidly leaves the bloodstream and gets into the brain. Because compound 5 does not possess the propensity for auto-oxidation that nor-BNI shows, its residence time and duration of action in the brain are also considerably shorter. Consequently, the effect of compound 5 on opioid receptors (i.e., binding, receptor desensitization, etc.) must be fundamentally different than for nor-BNI and other long-acting κ opioid receptor antagonists. Animals treated with compound 5 showed no residual effects after 24 hours and appeared to be normal from morphologic and behavioral standpoints. Administration of a dose of compound 5 to rats 500-fold greater than required for an ED50 dose for inhibition of alcohol self-administration did not show any detectable hepatotoxicity or renal toxicity or other toxicity. Long-term dosing of compound 5 in rats at 2 mg/kg for 7 days did not cause any detectable hepatotoxicity or other untoward clinical chemical abnormalities on the basis of analysis of plasma clinical chemical parameters taken at 7 days. The conclusion is that compound 5 is a relatively fast-acting opioid that is safe and relatively well tolerated in small animals.
Compared with naltrexone (ED50 500 µg/kg) or nalmefene (ED50 40 µg/kg), compound 5 (ED50 19 µg/kg) is a more potent inhibitor of alcohol self-administration in nondependent normal Wistar rats (Ghirmai et al., 2009). By use of P-rat and binge-like P-rat animals herein, we showed that compound 5 was even more efficacious at inhibiting alcohol self-administration (i.e., ED50 4–5 µg/kg and ED50 8 µg/kg, respectively). These data show that under a variety of experimental conditions compound 5 is an effective antagonist of responding maintained by large amounts of alcohol. We attribute this increase in efficacy to potent κ-opioid antagonism compared with naltrexone or nalmefene. As described above, it is also likely due to improved pharmaceutical properties of the compound and decreased interaction with the prominent P450 drug-metabolizing system.It may be that attenuation of the inhibitory potency of compound 5 toward P450 (Ghirmai et al., 2009) contributes to its safety. Compared with naltrexone, compound 5 showed decreased interaction with P450, and this may in part explain some of the metabolic stability observed for compound 5 and related compounds (MacDougall et al., 2004; Ghirmai et al., 2009), as well as some of the hepatoprotective properties. Substitution of an aryl amide moiety at the C-6 position of β-naltrexamine may also explain some of the hepatoprotective effects of compound 5. For example, at a dose of naltrexone that represents the ED50 for inhibition of alcohol self-administration (i.e., ED50 500 µg/kg), naltrexone exacerbates the hepatotoxicity of thiobenzamide in a rat model of hepatotoxicity. In contrast, at a dose of compound 5 that represents its ED50 (i.e., ED50 20 µg/kg), compound 5 protects against the hepatotoxicity of thiobenzamide in rats challenged with thiobenzamide, a potent hepatotoxin. Exacerbation of the hepatotoxicity of thiobenzamide by naltrexone is of considerable concern because, generally, the livers of individuals who abuse alcohol are severely compromised. It may be that decreasing the affinity of opioid derivatives for metabolic enzymes and increasing the metabolic stability results in compounds with less potential for increasing hepatotoxicity.
In a previous study (Ghirmai et al., 2009), we showed that compound 5 reduced alcohol self-administration in normal Wistar rats. We proposed that the mechanism of action of compound 5 involved its function as a κ-opioid receptor antagonist. In good agreement with those results, we show herein that compound 5 effectively decreases alcohol self-administration in a binge-like P-rat model as well as a binge-like Wistar rat model. Moreover, the reduction in alcohol self-administration seen with compound 5 was selective, because at efficacious doses, compound 5 did not affect consumption of water or Supersac. This is important because some opioid receptor antagonists decrease both ethanol and sucrose intake in rats (Pastor and Aragon, 2006) or inhibit energy-rich food consumption (Reid, 1985). It may be that opioid receptor antagonists prevent central reward mechanisms that may share common neural substrates responsible for the development of alcohol dependence (Yeomans and Gray, 2002).
On the basis of previously published opioid receptor binding data, compound 5 works as an partial agonist at the µ-opioid receptor and an antagonist at the δ- and κ-opioid receptors. However, the potency against the κ-opioid receptor is much greater than that against the δ-opioid receptor, and at the concentration of compound 5 that is efficacious in vivo at inhibiting alcohol self-administration, we conclude that κ is the pharmacologically prominent receptor. The finding from in vivo studies that compound 5 potently inhibits alcohol self-administration in P-rats and binge-like Wistar rats supports the idea that antagonism of κ-opioid receptors might be of utility for full alcohol cessation functional activity. However, compared with naltrexone, the in vivo efficacy of compound 5 may not only be dependent on interaction with the κ-opioid receptor but also partial agonism of the µ-opioid receptor. Presumably, the profile of opioid receptor binding coupled with the drug-like properties of compound 5 contributes to the optimal functional activity as an alcohol self-administration inhibition agent in vivo. This is in agreement with recent studies that show that an opioid with strong κ-opioid receptor antagonism, albeit possessing some opioid agonism (i.e., nalmefene) (Bart et al., 2005), was more effective at inhibition of alcohol self-administration than an opioid with broad opioid receptor antagonism (i.e., naltrexone) (Walker and Koob, 2008). Consequently, compound 5 and related agents may represent exciting leads for the next generation of opioid compounds useful in the treatment of alcohol abuse.
Acknowledgments
The authors thank Drs. Jarek Kalisiak and Marion Lanier for help with synthetic and analytical work; Dr. Sigeng Cheng for help with the animal work; and Michael Ly and David Johnson at Microconstants, Inc., for the pharmacokinetic analytical work.
Abbreviations
- BALs
blood alcohol levels
- BOP
(dimethylamino) phosphonium hexa-fluorophosphate
- compound 1
naltrexone
- compound 2
nalmefene hydrochloride
- compound 3
17-cyclopropylmethyl-3,14β-dihydroxy-4,5α-epoxy-6β-[(4′-bromo) benzamido]morphinan-hydrochloride
- compound 4
6-β-(4′-trifluoromethyl-2′,3′,5′,6′-tetradeutrio)benzamido-14-hydroxy-17-(cyclopropylmethyl)nordesmorphine
- compound 5
17-cyclopropylmethyl-3, 14β-dihydroxy-4, 5α-epoxy-6β-[(4′-trimethylfluoro)benzamido]morphinan-hydrochloride
- DCM
dichloromethane
- DIPEA
diisopropylethylamine
- GNTI
5′-guanidinonaltrindole
- [35S]GTPγS
5′-O-(3-[35S]thio)triphosphate
- HPLC
high-performance liquid chromatography
- JDTic
(3R)-7-hydroxy-N-[(2S)-1-[(3R,4R)-4-(3-hydroxyphenyl)-3,4-dimethylpiperidin-1-yl]-3-methylbutan-2-yl]-1,2,3,4-tetrahydroisoquinoline-3-carboxamide
- LC-MS
liquid chromatography–mass spectrometry
- LCMS-MS
liquid chromatography–tandem mass spectrometry
- NOP
nociceptin opioid receptor
- nor-BNI
norbinaltorphimine
- P-rat
alcohol-preferring rat
- P450
cytochrome P450
- PK
pharmacokinetics
- SGOT
serum glutamic oxaloacetic transaminase
- SGPT
serum glutamic-pyruvic transaminase
- t1/2
half-life
- Tmax
time to achieve maximum concentration
Authorship Contributions
Participated in research design: Cashman, Azar.
Conducted experiments: Cashman, Azar.
Contributed new reagents or analytic tools: Cashman.
Performed data analysis: Cashman, Azar.
Wrote or contributed to the writing of the manuscript: Cashman, Azar.
Footnotes
This work was financially supported by a grant from the National Institutes of Health [Grant AA016029] (to M.A.).
References
- Anton RF, Moak DH, Waid LR, Latham PK, Malcolm RJ, Dias JK. (1999) Naltrexone and cognitive behavioral therapy for the treatment of outpatient alcoholics: results of a placebo-controlled trial. Am J Psychiatry 156:1758–1764 [DOI] [PubMed] [Google Scholar]
- Bart G, Schluger JH, Borg L, Ho A, Bidlack JM, Kreek MJ. (2005) Nalmefene induced elevation in serum prolactin in normal human volunteers: partial kappa opioid agonist activity? Neuropsychopharmacology 30:2254–2262 [DOI] [PubMed] [Google Scholar]
- Benjamin D, Grant ER, Pohorecky LA. (1993) Naltrexone reverses ethanol-induced dopamine release in the nucleus accumbens in awake, freely moving rats. Brain Res 621:137–140 [DOI] [PubMed] [Google Scholar]
- Biggs TAG, Myers RD. (1998) Naltrexone and amperozide modify chocolate and saccharin drinking in high alcohol-preferring P rats. Pharmacol Biochem Behav 60:407–413 [DOI] [PubMed] [Google Scholar]
- Bouza C, Angeles M, Muñoz A, Amate JM. (2004) Efficacy and safety of naltrexone and acamprosate in the treatment of alcohol dependence: a systematic review. Addiction 99:811–828 [DOI] [PubMed] [Google Scholar]
- Brewer C, Wong VS. (2004) Naltrexone: report of lack of hepatotoxicity in acute viral hepatitis, with a review of the literature. Addict Biol 9:81–87 [DOI] [PubMed] [Google Scholar]
- Cashman JR, Hanzlik RP. (1981) Microsomal oxidation of thiobenzamide. A photometric assay for the flavin-containing monooxygenase. Biochem Biophys Res Commun 98:147–153 [DOI] [PubMed] [Google Scholar]
- Croop RS, Faulkner EB, Labriola DF, The Naltrexone Usage Study Group (1997) The safety profile of naltrexone in the treatment of alcoholism. Results from a multicenter usage study. Arch Gen Psychiatry 54:1130–1135 [DOI] [PubMed] [Google Scholar]
- Denton TT, Zhang X, Cashman JR. (2004) Nicotine-related alkaloids and metabolites as inhibitors of human cytochrome P-450 2A6. Biochem Pharmacol 67:751–756 [DOI] [PubMed] [Google Scholar]
- Ghirmai S, Azar MR, Polgar WE, Berzetei-Gurske I, Cashman JR. (2008) Synthesis and biological evaluation of α- and β-6-amido derivatives of 17-cyclopropylmethyl-3, 14β-dihydroxy-4, 5α-epoxymorphinan: potential alcohol-cessation agents. J Med Chem 51:1913–1924 [DOI] [PubMed] [Google Scholar]
- Ghirmai S, Azar MR, Cashman JR. (2009) Synthesis and pharmacological evaluation of 6-naltrexamine analogs for alcohol cessation. Bioorg Med Chem 17:6671–6681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianoulakis C, Krishnan B, Thavundayil J. (1996) Enhanced sensitivity of pituitary beta-endorphin to ethanol in subjects at high risk of alcoholism. Arch Gen Psychiatry 53:250–257 [DOI] [PubMed] [Google Scholar]
- Gilpin NW, Richardson HN, Koob GF. (2008) Effects of CRF1-receptor and opioid-receptor antagonists on dependence-induced increases in alcohol drinking by alcohol-preferring (P) rats. Alcohol Clin Exp Res 32:1535–1542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grisel JE, Grahame NJ, Mogil JS, Belknap JK, Low MJ. (1995) Decreased voluntary ethanol consumption in transgenic mice lacking β-endorphin (Abstract). Soc Neurosci Abst. 21:1699 [Google Scholar]
- Hanzlik RP, Cashman JR. (1983) Microsomal metabolism of thiobenzamide and thiobenzamide S-oxide. Drug Metab Dispos 11:201–205 [PubMed] [Google Scholar]
- Hanzlik RP, Cashman JR, Traiger GJ. (1980) Relative hepatotoxicity of substituted thiobenzamides and thiobenzamide-S-oxides in the rat. Toxicol Appl Pharmacol 55:260–272 [DOI] [PubMed] [Google Scholar]
- Hanzlik RP, Vyas KP, Traiger GJ. (1978) Substituent effects on the hepatotoxicity of thiobenzamide derivatives in the rat. Toxicol Appl Pharmacol 46:685–694 [DOI] [PubMed] [Google Scholar]
- Herz A. (1997) Endogenous opioid systems and alcohol addiction. Psychopharmacology (Berl) 129:99–111 [DOI] [PubMed] [Google Scholar]
- Horan P, Taylor J, Yamamura HI, Porreca F. (1992) Extremely long-lasting antagonistic actions of nor-binaltorphimine (nor-BNI) in the mouse tail-flick test. J Pharmacol Exp Ther 260:1237–1243 [PubMed] [Google Scholar]
- Ji D, Gilpin NW, Richardson HN, Rivier CL, Koob GF. (2008) Effects of naltrexone, duloxetine, and a corticotropin-releasing factor type 1 receptor antagonist on binge-like alcohol drinking in rats. Behav Pharmacol 19:1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- June HL, Cummings R, Eiler WJ, 2nd, Foster KL, McKay PF, Seyoum R, Garcia M, McCane S, Grey C, Hawkins SE, et al. (2004) Central opioid receptors differentially regulate the nalmefene-induced suppression of ethanol- and saccharin-reinforced behaviors in alcohol-preferring (P) rats. Neuropsychopharmacology 29:285–299 [DOI] [PubMed] [Google Scholar]
- June HL, Grey C, Warren-Reese C, Durr LF, Ricks-Cord A, Johnson A, McCane S, Williams LS, Mason D, Cummings R, et al. (1998) The opioid receptor antagonist nalmefene reduces responding maintained by ethanol presentation: preclinical studies in ethanol-preferring and outbred Wistar rats. Alcohol Clin Exp Res 22:2174–2185 [PubMed] [Google Scholar]
- Li TK, Lumeng L, McBride WJ, Murphy JM. (1987) Rodent lines selected for factors affecting alcohol consumption. Alcohol Alcohol Suppl 1:91–96 [PubMed] [Google Scholar]
- MacDougall JM, Zhang XD, Polgar WE, Khroyan TV, Toll L, Cashman JR. (2004) Design, chemical synthesis, and biological evaluation of thiosaccharide analogues of morphine- and codeine-6-glucuronide. J Med Chem 47:5809–5815 [DOI] [PubMed] [Google Scholar]
- Mason BJ, Salvato FR, Williams LD, Ritvo EC, Cutler RB. (1999) A double-blind, placebo-controlled study of oral nalmefene for alcohol dependence. Arch Gen Psychiatry 56:719–724 [DOI] [PubMed] [Google Scholar]
- Mitchell JE, Morley JE, Levine AS, Hatsukami D, Gannon M, Pfohl D. (1987) High-dose naltrexone therapy and dietary counseling for obesity. Biol Psychiatry 22:35–42 [DOI] [PubMed] [Google Scholar]
- Munro TA, Berry LM, Van’t Veer A, Béguin C, Carroll FI, Zhao Z, Carlezon WA, Jr, Cohen BM. (2012) Long-acting κ opioid antagonists nor-BNI, GNTI and JDTic: pharmacokinetics in mice and lipophilicity. BMC Pharmacol 12:1–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osa Y, Ida Y, Fujii H, Nemoto T, Hasebe K, Momen S, Mochizuki H, Nagase H. (2007) Catalytic aerobic oxidation of nor-binaltorphimine (nor-BNI) analogs without 4,5-epoxy bridge affords a more selective ligand for kappa opioid receptor than the representative kappa antagonist nor-BNI. Chem Pharm Bull (Tokyo) 55:1489–1493 [DOI] [PubMed] [Google Scholar]
- Oslin DW, Berrettini WH, O’Brien CP. (2006) Targeting treatments for alcohol dependence: the pharmacogenetics of naltrexone. Addict Biol 11:397–403 [DOI] [PubMed] [Google Scholar]
- Pastor R, Aragon CM. (2006) The role of opioid receptor subtypes in the development of behavioral sensitization to ethanol. Neuropsychopharmacology 31:1489–1499 [DOI] [PubMed] [Google Scholar]
- Pettinati HM, O’Brien CP, Rabinowitz AR, Wortman SP, Oslin DW, Kampman KM, Dackis CA. (2006) The status of naltrexone in the treatment of alcohol dependence: specific effects on heavy drinking. J Clin Psychopharmacol 26:610–625 [DOI] [PubMed] [Google Scholar]
- Rassnick S, Pulvirenti L, Koob GF. (1993) SDZ-205,152, a novel dopamine receptor agonist, reduces oral ethanol self-administration in rats. Alcohol 10:127–132 [DOI] [PubMed] [Google Scholar]
- Reid LD. (1985) Endogenous opioid peptides and regulation of drinking and feeding. Am J Clin Nutr 42(5, Suppl)1099–1132 [DOI] [PubMed] [Google Scholar]
- Roozen HG, de Waart R, van der Windt DA, van den Brink W, de Jong CA, Kerkhof AJ. (2006) A systematic review of the effectiveness of naltrexone in the maintenance treatment of opioid and alcohol dependence. Eur Neuropsychopharmacol 16:311–323 [DOI] [PubMed] [Google Scholar]
- Sax DS, Kornetsky C, Kim A. (1994) Lack of hepatotoxicity with naltrexone treatment. J Clin Pharmacol 34:898–901 [DOI] [PubMed] [Google Scholar]
- Stromberg MF, Mackler SA, Volpicelli JR, O’Brien CP. (2001) Effect of acamprosate and naltrexone, alone or in combination, on ethanol consumption. Alcohol 23:109–116 [DOI] [PubMed] [Google Scholar]
- Tabakoff B, Hoffman PL. (1983) Alcohol interactions with brain opiate receptors. Life Sci 32:197–204 [DOI] [PubMed] [Google Scholar]
- Traynor JR, Nahorski SR. (1995) Modulation by μ-opioid agonists of guanosine-5′-O-(3-[35S]thio)triphosphate binding to membranes from human neuroblastoma SH-SY5Y cells. Mol Pharmacol 47:848–854 [PubMed] [Google Scholar]
- Ulm RR, Volpicelli JR, Volpicelli LA. (1995) Opiates and alcohol self-administration in animals. J Clin Psychiatry 56 (Suppl 7):5–14 [PubMed] [Google Scholar]
- Walker BM, Koob GF. (2008) Pharmacological evidence for a motivational role of κ-opioid systems in ethanol dependence. Neuropsychopharmacology 33:643–652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker BM, Zorrilla EP, Koob GF. (2011) Systemic κ-opioid receptor antagonism by nor-binaltorphimine reduces dependence-induced excessive alcohol self-administration in rats. Addict Biol 16:116–119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson DA, Fan X, Buchwald SL. (2008) Carbonylation of aryl chlorides with oxygen nucleophiles at atmospheric pressure. Preparation of phenyl esters as acyl transfer agents and the direct preparation of alkyl esters and carboxylic acids. J Org Chem 73:7096–8001 [DOI] [PubMed] [Google Scholar]
- Wienkers LC, Heath TG. (2005) Predicting in vivo drug interactions from in vitro drug discovery data. Nat Rev Drug Discov 4:825–833 [DOI] [PubMed] [Google Scholar]
- Weiss F, Mitchiner M, Bloom FE, Koob GF. (1990) Free-choice responding for ethanol versus water in alcohol preferring (P) and unselected Wistar rats is differentially modified by naloxone, bromocriptine, and methysergide. Psychopharmacology (Berl) 101:178–186 [DOI] [PubMed] [Google Scholar]
- Yen M-H, Ko H-C, Tang F-I, Lu R-B, Hong J-S. (2006) Study of hepatotoxicity of naltrexone in the treatment of alcoholism. Alcohol 38:117–120 [DOI] [PubMed] [Google Scholar]
- Yeomans MR, Gray RW. (2002) Opioid peptides and the control of human ingestive behaviour. Neurosci Biobehav Rev 26:713–728 [DOI] [PubMed] [Google Scholar]