Abstract
Recent studies have identified AMP-activated kinase (AMPK) as a target of Ca2+/calmodulin-dependent kinase kinase (CaMKKβ) and a negative regulator of myosin light-chain (MLC) kinase (MLCK). The present study examined whether a change in expression or activity of AMPK is responsible for hypercontractility of intestinal longitudinal muscle during inflammation or in response to proinflammatory cytokines. In mouse colonic longitudinal muscle cells, acetylcholine (ACh) stimulated AMPK and MLCK phosphorylation and activity and induced MLC20 phosphorylation and muscle contraction. Blockade of CaMKKβ with STO609 (7-oxo-7H-benzimidazo[2,1-a]benz[de]isoquinoline-3-carboxylic acid acetate) inhibited AMPK and MLCK phosphorylation and augmented MLCK activity, MLC20 phosphorylation, and smooth muscle cell contraction. In muscle cells isolated from the colon of TNBS (2,4,6-trinitrobenzenesulfonic acid)-treated mice or from strips treated with interleukin-1β or tumor necrosis factor-α, nuclear factor κB was activated as indicated by an increase in p65 phosphorylation and IκBα degradation, and AMPK was phosphorylated at a cAMP-dependent protein kinase (PKA)–specific site (Ser485) that is distinct from the stimulatory CaMKKβ site (Thr172), resulting in attenuation of ACh-stimulated AMPK activity and augmentation of MLCK activity and muscle cell contraction. Inhibition of nuclear factor-κB activity with MG-132 (carbobenzoxy-l-leucyl-l-leucyl-l-leucinal Z-LLL-CHO) or PKA activity with myristoylated PKA inhibitor 14-22 amide blocked phosphorylation of AMPK at Ser485 and restored MLCK activity and muscle cell contraction to control levels. The results imply that PKA released from IκBα complex phosphorylated AMPK at a PKA-specific site and inhibited its activity, thereby relieving the inhibitory effect of AMPK on MLCK and increasing MLCK activity and muscle cell contraction. We conclude that hypercontractility of intestinal longitudinal muscle induced by inflammation or proinflammatory cytokines is mediated by nuclear factor κB/PKA-dependent inhibition of AMPK and activation of MLCK.
Introduction
Contraction of intestinal circular smooth muscle is initiated by sequential activation of Gq and phospholipase C-β1, resulting in inositol 1,4,5-trisphosphate (IP3)–stimulated Ca2+ release, Ca2+/calmodulin-dependent activation of myosin light-chain (MLC) kinase (MLCK), and phosphorylation of the 20-kDa myosin light chain (MLC20) (Kamm and Stull, 2001; Somlyo et al., 2004; Murthy, 2006; de Godoy and Rattan, 2011). The initial contraction is terminated, in part, by inactivation of Gαq by regulator of G protein signaling-4 (RGS4) and Ca2+ uptake into Ca2+ stores by the sarcoendoplasmic reticulum Ca2+-ATPase SERCA2 (Murthy et al., 1993; Colyer, 1998; Huang et al., 2005; Murthy, 2006; Hu et al., 2007). In intestinal longitudinal smooth muscle, however, there is minimal participation of Gq, RGS4, and IP3-dependent Ca2+ release (Murthy et al., 1991, 1995; Kuemmerle et al., 1994, 1998; Murthy, 2006). Contraction is initiated by cytoplasmic phospholipase A2 (cPLA2)–dependent Ca2+ influx leading to Ca2+- and cyclic ADP ribose–induced Ca2+ release via ryanodine receptors (Kuemmerle et al., 1994). Initial contraction is terminated, in part, by SERCA2-mediated Ca2+ uptake into Ca2+ stores. In both intestinal circular and longitudinal smooth muscle, direct inactivation of MLCK could contribute to termination of initial contraction. MLCK activity in vascular smooth muscle is negatively regulated by AMP-activated kinase (AMPK), a serine/threonine kinase that directly phosphorylates MLCK; phosphorylation decreases the affinity of MLCK for Ca2+/calmodulin (Horman et al., 2008; Sung and Choi, 2012; Lee and Choi, 2013). AMPK activity in smooth muscle is regulated via phosphorylation by Ca2+/calmodulin kinase kinase β (CaMKKβ) at Thr172 and at other sites by several kinases, including serine/threonine kinase 11 , protein kinase B, and cAMP-dependent protein kinase (PKA) (Hurley et al., 2006; Djouder et al., 2010; Hardie, 2011).
Smooth muscle cells act as both targets and source of inflammatory mediators. Receptors for interleukin-1β (IL-1β), tumor necrosis factor-α (TNF-α), and interferon-γ are expressed on vascular and visceral smooth muscle and have been shown to activate a variety of transcription factors [e.g., STAT4, nuclear factor-κB (NF-κB), activator protein-1] and other mediators (e.g., inducible nitric-oxide synthase and various regulatory kinases) (Akiho et al., 2002; Kinoshita et al., 2003; Cao et al., 2004; Shi and Sarna, 2005; Shi et al., 2005; Khan and Collins, 2006; Ihara et al., 2012; Shea-Donohue et al., 2012; Yang et al., 2013). The differences in signaling targets between circular and longitudinal muscle could result in different outcomes in response to inflammatory cytokines (Martinolle et al., 1997). We have previously shown that in circular muscle IL-1β and TNF-α acting via NF-κB cause an increase in RGS4 expression and activity resulting in rapid inactivation of Gαq, inhibition of IP3 formation, Ca2+ mobilization, and initial muscle contraction (Hu et al., 2007, 2008, 2009). Other studies have shown that exposure of longitudinal muscle to proinflammatory cytokines results in hypercontractility (Akiho et al., 2002; Zhao et al., 2005, 2006). The signaling targets that mediate hypercontractility of longitudinal muscle have not been identified. The present study shows that inflammatory cytokines (IL-1β and TNF-α) activate the canonical NF-κB pathway, causing release of the catalytic subunit of PKA from its binding to IκBα. Phosphorylation of AMPK at Ser485 by PKA relieves the inhibitory effect of AMPK on MLCK, causing further increase in MLCK activity, MLC20 phosphorylation and initial contraction.
Materials and Methods
Preparation of Dispersed Smooth Muscle Cells.
Smooth muscle cells were isolated from the longitudinal muscle of mouse colon by sequential enzymatic digestion, filtration, and centrifugation as described previously (Murthy et al., 1995; Huang et al., 2005; Hu et al., 2007). Muscle strips were incubated for 20 minutes in a smooth muscle buffer [NaCl 120 mM, KCl 4 mM, KH2PO4 2.6 mM, CaCl2 2.0 mM, MgCl2 0.6 mM, HEPES 25 mM, glucose 14 mM, and essential amino acid mixture 2.1% (pH 7.4)] at 31°C containing 0.1% collagenase (300 U/ml), and 0.01% soybean trypsin inhibitor (w/v). The partly digested tissues were washed twice with 50 ml of enzyme-free smooth muscle buffer, and the muscle cells were allowed to disperse spontaneously for 30 minutes. Cells were harvested by filtration through 500 μm Nitex and centrifuged twice at 350g for 10 minutes and resuspended in HEPES buffer. The cells were counted in a hemocytometer, and it is estimated that 95% of the cells excluded trypan blue. The experiments were done within 2–3 hours of cell dispersion.
Induction of Colitis and Preparation of Tissue.
Colitis was induced with TNBS (2,4,6-trinitrobenzene sulphonic acid) as described previously (Hazelgrove et al., 2009; Alkahtani et al., 2013). Briefly, 6- to 8-week-old male adult C57BL/6J (Charles River Laboratories, Wilmington, MA) mice were fasted for 24 hours and lightly anesthesized with isoflurane; 100 µl of TNBS solution [2.5% in 50% ethanol (v/v)] was injected via a catheter advanced to 3 cm proximal to the anus via 1-ml syringe fitted with a catheter. Age-matched control mice were treated with vehicle. Mice were euthanized 3 days after the induction of inflammation. Macroscopic examination of the distal colon and rectum 3 days after TNBS administration revealed multiple mucosal erosions and ulcerations, which were in sharp contrast to the appearance of the normal colon excised from control animals with intact smooth mucosa. Segments of the distal colon 2–3 cm long were obtained; muscle cells from longitudinal muscle layer were also obtained.
Kinase Assay for AMPKα and MLCK.
AMPKα and MLCK activities were determined on immunoprecipitates from cell extracts as described previously (Murthy et al., 2003; Hu et al., 2007). Briefly, AMPKα and MLCK were immunoprecipitated with specific antibodies, and the immunoprecipitates were washed three times with phosphorylation buffer containing 10 mM MgCl2 and 40 mM HEPES (pH 7.4). The immunoprecipitates were incubated with MLC20 (20 µM) for measurement of MLCK activity or MLCK (1 µg) for measurement of AMPK activity. The reaction was initiated with 10 µCi of [γ-32P]ATP (3000 Ci/ml) and 20 µM ATP and incubated for 15 minutes at 37°C. 32P-Labeled substrates were absorbed on to phosphocellulose disks, and free radioactivity was removed by repeated washing with 75 mM phosphoric acid. The extent of phosphorylation was determined from the radioactivity on phosphocellulose disks by liquid scintillation.
Phosphorylation of MLCK.
Phosphorylation of MLCK was determined from the amount of 32P incorporated into the enzyme after immunoprecipitation with specific antibody (Murthy et al., 2003). Smooth muscle cells (3 × 106 cell/ml) prelabeled with 0.5 Ci/ml of [32P]orthophosphate for 3 hours were treated with acetylcholine (ACh) for 1 minute in the presence or absence of CaMKK inhibitor STO609 (7-oxo-7H-benzimidazo[2,1-a]benz[de]isoquinoline-3-carboxylic acid acetate; 10 µM), and the reaction was terminated with medium containing 1% Triton X-100, 0.5% SDS, 10 mM EDTA, 1 mM phenylmethylsulfonyl fluoride, 10 µg/ml leupeptin, 100 µg/ml aprotinin, 10 mM sodium pyrophosphate, 50 mM NaF, and 0.2 mM sodium azide. The cell lysates were centrifuged at 13,000g for 15 minutes at 4°C, precleared with 40 µl of protein A/G PLUS agarose beads, and incubated with antibody to MLCK for 3 hours at 4°C, followed by another 1 hour of incubation with 40 µl of protein A/G PLUS agarose beads. The immunoprecipitates were washed with medium containing 10 mM Tris-HCl (pH 7.4), 150 mM NaCl, and 0.5% Triton X-100 and extracted with sample buffer. The samples were resolved by gel electrophoresis, and 32P-labeled MLCK was visualized by autoradiography, and the amount of radioactivity in the band was counted.
Western Blot Analysis.
Dispersed muscle cells were solubilized on ice for 1 hour in a medium containing 20 mM Tris/HCl (pH 8.0), 1 mM dithiothreitol, 100 mM NaCl, 0.5% SDS, 0.75% deoxycholate, 1 mM phenylmethylsulfonyl fluoride, 10 µg/ml leupeptin, and 100 µg/ml aprotinin. The homogenate was centrifuged and the supernatant was collected. Equal amounts (40 μg) of total protein were loaded on 10% SDS-PAGE gels and transferred to nitrocellulose membranes (Bio-Rad Laboratories, Hercules, CA). Membranes were subsequently blocked in blocking buffer for 90 minutes at room temperature followed by incubation overnight at 4°C with primary antibodies. After three 10-minute washes with Tris-buffered saline/Tween 20, membranes were incubated with horseradish peroxidase–labeled secondary antibodies (1:5000) at room temperature for 90 minutes, followed by an additional three washes with 0.1% Tris-buffered saline/Tween 20. Bands were subsequently visualized on film using SuperSignal Femto maximum sensitivity substrate kit and analyzed by densitometry. Densitometric values for protein expression were expressed in arbitrary units after normalization to β-actin.
Measurement of Contraction in Dispersed Smooth Muscle Cells.
Contraction in freshly dispersed smooth muscle cells was determined by scanning micrometry as previously described (Murthy et al., 1995; Huang et al., 2005; Hu et al., 2007). Aliquots (0.4 ml) of cells containing approximately 104 cells/ml were treated with 100 μl of HEPES buffer containing different concentrations of ACh (10 pM to 1 µM) for 30 seconds, and the reaction was terminated with 1% acrolein at a final concentration of 0.1%. The resting cell length was determined in control experiments in which muscle cells were incubated with 100 μl of buffer without ACh. The mean lengths of 50 muscle cells treated with various agonists was measured by scanning micrometry and compared with the mean lengths of untreated cells. The contractile response was expressed as the percentage of decrease in mean cell length from control cell length.
Chemicals.
[γ-32P]ATP and [32P]orthophosphate were from PerkinElmer Life Sciences (Boston, MA). Collagenase CLS type II and soybean trypsin inhibitor were from Worthington (Freehold, NJ); Western blotting material and protein assay kit and 10% Tris-HCl Ready Gels were from Bio-Rad Laboratories; antibodies to p65, IκBα, β-actin, AMPK, MLCK, and MLC20 were from Santa Cruz Biotechnology (Santa Cruz, CA); STO609 and IL-1β were obtained from Alexis Biochemicals (San Diego, CA). MG-132 (carbobenzoxy-l-leucyl-l-leucyl-l-leucinal Z-LLL-CHO) was from EMD Millipore (Billerica, MA) and was dissolved in dimethylsulfoxide; Dulbecco’s modified Eagle’s medium was from Thermo Fisher Scientific (Waltham, MA). All other chemicals were obtained from Sigma-Aldrich (St. Louis, MO).
Statistical Analysis.
The results were expressed as means ± S.E.M. of N experiments and analyzed for statistical significance using Student’s t test for paired and unpaired values. Each experiment was done on cells obtained from different animals. Five to six mice of each group were studied. A probability of P < 0.05 was considered significant.
Results
Negative Regulation of ACh-Stimulated MLCK Activity by AMPK in Longitudinal Smooth Muscle of the Colon.
Initial contraction in smooth muscle is mediated by Ca2+/CaM-dependent activation of MLCK. In vascular and gastric smooth muscle, MLCK is negatively regulated on phosphorylation of Ser815 by AMPK. AMPK is activated on phosphorylation at Thr172 by CaMKKβ (Horman et al., 2008).
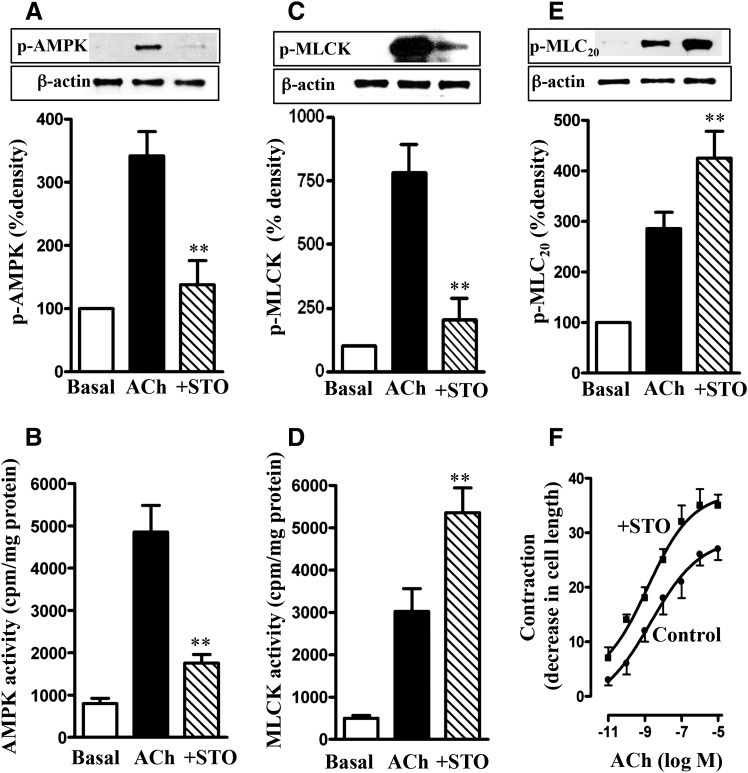
Treatment of longitudinal muscle cells of the colon with 1 µM ACh for 30 seconds stimulated AMPK activity and AMPK phosphorylation at Thr172; the increase in AMPK phosphorylation and activity was blocked by the CaMKKβ inhibitor STO609 (10 µM) (Tokumitsu et al., 2002) (Fig. 1, A and B). ACh stimulated also MLCK phosphorylation and activity. Treatment of cells with STO609 blocked phosphorylation of MLCK, leading to augmentation of its activity (Fig. 1, C and D). These results suggested that MLCK activity is negatively regulated by phosphorylation via AMPK. Consistent with this notion, ACh-induced MLC20 phosphorylation and muscle contraction were augmented in the presence of STO609 (Fig. 1, E and F). These results imply that in both colonic longitudinal and vascular smooth muscle, MLCK activity and muscle contraction in response to contractile agonists are negatively regulated via phosphorylation of MLCK by AMPK.
Fig. 1.
Regulation of MLCK activity and muscle contraction by AMPK. Longitudinal muscle cells isolated from control mice were treated with 1 µM ACh in the presence or absence of CaMKK inhibitor STO609 (STO; 10 µM) for 30 seconds. Cells were preincubated with STO609 for 15 minutes before the addition of ACh for 30 seconds. (A) Phosphorylation of AMPK at Thr172 by CaMKK. Phosphorylation of AMPK was analyzed by Western blot using phospho-specific antibody. (B) Stimulation of ACh-induced AMPK activity by CaMKK. AMPK activity was measured by immunokinase assay using MLCK as substrate and [32P]ATP. The results are expressed as cpm/mg protein. (C) Phosphorylation of MLCK by AMPK. Phosphorylation of MLCK was measured in cells labeled with 32P. (D) Feedback inhibition of ACh-induced MLCK activity by AMPK. MLCK activity was measured by immunokinase assay using MLC20 as substrate and [32P]ATP, and the results are expressed as cpm/mg protein. (E) Attenuation of ACh-induced MLC20 phosphorylation by AMPK. MLC20 phosphorylation at Ser19 was measured by immunoblot using phospho-specific antibody. (F) Attenuation of ACh-induced muscle contraction by AMPK. Muscle contraction was measured by scanning micrometry and is expressed as the percentage of decrease in cell length from the basal length (108 ± 7 µm). Values are means ± S.E.M. of four experiments. **P < 0.05, significantly different from response to ACh alone.
Augmentation of ACh-Induced Contraction in Colonic Longitudinal Smooth Muscle Cells Isolated from TNBS-Treated Mice or from Control Strips Treated with IL-1β or TNF-α.
The effect of proinflammatory cytokines on ACh-induced contraction of colonic longitudinal muscle cells isolated from longitudinal muscle strips treated for 24 hours with IL-1β (10 ng/ml) or TNF-α (1 nM) was examined. Parallel studies were done on smooth muscle cells isolated from TNBS-treated mice. The duration of TNBS treatment in vivo and the dose and the duration of treatment of muscle strips with IL-1β or TNF-α in vitro were based on our previous studies of colonic circular smooth muscle as described in Materials and Methods (Kuemmerle, 1998; Hu et al., 2007, 2008, 2009; Hazelgrove et al., 2009; Alkahtani et al., 2013).
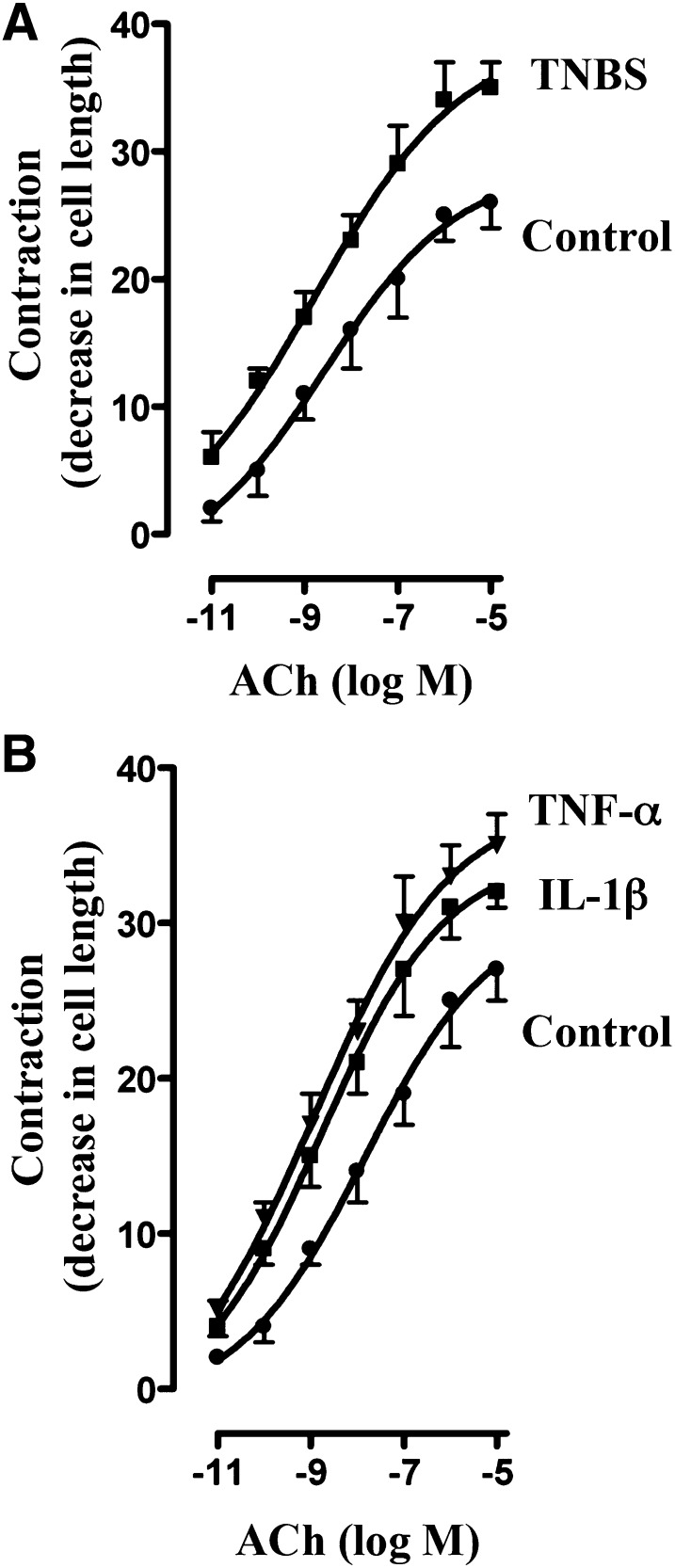
Initial muscle cell length was similar in control (vehicle-treated) and TNBS-treated mice (108 ± 5 µm versus 104 ± 6 µm), whereas the contractile response to ACh, expressed as percentage of decrease in cell length, was significantly greater in muscle cells isolated from TNBS-treated mice (Fig. 2A). The concentration response curve for muscle cells from TNBS-treated mice was shifted to the left (maximal contraction 35% ± 2% decrease in cell length; EC50 ∼1 nM) relative to that from vehicle-treated mice (26% ± 2% decrease in cell length; EC50 ∼10 nM) (Fig. 2A).
Fig. 2.
Augmentation of ACh-induced contraction by proinflammatory cytokines. Longitudinal muscle cells isolated from colon of control and TNBS-treated mice (A) or from muscle strips cultured in the absence (control) or presence of IL-1β (10 ng/ml) or TNF-α (1 nM) for 24 hours (B) were treated with different concentrations of ACh for 30 seconds to measure initial Ca2+-dependent contraction. Muscle contraction was measured by scanning micrometry and expressed as percentage of decrease in cell length before ACh treatment. Basal cell lengths were not significantly different between control and TNBS-treated mice or between control and cytokine-treated muscle strips (115 ± 4 µm to 121 ± 6 µm). Values are means ± S.E.M. of six experiments.
Initial cell length (range: 102 ± 4 to 108 ± 7 μm) and the pattern of response to ACh in muscle cells isolated from muscle strips treated for 24 hours with IL-1β or TNF-α were identical to those obtained in muscle cells isolated from TNBS-treated mice (control: 26% ± 3% decrease in cell length, EC50 ∼10 nM; IL-1β: 35% ± 3% decrease in cell length, EC50 ∼1 nM; TNF-α: 36% ± 2% decrease in cell length, EC50 ∼1 nM) (Fig. 2B).
Augmentation of ACh-Induced MLCK Activity and MLC20 Phosphorylation in Colonic Longitudinal Smooth Muscle Cells Isolated from TNBS-Treated Mice or from Control Strips Treated with IL-1β or TNF-α.
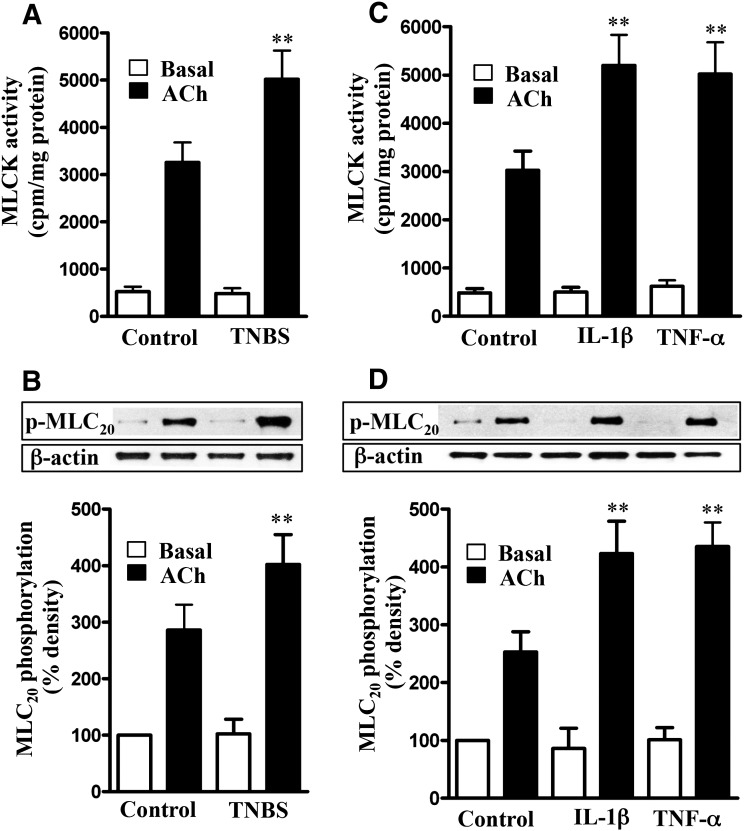
Treatment of colonic longitudinal smooth muscle cells isolated from vehicle- or TNBS-treated mice with 1 µM ACh for 30 seconds significantly stimulated MLCK activity and MLC20 phosphorylation (Fig. 3, A and B). The increase in MLCK activity and MLC20 phosphorylation, however, was significantly greater in muscle cells isolated from TNBS-treated mice (Fig. 3, A and B). Basal MLCK activity and MLC20 phosphorylation were not significantly different between vehicle- and TNBS-treated mice.
Fig. 3.
Augmentation of ACh-induced MLCK activity and MLC20 phosphorylation by proinflammatory cytokines. Longitudinal muscle cells isolated from colon of control and TNBS-treated mice (A and B) or from muscle strips cultured in the absence (control) or presence of IL-1β (10 ng/ml) or TNF-α (1 nM) for 24 hours (C and D) were treated with 1 µM of ACh for 30 seconds to measure initial Ca2+/calmodulin-dependent MLCK activity (A and C) and MLC20 phosphorylation (B and D). MLCK activity was measured by immunokinase assay, and MLC20 phosphorylation at Ser19 was measured by immunoblot using phospho-specific antibody. Basal MLCK activity was not significantly different between control and TNBS-treated mice or between control and cytokine-treated muscle strips. Values are means ± S.E.M. of six experiments. **P < 0.01, significantly different from control response to ACh.
Similarly, ACh-stimulated MLCK activity and MLC20 phosphorylation were significantly greater in muscle cells isolated from strips treated for 24 hours with IL-1β (10 ng/ml) or TNF-α (1 nM) relative to MLCK activity and MLC20 phosphorylation in muscle cells isolated from strips cultured in control medium (Fig. 3, C and D). Basal MLCK activity and MLC20 phosphorylation were not significantly different between the different groups (Fig. 3, C and D).
Inhibition of ACh-Induced AMPK Activity and AMPK-Mediated MLCK Phosphorylation in Colonic Longitudinal Smooth Muscle Cells Isolated from TNBS-Treated Mice or from Control Strips Treated with IL-1β or TNF-α.
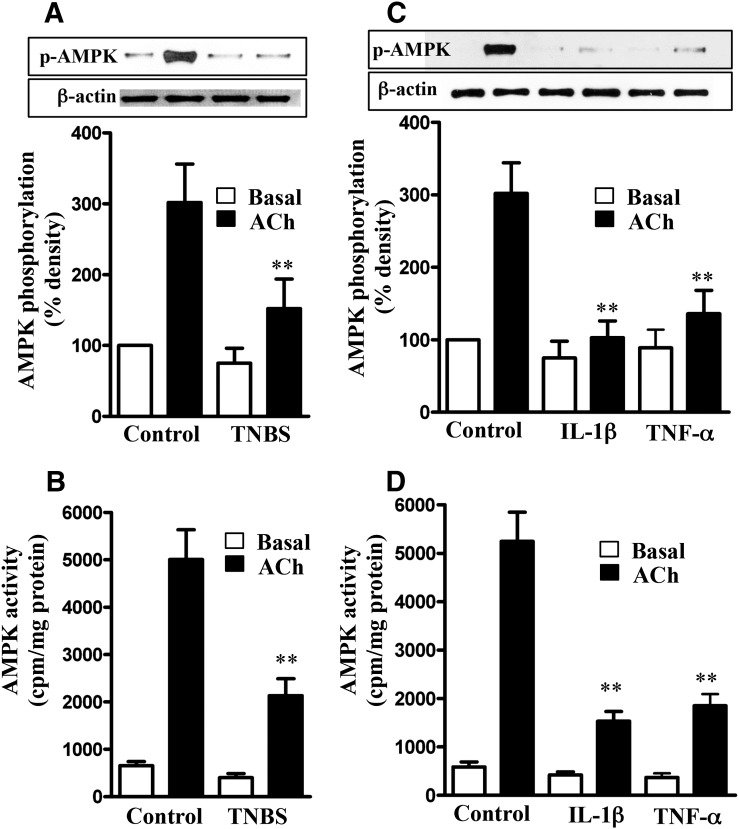
We tested the hypothesis that suppression of AMPK activity by proinflammatory cytokines masks the inhibitory effect of AMPK on MLCK, leading to an increase in ACh-stimulated MLCK activity, MLC20 phosphorylation, and muscle contraction. ACh-induced stimulation of AMPK phosphorylation at Thr172 and AMPK activity were significantly attenuated in muscle cells isolated from TNBS-treated mice compared with muscle cells from control mice (Fig. 4, A and B). Similarly, ACh-induced stimulation of AMPK phosphorylation at Thr172 and AMPK activity were significantly attenuated in cells isolated from muscle strips cultured for 24 hours with IL-1β or TNF-α (Fig. 4, C and D). Basal AMPK activity was not significantly different between different groups (Fig. 4).
Fig. 4.
Suppression of ACh-induced AMPK phosphorylation at Thr172 and AMPK activity by proinflammatory cytokines. Longitudinal muscle cells isolated from colon of control and TNBS-treated mice (A and B) or from muscle strips cultured in the absence (control) or presence of IL-1β (10 ng/ml) or TNF-α (1 nM) for 24 hours (C and D) were treated with 1 µM of ACh for 30 seconds to measure initial Ca2+/calmodulin-dependent AMPK phosphorylation (A and C) and AMPK activity (B and D). AMPK phosphorylation at Thr172 was measured by immunoblot using phospho-specific antibody, and AMPK activity was measured by immunokinase assay. Basal AMPK activity was not significantly different between control and TNBS-treated mice or between control and cytokine-treated muscle strips. Values are means ± S.E.M. of five experiments. **P < 0.01, significantly different from control response to ACh.
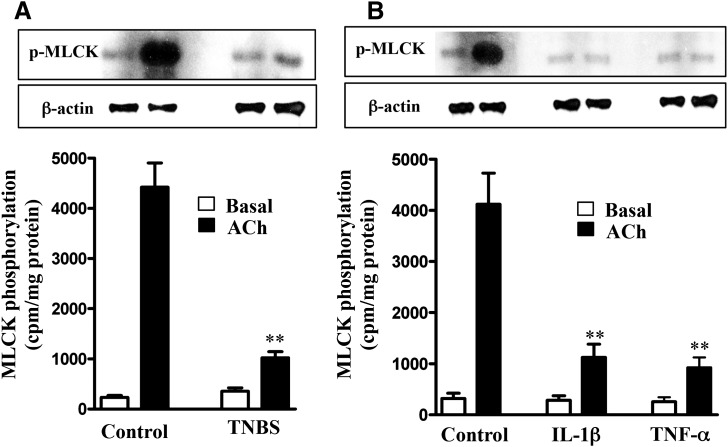
Consistent with the decrease in AMPK activity, AMPK-dependent phosphorylation of MLCK decreased in muscle cells isolated from TNBS-treated mice or from muscle strips treated with IL-1β or TNF-α (Fig. 5, A and B). These results implied that the increase in MLCK activity, MLC20 phosphorylation, and muscle contraction in muscle cells isolated from TNBS-treated mice or from strips treated with IL-1β or TNF-α was due to suppression of AMPK activity and AMPK-mediated inhibitory phosphorylation of MLCK. Basal cell lengths and MLCK activity were not significantly different in muscle cells isolated from strips incubated in the presence or absence of IL-1β or TNF-α, and this could be due to recovery of cell length during muscle cell isolation protocol.
Fig. 5.
Suppression of ACh-induced MLCK phosphorylation by proinflammatory cytokines. Longitudinal muscle cells isolated from colon of control and TNBS-treated mice (A) or from muscle strips cultured in the absence (control) or presence of IL-1β (10 ng/ml) or TNF-α (1 nM) for 24 hours (B) were treated with 1 µM of ACh for 30 seconds to measure MLCK phosphorylation. MLCK phosphorylation was measured in cells labeled with 32P, and results are expressed as cpm/mg protein. Values are means ± S.E.M. of five experiments. **P < 0.01, significantly different from control response to ACh.
Signaling Pathways That Mediate Inhibition of AMPK Activity in Colonic Longitudinal Smooth Muscle Cells Isolated from TNBS-Treated Mice or from Control Strips Treated with IL-1β or TNF-α.
As shown in Fig. 1, phosphorylation of the α subunit of AMPK at Thr172 by CaMKKβ stimulates AMPK activity. However, phosphorylation of AMPK at Ser485 by PKA inhibits AMPK activity (Djouder et al., 2010; Hardie, 2011). Previous studies have shown that proinflammatory cytokines (IL-1β or TNF-α) cause activation of NF-κB (Hu et al., 2008, 2009). The canonical pathway for activation of NF-κB involves IκBα kinase–mediated phosphorylation of IκBα, leading to degradation of IκBα and nuclear translocation of p65/p50 proteins. Degradation of IκBα releases the catalytic subunit of PKA from its binding to IκBα (Sriwai et al., 2013). An obvious candidate for mediating inhibition of AMPK activity is PKA derived from NF-κB activation. This notion was examined in colonic longitudinal muscle cells isolated from TNBS-treated mice and from muscle strips incubated with IL-1β or TNF-α for 24 hours in the presence or absence of inhibitors of NF-κB (MG-132) and PKA (myristoylated PKA inhibitor 14-22 amide [PKI]).
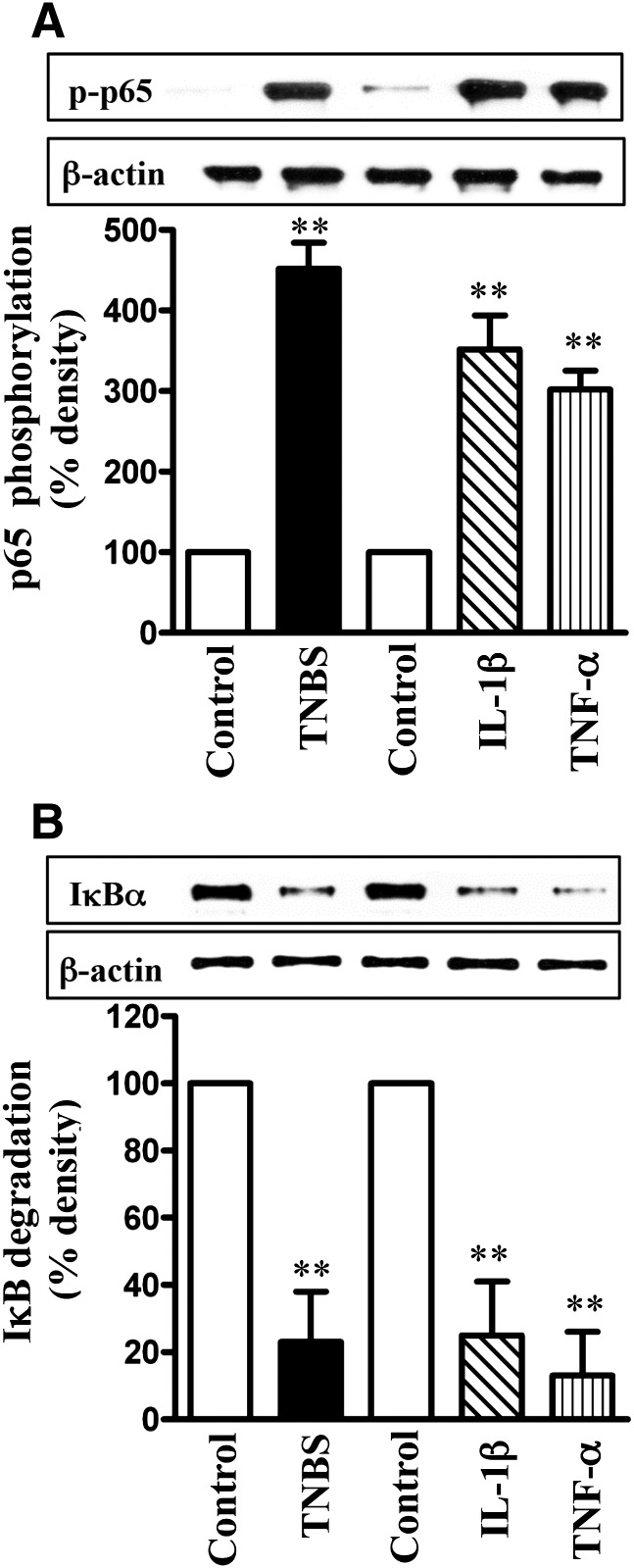
NF-κB activity, measured as an increase in p65 subunit phosphorylation or IκBα degradation (decrease in IκBα protein levels), was significantly stimulated in colonic longitudinal muscle cells isolated from TNBS-treated mice, as well as in muscle cells isolated from muscle strips treated with IL-1β or TNF-α for 24 hours (Fig. 6, A and B).
Fig. 6.
Activation of NF-κB pathway by proinflammatory cytokines. Longitudinal muscle cells isolated from colon of control and TNBS-treated mice or from muscle strips cultured in the absence (control) or presence of IL-1β (10 ng/ml) or TNF-α (1 nM) for 24 hours were used for measurement of p65 subunit phosphorylation (i.e., activation) by Western blot using antibody to phospho-specific (Ser177/181) antibody (A) and IκBα degradation using antibody to IκBα (B). Western blot of the β-actin protein is shown for a control loading. Values are means ± S.E.M. of four experiments **P < 0.01, significantly different from control.
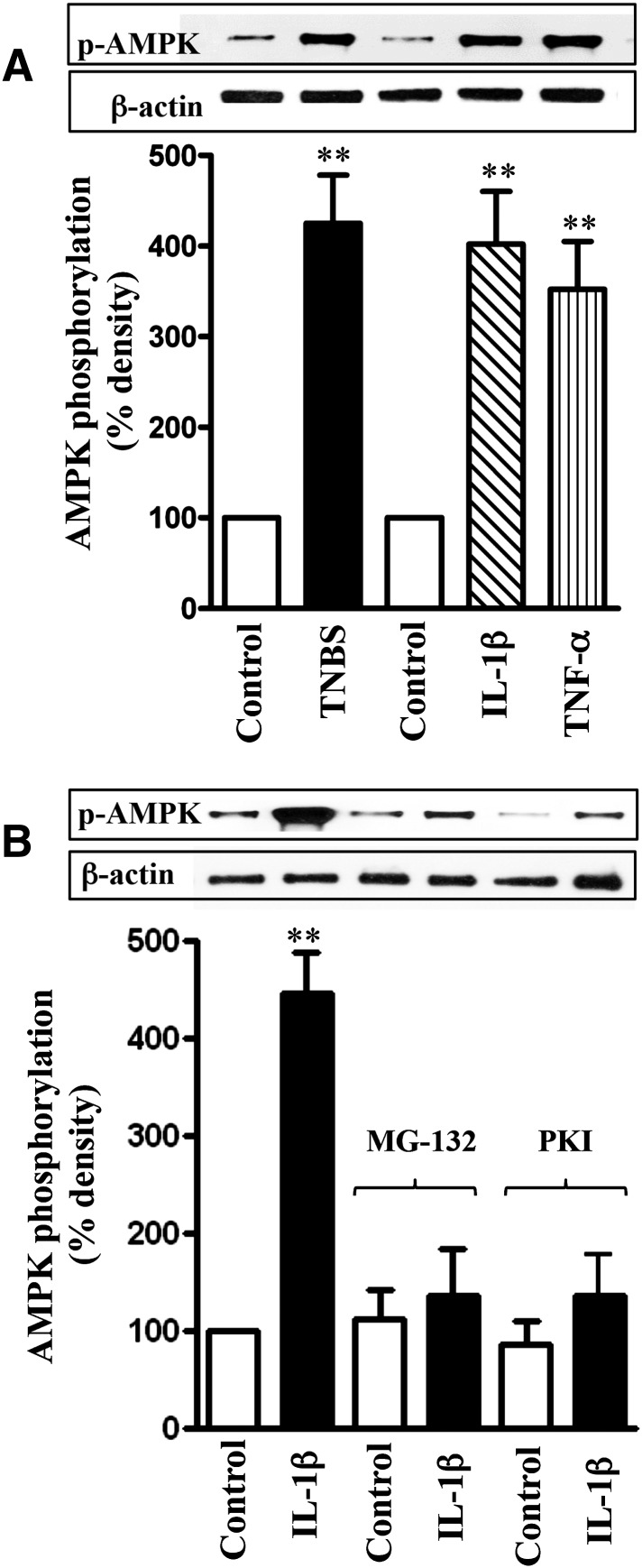
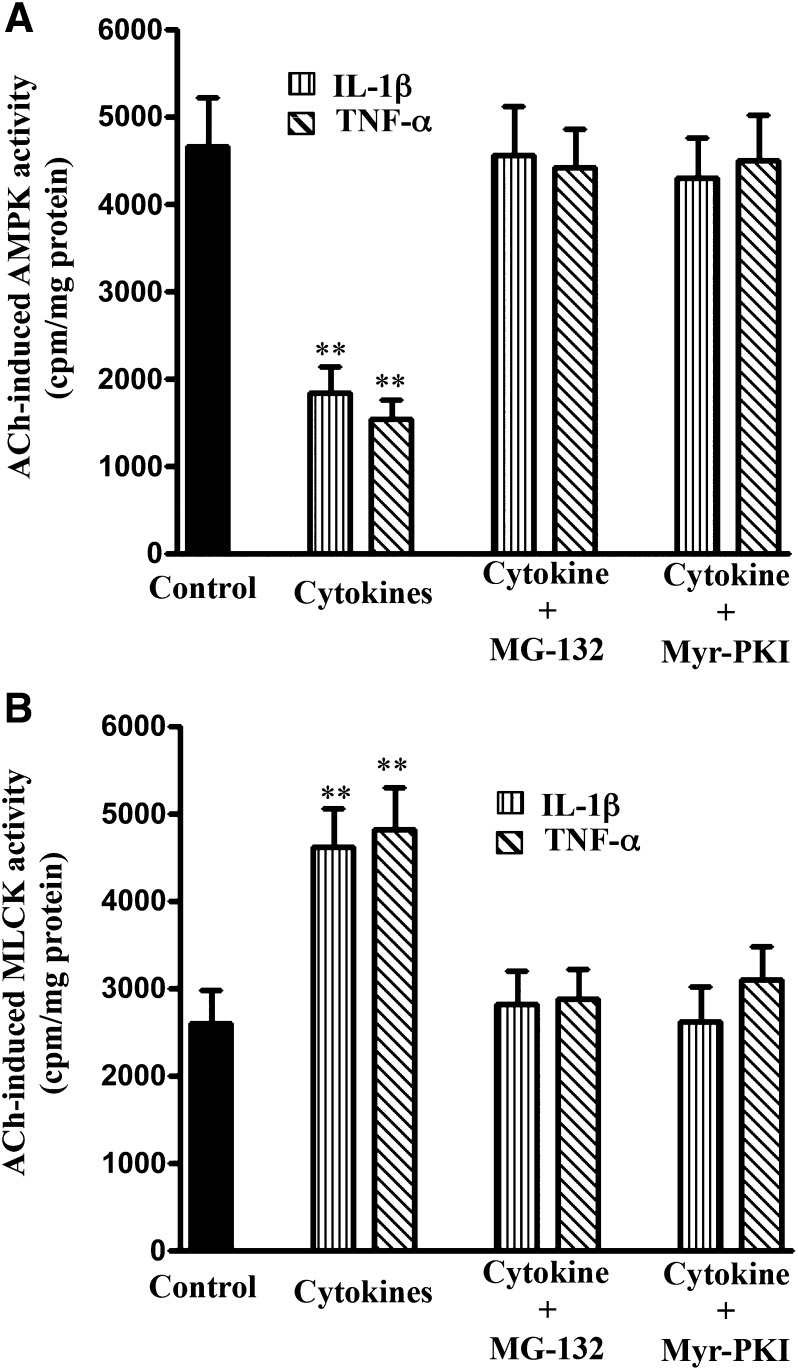
The increase in NF-κB activity in TNBS-treated mice and cytokine-treated muscle strips was accompanied by an increase in AMPK phosphorylation at Ser485, measured using phospho-specific antibody (Fig. 7A). Phosphorylation of AMPK at Ser485 was blocked when muscle strips were incubated with IL-1β in the presence of the NF-κB inhibitor, MG-132 (10 µM), or the PKA inhibitor PKI (1 μM) (Fig. 7B). Treatment of muscle strips with the MG-132 or PKI also blocked the inhibitory effect of IL-1β or TNF-α on ACh-stimulated AMPK activity (Fig. 8A). These results suggest that activation of NF-κB causes phosphorylation of AMPK via PKA leading to attenuation of AMPK activity.
Fig. 7.
Cytokine-induced phosphorylation of AMPK at Ser485 by PKA derived from NF-κB activation. Longitudinal muscle cells were isolated from colon of control and TNBS-treated mice or from muscle strips cultured in the absence (control) or presence of IL-1β (10 ng/ml) or TNF-α (1 nM) for 24 hours (A). In some experiments, muscle strips were incubated with IL-1β in the presence of inhibitors of NF-κB pathway (MG-132, 10 µM) or PKA (myristoylated PKI, 1 µM) (B). Phosphorylation of AMPK at Ser485 was measured using phospho-specific antibody. Values are means ± S.E.M. of four experiments. **P < 0.01, significantly different from control.
Fig. 8.
Blockade of NF-κB or PKA reverses the effect of cytokines on ACh-stimulated AMPK and MLCK activity. Longitudinal muscle cells were isolated from colonic muscle strips cultured with IL-1β (10 ng/ml) or TNF-α (1 nM) for 24 hours in the presence or absence of inhibitors of NF-κB pathway (MG-132, 10 µM) or PKA (PKI, 1 µM). (A) Stimulation of AMPK in response to ACh was measured by immunokinase assay using MLCK as substrate and [32P]ATP. (B) Stimulation of MLCK activity in response to ACh was measured by immunokinase assay using specific substrates and [32P]ATP. Results are expressed as cpm/mg protein. Values are means ± S.E.M. of four experiments. **P < 0.01, significantly different from control response to ACh.
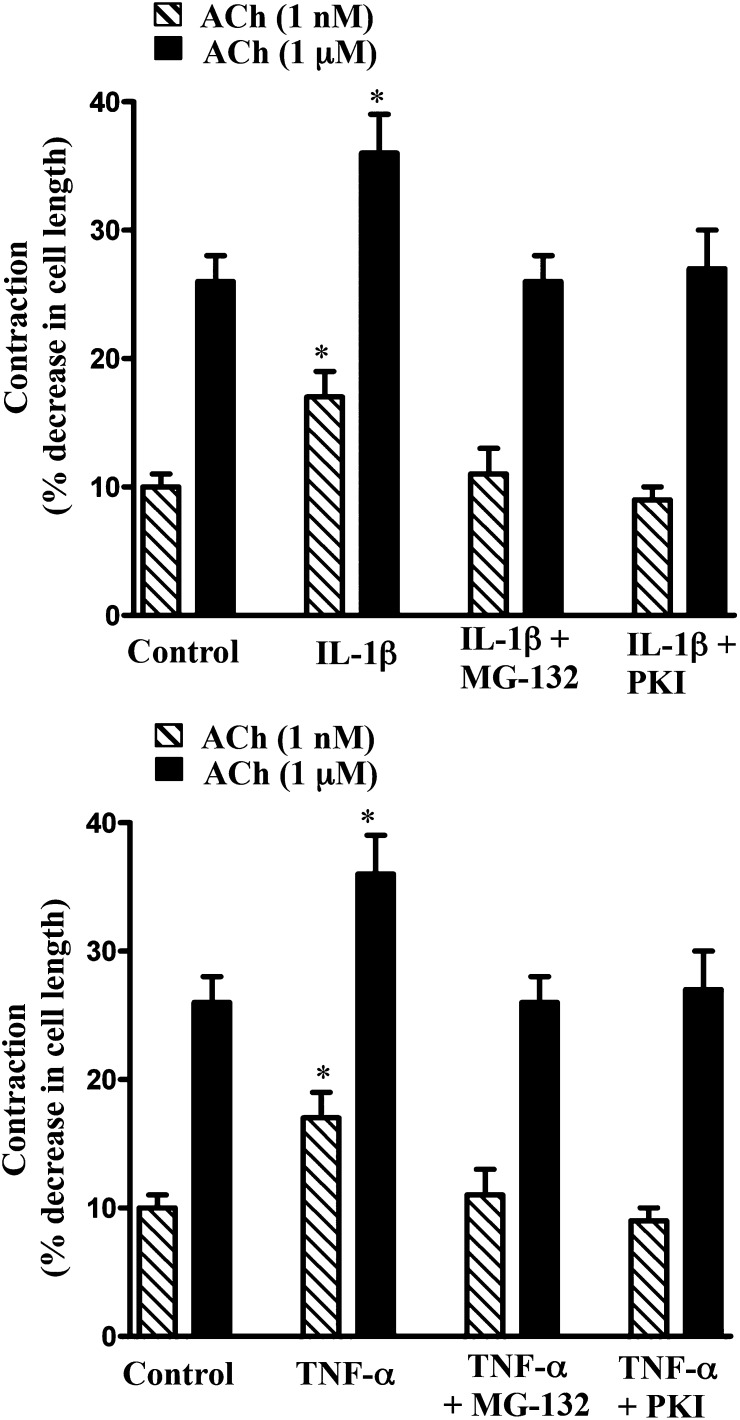
The involvement of NF-κB/PKA/AMPK pathway in cytokine-induced augmentation of MLCK activity and muscle contraction was further examined in smooth muscle cells isolated from muscle strips treated with IL-1β or TNF-α in the presence of MG-132 or PKI. As shown in Figs. 8B and 9, augmentation of MLCK activity and muscle contraction induced by IL-1β or TNF-α was blocked when the strips were incubated in the presence of MG-132 or PKI, implying that the increase in MLCK activity and contraction resulted from inhibition of AMPK activity on phosphorylation of AMPK by the catalytic subunit of PKA released from IκBα.
Fig. 9.
Blockade of NF-κB or PKA reverses the effect of cytokines on augmentation of contraction in response to ACh. Longitudinal muscle cells were isolated from colonic muscle strips cultured with IL-1β (10 ng/ml) or TNF-α (1 nM) for 24 hours in the presence or absence of inhibitors of NF-κB pathway (MG-132, 10 µM) or PKA (PKI, 1 µM). Muscle contraction in response to ACh (1 nM and 1 µM) was measured by scanning micrometry and is expressed as the percentage of decrease in cell length before ACh treatment (106 ± 5 µM to 108 ± 4 µm). Values are means ± S.E.M. of four experiments. *P < 0.05, significantly different from control response to ACh.
Discussion
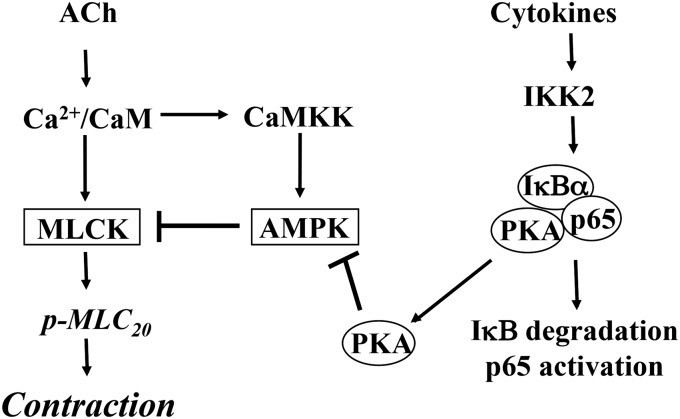
The present study characterized the signaling pathways mediating hypercontractility of colonic longitudinal muscle during inflammation in vivo and in response to proinflammatory cytokines in vitro. We measured ACh-stimulated initial contraction in colonic longitudinal muscle cells isolated from TNBS-treated mice and from muscle strips cultured for 24 hours with IL-1β or TNF-α. We demonstrated that inflammation in vivo and IL-1β or TNF-α in vitro significantly augmented ACh-stimulated MLCK activity, MLC20 phosphorylation, and initial Ca2+-dependent contraction. In contrast, our previous studies had shown that in colonic circular muscle cells isolated from TNBS-treated mice and from muscle strips cultured for 24 hours with IL-1β or TNF-α, ACh-stimulated MLC20 phosphorylation and initial Ca2+-dependent contraction were significantly decreased (Hu et al., 2007, 2008, 2009). Initial contraction in circular smooth muscle is mediated by activation of Gq and phospholipase C-β1, IP3-induced Ca2+ release, Ca2+/calmodulin-dependent activation of MLCK, and phosphorylation of MLC20. The signal for the initial phase is rapidly terminated by regulator of G protein signaling 4 (RGS4), which accelerates inactivation of Gαq. RGS4, an endogenous regulator of Gq signaling, was identified as the target of proinflammatory cytokines acting via NF-κB (Hu et al., 2007, 2008, 2009). An increase in RGS4 expression resulted in rapid inactivation of Gαq, inhibition of IP3 formation, Ca2+ mobilization, and initial muscle contraction. In contrast, as previously shown, initial contraction in longitudinal smooth muscle is initiated by Ca2+ influx and Ca2+- and cyclic ADP ribose–induced Ca2+ release via ryanodine receptors/Ca2+ channels (Murthy et al., 1991, 1995; Kuemmerle et al., 1994, 1998; Murthy, 2006). There is minimal participation of Gq, RGS4, and IP3-dependent Ca2+ release in this muscle. In longitudinal smooth muscle, as shown in the present study, initial contraction is terminated by inactivation of MLCK via a novel pathway involving sequential activation of CaMKKβ and AMPK. This pathway is highly expressed in longitudinal muscle and appears to be the dominant pathway for inactivation of MLCK and termination of initial contraction. The present study demonstrates that inflammatory cytokines, acting via NF-κB, induce hypercontractility in longitudinal smooth muscle by attenuating AMPK activity (Fig. 10).
Fig. 10.
Schematic diagram demonstrating the effects of cytokines on signaling pathways mediating initial Ca2+-dependent contraction in colonic smooth muscle cells. Initial contraction in response to ACh is mediated by Ca2+ influx and Ca2+/calmodulin-dependent activation of MLCK and MLC20 phosphorylation. Activation of AMPK via CaMKK provides a negative feedback regulation of MLCK activity. IL-1β or TNF-α stimulates NF-κB pathway involving phosphorylation of IκBα by IκB kinase (IKK) 2 leading to degradation of IκBα and nuclear translocation of p65/p50 proteins. Degradation of IκBα releases the catalytic subunit of PKA. Phosphorylation of AMPK at Ser485 by PKA causes decrease in phosphorylation at Thr172 by CaMKK and suppression of its activity in response to ACh. Inhibition of AMPK activity augments MLCK activity, MLC20 phosphorylation and muscle contraction in response to ACh.
MLCK activity in longitudinal muscle is negatively regulated via phosphorylation of MLCK by AMPK. Activation of NF-κB activity in response to cytokines releases the catalytic subunit of PKA from its binding to IκBα; phosphorylation of AMPK at Ser485 by PKA inhibits AMPK activity and attenuates its inhibitory effect on MLCK. The evidence supporting this conclusion can be summarized as follows: 1) ACh induced phosphorylation of AMPK (at Thr172) and MLCK and stimulated their activities leading to MLC20 phosphorylation and muscle contraction. 2) STO609, an inhibitor of CaMKKβ, blocked phosphorylation of AMPK and MLCK, resulting in attenuation of AMPK activity and augmentation of MLCK activity, MLC20 phosphorylation, and muscle contraction. The results implied that agonist-induced MLC20 phosphorylation and muscle contraction were negatively regulated via phosphorylation of MLCK by AMPK. 3) ACh-stimulated AMPK activity and phosphorylation at Thr172 were inhibited, whereas MLCK activity was augmented in muscle cells isolated from TNBS-treated mice or muscle strips treated with IL-1β or TNF-α. 4) NF-κB activity was stimulated and AMPK was phosphorylated at the PKA site (Ser485) in muscle cells isolated from TNBS-treated mice or muscle strips treated with IL-1β or TNF-α. 5) Phosphorylation of AMPK at Ser485 and attenuation of AMPK activity induced by IL-1β or TNF-α was blocked by inhibitors of NF-κB and PKA. 6) Augmentation of MLCK activity and muscle contraction induced by IL-1β or TNF-α was blocked by inhibitors of NF-κB and PKA. These results imply that the increase in ACh-stimulated MLCK activity, MLC20 phosphorylation, and muscle contraction induced by cytokines resulted from inhibition of AMPK by PKA derived from NF-κB activity, thereby relieving the inhibitory effect of AMPK on MLCK activity.
AMPK, a serine/threonine kinase, is activated in an AMP-dependent manner via phosphorylation at Thr172 by LKB (Hardie, 2011). AMPK is also activated in an AMP-independent manner via phosphorylation at Thr172 by CAMKKβ to regulate functions other than energy sensing. In addition to phosphorylation of AMPK at the activation site (Thr172), phosphorylation at Ser485/491 has been identified as both an autophosphorylation site and a target site for PKA and Akt (Woods et al., 2003; Horman et al., 2006; Omar et al., 2009). Stoichiometric phosphorylation of AMPK at Ser485 has been reported in vitro by the recombinant catalytic subunit of PKA. Phosphorylation of AMPK at Thr172 by CaMKKβ was shown to be greatly enhanced in COS cells expressing AMPK lacking the Ser485 site (Djouder et al., 2010). This finding suggests that the decrease in phosphorylation of AMPK at Thr172 in muscle cells isolated from TNBS-treated mice and from muscle strips treated with cytokines may reflect concurrent phosphorylation of AMPK at Ser485. However, a direct inhibitory effect of cytokines on CaMKKβ activity cannot be ruled out.
In longitudinal muscle cells, as previously noted herein, agonist-mediated activation of cPLA2 and generation of arachidonic acid are the triggers for Ca2+ mobilization and initial muscle contraction (Murthy et al., 1991, 1995; Kuemmerle et al., 1994, 1998). Previous studies had shown that both cAMP- and cGMP-dependent protein kinases phosphorylate cPLA2 and inhibit Ca2+ mobilization and contraction (Murthy and Makhlouf, 1998), in contrast to the augmentation of contraction induced by the catalytic subunit of PKA on release from binding to IκBα. Preliminary studies show that ACh-stimulated cPLA2 activity was not affected in muscle cells isolated from TNBS-treated mice or from muscle strips treated with IL-1β and TNF-α, suggesting that targets for cAMP-dependent PKA and cAMP-independent PKA are distinct.
Several studies have examined the changes in signaling mechanisms that result in hypocontractility of intestinal circular smooth muscle during inflammation and in response to proinflammatory cytokines (Ohama et al., 2003; Shi et al., 2003, 2005; Cao et al., 2004; Jin et al., 2004; Shi and Sarna, 2005; Hu et al., 2007; Ross et al., 2007). Hypercontractility of longitudinal muscle has been observed in response to parasitic infestation, which leads to activation of STAT-6 and upregulation of protease-activated receptor-1 and 5-HT2A receptors via IL-4 and IL-13 (Akiho et al., 2002; Zhao et al., 2005, 2006; Shea-Donohue et al., 2012). However, the changes in expression or activity of signaling targets that result in hypercontractility of longitudinal muscle have not been examined. The present study provides evidence for specific changes in signaling targets that mediate initial Ca2+/MLCK-dependent contraction in longitudinal smooth muscle exposed to proinflammatory cytokines. The pathways that mediate sustained contraction are distinct and involve G12/RhoGEF-mediated activation of RhoA and Rho kinase–mediated inhibition of MLC phosphatase (Murthy, 2006; de Godoy and Rattan 2011). Preliminary studies suggest that inflammatory cytokines stimulate expression of RhoGEF and inhibit expression of telokin, an endogenous activator of MLC phosphatase leading to increase in sustained muscle contraction. The disparate effects of inflammation on circular and longitudinal muscle disrupt the functional coordination of the two muscle layers.
Abbreviations
- ACh
acetylcholine
- AMPK
AMP-activated kinase
- CaMKK
calmodulin kinase kinase
- cPLA2
cytosolic phospholipase A2
- IL-1β
interleukin 1β
- IP3
inositol 1,4,5-trisphosphate
- MG-132
carbobenzoxy-l-leucyl-l-leucyl-l-leucinal Z-LLL-CHO
- MLC
myosin light chain
- MLCK
myosin light chain kinase
- NF-κB
nuclear factor κ B
- PKA
cAMP-dependent protein kinase
- PKI
myristoylated PKA inhibitor 14-22 amide
- RGS4
regulator of G protein signaling-4
- STO609
7-oxo-7H-benzimidazo[2,1-a]benz[de]isoquinoline-3-carboxylic acid acetate
- TNBS
2,4,6-trinitrobenzenesulfonic acid
- TNF-α
tumor necrosis factor-α
Authorship Contributions
Participated in research design: Grider, Kuemmerle, Murthy.
Conducted experiments: Mahavadi, Nalli, Kumar, Al-Shboul, Alkahtani.
Contributed new reagents or analytic tools: Kuemmerle.
Performed data analysis: Mahavadi, Murthy.
Wrote or contributed to the writing of the manuscript: Mahavadi, Grider, Murthy.
Footnotes
This work was supported by the National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases [Grant R01-DK15564] (to K.S.M.).
References
- Akiho H, Blennerhassett P, Deng Y, Collins SM. (2002) Role of IL-4, IL-13, and STAT6 in inflammation-induced hypercontractility of murine smooth muscle cells. Am J Physiol Gastrointest Liver Physiol 282:G226–G232 [DOI] [PubMed] [Google Scholar]
- Alkahtani R, Mahavadi S, Al-Shboul O, Alsharari S, Grider JR, Murthy KS. (2013) Changes in the expression of smooth muscle contractile proteins in TNBS- and DSS-induced colitis in mice. Inflammation 36:1304–1315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao W, Vrees MD, Potenti FM, Harnett KM, Fiocchi C, Pricolo VE. (2004) Interleukin 1β-induced production of H2O2 contributes to reduced sigmoid colonic circular smooth muscle contractility in ulcerative colitis. J Pharmacol Exp Ther 311:60–70 [DOI] [PubMed] [Google Scholar]
- Colyer J. (1998) Phosphorylation states of phospholamban. Ann N Y Acad Sci 853:79–91 [DOI] [PubMed] [Google Scholar]
- de Godoy MA, Rattan S. (2011) Role of rho kinase in the functional and dysfunctional tonic smooth muscles. Trends Pharmacol Sci 32:384–393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djouder N, Tuerk RD, Suter M, Salvioni P, Thali RF, Scholz R, Vaahtomeri K, Auchli Y, Rechsteiner H, Brunisholz RA, et al. (2010) PKA phosphorylates and inactivates AMPKalpha to promote efficient lipolysis. EMBO J 29:469–481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardie DG. (2011) AMP-activated protein kinase: a cellular energy sensor with a key role in metabolic disorders and in cancer. Biochem Soc Trans 39:1–13 [DOI] [PubMed] [Google Scholar]
- Hazelgrove KB, Flynn RS, Qiao LY, Grider JR, Kuemmerle JF. (2009) Endogenous IGF-I and alpha v beta3 integrin ligands regulate increased smooth muscle growth in TNBS-induced colitis. Am J Physiol Gastrointest Liver Physiol 296:G1230–G1237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horman S, Morel N, Vertommen D, Hussain N, Neumann D, Beauloye C, El Najjar N, Forcet C, Viollet B, Walsh MP, et al. (2008) AMP-activated protein kinase phosphorylates and desensitizes smooth muscle myosin light chain kinase. J Biol Chem 283:18505–18512 [DOI] [PubMed] [Google Scholar]
- Horman S, Vertommen D, Heath R, Neumann D, Mouton V, Woods A, Schlattner U, Wallimann T, Carling D, Hue L, et al. (2006) Insulin antagonizes ischemia-induced Thr172 phosphorylation of AMP-activated protein kinase α-subunits in heart via hierarchical phosphorylation of Ser485/491. J Biol Chem 281:5335–5340 [DOI] [PubMed] [Google Scholar]
- Hu W, Mahavadi S, Li F, Murthy KS. (2007) Upregulation of RGS4 and downregulation of CPI-17 mediate inhibition of colonic muscle contraction by interleukin-1β. Am J Physiol Cell Physiol 293:C1991–C2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu W, Li F, Mahavadi S, Murthy KS. (2008) Interleukin-1beta up-regulates RGS4 through the canonical IKK2/IkappaBalpha/NF-kappaB pathway in rabbit colonic smooth muscle. Biochem J 412:35–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu W, Li F, Mahavadi S, Murthy KS. (2009) Upregulation of RGS4 expression by IL-1β in colonic smooth muscle is enhanced by ERK1/2 and p38 MAPK and inhibited by the PI3K/Akt/GSK3β pathway. Am J Physiol Cell Physiol 296:C1310–C1320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, Zhou H, Mahavadi S, Sriwai W, Lyall V, Murthy KS. (2005) Signaling pathways mediating gastrointestinal smooth muscle contraction and MLC20 phosphorylation by motilin receptors. Am J Physiol Gastrointest Liver Physiol 288:G23–G31 [DOI] [PubMed] [Google Scholar]
- Hurley RL, Barré LK, Wood SD, Anderson KA, Kemp BE, Means AR, Witters LA. (2006) Regulation of AMP-activated protein kinase by multisite phosphorylation in response to agents that elevate cellular cAMP. J Biol Chem 281:36662–36672 [DOI] [PubMed] [Google Scholar]
- Ihara E, Chappellaz M, Turner SR, MacDonald JA. (2012) The contribution of protein kinase C and CPI-17 signaling pathways to hypercontractility in murine experimental colitis. Neurogastroenterol Motil 24:e15–e26 [DOI] [PubMed] [Google Scholar]
- Jin X, Malykhina AP, Lupu F, Akbarali HI. (2004) Altered gene expression and increased bursting activity of colonic smooth muscle ATP-sensitive K+ channels in experimental colitis. Am J Physiol Gastrointest Liver Physiol 287:G274–G285 [DOI] [PubMed] [Google Scholar]
- Kamm KE, Stull JT. (2001) Dedicated myosin light chain kinases with diverse cellular functions. J Biol Chem 276:4527–4530 [DOI] [PubMed] [Google Scholar]
- Khan WI, Collins SM. (2006) Gut motor function: immunological control in enteric infection and inflammation. Clin Exp Immunol 143:389–397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita K, Sato K, Hori M, Ozaki H, Karaki H. (2003) Decrease in activity of smooth muscle L-type Ca2+ channels and its reversal by NF-kappaB inhibitors in Crohn’s colitis model. Am J Physiol Gastrointest Liver Physiol 285:G483–G493 [DOI] [PubMed] [Google Scholar]
- Kuemmerle JF. (1998) Synergistic regulation of NOSII expression by IL-1β and TNF-α in cultured rat colonic smooth muscle cells. Am J Physiol Gastrointest Liver Physiol 274:G178–G185 [DOI] [PubMed] [Google Scholar]
- Kuemmerle JF, Murthy KS, Makhlouf GM. (1994) Agonist-mediated influx activates ryanodine-sensitive, IP3-insensitive Ca2+ release channels in longitudinal muscle of intestine. Am J Physiol Cell Physiol 266:C1421–C1431 [DOI] [PubMed] [Google Scholar]
- Kuemmerle JF, Murthy KS, Makhlouf GM. (1998) Longitudinal smooth muscle of the mammalian intestine: a model for Ca2+ signaling by cADPR. Cell Biochem Biophys 28:31–44 [DOI] [PubMed] [Google Scholar]
- Martinolle JP, Garcia-Villar R, Fioramonti J, Bueno L. (1997) Altered contractility of circular and longitudinal muscle in TNBS-inflamed guinea pig ileum. Am J Physiol 272:G1258–G1267 [DOI] [PubMed] [Google Scholar]
- Murthy KS. (2006) Signaling for contraction and relaxation in smooth muscle of the gut. Annu Rev Physiol 68:345–374 [DOI] [PubMed] [Google Scholar]
- Murthy KS, Grider JR, Makhlouf GM. (1991) InsP3-dependent Ca2+ mobilization in circular but not longitudinal muscle cells of intestine. Am J Physiol 261:G937–G944 [DOI] [PubMed] [Google Scholar]
- Murthy KS, Kuemmerle JF, Makhlouf GM. (1995) Release of arachidonic acid by agonist-mediated activation of PLA2 initiates Ca2+ mobilization in intestinal smooth muscle. Am J Physiol Gastrointest Liver Physiol 269:G93–G102 [DOI] [PubMed] [Google Scholar]
- Murthy KS, Makhlouf GM. (1998) Differential regulation of phospholipase A2 (PLA2)-dependent Ca2+ signaling in smooth muscle by cAMP- and cGMP-dependent protein kinases. Inhibitory phosphorylation of PLA2 by cyclic nucleotide-dependent protein kinases. J Biol Chem 273:34519–34526 [DOI] [PubMed] [Google Scholar]
- Murthy KS, Severi C, Grider JR, Makhlouf GM. (1993) Inhibition of IP3 and IP3-dependent Ca2+ mobilization by cyclic nucleotides in isolated gastric muscle cells. Am J Physiol 264:G967–G974 [DOI] [PubMed] [Google Scholar]
- Murthy KS, Zhou H, Grider JR, Brautigan DL, Eto M, Makhlouf GM. (2003) Differential signalling by muscarinic receptors in smooth muscle: m2-mediated inactivation of myosin light chain kinase via Gi3, Cdc42/Rac1 and p21-activated kinase 1 pathway, and m3-mediated MLC20 (20 kDa regulatory light chain of myosin II) phosphorylation via Rho –associated kinase/myosinj phosphatase targeting subunit 1 and protein kinase CPI-17 pathway. Biochem J 374:145–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KY, Choi HC. (2013) Acetylcholine-induced AMP-activated protein kinase activation attenuates vasoconstriction through an LKB1-dependent mechanism in rat aorta. Vascul Pharmacol 59:96–102 [DOI] [PubMed] [Google Scholar]
- Ohama T, Hori M, Sato K, Ozaki H, Karaki H. (2003) Chronic treatment with interleukin-1beta attenuates contractions by decreasing the activities of CPI-17 and MYPT-1 in intestinal smooth muscle. J Biol Chem 278:48794–48804 [DOI] [PubMed] [Google Scholar]
- Omar B, Zmuda-Trzebiatowska E, Manganiello V, Göransson O, Degerman E. (2009) Regulation of AMP-activated protein kinase by cAMP in adipocytes: roles for phosphodiesterases, protein kinase B, protein kinase A, Epac and lipolysis. Cell Signal 21:760–766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross GR, Kang M, Shirwany N, Malykhina AP, Drozd M, Akbarali HI. (2007) Nitrotyrosylation of Ca2+ channels prevents c-Src kinase regulation of colonic smooth muscle contractility in experimental colitis. J Pharmacol Exp Ther 322:948–956 [DOI] [PubMed] [Google Scholar]
- Shea-Donohue T, Notari L, Sun R, Zhao A. (2012) Mechanisms of smooth muscle responses to inflammation. Neurogastroenterol Motil 24:802–811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi XZ, Lindholm PF, Sarna SK. (2003) NF-kappa B activation by oxidative stress and inflammation suppresses contractility in colonic circular smooth muscle cells. Gastroenterology 124:1369–1380 [DOI] [PubMed] [Google Scholar]
- Shi XZ, Pazdrak K, Saada N, Dai B, Palade P, Sarna SK. (2005) Negative transcriptional regulation of human colonic smooth muscle Cav1.2 channels by p50 and p65 subunits of nuclear factor-kappaB. Gastroenterology 129:1518–1532 [DOI] [PubMed] [Google Scholar]
- Shi XZ, Sarna SK. (2005) Transcriptional regulation of inflammatory mediators secreted by human colonic circular smooth muscle cells. Am J Physiol Gastrointest Liver Physiol 289:G274–G284 [DOI] [PubMed] [Google Scholar]
- Somlyo AV, Khromov AS, Webb MR, Ferenczi MA, Trentham DR, He ZH, Sheng S, Shao Z, Somlyo AP. (2004) Smooth muscle myosin: regulation and properties. Philos Trans R Soc Lond B Biol Sci 359:1921–1930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sriwai W, Mahavadi S, Al-Shboul O, Grider JR, Murthy KS. (2013) Distinctive G protein-dependent signaling by protease-activated receptor 2 (PAR2) in smooth muscle: feedback inhibition of RhoA by cAMP-independent PKA. PLoS ONE 8:e66743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung JY, Choi HC. (2012) Metformin-induced AMP-activated protein kinase activation regulates phenylephrine-mediated contraction of rat aorta. Biochem Biophys Res Commun 421:599–604 [DOI] [PubMed] [Google Scholar]
- Tokumitsu H, Inuzuka H, Ishikawa Y, Ikeda M, Saji I, Kobayashi R. (2002) STO-609, a specific inhibitor of the Ca(2+)/calmodulin-dependent protein kinase kinase. J Biol Chem 277:15813–15818 [DOI] [PubMed] [Google Scholar]
- Woods A, Vertommen D, Neumann D, Turk R, Bayliss J, Schlattner U, Wallimann T, Carling D, Rider MH. (2003) Identification of phosphorylation sites in AMP-activated protein kinase (AMPK) for upstream AMPK kinases and study of their roles by site-directed mutagenesis. J Biol Chem 278:28434–28442 [DOI] [PubMed] [Google Scholar]
- Yang Z, Sun R, Grinchuk V, Fernández-Blanco JA, Notari L, Bohl JA, McLean LP, Ramalingam TR, Wynn TA, Urban JF, Jr, et al. (2013) IL-33-induced alterations in murine intestinal function and cytokine responses are MyD88, STAT6, and IL-13 dependent. Am J Physiol Gastrointest Liver Physiol 304:G381–G389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao A, Morimoto M, Dawson H, Elfrey JE, Madden KB, Gause WC, Min B, Finkelman FD, Urban JF, Jr, Shea-Donohue T. (2005) Immune regulation of protease-activated receptor-1 expression in murine small intestine during Nippostrongylus brasiliensis infection. J Immunol 175:2563–2569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao A, Urban JF, Jr, Morimoto M, Elfrey JE, Madden KB, Finkelman FD, Shea-Donohue T. (2006) Contribution of 5-HT2A receptor in nematode infection-induced murine intestinal smooth muscle hypercontractility. Gastroenterology 131:568–578 [DOI] [PubMed] [Google Scholar]