HIV infection in macrophages is enhanced by substance P through a mechanism dependent on the haptoglobinhemoglobin scavenger receptor CD163.
Keywords: AIDS, neurocognitive impairment, monocytes, hemoglobin, haptoglobin
Abstract
Activation of NK1R by SP contributes to increased HIV-1 infection in macrophages. The scavenger receptor CD163 is expressed on cells of monocyte-macrophage origin. Our main goal was to determine if there is interplay among SP, CD163 expression, and HIV infection in macrophages. We showed that SP triggers intracellular calcium elevation and increased CD163 expression in human monocytes in a time- and concentration-dependent manner. The role of CD163 on HIV infection was examined by RT-PCR in sorted monocytes (CD163low and CD163high) and in macrophages having CD163 knocked down using siRNA. We found that the productivity of HIV infection was higher in CD163high cells. Additionally, in macrophages with CD163 expression knocked down, we found a significant decrease of HIV infection. Furthermore, Hb-Hp complexes, which function as an endogenous ligand for CD163, decreased HIV infection in macrophages in a dose-dependent manner. Thus, we demonstrate that SP induces higher levels of CD163 in monocytes and that high expression of CD163 is associated with increases HIV infection in macrophages. Thus, in addition to being a prognostic marker of HIV infection, the expression of CD163 on macrophages may be critical in HIV immunopathogenesis.
Introduction
SP is an undecapeptide that functions as a neurotransmitter in the central and peripheral nervous system and has immunomodulatory properties. SP belongs to the tachykinin family of neuropeptides, which share a common C-terminal sequence, Phe-X-Gly-Leu-Met-NH2, that is essential for interaction with their receptors [1]. Two isoforms of NK1R are generated through alternative splicing: a full-length NK1R that includes in its primary structure 407 aa residues and a truncated NK1R that lacks the last 96-aa residues at the C-terminus intracellular domain [2–4].
Our group has provided compelling evidence that SP and NK1R are important in the pathogenesis of HIV/AIDS [5–8]. Plasma SP levels are elevated in HIV-infected individuals [5, 9, 10], and SP treatment of MDM promotes HIV infection, whereas treatment with NK1R antagonists inhibits HIV infection [7, 8, 11]. However, the molecular mechanisms responsible for the effect of SP on promoting HIV replication in macrophages are not understood completely.
CD163, the scavenger receptor for Hb-Hp complexes, has been linked to HIV infection [12–17]. CD163 is expressed exclusively in cells of the monocyte/macrophage lineage [18–21]. Hemolysis is caused by infectious and inflammatory conditions, and the clearance of Hb-Hp complexes by CD163-linked mechanisms is an important process in the resolution of inflammation [22]. CD163 expression is up-regulated by anti-inflammatory mediators, including glucocorticoids [23] and IL-10 [24], and it is down-regulated by proinflammatory molecules, such as IFN-γ, TNF-α, and LPS [24]. Higher levels of CD163 are detected on macrophages with an anti-inflammatory potential, such as alternatively activated and deactivated macrophages.
High expression of CD163 was detected on CNS macrophages in brains of HIV-positive individuals [25–28]. Increased levels of CD163 may be a biomarker reflecting efforts of the immune system to resolve immune activation and inflammation in HIV-infected individuals [13]. Higher frequency of CD163+/CD16+ cells in HIV-positive individuals is associated with a decrease in CD4+ T cells and increase in viral loads [25, 28]. CD163+/CD16+ macrophages are associated with CNS and other tissue invasion in humans and in nonhuman primates [25, 28].
Proinflammatory stimuli not only inhibit production of CD163 but also result in shedding of the extracellular portion of CD163, which circulates in blood as a soluble protein (sCD163). Although the functions of sCD163 are unknown, increased levels of sCD163 occur in several chronic inflammatory diseases, including HIV infection [12, 15, 29]. Monocyte- and macrophage-derived sCD163 may be a marker of HIV activity that links viral replication with monocyte and macrophage activation [12, 15]. Inverse correlation was found between membrane CD163 expression in human monocytes and sCD163 levels in plasma [30], suggesting that plasma sCD163 is derived from circulating monocytes and tissue macrophages. There is no direct evidence, however, that sCD163 or membrane-bound CD163 influences HIV entry or replication.
Our study demonstrates that SP induces a NK1R-mediated intracellular calcium increase in freshly isolated human monocytes and triggers the alternative pathway of macrophage differentiation, with up-regulation of membrane-bound CD163. We also demonstrate that the productivity of infection is increased in macrophages with high levels of expression of CD163. Our findings provide novel insights into the cellular mechanisms responsible for SP enhancement of HIV infection.
MATERIALS AND METHODS
Reagents
Emend capsules (Merck & Co., Whitehouse Station, NJ, USA) were purchased, and aprepitant was purified by chromatography. SP, fMLP, human Hb, and human Hp from pooled plasma were purchased from Sigma-Aldrich (St. Louis, MO, USA). NKA and NKB were purchased from Phoenix Pharmaceuticals (Burlingame, CA, USA).
Viruses
HIV-1 Clone BaL.01 (Cat. #11445), from Dr. John R. Mascola, was from the NIH AIDS Reagent Program, Division of AIDS, National Institute of Allergy and Infectious Diseases (Bethesda, MD, USA) [31]. HIV tissue-culture infectious dose 50 was determined using membrane-associated guanylate kinase with inverted orientation cells expressing CD4 and CCR5. Cells were infected with 5 ng p24/well, corresponding to a MOI of 0.02. The HIV-GFP-tagged reporter virus pSF162R3 Nef+ was a gift from Dr. Amanda Brown (The John Hopkins School of Medicine, Baltimore, MD, USA) [32].
Cells.
Deidentified, fresh monocytes (purity ≥95%) or PBMCs from HIV-negative donors were purchased from the Human Immunology Core Facility of the University of Pennsylvania School of Medicine (Philadelphia, PA, USA).
Intracellular calcium measurements.
Fresh PBMCs were loaded with Fluo-4-AM (2 μM) and Fura-Red-AM (2 μM; Invitrogen, Eugene, OR, USA). Ratiometric intracellular calcium recordings were performed on an Accuri C6 cytometer (BD Biosciences, San Jose, CA, USA), as described previously, at room temperature [33].
CD163 measurement by flow cytometry
For time-course experiments, freshly isolated monocytes were stimulated with IL-10 (20 ng/ml) or SP (10 μM), and after 0, 2, 4, and 6 days of culture, the cells were detached, and the expression of membrane-bound CD163 was quantified by flow cytometry. For the concentration-response experiments, monocytes were stimulated with IL-10 (0.2, 0.6, 2, 6, or 20 ng/ml) or SP (0.3, 1, 3, 10, or 30 μM), and after 4 days, the cells were detached for CD163 measurements using anti-human CD163 antibody (Clone R-20) conjugated with PE (Trillium Diagnostics, Scarborough, ME, USA). Flow cytometry experiments were performed on an Accuri C6 analyzer (BD Biosciences) in the Flow Cytometry Core Laboratory of the Children's Hospital of Philadelphia Research Institute.
To validate the CD163 assay by flow cytometry, we performed preliminary experiments in which whole blood and isolated monocytes were stained in parallel with CD163-PE (Trillium Diagnostics) and CD14-allophycocyanin (Biolegend, San Diego, CA). Flow cytometry analysis was performed to determine the percentage of CD163+ cells, and we found that for both types of samples, >95% of CD14-positive cells (i.e., monocytes) were also positive for CD163 (n=5; P=0.57). However, we found that only 83.4% of CD14+ cells from cryopreserved PBMCs expressed CD163, and this percentage was significantly different from monocytes stained in whole blood (P<0.01; data not shown). As the data suggest that cryopreservation reduces CD163 expression, we used freshly isolated cells in all experiments.
Cell sorting
Monocytes were cultured overnight, then labeled with anti-human CD163-PE, and sorted, based on the expression level of CD163 using a MoFlo XDP cell sorter (Beckman Coulter, Brea, CA, USA). After 4 h, cells were infected with HIV-BaL.
HIV infection
Macrophages were incubated with the HIV-BaL virus overnight and then washed to remove unbound virus. The residual HIV RNA level was <1% of viral RNA detected at Day 7 after infection. At Days 5, 7, 9, and 11 post-HIV infection, RNA was extracted from the MDM for assessment of HIV-GAG mRNA expression using real-time RT-PCR assays.
RNA extraction and real-time RT-PCR assays
Total RNA was extracted from macrophages using the RNeasy kit (Qiagen, Valencia, CA, USA), and the potential DNA contamination was eliminated by on-column DNase digestion. Total RNA (1 μg) was reverse-transcribed using the AffinityScript QPCR cDNA Synthesis kit (Stratagene, Cedar Creek, TX, USA) with random primer, as instructed by the manufacturer. RT-negative controls were used to control for genomic DNA contamination. The resulting cDNA (1 μl) was used as a template for real-time PCR amplification.
The sequences of the primers and probes for GAPDH and HIV-GAG (Integrated DNA Technologies, Coralville, IA, USA) were reported previously [34, 35]. For experiments involving detection of viral LTR, HIV stock was treated with DNase (50 μg/ml) for 30 min before infection. Absence of viral DNA after DNase treatment was confirmed by real-time PCR. For PCR quantification of early stages of viral infection, total cellular DNA was isolated 48 h after MDM infection using the Qiagen DNeasy kit. LTR was measured using real-time PCR, using primers and probe reported [36], and normalized to total cellular DNA measured, using the GAPDH-DNA qPCR Assay (Hs.PT.56a.589810.g; Integrated DNA Technologies).
RNAi
RNAi CD163 pool and control RNAi were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA) and were transfected into macrophages, and experiments were carried out 48 h after transfection. Viability of siRNA-treated cells was >95% (Trypan blue method).
Microscopy
Macrophages were infected with HIV-GFP-tagged, reported virus pSF162R3 Nef+. Infected cells were observed on a system, including an Olympus IX51 fluorescence microscope (Olympus America, Center Valley, PA, USA), and green fluorescent spots were counted using ImageJ software (NIH).
Statistical analysis
Three to six independent experiments were performed for each condition, and mean values and se were calculated. One-way ANOVA and a two-tailed t-test were used to determine the statistical significance of differences between means (*P<0.05).
RESULTS AND DISCUSSION
SP induces intracellular calcium increase in human monocytes
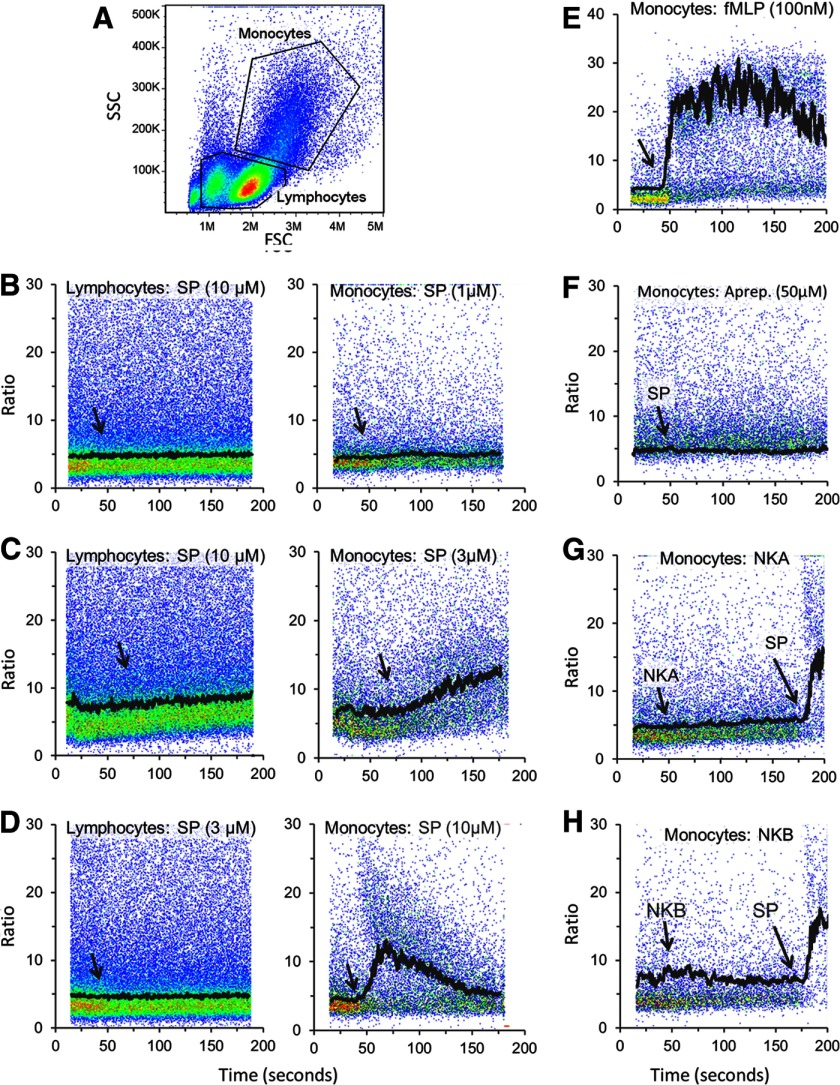
Our previous studies have shown that in the U373MG cell, NK1R stimulates the release of calcium from intracellular stores though activation of PLC. To assess whether the effect of SP on monocytes is a result of activation of NK1R, we examined the levels of intracellular calcium. We used a ratiometric calcium assay, where PBMCs were loaded with Fluo-4 and Fura-Red and then stimulated with SP or the appropriate agonist. Gating on lymphocyte and monocyte populations was performed based on light-scattering properties of the cells (Fig. 1A). SP failed to induce any detectable change in intracellular calcium in monocytes at concentrations of 1 μM or lower (Fig. 1B), whereas 3 or 10 μM SP induced an intracellular calcium increase (Fig. 1C and D). This pharmacology profile of NK1R, with high concentrations of SP required to elicit a response, is consistent with the presence of only the truncated form of NK1R on human monocytes. SP did not induce intracellular calcium increase in lymphocytes (Fig. 1A–C).
Figure 1. SP-induced intracellular calcium increase in human monocytes is mediated by NK1R.
Freshly isolated monocytes were loaded with Fluo-4-AM and Fura-Red-AM and used for intracellular calcium measurements by flow cytometry. (A) A typical forward- and side-scatter (FSC and SSC, respectively) plot of PBMCs with the gating of monocytes and lymphocytes is shown. (B–H) MFI line graphs (black tracings) were overlapped with pseudocolor dot plots (ratio fluorescence intensities Fluo 4/Fura Red vs. time). (B–D) PBMCs were stimulated with 1, 3, or 10 μM SP, (E) 100 nM fMLP, or (F) pretreated with 50 μM aprepitant (Aprep.) for 15 min followed by 10 μM SP. PBMCs were treated with 10 μM NKA (G) or 10 μM NKB (H), and SP was added where indicated, simply to demonstrate that cells were responsive to SP. (B–D, left) Intracellular calcium recordings in gated lymphocytes are shown. Similar results were obtained in three independent experiments with PBMCs from different donors. Arrows indicate the time when SP or neurokinins were added to cells.
We also used fMLP as a positive control to show that the monocytes treated with aprepitant were capable of a calcium response, as shown in Fig. 1E. We next confirmed that NK1R mediates the response to SP by pretreating PBMCs with the selective NK1R antagonist aprepitant (50 μM) for 15 min before adding SP. Aprepitant abrogated the SP-induced calcium response (Fig. 1F). We have shown previously that aprepitant blocks the responses mediated by the truncated NK1R [37]. Additionally, to ensure that the response to SP is not mediated by NK2R or NK3R, we used the agonists NKA and NKB, respectively. NKA (10 μM) and NKB (10 μM) both failed to induce calcium responses, whereas addition of SP induced a response in the same samples (Fig. 1G and H).
SP increases expression of CD163 in human monocytes
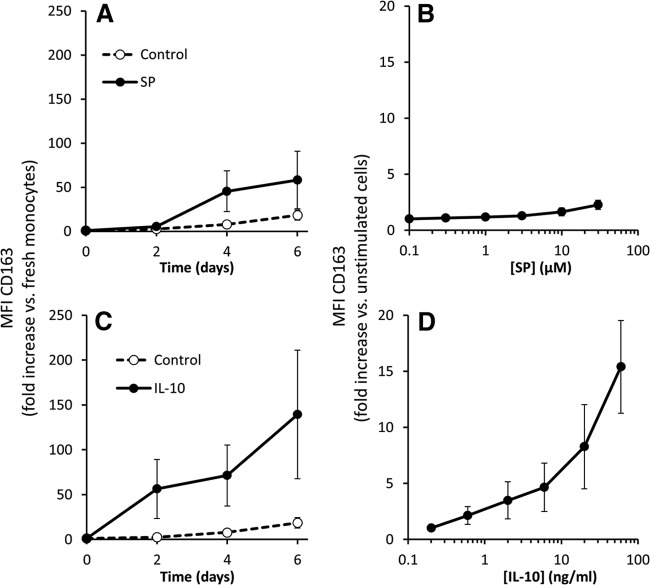
We performed a time-course study with 10 μM SP, and we also determined the concentration-response relationship after 4 days stimulation with SP. The time course of SP stimulation of monocytes is shown in Fig. 2A. SP increased CD163 expression at 4 days and 6 days. Fig. 2B shows the concentration response for the effect of SP on CD163 expression as measured after 4 days. SP concentrations from 0.3 to 10 μM all showed significant increase in CD163 expression.
Figure 2. SP and IL-10 increase expression of CD163 is human macrophages.
Freshly isolated monocytes were treated with SP or IL-10, then stained for CD163, and analyzed by flow cytometry. (A) Time-course experiments on monocytes treated with 10 μM SP for 2, 4, or 6 days. (B) Concentration-response experiments on monocytes cultured for 4 days in the presence of 0, 0.1, 0.3, 1, 3, 10, or 30 μM SP. (C) Time course of monocytes treated with 20 ng/ml IL-10 for 2, 4, or 6 days. (D) Concentration- response experiments on monocytes cultured for 4 days in the presence of 0, 0.2, 0.6, 2, 6, 20, or 60 ng/ml IL-10. For time-course experiments (A and C), the change in MFI compared with the corresponding values at Time 0 (freshly isolated monocytes) are shown. For concentration-response experiments (B and D), MFI values compared with unstimulated monocytes at Day 4 are shown. Results from five independent experiments are presented as mean ± sem.
IL-10 increases expression of CD163 in human monocytes
As IL-10 is known to induce CD163 expression in monocytes, we examined for comparison the expression of CD163 in response to IL-10 in cultured human monocytes. A time course of IL-10 stimulation of monocytes is shown in Fig. 2C. Notably, there is a significant increase in the expression of CD163 in as little as 2 days, and this elevation is higher at Days 4 and 6. Nontreated cells also show an increase in CD163 over time but to much lower levels compared with cells treated with IL-10. A concentration-response curve for the effect of IL-10 on CD163 expression, as measured after 4 days of culture, is shown in Fig. 2D. As little as 0.2 ng/ml IL-10 induced a significant increase in CD163 expression, and treatment with as high as 60 ng/ml IL-10 did not induce a maximal increase in CD163 expression. Taken together, our results indicate that SP induces expression of CD163, although at a lower extent than that induced by IL-10.
Macrophages derived from monocytes with high levels of membrane-bound CD163 have increased susceptibility to HIV infection
To determine if susceptibility to HIV infection correlates with the levels of expression of CD163 on a cell membrane, we stained monocytes with anti-CD163-PE antibody and performed cell sorting in two populations: CD163high and CD163low (Fig. 3A). Cells were kept overnight under standard cell-culture conditions and then infected with HIV-BaL in 24-well plates, and HIV infection was assayed by RT-PCR. We found that the productivity of infection in CD163high macrophages was much higher compared with CD163low cells (Fig. 3B). To bring additional evidence that high levels of membrane-bound CD163 promote HIV infection, we used siRNA to knock down the expression of CD163 in MDMs (Fig. 4A), and we measured HIV infection by RT-PCR in siRNA-treated cells. We have found that the HIV infection of cells treated with siRNA targeting CD163 was inhibited almost completely, whereas control siRNA had a relatively modest, albeit statistically significant, inhibitory effect on macrophage infection (Fig. 4B).
Figure 3. Monocytes expressing high levels of CD163 are more susceptible to HIV infection.
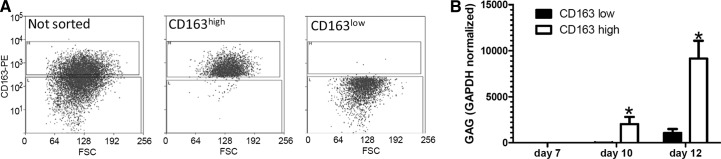
(A) Monocytes were sorted based on expression level of CD163 using a MoFlo XDP (Beckman Coulter) cell sorter and infected with HIV-BaL, 4 h later. Cell purity was checked after each cell-sorting experiment and found >95%. (B) HIV-GAG normalized to GAPDH assayed by real-time RT-PCR. Average results of three independent experiments are presented as mean ± sd. *P < 0.01 CD163high versus CD163low.
Figure 4. Decreased expression of CD163 on macrophages leads to inhibition of HIV infection.
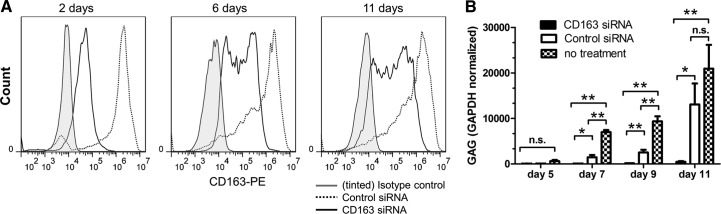
Macrophages were cultured in vitro, and siRNA was used to decrease the expression of CD163. (A) Flow cytometry data showing CD163 expression in macrophages, 2, 6, and 11 days after transfection with CD163 siRNA (solid lines) or control siRNA (dotted lines). Control cells stained with an isotype control antibody are also shown (gray-tinted histograms). (B) Macrophages, transfected with CD163 siRNA, control siRNA, or untransfected control, were infected after 48 h with HIV-BaL. RNA was collected at indicated time-points and assayed for HIV-GAG by real-time RT-PCR. Average results of three independent experiments are presented as mean ± sd. *P < 0.05, and **P < 0.01; n.s., not significant by Student's t-test.
Hb-Hp complexes inhibit HIV infection in macrophages
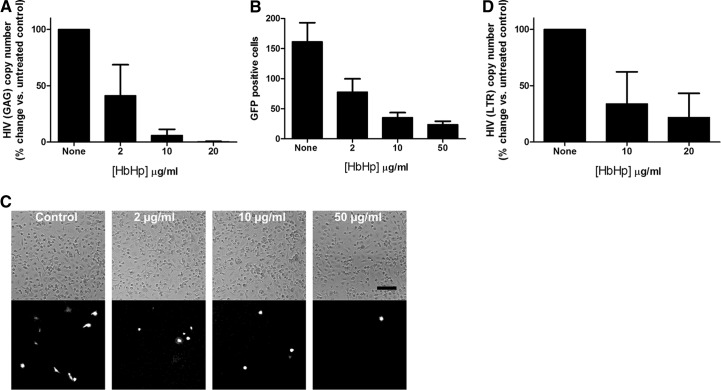
As CD163 is the prototypic scavenger receptor for Hb-Hp complexes, we determined whether HIV infection is influenced by the presence of these complexes in culture media. For this set of experiments, we used nontagged HIV-BaL virus (Fig. 5A) and the HIV-GFP-tagged virus pSF162R3 Nef+ [32], developed from the HIV-SF162 strain (Fig. 5B and C). We have found that Hb-Hp complexes have an inhibitory effect on the infection with BaL-HIV, as detected by real-time RT-PCR (Fig. 5A). We have also found that Hb-Hp complexes caused a concentration-dependent decrease in the number of HIV pSF162R3 Nef+-infected cells, counted as GFP-positive cells (Fig. 5B and C). Furthermore, treatment of MDMs with Hb-Hp complexes reduced early stages of HIV infection, measured 48 h after infection, although to lesser extent than production of viral RNA, measured 7 days postinfection (Fig. 5D).
Figure 5. Effect of Hb-Hp complexes on HIV infection in macrophages.
Hb and Hp were added to macrophages in a 1:1-M ratio and incubated for 2 h at 37°C. (A) Macrophages, treated with 0, 2, 10, or 20 μg/ml Hb-Hp complex and infected with HIV-BaL, were assayed 7 days postinfection for HIV-GAG by real-time RT-PCR. (B) Macrophages, treated with 0, 2, 10, or 50 μg/ml Hb-Hp complex and infected with HIV-GFP-tagged reported virus, were assayed 7 days postinfection. The average number of green fluorescent spots counted in each well is shown on the y-axis. (C) Representative phase-contrast (upper) and fluorescence (lower) micrographs of macrophages, treated with 0, 2, 10, or 50 μg/ml Hb-Hp complex and infected with HIV-GFP-tagged reported virus, 7 days postinfection. Bar, 100 μm. (D) Macrophages were treated with 0, 10, or 20 μg/ml Hb-Hp complex and infected with HIV-BaL, 1 hr later. DNA was extracted 2 days postinfection for HIV LTR assay by real-time RT-PCR. (A, B, and D) Mean values ± sd, obtained from three independent experiments, are presented.
CD163 was described initially as a receptor responsible for binding and internalizing Hb-Hp complexes through endocytosis. More recently, it has been found that CD163 is also a binding target for ASFV [38] and for PRRSV [39]. Although CD163 seems to mediate ASFV entry effectively, it has been suggested that PRRSV and CD163 do not interact at the cell surface but only intracellularly in early endosomes where CD163 facilitates PRRSV uncoating. CD163 can also mediate cytokine secretion in response to bacteria or to initiate apoptosis via TNF-like weak inducer of apoptosis [39].
It has been shown that SP enhances HIV infection in MDMs, and this effect may be mediated by an enhanced expression of the HIV coreceptor CCR5 [7, 11] or by the activation of the HIV LTR promoter [7]. However, none of the mechanisms proposed until now completely explain the effect of SP, and it is more likely that multiple mechanisms are concurrently implicated in this phenomenon. In the present study, we examined the effect of membrane-bound CD163 on HIV infection of macrophages, which contributes to the promoting effect of SP on HIV infection. This is consistent with the elevated levels of CD163 in HIV-positive individuals [25–27]. Our findings suggest that blocking SP stimulation or reducing the levels of CD163 expression result in reducing overall HIV infection.
This is the first study showing that high concentrations of SP (10 μM) induce intracellular calcium mobilization in monocytes. Previous work had only shown that low concentrations of SP potentiated CCR5-mediated calcium mobilization [37]. We determined that SP stimulation of monocytes leads to intracellular calcium increase through activation of NK1R. Previous work from our lab showed that SP triggers an intracellular calcium increase in HEK293 cells transfected with the NK1R receptor [3] and also in U373MG cells, which endogenously express the receptor [40], but SP (1 μM) failed to trigger a calcium increase in HEK293 cells expressing the truncated isoform of NK1R, consistent with the view that SP has much lower affinity for the truncated NK1R than for the full-length receptor.
Although plasma concentrations of SP are in the picomolar range, it is conceivable that in certain microenvironments (e.g., in the brain, where SP is produced in large amounts in neurons), SP can reach much higher concentrations. To demonstrate further that NK1R mediates a SP-induced calcium increase in monocytes, we showed that treatment with aprepitant, an NK1R-selective antagonist, abolished the SP-stimulated calcium response.
Aprepitant has been shown to block full-length and truncated NK1R effectively [37]. Although NK2R and NK3R can be activated by large concentrations of SP, we have shown that NK2R and NK3R agonists NKA and NKB, respectively, did not elicit any change in intracellular calcium. Thus, our findings strongly suggest that monocytes express a functional truncated NK1R that triggers an intracellular calcium increase in response to high concentrations of SP.
Freshly isolated monocytes express relatively high, homogenous levels of membrane-bound CD163. However, we found that the levels of CD163 vary in mature macrophages cultured under standard conditions in the presence of FBS. In experiments on sorted CD163high and CD163low macrophages, we found that CD163high monocytes expressed levels of HIV-GAG significantly higher after 10 and 12 days postinfection with HIV, suggesting that the presence of high levels of CD163 on macrophages facilitates HIV infection.
Simply classifying the monocytes into two groups of high and low expression of CD163 and measuring a difference in HIV infectivity does not prove that the expression of CD163 is the cause of the difference. To demonstrate the hypothesis clearly—that membrane-bound CD163 plays a role in facilitating HIV infection—we knocked down the expression of CD163 using siRNA. The finding that the infection of cells treated with siRNA-targeting CD163 was significantly lower compared with cells treated with control siRNA strongly supports our hypothesis. Thus, the fact that SP induces enhanced HIV infection of macrophages can be explained by increased CD163 expression.
We have also found that HIV infection is significantly attenuated in the presence of Hb-Hp complexes. There are several explanations for this finding. It is possible that CD163 facilitates HIV entry, and the binding of HIV to the CD163 molecule is competitively inhibited in the presence of Hb-Hp complexes. However, we cannot rule out an alternative mechanism that can explain our finding based on the intracellular signaling capability of CD163. Thus, it is also possible that during the internalization of Hb-Hp bound to CD163, some of the molecules that are critical for HIV entry, such as CCR5 or CD4, are also internalized, hence rendering cells less susceptible to HIV infection. It is also possible that Hb-Hp complexes bound to CD163 hinder the binding of HIV to CD4 and CCR5, thus inhibiting viral entry.
In conclusion, our study brings solid evidence that SP enhances the expression of membrane-bound CD163 in monocytes, thus shifting the differentiation of this cell type toward a macrophage phenotype that is more susceptible to HIV infection. We clearly show using multiple approaches that high levels of CD163 are associated with enhanced HIV infection of macrophages. Thus, this mechanism is likely to be responsible, at least in part, for the facilitating effect of SP on HIV infection. Additional studies, which are beyond the scope of this report, will be required to pinpoint specifically the molecular mechanisms that explain the role of CD163 in enhancing HIV infection. Furthermore, it will also be important to demonstrate that the phenomenon that we observed in MDMs ex vivo accurately reflects the mechanism of infection of macrophages in vivo.
The current study raises awareness of a possible role of CD163 in the maintenance of HIV reservoirs in the CNS, where HIV-infected, CD163-positive macrophages may be exposed to high concentrations of SP. Targeting CD163 for the development of novel HIV therapies aimed at depleting HIV reservoirs in the brain and for HIV-associated neurocognitive disorders may become a novel therapeutic strategy in the future.
ACKNOWLEDGMENTS
This work was supported by U.S. National Institutes of Health Grants U01 MH 090325, R01-MH 049981, and R21 AI 108298 (to S.D.D.) and by the Penn Center for AIDS Research (CFAR) at the University of Pennsylvania (U.S. National Institutes of Health Grant P30 AI45008).
We thank Dr. Amanda Brown (The Johns Hopkins University School of Medicine) for kindly providing the HIV-GFP-tagged reporter virus.
Footnotes
- ASFV
- African swine fever virus
- HEK293
- human embryonic kidney 293
- Hp
- haptoglobin
- MFI
- median fluorescence intensity
- Nef
- negative regulatory factor
- NK1/2/3R
- neurokinin-1/2/3 receptor
- NKA/B
- neurokinin A/B
- PRRSV
- porcine reproductive and respiratory syndrome virus
- qPCR
- quantitative PCR
- RNAi
- RNA interference
- sCD163
- soluble CD163
- siRNA
- small interfering RNA
- SP
- substance P
AUTHORSHIP
All authors participated in the design of experiments, interpretation of data, and writing of the manuscript. F.T., J.M., and S.S. performed the experiments and analyzed the data.
REFERENCES
- 1. Severini C., Improta G., Falconieri-Erspamer G., Salvadori S., Erspamer V. (2002) The tachykinin peptide family. Pharmacol. Rev. 54, 285–322 [DOI] [PubMed] [Google Scholar]
- 2. Lai J. P., Ho W. Z., Kilpatrick L. E., Wang X., Tuluc F., Korchak H. M., Douglas S. D. (2006) Full-length and truncated neurokinin-1 receptor expression and function during monocyte/macrophage differentiation. Proc. Natl. Acad. Sci. USA 103, 7771–7776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lai J. P., Lai S., Tuluc F., Tansky M. F., Kilpatrick L. E., Leeman S. E., Douglas S. D. (2008) Differences in the length of the carboxyl terminus mediate functional properties of neurokinin-1 receptor. Proc. Natl. Acad. Sci. USA 105, 12605–12610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fong T. M., Anderson S. A., Yu H., Huang R. R., Strader C. D. (1992) Differential activation of intracellular effector by two isoforms of human neurokinin-1 receptor. Mol. Pharmacol. 41, 24–30 [PubMed] [Google Scholar]
- 5. Douglas S. D., Ho W. Z., Gettes D. R., Cnaan A., Zhao H., Leserman J., Petitto J. M., Golden R. N., Evans D. L. (2001) Elevated substance P levels in HIV-infected men. AIDS (London, England) 15, 2043–2045 [DOI] [PubMed] [Google Scholar]
- 6. Ho W. Z., Douglas S. D. (2004) Substance P and neurokinin-1 receptor modulation of HIV. J. Neuroimmunol. 157, 48–55 [DOI] [PubMed] [Google Scholar]
- 7. Lai J. P., Ho W. Z., Zhan G. X., Yi Y., Collman R. G., Douglas S. D. (2001) Substance P antagonist (CP-96,345) inhibits HIV-1 replication in human mononuclear phagocytes. Proc. Natl. Acad. Sci. USA 98, 3970–3975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wang X., Douglas S. D., Lai J. P., Tuluc F., Tebas P., Ho W. Z. (2007) Neurokinin-1 receptor antagonist (aprepitant) inhibits drug-resistant HIV-1 infection of macrophages in vitro. J. Neuroimmune Pharmacol. 2, 42–48 [DOI] [PubMed] [Google Scholar]
- 9. Douglas S. D., Cnaan A., Lynch K. G., Benton T., Zhao H., Gettes D. R., Evans D. L. (2008) Elevated substance P levels in HIV-infected women in comparison to HIV-negative women. AIDS Res. Hum. Retroviruses 24, 375–378 [DOI] [PubMed] [Google Scholar]
- 10. Douglas S. D., Leeman S. E. (2011) Neurokinin-1 receptor: functional significance in the immune system in reference to selected infections and inflammation. Ann. N. Y. Acad. Sci. 1217, 83–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Manak M. M., Moshkoff D. A., Nguyen L. T., Meshki J., Tebas P., Tuluc F., Douglas S. D. (2010) Anti-HIV-1 activity of the neurokinin-1 receptor antagonist aprepitant and synergistic interactions with other antiretrovirals. AIDS (London, England) 24, 2789–2796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Burdo T. H., Lentz M. R., Autissier P., Krishnan A., Halpern E., Letendre S., Rosenberg E. S., Ellis R. J., Williams K. C. (2011) Soluble CD163 made by monocyte/macrophages is a novel marker of HIV activity in early and chronic infection prior to and after anti-retroviral therapy. J. Infect. Dis. 204, 154–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tippett E., Cheng W. J., Westhorpe C., Cameron P. U., Brew B. J., Lewin S. R., Jaworowski A., Crowe S. M. (2011) Differential expression of CD163 on monocyte subsets in healthy and HIV-1 infected individuals. PloS One 6, e19968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hearps A. C., Maisa A., Cheng W. J., Angelovich T. A., Lichtfuss G. F., Palmer C. S., Landay A. L., Jaworowski A., Crowe S. M. (2012) HIV infection induces age-related changes to monocytes and innate immune activation in young men that persist despite combination antiretroviral therapy. AIDS (London, England) 26, 843–853 [DOI] [PubMed] [Google Scholar]
- 15. Burdo T. H., Lo J., Abbara S., Wei J., DeLelys M. E., Preffer F., Rosenberg E. S., Williams K. C., Grinspoon S. (2011) Soluble CD163, a novel marker of activated macrophages, is elevated and associated with noncalcified coronary plaque in HIV-infected patients. J. Infect. Dis. 204, 1227–1236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Subramanian S., Tawakol A., Burdo T. H., Abbara S., Wei J., Vijayakumar J., Corsini E., Abdelbaky A., Zanni M. V., Hoffmann U., Williams K. C., Lo J., Grinspoon S. K. (2012) Arterial inflammation in patients with HIV. JAMA 308, 379–386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Knudsen T. B., Gustafson P., Kronborg G., Kristiansen T. B., Moestrup S. K., Nielsen J. O., Gomes V., Aaby P., Lisse I., Moller H. J., Eugen-Olsen J. (2005) Predictive value of soluble haemoglobin scavenger receptor CD163 serum levels for survival in verified tuberculosis patients. Clin. Microbiol. Infect. 11, 730–735 [DOI] [PubMed] [Google Scholar]
- 18. Gordon S. (2001) Homeostasis: a scavenger receptor for haemoglobin. Curr. Biol. 11, R399–R401 [DOI] [PubMed] [Google Scholar]
- 19. Graversen J. H., Madsen M., Moestrup S. K. (2002) CD163: a signal receptor scavenging haptoglobin-hemoglobin complexes from plasma. Int. J. Biochem. Cell. Biol. 34, 309–314 [DOI] [PubMed] [Google Scholar]
- 20. Kristiansen M., Graversen J. H., Jacobsen C., Sonne O., Hoffman H. J., Law S. K., Moestrup S. K. (2001) Identification of the haemoglobin scavenger receptor. Nature 409, 198–201 [DOI] [PubMed] [Google Scholar]
- 21. Moller H. J., Peterslund N. A., Graversen J. H., Moestrup S. K. (2002) Identification of the hemoglobin scavenger receptor/CD163 as a natural soluble protein in plasma. Blood 99, 378–380 [DOI] [PubMed] [Google Scholar]
- 22. Moreno J. A., Ortega-Gomez A., Delbosc S., Beaufort N., Sorbets E., Louedec L., Esposito-Farese M., Tubach F., Nicoletti A., Steg P. G., Michel J. B., Feldman L., Meilhac O. (2012) In vitro and in vivo evidence for the role of elastase shedding of CD163 in human atherothrombosis. Eur. Heart J. 33, 252–263 [DOI] [PubMed] [Google Scholar]
- 23. Hogger P., Dreier J., Droste A., Buck F., Sorg C. (1998) Identification of the integral membrane protein RM3/1 on human monocytes as a glucocorticoid-inducible member of the scavenger receptor cysteine-rich family (CD163). J. Immunol. 161, 1883–1890 [PubMed] [Google Scholar]
- 24. Buechler C., Ritter M., Orso E., Langmann T., Klucken J., Schmitz G. (2000) Regulation of scavenger receptor CD163 expression in human monocytes and macrophages by pro- and antiinflammatory stimuli. J. Leukoc. Biol. 67, 97–103 [PubMed] [Google Scholar]
- 25. Fischer-Smith T., Tedaldi E. M., Rappaport J. (2008) CD163/CD16 coexpression by circulating monocytes/macrophages in HIV: potential biomarkers for HIV infection and AIDS progression. AIDS Res. Hum. Retroviruses 24, 417–421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kim W. K., Alvarez X., Fisher J., Bronfin B., Westmoreland S., McLaurin J., Williams K. (2006) CD163 identifies perivascular macrophages in normal and viral encephalitic brains and potential precursors to perivascular macrophages in blood. Am. J. Pathol. 168, 822–834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Soulas C., Conerly C., Kim W. K., Burdo T. H., Alvarez X., Lackner A. A., Williams K. C. (2011) Recently infiltrating MAC387(+) monocytes/macrophages a third macrophage population involved in SIV and HIV encephalitic lesion formation. Am. J. Pathol. 178, 2121–2135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Fischer-Smith T., Bell C., Croul S., Lewis M., Rappaport J. (2008) Monocyte/macrophage trafficking in acquired immunodeficiency syndrome encephalitis: lessons from human and nonhuman primate studies. J. Neurovirol. 14, 318–326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Moller H. J. (2012) Soluble CD163. Scand. J. Clin. Lab. Invest. 72, 1–13 [DOI] [PubMed] [Google Scholar]
- 30. Davis B. H., Zarev P. V. (2005) Human monocyte CD163 expression inversely correlates with soluble CD163 plasma levels. Cytometry 63, 16–22 [DOI] [PubMed] [Google Scholar]
- 31. Li Y., Svehla K., Mathy N. L., Voss G., Mascola J. R., Wyatt R. (2006) Characterization of antibody responses elicited by human immunodeficiency virus type 1 primary isolate trimeric and monomeric envelope glycoproteins in selected adjuvants. J. Virol. 80, 1414–1426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Brown A. M. (2009) Use of a macrophage-tropic GFP-tagged human immunodeficiency virus type 1 (HIV-1) to study viral reservoirs. Methods Mol. Biol. 515, 165–175 [DOI] [PubMed] [Google Scholar]
- 33. Chen P., Douglas S. D., Meshki J., Tuluc F. (2012) Neurokinin 1 receptor mediates membrane blebbing and sheer stress-induced microparticle formation in HEK293 cells. PloS One 7, e45322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Palmer S., Wiegand A. P., Maldarelli F., Bazmi H., Mican J. M., Polis M., Dewar R. L., Planta A., Liu S., Metcalf J. A., Mellors J. W., Coffin J. M. (2003) New real-time reverse transcriptase-initiated PCR assay with single-copy sensitivity for human immunodeficiency virus type 1 RNA in plasma. J. Clin. Microbiol. 41, 4531–4536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Spitsin S., Stevens K. E., Douglas S. D. (2013) Expression of substance P, neurokinin-1 receptor and immune markers in the brains of individuals with HIV-associated neuropathology. J. Neurol. Sci. 334, 18–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Yi Y., Shaheen F., Collman R. G. (2005) Preferential use of CXCR4 by R5X4 human immunodeficiency virus type 1 isolates for infection of primary lymphocytes. J. Virol. 79, 1480–1486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Chernova I., Lai J. P., Li H., Schwartz L., Tuluc F., Korchak H. M., Douglas S. D., Kilpatrick L. E. (2009) Substance P (SP) enhances CCL5-induced chemotaxis and intracellular signaling in human monocytes, which express the truncated neurokinin-1 receptor (NK1R). J. Leukoc. Biol. 85, 154–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sanchez-Torres C., Gomez-Puertas P., Gomez-del-Moral M., Alonso F., Escribano J. M., Ezquerra A., Dominguez J. (2003) Expression of porcine CD163 on monocytes/macrophages correlates with permissiveness to African swine fever infection. Arch. Virol. 148, 2307–2323 [DOI] [PubMed] [Google Scholar]
- 39. Van Gorp H., Van Breedam W., Van Doorsselaere J., Delputte P. L., Nauwynck H. J. (2010) Identification of the CD163 protein domains involved in infection of the porcine reproductive and respiratory syndrome virus. J. Virol. 84, 3101–3105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Meshki J., Douglas S. D., Hu M., Leeman S. E., Tuluc F. (2011) Substance P induces rapid and transient membrane blebbing in U373MG cells in a p21-activated kinase-dependent manner. PloS One 6, e25332. [DOI] [PMC free article] [PubMed] [Google Scholar]