MCs and histamine in survival, proliferation, and gene expression of MDSCs in mouse models of Th2 disease.
Keywords: allergic disease, parasite, Nippostrongylus brasiliensis, cimetidine, cetirizine, Th2
Abstract
It has been shown recently that MCs are required for differential regulation of the immune response by granulocytic versus monocytic MDSCs. Granulocytic MDSCs promoted parasite clearance, whereas monocytic MDSCs enhanced tumor progression; both activities were abrogated in MC-deficient mice. Herein, we demonstrate that the lack of MCs also influences MDSC trafficking. Preferential trafficking to the liver was not seen in MC-deficient mice. In addition, evidence that the MC mediator histamine was important in MDSC trafficking and activation is also shown. MDSCs express HR1–3. Blockade of these receptors by HR1 or HR2 antagonists reversed the histamine enhancement of MDSC survival and proliferation observed in cell culture. In addition, histamine differentially influenced Arg1 and iNOS gene expression in MDSCs and greatly enhanced IL-4 and IL-13 message, especially in granulocytic MDSCs. Evidence that histamine influenced activity seen in vitro translated to in vivo when HR1 and HR2 antagonists blocked the effect of MDSCs on parasite expulsion and tumor metastasis. All of these data support the MDSC-mediated promotion of Th2 immunity, leading to the suggestion that allergic-prone individuals would have elevated MDSC levels. This was directly demonstrated by looking at the relative MDSC levels in allergic versus control patients. Monocytic MDSCs trended higher, whereas granulocytic MDSCs were increased significantly in allergic patients. Taken together, our studies indicate that MCs and MC-released histamine are critical for MDSC-mediated immune regulation, and this interaction should be taken into consideration for therapeutic interventions that target MDSCs.
Introduction
Acute inflammatory reactions stimulate the development and recruitment of cells of myeloid lineage. Unresolved chronic inflammation can induce hematopoietic dysregulation, favoring the accumulation of a heterogeneous population of mononuclear and polymorphonuclear cells, known as MDSCs [1, 2]. The proneoplastic role of MDSCs has been well-established, with MDSCs exerting effects through the release of small soluble oxidizers, the impairment of T cell antigen recognition, and the depletion of essential amino acids from the local extracellular environment, ultimately leading to T cell suppression [3]. MDSC immunosuppression additionally serves a beneficial role in transplantation, parasitic infection, autoimmunity, and sepsis [4–7]. Recent findings are beginning to suggest paradoxical and immune-supportive roles of these cells in disease states, as well as their interaction with other cells of the immune system, such as MCs [8, 9].
MCs are long-lived cells that typically reside in the skin and mucous membranes. MCs are primarily implicated in the pathogenesis of allergic disease and identified as critical regulators of the antiparasitic immune response [10–12]. We reported previously a novel collaboration between MDSCs and MCs using infection with a Th2 helminth, Nb, similar to the human hookworm [9]. Mice infected with Nb and receiving AT MDSCs had enhanced parasitic clearance, largely dependent on granulocytic (Ly6G+) but not monocytic (Ly6C+) MDSCs [9]. Intriguingly, AT of MDSCs into MC-deficient mice did not mediate parasite clearance. This abrogated response in MC-deficient mice stimulated us to elucidate the MC/MDSC collaboration further.
The secretion of chemokines and cytokines by MCs generates a critical cytokine milieu for polarization of the immune system and recruitment of effector cells [13]. Activated MCs secrete histamine, proteases, and lipid-derived mediators. The biogenic amine, histamine, is considered a major mediator released by MCs. Histamine acts on a spectrum of cell types through GPCRs, known as HR1–4. The expression profile of the HRs determines the net effect on cell growth, motility, phenotypic alterations, and signaling mechanisms [14–17]. Through effects on target cells, histamine induces vasodilation, vasopermeability, smooth muscle contraction, and mucus production [18]. Numerous studies have linked excessive histamine production to allergic reactions, infections, and tumor growth [19, 20]. Myeloid cells have been reported to express HRs and HDC, the enzyme required for histamine synthesis [21]. Histamine, in turn, protects myeloid cells against apoptosis and promotes cytokine production. In fact, coculture of monocytes with histamine has been shown to increase IL-10 and inhibit IL-12, thus skewing toward Th2 immunity [15, 22]. Thus, histamine can alter the cytokine milieu, transcription factors, and signaling pathways important for MDSC accumulation [23].
Herein, we show that histamine is a strong contributor to the MC/MDSC interaction. MDSCs express HR1–3 and are sensitive to the proliferation-promoting and prosurvival effects of histamine, which induced differential gene expression in monocytic and granulocytic MDSCs. The enhanced anti-Nb immunity, induced by AT of MDSCs, is abrogated with histamine blockade in WT mice. In addition, MDSCs traffic to the liver during Nb infection. This hepatic trafficking is reduced in MC-deficient mice. Finally, symptomatic allergic patients showed increased MDSC levels in circulation. These studies indicate that histamine is a potential mediator, by which MCs regulate the survival and activity of MDSCs, and is critical for MDSC-mediated immune regulation. This interaction should be taken into consideration for disorders involving elevated levels of histamine or therapeutic interventions targeting MDSCs.
MATERIALS AND METHODS
Mice
Mice were maintained at the Virginia Commonwealth University Animal Facility in accordance with guidelines by the U.S. National Institutes of Health and American Association for the Accreditation of Laboratory Animal Care. C57BL/6 A10Tgs were generated as described previously [24]. C57BL/6 WT mice were purchased from The Jackson Laboratory (Bar Harbor, ME, USA). KitWsh/Wsh mice were progeny of breeding pairs purchased from The Jackson Laboratory. Cpa3cre; Mcl-1fl/fl mice [25] (C57BL/6 background) were generously provided by Dr. Stephen Galli (Stanford University, Stanford, CA, USA). All mouse protocols were approved by the Virginia Commonwealth University Institutional Animal Care and Use Committee.
Cell culture
BMMCs were derived from femurs of WT mice and cultured in cRPMI 1640 containing 10% FBS, 2 mM L-glutamine, 100 U/mL penicillin, 100 μg/mL streptomycin, 1 mM HEPES (Quality Biological, Gaithersburg, MD, USA), and 1 mM sodium pyruvate (Cellgro, Herndon, VA, USA). Cultures were supplemented with IL-3-containing supernatant from WEHI-3 cells (1 ng/mL) and stem cell factor containing supernatant from BHK-MKL cells (10 ng/mL). Mature BMMCs were used after 28 days of culture. The B16-melanoma cell line was maintained in DMEM with 10% FBS, 1% penicillin, 1% streptomycin, and 1% L-glutamine.
Isolation of MDSCs, AT, and dye labeling
Spleens were harvested from A10Tg mice. They were then dispersed into single-cell suspensions. Erythrocytes were lysed using ammonium-chloride-potassium lysing buffer (Quality Biological). T cells were depleted using CD90.2 magnetic depletion (Miltenyi Biotec, Auburn, CA, USA), according to the manufacturer's protocol. Gr-1+, Ly6G+, or Ly6C+ cells were purified using EasySep PE-selection kit (Stemcell Technologies, Vancouver, BC, Canada), according to the manufacturer's protocol. For AT studies, 10 × 106 MDSCs were injected into tail veins every 3 days. For AT of dye-labeled MDSCs, cells were stained with the PKH26GL dye-linker kit (Sigma-Aldrich, St. Louis, MO, USA), according to the manufacturer's protocol.
Migration assays
B16 (2×105), BMMC, or media alone were loaded into the lower well of 8 μm transwell plates (Corning, Herndon, VA, USA). MDSCs (2×105) were loaded into the upper well. Plates were incubated for 4 h at 37°C, and the lower well was harvested for flow cytometry.
Survival curve and proliferation assays
MDSCs were cultured in cRPMI with 1, 5, 10, or 100 μM histamine (Calbiochem, San Diego, CA, USA). For survival curves, counts were made with trypan blue dead cell exclusion, and cell concentration was 1 × 106/mL in 5 mL. For proliferation, MDSCs were precultured for 15 min with 10μM CT, CIM, or alone, before histamine addition. Cell concentrations were 50,000 cells/well in a 96-well plate. After 48 h, a 24-h pulse of [H3]-thymidine, 1 mCi/mL (Perkin Elmer, Waltham, MA, USA), was used. Plates were harvested using a Filtermate cell harvester onto GF/C plates and read using a TopCount plate counter (Perkin Elmer).
qPCR
MDSCs were isolated, as described previously, and cultured with 100 μM histamine for 24 h. Total RNA was extracted using TRIzol reagent (Invitrogen Life Technologies, Grand Island, NY, USA). RNA (400 ng/μl) was reverse-transcribed using the iScript cDNA synthesis kit (Bio-Rad, Hercules, CA, USA). Primers for running an iQ SYBR Green Supermix (Bio-Rad) qPCR assay were purchased from Integraded DNA Technologies (Coralville, IA, USA). Primers are as listed for Arg1 forward 5′-GAC CAC AGT CTG GCA GTT GG-3′, Arg1 reverse 5′-TGG TTG TCA GGG GAG TGT TG-3′; iNOS forward 5′-CAC CCC AAG TTC GAC TGG TT-3′, iNOS reverse 5′-CTA AAG GGA CAG GCG CTG AA-3′. Probes (Applied Biosystems, Life Technologies, Grand Island, NY, USA) were as follows: IL-13 (Mm00434206), IL-4 (Mm00445259), and GAPDH (Mm99999915). Results were analyzed with Bio-Rad iQ5 Real-Time PCR software (version 2.0). Fold variation was determined using ΔΔCt method of analysis.
Flow cytometry
Cell isolation and labeling were conducted, as described previously [24]. Antibodies included purified rabbit anti-mouse Arg1 (9819S; Cell Signaling Technology, Danvers, MA, USA); anti-HRH2 (M-19), H3 (C-20), and H4 (M-12; Santa Cruz Biotechnology, Dallas, TX, USA); FITC-conjugated anti-mouse Gr-1 (RB6-8C5), PE-conjugated anti-mouse Gr-1 (RB6-8C5), APC-labeled anti-mouse Ly6C (HK1.4), PerCP/Cy5.5 anti-human CD15 (W6D3), PE/Cy7-conjugated anti-mouse CD11b, and anti-human CD33 (WM-53; BioLegend, San Diego, CA, USA); PE/Cy5-conjugated anti-mouse HRH1 (polyclonal; Bioss, Woburn, MA, USA); APC anti-human CD11b (ICRF44) and PE anti-human CD14 (61D3; eBioscience, San Diego, CA, USA); and anti-mouse Ly6G-FITC (1A8; BD Biosciences, San Jose, CA, USA). Secondary antibodies included donkey anti-goat IgG-FITC (Santa Cruz Biotechnology) and Dylight 649 donkey anti-rabbit IgG (Poly 4064; BioLegend). Flow cytometric analysis was performed using FACSCanto (BD Biosciences). Data analysis was conducted using FlowJo v7.6.5.
Nb infection, B16 melanoma, and CIM or CT administration
Nb larvae were generously provided by Joe Urban (Agriculture Research Station, Beltsville, MD, USA) and were maintained as described previously [26]. Each experimental group of mice was infected with ∼650 L3 Nb. Fecal egg burdens were enumerated on Days 5–13, as described [26]. Mice were killed on Day 7 or 14 postinfection. For B16 melanoma, mice were injected i.v. with 250,000 cells, monitored daily, and killed after 3 weeks; lungs were harvested and weighed to determine degree of colonization. For mice treated with CT, 0.5 mg/kg was injected i.p., starting on Day −1 and repeated daily. For mice injected with CIM, 20 mg/kg was injected i.p., starting on Day −1 and repeated every 2 days.
Human MDSCs
Human studies were conducted under appropriate Institutional Review Board-approved protocols. All patients gave informed consent for this research. Blood (20 mL) was collected in ETDA-coated vacuum tubes from allergic patients, identified as symptomatic or nonallergic controls, and PBL were isolated using Ficoll-Paque separation medium (GE Healthcare, Buckinghamshire, UK).
Statistical analysis
P values were calculated using unpaired two-tailed Student's t-tests in GraphPad Prism v5. Error bars represent the sd between samples. P < 0.05 is considered statistically significant.
RESULTS
MCs promote MDSC trafficking and parasite clearance
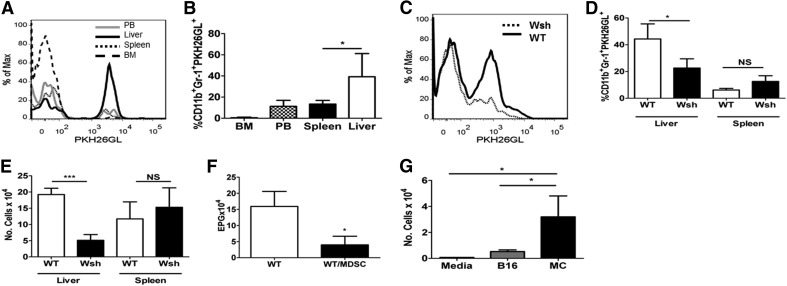
Given the literature highlighting MCs as attractants of MDSCs and the importance of MDSC recruitment in an immune response, the trafficking behavior of MDSCs was analyzed after AT in the presence or absence of MCs. MDSCs were labeled with the PKH26GL lipid dye and AT by i.v. injection into naive mice. As shown in Fig. 1A and B, 18 h post-AT, MDSCs preferentially trafficked to the liver; this agrees with other published results [27]. To determine whether MDSCs exhibit similar trafficking patterns during the course of Nb infection, MDSCs were labeled and infused concomitant with Nb challenge in WT and MC-deficient KitWsh/Wsh mice. As with naive mice, MDSCs trafficked to liver in infected WT mice. However, the accumulation of MDSCs in the liver was reduced significantly in KitWsh/Wsh mice, indicating the role of MCs in MDSC recruitment (Fig. 1C–E). The PKH26GL dye used for labeling does not affect MDSC functionality, as labeled cells were fully capable of reducing parasite egg burden in the feces (Fig. 1F). These results were supported by in vitro migration assays, in which MDSCs exhibited a high degree of migration toward MCs (Fig. 1G). This is consistent with published literature, indicating that MDSCs traffic to the liver in tumor models and that MCs secrete mediators from the liver through the bile [28].
Figure 1. MDSCs preferentially migrate to the liver in a MC-dependent manner.
(A) WT mice given labeled MDSCs, analyzed 18 h later. MDSC in liver, peripheral blood (PB), spleen, and bone marrow (BM). Data compiled in B. (C) Wsh or WT was given labeled MDSCs, infected with Nb, and examined for MDSC staining on Day 7. Cell percentage of PKH26GL+ cells out of total Gr1+CD11b+cells (D) and number (E) from C. (F) Eggs/gm feces (EPG) determined on Day 7. MDSCs given Days −1, 2, and 5. (G) Four-hour MDSC migration in response to B16 melanoma, MCs, or media alone. *P < 0.05; ***P < 0.0005. Mean ± sd; n ≥ 5/group.
To confirm that this was not unique to KitWsh/Wsh mice, which have other hematopoietic issues, two studies were done. KitWsh/Wsh mice, which were reconstituted with WT MCs, responded to AT of MDSC with enhanced parasite clearance (Supplemental Figure 1A). In addition, another strain of MC-deficient mice, Cpa3cre; Mcl-1fl/fl (C57BL/6 background) was tested. These mice express Cre recombinase under the control of the Cpa3 promoter. C57BL/6-Cpa3cre; Mcl-1fl/fl mice are severely deficient in MCs and basophils with no other apparent hematologic changes [25]. Similar to KitWsh/Wsh mice, AT of MDSCs also failed to clear Nb in the Cpa3cre; Mcl-1fl/fl mice (Supplemental Fig. 1B). These studies further confirm that MCs are required for MDSC-mediated parasite clearance.
We next wanted to see if MDSC accumulation and migration to the liver are MC-dependent during tumor development. A model of B16 melanoma metastasis was used. In B16 melanoma, WT lung colonization is exacerbated with the AT of MDSCs. While in Cpa3cre; Mcl-1fl/fl MC-deficient mice, AT of MDSCs did not cause increased lung colonization, confirming previous data published with the KitWsh/Wsh mice (Supplemental Fig. 1C) [9]. Additionally, MDSCs are reduced in liver but not in spleen over WT mice, with or without AT of MDSCs (Supplemental Fig. 1D).
MDSCs express HR1 and HR2
Given the contribution of histamine to MDSC activity, cell proliferation, and Th2-skewed immune responses [15, 21], we examined whether histamine could serve as a potential mediator in the MC/MDSC cross-communication. Liver MDSCs were examined, as they were the population most affected in MC-deficient mice. They express HR1–3 but not 4 (Supplemental Fig. 2A). Splenic MDSCs express high levels of HR1, as shown in Supplemental Fig. 2B. Additionally, purified MDSCs express detectable HR2 and 3 but not 4 (Supplemental Fig. 2C).
Histamine induces MDSC proliferation and differential gene expression in monocytic versus granulocytic MDSCs
As shown in Fig. 2A and B, in vitro exposure to histamine promoted survival and proliferation of MDSCs in a dose-dependent manner. The contribution of histamine was confirmed using CT, CIM, HR1, and HR2 antagonists, respectively. As shown in Fig. 2C, CT and CIM inhibited histamine-stimulated cell proliferation. Both subtypes of MDSCs are responsive to histamine; however, the monocytic subset is significantly more sensitive to the proliferative effects of histamine than are granulocytic MDSCs (Fig. 2D).
Figure 2. Histamine increases MDSC survival and proliferation.
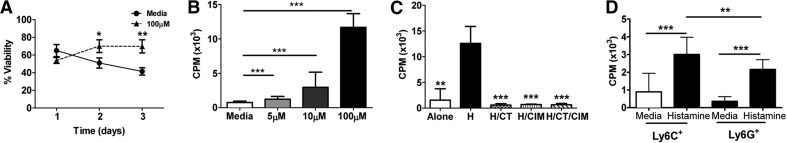
(A) Survival assessed by trypan blue exclusion over time course of MDSCs alone (circle) or with histamine (triangle). (B and C) Proliferation assays of MDSCs cultured alone, with histamine (H), or in combination with indicated agents, as measured by counts per minute (CPM). (D) MDSCs were cultured with histamine or media. *P < 0.05; **P < 0.005; ***P < 0.0005. Mean ± sd; n ≥ 10/group and at least two independent experiments.
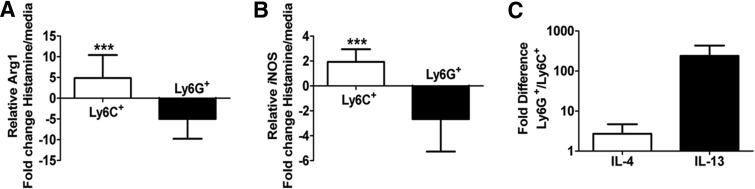
Given the ability of histamine to induce MDSC proliferation in vitro, a logical step was to examine its role in modulating markers of MDSC activity. Accordingly, monocytic or granulocytic MDSCs were cultured with 100 μM histamine, as in Fig. 2A, and analyzed for expression of Arg1 and iNOS via qPCR. It has been documented that MDSC immunosuppression largely results from impairment of T cell activity modulated by these enzymes [1, 29]. In the presence of histamine, we observed a fivefold increase (ΔΔCt method) [30] in the expression of Arg1 and a twofold increase in the expression of iNOS in monocytic MDSCs over granulocytic MDSCs. However, the granulocytic MDSCs exhibit a fivefold decrease in the expression of Arg1 and a threefold decrease in the expression of iNOS, exhibiting striking differential gene expression between the two populations (Fig. 3A and B).These data are reinforced by intracellular flow cytometry, showing increased protein expression of Arg1 by monocytic MDSCs when exposed to histamine in vitro but unchanged expression by the granulocytic MDSC (Supplemental Fig. 3A and B).
Figure 3. Histamine influences MDSC gene expression.
Ly6C+ or Ly6G+ MDSCs were cultured ± histamine and analyzed (48 h) for Arg1 (A) or iNOS (B) message. Fold change of histamine-treated/media alone (A and B). Fold change of IL-13 and IL-4 message from histamine-treated Ly6G+/Ly6C+ cells (C). After normalization to GAPDH, data were analyzed using the ΔΔCt method.***P < 0.0005. Mean ± sd; n ≥ 5 from two experiments.
Arg1 and iNOS are essential for classical MDSC-mediated T cell suppression, whereas IL-13 and IL-4 are important Th2 cytokines involved in Nb clearance. With addition of histamine, granulocytic MDSCs—the population important for augmented parasitic clearance—increased IL-4 expression fourfold and IL-13 expression over 200-fold compared with monocytic MDSCs (Fig 3C).
Histamine antagonist prevents MDSC-mediated Nb expulsion and tumor progression
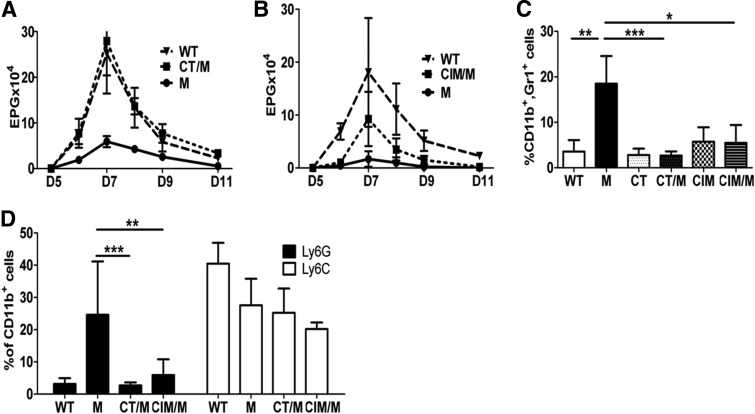
To confirm the effects of histamine on MDSC activity in vivo, mice were infected with 650 L3 Nb, a Th2 helminth. MDSCs were AT i.v. in conjunction with CT, CIM, HR1, or HR2 antagonists, respectively. Administration of CT or CIM significantly abrogated the ability of MDSCs to increase the clearance of Nb (Fig. 4A and B). This was correlated with reduced-infiltrating MDSCs in the liver (Fig. 4C) and diminished phenotypic conversion toward granulocytic MDSCs (Fig. 4D), which is the important phenotype for Nb clearance [9].
Figure 4. HR1 and HR2 antagonists block MDSC-mediated Nb clearance.
(A and B) Eggs/gm feces in Nb-infected ± MDSCs (M) on indicated days (D) and ± daily CT treatment (A) or every other day CIM treatment (B). (C) Day 14 analysis of total MDSCs. (D) Flow cytometric determination of liver monocytic or granulocytic populations. *P < 0.05; **P < 0.005; ***P < 0.0005. Mean ± sd; n ≥ 5 with two independent experiments.
As histamine induces increased iNOS and Arg1 message (Fig 3A and B), both important for MDSC-induced T cell suppression, we analyzed whether histamine blockade affected MDSC-induced tumor progression. A mouse model of melanoma was used (B16). This model does not naturally accumulate large numbers of MDSCs, so AT of MDSCs was used to increase MDSC numbers. Mice receiving AT with MDSCs and treated with CIM had significantly reduced colonization to the lungs with B16 melanoma compared with mice that received MDSCs but no CIM, as determined by lung weight (Supplemental Fig. 4). CIM alone had no significant effect on tumor burden in the absence of AT of MDSCs. This is consistent with data showing similar results using a model of murine Lewis lung carcinoma, a model with natural accumulation of MDSCs, where CIM treatment resulted in reduced tumor size [31].
MDSCs are increased in allergic patients
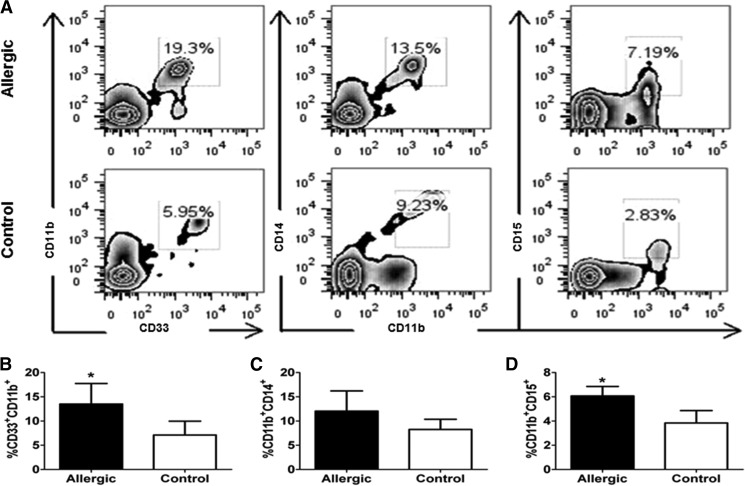
Allergy is a MC-mediated, Th2-dependent immune response. Allergic patients experiencing symptoms have increased levels of circulating MC-derived histamine [32]. Additionally, Deshane et al. [33] describes MDSCs as being involved in Th2-driven airway hyper-responsiveness. To elucidate the translational relevance of our findings, we isolated PBL from patients currently experiencing allergy symptoms and nonallergic controls, as determined by a patient questionnaire. Patients that reported taking antihistamines were excluded from the study. PBL was examined by flow cytometry; human MDSCs are defined as CD34−CD33+CD11b+ cells [34]. Monocytic MDSCs are defined as CD34−CD11b+CD14+ and granulocytic MDSCs as CD34−CD11b+CD15+ [34]. Allergic patients were found to exhibit >40% more circulating MDSCs than controls (Fig. 5A and B). Interestingly, the monocytic subset of MDSCs appeared to be the dominant subpopulation in the controls but were not elevated significantly in allergic patients (Fig. 5C). However, the granulocytic MDSCs, which are the population implicated to be more important in Th2 responses, such as helminth immunity, were increased significantly with >20% more in the allergic patients compared with controls (Fig. 5D).
Figure 5. Allergic patients have increased circulating MDSCs.
Symptomatic allergic patients were compared with nonallergic controls. Cells were isolated from peripheral blood gating strategy, presented in Supplemental Fig. 5. Percent total MDSCs (B) and subpopulations, CD14+ (C) or CD15+ (D), were determined by flow cytometry. (A) Representative allergic patient versus control and (B–D) compiled.*P < 0.05. Mean ± sd. Allergic patients, n = 6; controls, n = 6.
DISCUSSION
We previously reported a mouse model, in which overexpression of a disintegrin and metalloprotease 10 results in the accumulation of MDSCs in a tumor-free environment. These A10Tg mice provide a novel and consistent tool for studying MDSCs [24]. Both subpopulations of A10Tg MDSCs are functionally and phenotypically analogous to tumor-derived MDSCs. Thus, this model provides the flexibility to address the activity of each subset of MDSCs outside of neoplasia as well as their interactions with other cells of the immune system. With the use of this valuable tool, we have demonstrated that the monocytic and granulocytic populations of MDSCs function differently depending on the disease state, and that antiparasitic and tumor growth-promoting activities require the presence of MCs [9].
Here, we further examine the importance and mechanism of the MC/MDSC interaction. The use of AT of labeled MDSCs allowed examination of their trafficking pattern and migration during parasitic infection. Previous literature indicates that during tumor progression, MDSCs traffic and accumulate in the liver in addition to the tumor site, where they inhibit Kupffer cells and T cells to dampen anti-tumor immune responses [27, 35, 36]. Similarly, in our Nb infection model, MDSCs preferentially migrated to the liver (Fig. 1A and B). Previous work has shown that liver-accumulated MDSCs produce an array of proinflammatory and regulatory cytokines and chemokines in response to tumor challenge [27]. This would argue that the liver is a critical site for MDSC function. In models of MC deficiency, where MDSCs do not traffic to the liver, MDSCs do not function as efficiently [9]. It has been reported that hepatic MCs release histamine and other mediators through the portal bile ducts and into the bile [28]. Therefore, we hypothesized that MDSCs may interact with hepatic MCs in the liver and become further activated. The attraction between these two cells may explain that in the absence of MCs, MDSC migration to the liver and parasite expulsion are reduced significantly (Fig. 1F).
MCs and histamine have been shown to play an important role in parasitic clearance. Increased numbers of MCs and concentrations of histamine are positively correlated with natural helminth resistance [37]. The role of histamine in parasitic infection has been ascribed to smooth muscle contraction to enhance the “sweep” portion of the “weep and sweep” method of parasitic clearance, acting in conjunction with IL-13/IL-4 [38, 39]. Taken in conjunction with the positive correlation of histamine levels and parasitic resistance, this could imply that natural immunity in these animals may not only be a result of the effect of histamine on smooth muscle cells but also effects via MDSC activation and proliferation. Whereas potentially, this could be tested using mice deficient in HDC, the secondary increase in goblet cells seen in these mice would be a complicating issue [40]. To our knowledge, the response to helminth infection has not been reported in these mice.
The addition of histamine to MDSCs protected against cell death and increased cellular proliferation in vitro (Fig. 2A and B). Furthermore, histamine enhanced expression of T cell suppression markers Arg1 and iNOS in monocytic MDSCs and decreased expression of these markers in granulocytic MDSCs (Fig. 3A and B). As a result of these findings, T cell suppression by monocytic MDSCs should be increased after exposure to histamine or culture with MCs. These studies are currently underway. The granulocytic MDSCs increase IL-13 and IL-4 expression over monocytic MDSCs when exposed to histamine. IL-13 is essential in Nb infection for worm expulsion responsible for increased mucus production and enteric nerve stimulation [41, 42]. This expression reinforces the differential roles that both subsets of MDSCs play [9, 43]. These studies are supported by a recent publication, in which histamine blockade with CIM increased MDSC apoptosis, reduced Arg1 and iNOS enzyme expression, and decreased MDSC accumulation in a mouse model of LLC [31].
These results indicate that MCs enhance site-specific migration and as a result of histamine secretion, induce increased MDSC proliferation and differential gene expression. We hypothesize that in liver, as well as other sites of immune response, resident MCs attract MDSCs and activate them via histamine release. This affords the production of soluble mediators that can act locally and distally by secretion into the bile in our parasitic model. Given our findings and other published studies on MDSCs, the following model is suggested (Fig. 6). MDSCs secrete Th2 cytokines, notably IL-4 and IL-13, which promote Th2 differentiation, along with IL-6 and TGF-β that chemoattract additional MCs. The T cells not only contribute to the pool of IL-4 and IL-13 but also increase IgE synthesis. IgE, in turn, further activates MCs. These events afford a self-sustaining and synergistic cycle of MC/MDSC activation, resulting in increased survival and proliferation/activation of MDSCs, in addition to a Th2-skewed immune response. This Th2-skewed immune response is detrimental in the setting of allergic disease or neoplasia but beneficial for parasite clearance.
Figure 6. Model of MDSC/MC interaction.
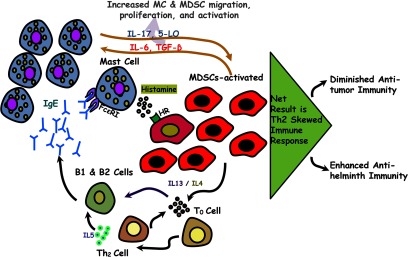
MCs are required for MDSC activity. MCs release mediators, such as histamine, that induce MDSC activation, proliferation, and Th2 cytokine production. This enhanced cytokine production culminates in Th2-skewed immune responses that promote allergy and parasitic clearance and diminish anti-tumor responses.
The importance of the histamine/MDSC interaction was demonstrated dramatically in that histamine blockade, with HR1 and HR2 antagonists, reversed in vitro the proliferative effects of histamine (Fig. 2C) and reversed in vivo MDSC-mediated clearance of parasites, similar to the deficit in MDSC functions found in MC-deficient mouse models (Fig. 4A and D). Impressively, HR2 blockade also decreased lung tumor burden in a B16 melanoma model (Supplemental Fig. 4) and other tumor models [31]. In addition to having the same effect on parasite clearance, HR1 and HR2 blockade decreased liver MDSC accumulation significantly (Fig. 4B). This suggests that HR1 and HR2 have overlapping roles in the MDSC/MC interaction and when blocked, result in reduced total MDSC function. The HR1 antagonist, CT, has been shown previously to affect migration of other cells of myeloid lineage, such as monocytes, in vitro and could be responsible for the reduced trafficking when the drug is used [44, 45]. HR2 antagonists have been shown to affect production in T cells of IL-13, IL-4, and IL-5—cytokines important in Nb infection [41, 42]. In this manuscript, we show that granulocytic MDSCs are producing IL-4 and IL-13 message after histamine addition (Fig. 3C), and this finding suggests that CIM may work by reducing MDSC IL-4 and IL-13 production. Whereas the MC/MDSC interaction is multifaceted, we propose that MC-derived histamine drives distinct MDSC subpopulations to differential gene expression, inducing phenotypic activation that further directs the activity of the MDSC.
Up-regulation of MDSCs in patient peripheral blood has been detected in many cancers [46–48] and suggested as a marker of poor prognosis [49]. The link between inflammation and cancer has been studied heavily in the last decade. MC and MDSC interactions can potentially serve as a bridge between allergic inflammation and tumorigenic host responses [13, 50, 51]. This is the first report that patients with allergic inflammation have increased circulating MDSCs (Fig. 5A and B). Taken together, our results have important implications for regulation of MDSC survival, trafficking, and activity via antihistamines for disease states, in which the accumulation of MDSCs is detrimental to the host.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by U.S. National Institutes of Health Grants RO1AI18697 and U19AI077435, Project 2 (to D.H.C.), and F31CA159877 (to S.J.S.) and a pilot grant from the Massey Cancer Center. This work was additionally supported by the American Asthma Foundation Grant 11-0094 (to D.H.C.). Flow cytometry was supported, in part, by U.S. National Institutes of Health Grant P30 CA16059.
The authors give special thanks to Janet Cross for manuscript comments.
The online version of this paper, found at www.jleukbio.org, includes supplemental information.
- A10Tg
- a disintegrin and metalloprotease 10 transgenic
- APC
- allophycocyanin
- Arg1
- arginase 1
- AT
- adoptive transfer
- BMMC
- bone marrow-derived mast cell
- CIM
- cimetidine
- Cpa3
- carboxypeptidase A3
- cRPMI
- complete RPMI
- Ct
- comparative threshold
- CT
- cetirizine
- HDC
- histamine decarboxylase
- HR
- histamine receptor
- L3
- Stage 3 larvae
- MC
- mast cell
- Mcl-1
- myeloid cell leukemia sequence 1
- MDSC
- myeloid-derived suppressor cell
- Nb
- Nippostrongylus brasiliensis
- PBL
- peripheral blood leukocytes
- qPCR
- quantitative PCR
- Wsh
- W-sash
AUTHORSHIP
R.K.M. and S.J.S. equally participated in experimental design, conducting experiments, and manuscript preparation. L.F., H.B.Z., S.R.D., and G-K.T.N. conducted experiments. J.J.R., H.D.B., and A-M.I. assisted in experimental design. D.H.C. assisted in experimental design and manuscript preparation.
DISCLOSURES
The authors declare no conflicts of interest.
REFERENCES
- 1. Gabrilovich D. I., Nagaraj S. (2009) Myeloid-derived suppressor cells as regulators of the immune system. Nat. Rev. Immunol. 9, 162–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Saleem S. J., Conrad D. H. (2011) Hematopoietic cytokine-induced transcriptional regulation and Notch signaling as modulators of MDSC expansion. Int. Immunopharmacol. 11, 808–815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gabrilovich D. I., Ostrand-Rosenberg S., Bronte V. (2012) Coordinated regulation of myeloid cells by tumours. Nat. Rev. Immunol. 12, 253–268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Goni O., Alcaide P., Fresno M. (2002) Immunosuppression during acute Trypanosoma cruzi infection: involvement of Ly6G (Gr1(+))CD11b(+) immature myeloid suppressor cells. Int. Immunol. 14, 1125–1134 [DOI] [PubMed] [Google Scholar]
- 5. Guilliams M., Movahedi K., Bosschaerts T., VandenDriessche T., Chuah M. K., Herin M., Acosta-Sanchez A., Ma L., Moser M., Van Ginderachter J. A., et al. (2009) IL-10 dampens TNF/inducible nitric oxide synthase-producing dendritic cell-mediated pathogenicity during parasitic infection. J. Immunol. 182, 1107–1118 [DOI] [PubMed] [Google Scholar]
- 6. Narita Y., Wakita D., Ohkur T., Chamoto K., Nishimura T. (2009) Potential differentiation of tumor bearing mouse CD11b+Gr-1+ immature myeloid cells into both suppressor macrophages and immunostimulatory dendritic cells. Biomed. Res. 30, 7–15 [DOI] [PubMed] [Google Scholar]
- 7. Van Ginderachter J. A., Beschin A., De B. P., Raes G. (2010) Myeloid-derived suppressor cells in parasitic infections. Eur. J. Immunol. 40, 2976–2985 [DOI] [PubMed] [Google Scholar]
- 8. Cuenca A. G., Delano M. J., Kelly-Scumpia K. M., Moreno C., Scumpia P. O., LaFace D. M., Heyworth P. G., Efron P. A., Moldawer L. L. (2011) A paradoxical role for myeloid-derived suppressor cells in sepsis and trauma. Mol. Med. 17, 281–292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Saleem S. J., Martin R. K., Morales J. K., Sturgill J. L., Gibb D. R., Graham L., Bear H. D., Manjili M. H., Ryan J. J., Conrad D. H. (2012) Cutting edge: mast cells critically augment myeloid-derived suppressor cell activity. J. Immunol. 189, 511–515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Huang B., Lei Z., Zhang G. M., Li D., Song C., Li B., Liu Y., Yuan Y., Unkeless J., Xiong H., et al. (2008) SCF-mediated mast cell infiltration and activation exacerbate the inflammation and immunosuppression in tumor microenvironment. Blood 112, 1269–1279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Miller H. R. (1996) Mucosal mast cells and the allergic response against nematode parasites. Vet. Immunol. Immunopathol. 54, 331–336 [DOI] [PubMed] [Google Scholar]
- 12. Oldford S. A., Haidl I. D., Howatt M. A., Leiva C. A., Johnston B., Marshall J. S. (2010) A critical role for mast cells and mast cell-derived IL-6 in TLR2-mediated inhibition of tumor growth. J. Immunol. 185, 7067–7076 [DOI] [PubMed] [Google Scholar]
- 13. Ribatti D., Crivellato E. (2009) The controversial role of mast cells in tumor growth. Int. Rev. Cell. Mol. Biol. 275, 89–131 [DOI] [PubMed] [Google Scholar]
- 14. El-Agamy D. S. (2012) Targeting c-kit in the therapy of mast cell disorders: current update. Eur. J. Pharmacol. 690, 1–3 [DOI] [PubMed] [Google Scholar]
- 15. Elenkov I. J., Webster E., Papanicolaou D. A., Fleisher T. A., Chrousos G. P., Wilder R. L. (1998) Histamine potently suppresses human IL-12 and stimulates IL-10 production via H2 receptors. J. Immunol. 161, 2586–2593 [PubMed] [Google Scholar]
- 16. Hsu C. L., Bryce P. J. (2012) Inducible IL-33 expression by mast cells is regulated by a calcium-dependent pathway. J. Immunol. 189, 3421–3429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Suzukawa M., Morita H., Nambu A., Arae K., Shimura E., Shibui A., Yamaguchi S., Suzukawa K., Nakanishi W., Oboki K., et al. (2012) Epithelial cell-derived IL-25, but not Th17 cell-derived IL-17 or IL-17F, is crucial for murine asthma. J. Immunol. 189, 3641–3652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Galli S. J., Tsai M., Piliponsky A. M. (2008) The development of allergic inflammation. Nature 454, 445–454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Packard K. A., Khan M. M. (2003) Effects of histamine on Th1/Th2 cytokine balance. Int. Immunopharmacol. 3, 909–920 [DOI] [PubMed] [Google Scholar]
- 20. Skokos D., Botros H. G., Demeure C., Morin J., Peronet R., Birkenmeier G., Boudaly S., Mecheri S. (2003) Mast cell-derived exosomes induce phenotypic and functional maturation of dendritic cells and elicit specific immune responses in vivo. J. Immunol. 170, 3037–3045 [DOI] [PubMed] [Google Scholar]
- 21. Yang X. D., Ai W., Asfaha S., Bhagat G., Friedman R. A., Jin G., Park H., Shykind B., Diacovo T. G., Falus A., et al. (2011) Histamine deficiency promotes inflammation-associated carcinogenesis through reduced myeloid maturation and accumulation of CD11b+Ly6G+ immature myeloid cells. Nat. Med. 17, 87–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Van der Pouw Kraan T. C., Snijders A., Boeije L. C., de Groot E. R., Alewijnse A. E., Leurs R., Aarden L. A. (1998) Histamine inhibits the production of interleukin-12 through interaction with H2 receptors. J. Clin. Invest. 102, 1866–1873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ishikawa T., Kanda N., Hau C. S., Tada Y., Watanabe S. (2009) Histamine induces human β-defensin-3 production in human keratinocytes. J. Dermatol. Sci. 56, 121–127 [DOI] [PubMed] [Google Scholar]
- 24. Gibb D. R., Saleem S. J., Kang D. J., Subler M. A., Conrad D. H. (2011) ADAM10 overexpression shifts lympho- and myelopoiesis by dysregulating site 2/site 3 cleavage products of Notch. J. Immunol. 186, 4244–4252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lilla J. N., Chen C. C., Mukai K., BenBarak M. J., Franco C. B., Kalesnikoff J., Yu M., Tsai M., Piliponsky A. M., Galli S. J. (2011) Reduced mast cell and basophil numbers and function in Cpa3-Cre; Mcl-1fl/fl mice. Blood 118, 6930–6938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Camberis M., Le G. G., Urban J., Jr. (2003) Animal model of Nippostrongylus brasiliensis and Heligmosomoides polygyrus. Curr. Protoc. Immunol. Chapter 19, Unit 19.12 [DOI] [PubMed] [Google Scholar]
- 27. Connolly M. K., Mallen-St C. J., Bedrosian A. S., Malhotra A., Vera V., Ibrahim J., Henning J., Pachter H. L., Bar-Sagi D., Frey A. B., et al. (2010) Distinct populations of metastases-enabling myeloid cells expand in the liver of mice harboring invasive and preinvasive intra-abdominal tumor. J. Leukoc. Biol. 87, 713–725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Collins A. M., Leach S., Payne J., Mitchell A., Dai Y., Jackson G. D. (1999) A role for the hepatobiliary system in IgE-mediated intestinal inflammation in the rat. Clin. Exp. Allergy 29, 262–270 [DOI] [PubMed] [Google Scholar]
- 29. Condamine T., Gabrilovich D. I. (2011) Molecular mechanisms regulating myeloid-derived suppressor cell differentiation and function. Trends Immunol. 32, 19–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Dussault A. A., Pouliot M. (2006) Rapid and simple comparison of messenger RNA levels using real-time PCR. Biol. Proced. Online 8, 1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zheng Y., Xu M., Li X., Jia J., Fan K., Lai G. (2013) Cimetidine suppresses lung tumor growth in mice through proapoptosis of myeloid-derived suppressor cells. Mol. Immunol. 54, 74–83 [DOI] [PubMed] [Google Scholar]
- 32. Bush R. K. (2004) Etiopathogenesis and management of perennial allergic rhinitis: a state-of-the-art review. Treat. Respir. Med. 3, 45–57 [DOI] [PubMed] [Google Scholar]
- 33. Deshane J., Zmijewski J. W., Luther R., Gaggar A., Deshane R., Lai J. F., Xu X., Spell M., Estell K., Weaver C. T., et al. (2011) Free radical-producing myeloid-derived regulatory cells: potent activators and suppressors of lung inflammation and airway hyperresponsiveness. Mucosal Immunol. 4, 503–518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Greten T. F., Manns M. P., Korangy F. (2011) Myeloid derived suppressor cells in human diseases. Int. Immunopharmacol. 11, 802–807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chan T., Wiltrout R. H., Weiss J. M. (2011) Immunotherapeutic modulation of the suppressive liver and tumor microenvironments. Int. Immunopharmacol. 11, 879–889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ilkovitch D., Lopez D. M. (2009) The liver is a site for tumor-induced myeloid-derived suppressor cell accumulation and immunosuppression. Cancer Res. 69, 5514–5521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Saddiqi H. A., Sarwar M., Iqbal Z., Nisa M., Shahzad M. A. (2012) Markers/parameters for the evaluation of natural resistance status of small ruminants against gastrointestinal nematodes. Animal 6, 994–1004 [DOI] [PubMed] [Google Scholar]
- 38. Nawa Y., Ishikawa N., Tsuchiya K., Horii Y., Abe T., Khan A. I., Bing S., Itoh H., Ide H., Uchiyama F. (1994) Selective effector mechanisms for the expulsion of intestinal helminths. Parasite Immunol. 16, 333–338 [DOI] [PubMed] [Google Scholar]
- 39. Shin E. H., Kim T. H., Hong S. J., Park J. H., Guk S. M., Chai J. Y. (2003) Effects of anti-allergic drugs on intestinal mastocytosis and worm expulsion of rats infected with Neodiplostomum seoulense. Korean J. Parasitol. 41, 81–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Yamauchi K., Piao H. M., Nakadate T., Shikanai T., Nakamura Y., Ito H., Mouri T., Kobayashi H., Maesawa C., Sawai T., et al. (2009) Enhanced goblet cell hyperplasia in HDC knockout mice with allergic airway inflammation. Allergol. Int. 58, 125–134 [DOI] [PubMed] [Google Scholar]
- 41. Elliott K. A., Osna N. A., Scofield M. A., Khan M. M. (2001) Regulation of IL-13 production by histamine in cloned murine T helper type 2 cells. Int. Immunopharmacol. 1, 1923–1937 [DOI] [PubMed] [Google Scholar]
- 42. Finkelman F. D., Shea-Donohue T., Morris S. C., Gildea L., Strait R., Madden K. B., Schopf L., Urban J. F., Jr., (2004) Interleukin-4- and interleukin-13-mediated host protection against intestinal nematode parasites. Immunol. Rev. 201, 139–155 [DOI] [PubMed] [Google Scholar]
- 43. Schmidt J., Fleissner S., Heimann-Weitschat I., Lindstaedt R., Szelenyi I. (1994) Histamine increases anti-CD3 induced IL-5 production of TH2-type T cells via histamine H2-receptors. Agents Actions 42, 81–85 [DOI] [PubMed] [Google Scholar]
- 44. Jinquan T., Reimert C. M., Deleuran B., Zachariae C., Simonsen C., Thestrup-Pedersen K. (1995) Cetirizine inhibits the in vitro and ex vivo chemotactic response of T lymphocytes and monocytes. J. Allergy Clin. Immunol. 95, 979–986 [DOI] [PubMed] [Google Scholar]
- 45. Walsh G. M., Moqbel R., Hartnell A., Kay A. B. (1991) Effects of cetirizine on human eosinophil and neutrophil activation in vitro. Int. Arch. Allergy Appl. Immunol. 95, 158–162 [DOI] [PubMed] [Google Scholar]
- 46. Basso D., Fogar P., Falconi M., Fadi E., Sperti C., Frasson C., Greco E., Tamburrino D., Teolato S., Moz S., et al. (2013) Pancreatic tumors and immature immunosuppressive myeloid cells in blood and spleen: role of inhibitory co-stimulatory molecules PDL1 and CTLA4. An in vivo and in vitro study. PLoS One 8, e54824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Jiao Z. J., Gao J. J., Hua S. H., Chen D. Y., Wang W. H., Wang H., Wang X. H., Xu H. X. (2012) Correlation between circulating myeloid-derived suppressor cells and Th17 cells in esophageal cancer. World J. Gastroenterol. 18, 5454–5461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Sun H. L., Zhou X., Xue Y. F., Wang K., Shen Y. F., Mao J. J., Guo H. F., Miao Z. N. (2012) Increased frequency and clinical significance of myeloid-derived suppressor cells in human colorectal carcinoma. World J. Gastroenterol. 18, 3303–3309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Diaz-Montero C. M., Salem M. L., Nishimura M. I., Garrett-Mayer E., Cole D. J., Montero A. J. (2009) Increased circulating myeloid-derived suppressor cells correlate with clinical cancer stage, metastatic tumor burden, and doxorubicin-cyclophosphamide chemotherapy. Cancer Immunol. Immunother. 58, 49–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Hanahan D., Coussens L. M. (2012) Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell 21, 309–322 [DOI] [PubMed] [Google Scholar]
- 51. Ruffell B., Coussens L. M. (2011) Histamine restricts cancer: nothing to sneeze at. Nat. Med. 17, 43–44 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.