Abstract
Pain that recurs or persists is unfortunately a common experience for children. One of the unique considerations in pediatric chronic pain management is the bidirectional influences of children’s pain experiences and parental and family factors. In this review we present a developmental perspective on understanding pediatric chronic pain and disability, highlighting factors relevant from infancy to adolescence, and family and parent influences. Preliminary evidence indicates that developmental processes are influenced and may also shape the pediatric pain experience. Parent emotions, behaviors, and health also play a role in children’s pain experiences, where overly protective parent behaviors, increased distress, and history of chronic pain are important parent level influences. Research on family level influences has revealed that families of children with chronic pain have poorer family functioning (e.g., more conflict, less cohesion) than families of healthy children. Several important gaps exist in this research, such as in understanding basic developmental processes in children with chronic pain and how they influence children’s perception of and responses to pain. Also, there is a lack of longitudinal data on family relationships and individual adjustment to allow for understanding of whether changes occur in parenting over the course of the child’s chronic pain experience. Although parent interventions have been successfully incorporated into many cognitive-behavioral treatments for children with chronic pain conditions, little guidance exists for adapting intervention strategies to be developmentally appropriate. Additional research is needed to examine whether parent interventions are effective at different developmental stages and the best way to incorporate developmental goals into treatment.
Keywords: child, pediatric, chronic pain, developmental, family, parenting
Chronic pain is defined as recurrent or persistent pain lasting longer than the normal tissue healing time, approximately 3 to 6 months (American Pain Society, 2012). Children may experience pain related to injury (e.g., burns), to a chronic or underlying disease process (e.g., cancer, arthritis), or pain can also be the chronic condition itself (e.g., complex regional pain syndrome, fibromyalgia) and may be present without any specific, identifiable injury or disease (e.g., functional abdominal pain). For a complete description of types of chronic pain in childhood see Schechter, et al. (2009). During childhood, abdominal, musculoskeletal, and headache pain are the most frequently occurring conditions (Perquin, et al., 2000). However, definitions of chronic pain do not take into account standard criteria for assessing particular pain symptoms or for evaluating the intensity or impact of pain, and therefore includes individuals with varying symptoms and experiences. Consequently, in epidemiological surveys, prevalence estimates vary widely. Overall prevelence rates for different childhood pains range from 4% to 88% (King, et al., 2011). For example, an average of 13.5% to 31.8% of adolescents in a community sample reported having weekly abdominal, headache, or musculoskeletal pains (e.g., Stanford, Chambers, Biesanz, & Chen, 2008). Most epidemiological studies report prevalence and do not report the severity or impact of the pain. Research indicates that only a subset of children and adolescents with chronic pain (approximately 5%) experience moderate-severe disability (Huguet & Miro, 2008), and this likely better represents the estimated population for whom help is needed to treat pain and associated problems.
It is well appreciated that parent and family factors may influence the pain experience of children (Palermo & Chambers, 2005). Research has shown that parenting a child with chronic pain can disrupt parents and family life (Palermo & Eccleston, 2009). Parents of children with chronic pain describe social restrictions and high levels of parenting stress, anger, depressive symptoms, and anxiety (Eccleston, Crombez, Scotford, Clinch, & Connell, 2004). Parents also experience the financial burden of evaluation and management of recurrent and chronic pain, including the cost of diagnostic exams, hospitalization, doctors’ visits, and medications. In addition, parents incur costs related to taking time off from work, transportation, and additional childcare for siblings (Sleed, Eccleston, Beecham, Knapp, & Jordan, 2005). Further, chronic pain may aggregate in families, and parental pain status has been identified as a potential contributor to childhood chronic pain (Boey & Goh, 2001; Lester, Lefebvre, & Keefe, 1994; Anttila, Metsahonkala, Helenius, & Sillanpaa, 2000; Goodman, McGrath, & Forward, 1997). Specific parent behaviors such as social reinforcement of pain behaviors (e.g., granting permission to avoid regular activities) have also been recognized as important in children’s response to pain (e.g., Walker & Zeman, 1992). The broader influence of the overall family unit, in regards to their level of organization, conflict, and cohesion, may also contribute to inter-individual variability in children’s experience of pain (Lewandowski, Palermo, Stinson, Handley, & Chambers, 2010).
Although the context of the parent and family has been incorporated into psychosocial pain research, very little attention has been paid to how pain may interrupt normal developmental processes or how familial and parental factors may influence adjustment to chronic pain differentially during distinct developmental periods. In addition, consideration of developmental processes has yet to guide understanding of children’s experiences and reactions to pain or treatment efforts. Thus, the aim of this review is to present a developmental perspective on pediatric chronic pain and disability and on family and parent influences that may guide and stimulate understanding and treatment development. We accomplish this aim by discussing the experience of pain at the child level and how it interacts with normal developmental processes across the pediatric age span. Then, a review of the evidence for parent and family level influences on children’s pain is provided. Next, a conceptual model of the bidirectional relationship of children’s pain and disability within the context of children’s development and parent and family influences is presented. Lastly, the evidence base for parent and family interventions for children with chronic pain is summarized with suggestions for future treatment research to incorporate a developmental perspective.
Child Level Influences within a Developmental Context
The following sections present information on children’s pain experiences during three developmental phases: the infant and preschool years, the school-age years, and adolescence. Though a comprehensive discussion of important developmental milestones during each stage is beyond the scope of this review, key milestones and goals that may be particularly important to pediatric pain are highlighted in each section. Unique family and parental factors that may influence, or be influenced by, children’s experiences and adjustment to chronic pain and their development are also highlighted.
Infancy and Preschool-age Children
Estimates of chronic pain during early childhood indicate that somatic complaints (e.g., abdominal pain, headache) occur with some regularity in pre-school age children. For example, Perquin et al (2000) found that 11.8% of parents of children aged 0 to 3 years reported that their child experienced continuous or recurrent pain lasting for at least three months. However, chronic pain is poorly characterized in very young children (Pillai Riddell et al., 2009). Young children’s caregivers serve as the primary interpreters and reporters of their pain experiences. Thus, as posited in frameworks for understanding psychological and social determinants of pain through pain communication, young children’s pain cannot be understood outside the context of the primary caregiver (Hadjistavropoulos, Craig, Duck, Cano, Goubert, et al., 2011). Moreover, young children’s ways of expressing pain and their caregivers’ interpretations and reactions to the children’s pain are separately influenced by a range of socio-cultural systems, including the overall family.
Normal developmental processes during the infant and preschool years include the regulation of sleep/wake patterns, the achievement of motor milestones, the development of language abilities, and early emotional development (Oates, Wood, & Grayson, 2005). Few studies focus on developmental processes in young children with chronic pain. One study (Grunau, et al, 2009) found that repeated procedural pain during infancy was related to poorer motor function in a sample of infants born preterm and full term. In addition, in a study of preschool and early school-age children with juvenile idiopathic arthritis (JIA), earlier disease onset and longer disease duration were negatively related to motor development (van der Net et al., 2008), although the association to pain was not evaluated.
The area of temperament and attachment in young children with chronic pain has also received very little attention. In the acute pain literature, during immunization procedures and everyday pain incidents, an ambivalent or controlling attachment style was related to greater pain reaction while a controlling attachment style was related to greater anger response in young children (Walsh, McGrath, Symons, 2008). Positive temperament attributes such as adaptability and persistence are related to decreased child pain and distress during painful medical procedures in young children with cancer (Harper, Penner, Peterson, Albrecht, & Taub, 2012). In the chronic pain literature, research indicates that school-age children and adolescents with chronic pain evidence more difficult temperaments than their peers (Campo et al., 2004; Conte, Walco, & Kimura, 2003). Also, children with more difficult temperaments tend to be more reactive to stress and more sensitive to pain than other children (Ramchandani, Stein, Hotopf, & Wiles, 2006). Further, infants with difficult temperaments are more likely to evidence insecure attachment styles with their primary caregiver (van den Boom, 1994), which has been postulated as potentially leading to problems in learning how to effectively communicate pain to others. Overall, the current research suggests a potential interaction between temperament and chronic pain in childhood, but more research is needed. Of note, parent and family influences during this stage may be important for the development of early pain management skills that may be useful upon onset of school for managing academic demands and interacting with peers while coping with chronic pain. Social skill development will be discussed further in the section on school-age children.
School-age Children
By the school-age years, children are able to provide consistent and accurate reports of their pain to caregivers and health care providers (Azize, Humphreys, & Cattani, 2011). Chronic pain prevalence is higher than in younger years. In a community sample of 5,423 Dutch children aged 0 to 18 years, 19.3% of four to seven year olds and 23.7% of children aged eight to 11 years reported chronic pain (Perquin, et al., 2000).
Major developmental milestones center on social skills and developing meaningful friendships. Children’s interactions with parents and family members teach them the basic social skills they take into their interactions with peers (Rubin, Bukowski, & Laursen, 2009). For instance, research indicates that parents with more extensive social networks tend to have children with more extensive social networks (Romano, Hubbard, McAuliffe, & Morrow, 2009). Children with chronic pain, however, evidence poor social functioning, as supported by reports that they have fewer friends, suffer more peer victimization and isolation, and receive less positive ratings by peers compared to children without chronic pain (for a review, see Forgeron et al., 2010).
Research also indicates that children with chronic pain evidence impaired school functioning (Logan, Simons, Stein, & Chastain, 2008). For instance, one area of school functioning consistently impacted by chronic pain is school attendance (Kashikar-Zuck, Johnston, et al., 2010; Logan, et al., 2008). A study of 149 children and adolescents with unexplained chronic pain (Konijnenberg et al, 2005) found that 51% of children reported missing school due to pain. Despite this, children with chronic pain tend to perform as well as their peers in relation to processing information and academic achievement (Ho, Bennett, Cox, & Poole, 2009), unless there are neurological consequences associated with the disease, such as sickle cell disease (SCD; Schatz, Finke, Kellett, & Kramer, 2002). In a recent review of cognitive function in children with chronic pain, some preliminary evidence was noted for possible specific effects of pain on attentional disruption, which will require further research to understand (Dick & Pillai Riddell, 2010). While family endorsement of illness behavior has been linked to more school absences in adolescents with chronic pain (Brace, Scott Smith, McCauley, & Sherry, 2000), family and parental influences on school functioning have not been extensively studied in school-aged children. More research is needed to understand whether school functioning, performance, and absenteeism is related to family organization and overall functioning.
Adolescents
Chronic pain and pain-related disability peak during adolescence (e.g., Roth-Isigkeit, et al., 2005). This increase in chronic pain may be related to pubertal development and the associated physical, cognitive, emotional, and social changes that accompany development during adolescence. For example, in a cross-sectional national study of children aged four to 18 years, reports of frequent headache and migraine increased steadily as age increased, with estimates of frequent headache highest for adolescents aged 16 to 18 at 27.4% (Lateef et al., 2009). Similarly, for other types of chronic pain (e.g., abdominal, musculoskeletal pain), incidence rates peak at 14 to 15 years of age (Stanford, et al., 2008).
A major developmental outcome of adolescence is achieving autonomy or separateness from parents emotionally, in decision making, and in ideas. The balance between parental involvement and autonomy appears to be a complex and critical issue for adolescents with chronic pain (e.g., Evans, Meldrum, Tsao, Fraynt, & Zeltzer, 2010). For instance, in samples of adolescents with chronic headache, researchers found that higher levels of family conflict and lower levels of adolescent autonomy were associated with increased youth functional impairment and increased symptoms of depression (Lewandowski & Palermo, 2009; Palermo, Putnam, Armstrong, & Daily, 2007).
The transition of pain and disease self-management activities from parents to adolescents is a critical issue for adolescents with chronic pain, as well as their families. Adolescents commonly report increasing independence around self-administration of medication for pain (Fichtel & Larsson, 2002; Hansen, Holstein, Due, & Currie, 2003). However, research indicates that maternal communication and maternal modeling of pain management remain significant influences on adolescents’ pain management choices (Beyer & Simmons, 2004; Hatchette, McGrath, Murray, & Finley, 2008). Parental involvement may also relate to youth pain experiences; for example, in a preliminary study with adolescents with SCD, higher parental involvement in pain and disease management activities was associated with higher levels of youth disability (Oliver-Carpenter, Barach, Crosby, Valenzuela, & Mitchell, 2011). Longitudinal research is needed to understand bi-directional and temporal relationships in order to help identify optimal times for transitioning adolescents into assuming pain and disease management activities, and to identify specific family and parental practices that may promote autonomy and effective pain self-management.
For adolescents with chronic pain conditions, the transition from pediatric to adult health care is another important developmental task (e.g., Telfair, Myers, & Drezner, 1994). Qualitative research suggests parents continue to play a significant role in adolescents’ and young adults’ medical care (Oliver-Carpenter, et al., 2011), and has mostly focused on identifying concerns and needs of youth with health conditions who report feeling inadeqautely prepared to properly manage the transition to new roles and responsibililties regarding self-management (e.g., DeBaun & Telfair, 2012; van Staa, Jedeloo, van Meeteren, & Latour, 2011). However, perhaps due to this time period being characterized by major changes in occupational and living situations, there is limited empirical research investigating the influence of family and parent factors on the transition from pediatric to adult health care for persons with chronic pain conditions, and thus, this is an important area for future research.
The following sections discuss parent and family level influences on children’s experiences and reactions to chronic pain.
Parent Level Influences
Parent Emotions and Behaviors
Research indicates that a sizeable number of parents of children and adolescents with chronic pain experience clinically significant distress including anxiety and depressive symptoms and parental role stress (e.g., Eccleston, et al., 2004), although it is unknown whether these symptoms predate the pain condition or develop in reaction to parenting a child with chronic pain. Specifically, depression and anxiety disorders are particularly prevalent among mothers of children with chronic pain conditions (Campo et al., 2007; Walker & Greene, 1989). For example, in one clinic-based sample, mothers of children with functional abdominal pain were 4.9 times more likely to have a lifetime history of depressive disorders and 4.8 times more likely to have a lifetime history of anxiety disorders compared to mothers of healthy children (Campo, et al., 2007). Moreover, mothers of adolescents with chronic pain with greater disability tend to endorse more symptoms of stress, anxiety, and depression than do mothers of less impaired adolescents with chronic pain (Cohen, Vowles, & Eccleston, 2010). Little is known about the psychological functioning of fathers in this population.
Research also indicates that maternal distress and childhood chronic pain are likely bidirectional in nature. In a study that used path analysis to examine the relationship between caregiver variables and pediatric chronic pain and illness, researchers found that maternal depression was a direct predictor of child depression in children with chronic pain, while maternal use of active coping strategies was related to lower levels of depression in children with chronic pain (Williamson, Walters, & Shaffer, 2002). In a more recent population-based birth cohort study in the Netherlands, maternal symptoms of anxiety during pregnancy independently predicted increased child somatic complaints at 18 months of age (Wolff et al., 2010). Such studies emphasize the possible bidirectional nature of the parent-child relationship, underscoring the relationship between maternal emotional functioning and children’s experience of chronic pain and disability.
Parent cognitive and behavioral functioning in reaction to children’s pain has also been shown to play an important role (Rhee, 2003). Parental pain catastrophizing is a particularly important cognitive process (involving rumination, magnification, and helplessness about the child’s pain) that has been studied. For example, research has demonstrated that maternal but not paternal catastrophizing is significantly related to the child’s pain intensity, whereas neither mothers’ nor fathers’ catastrophizing were significantly related to the child’s disability (Hechler, Vervoort, Hamann, et al., 2011). Moreover, parents who reported a high level of catastrophic thinking experienced more distress and wanted to stop their child’s pain-inducing activities more than parents with lower pain catastrophizing (Caes, Vervoort, Eccleston, Vandenhende, & Goubert, 2011). Attention to pain (i.e., solicitous or protective responses) has been the most studied parental behavior. This concept is theoretically derived from social learning theory and is demonstrated by more solicitous responses from parents (i.e., frequent attending to pain symptoms, granting permission to avoid regular activities) toward their children’s pain behaviors being related to increased disability in children with recurrent and chronic pain (e.g., Claar, Simons, & Logan, 2008; Lipani & Walker, 2006; Walker & Zeman, 1992).
Parent Health Status
Parental health status is also an important influence on childhood chronic health conditions, including chronic pain. The vast majority of this research has examined maternal health. For example, maternal poor health and function are associated with poorer health and function in children with chronic conditions (e.g., Daniels, Moos, Billings, & Miller, 1987). Also, studies have demonstrated that many types of chronic pain conditions cluster in families (Boey & Goh, 2001; Lester et al., 1994). For example, children with headache are more likely to have a parent with headache or other recurrent pains than children without headache (Anttila et al., 2000). In a large community study of families, parental pain and disability were significantly associated with the child’s level of pain and disability (Goodman et al., 1997). In one of the few studies to include fathers using an innovative family linkage study design, Hoftun and colleagues (2013) found that maternal and paternal chronic pain increased the odds of chronic pain in adolescents, and that associations were even stronger when both parents reported chronic pain. Conceptually, these connections might represent shared genetics of chronic pain and/or modeling of pain behavior and coping. More research is needed to elucidate the mechanisms of influence between maternal and paternal health and childhood chronic pain. Further inclusion of mothers and fathers in pediatric pain research will expand understanding of the relationship between parental factors (and potential differences between mothers and fathers) and the child’s pain experience during different developmental stages.
Family Level Influences
One of the primary tenets of family systems theories is that families are complicated, interrelated systems that act as a unit to maintain internal balance and order (Goldenberg & Goldenberg, 2008). Thus, when a child experiences chronic pain, there are consequences both for the child and the overall family system of the child (Kazak, 1989). A child’s pain experiences may be distressing for other family members or the regimen for dealing with a child’s pain may lead to disruptions in planned family events, thus disrupting the overall family system. Conversely, a lack of communication within the family may lead to poorer pain management for the child. Also, families transition through different life cycle stages that are generally dependent upon the characteristics of the members, such as developmental stage of the children, and that influence the overall goals of the system (McGoldrick, Carter, & Garcia-Preto, 2011). For example, families with young children may be focused on defining parenting roles and assisting children in developing early socio-emotional skills. Comparatively, families with adolescents may be focused on promoting adolescent autonomy.
Five characteristics of family functioning commonly assessed in family systems theories are organization, cohesion, communication, affective environment, and problem solving (Alderfer et al., 2008). Well-functioning families are generally defined as those with well-defined roles and structure; those whose family members are connected and supportive of one another; those who demonstrate clear and open communication; and those who help each other express and control their emotions to create an environment with minimal conflict. In contrast, poorly functioning families can be those that are highly disorganized, with unclear communication and high expressions of conflict or negative affect that only become more disrupted when faced with a stressor. Poorly functioning families also can be characterized by being overly restrictive and ordered, limiting the adaptability of the system to deal with stressors, and limiting individual members’ ability to express their emotions or modify maladaptive roles.
In a review by Lewandowski and colleagues (2010), there was evidence that families of children and adolescents with chronic pain have poorer general family functioning, as indicated by less organization, less cohesion, and more conflict than families of healthy children and adolescents. In addition, poorer general family functioning was related to increased pain-related disability in children and adolescents. However, findings concerning the relationship between family functioning and pain perception were equivocal. Also, there is a limited research base on specific aspects of family functioning (e.g., problem solving, family cohesion), and no consensus on which are most relevant to children’s experience of pain and disability. Research to date does not contribute to an understanding of causality as studies are cross-sectional. Additional research, and in particular longitudinal research, is needed focusing on the relation between family factors and the pediatric pain experience during different child developmental stages.
Conceptual Framework for Understanding Pediatric Chronic Pain in the Context of Development and Parent and Family Influences
Over the past few decades, two primary frameworks: biopsychosocial models and ecological models, have been applied to the understanding of chronic pain and its associated disability. The biopsychosocial model builds from social learning theory and was first applied to adults with chronic pain (Fordyce et al., 1973). In pediatric chronic pain models, social reinforcement refers to those parent behaviors, such as attention to pain complaints, that may serve to inadvertently reinforce maladaptive child pain and coping behaviors (e.g., activity restriction and catastrophizing; Walker, Baber, Garber, & Smith, 2008). One prior review has applied the biopsychosocial model to understanding parent and family factors in pediatric chronic pain (Palermo & Chambers, 2005). However, this model was limited in that it did not provide a link to understanding how normative developmental processes shape both the child’s individual experience of and response to pain and the role of family and parent influences. Ecological models, such as Bronfenbrenner’s Ecological Model (1979), also provides a strong framework for understanding the experience of chronic pain, and has been applied to pediatric pain in prior work (e.g., Logan, Engle, Feinstein, Sieberg, Sparling, et al., 2012). The model proposes that individual development is influenced by five interacting levels of systems branching out from individuals’ interactions with their immediate settings, such as the interactions of children with their parents or with their families as a whole, to interactions within the context of external settings (e.g., culture, sociohistorical changes).
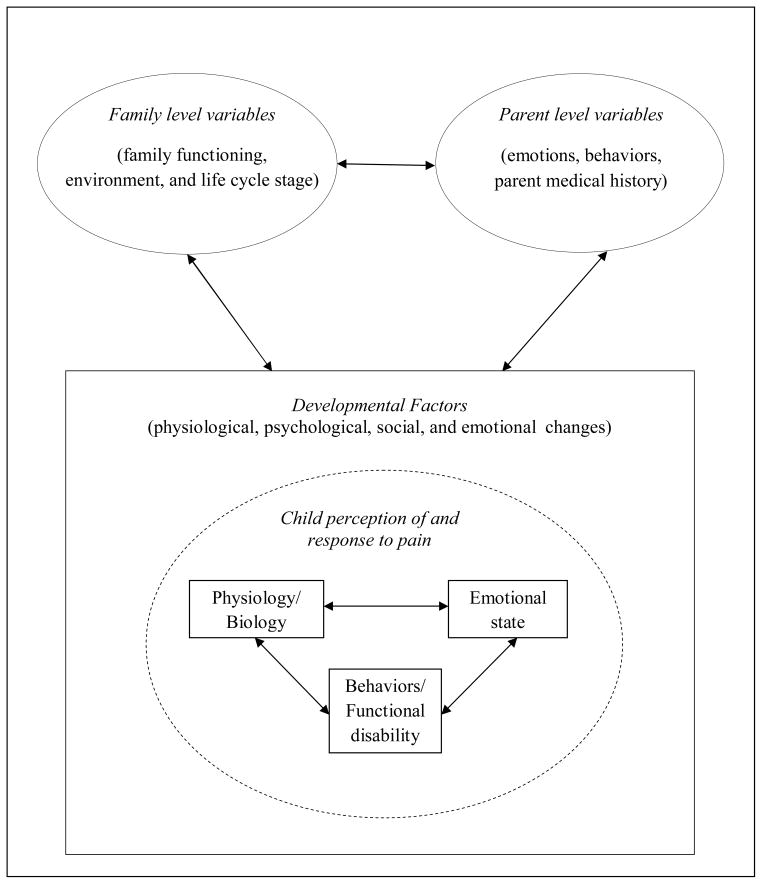
The model presented in this paper (see Figure 1) integrates these predominant frameworks to exclusively focus on the fluid and bidirectional relationships between children’s pain experiences and responses as shaped by a developmental framework, as well as the influence of parent’s emotions, behaviors, and health and family level influences. At the child individual level, the child’s perception of pain is related to his or her physiology/biology, current emotional state, pain behaviors, and functional disability. All of these factors and their interactions are shaped by the physiological, psychological, social, and emotional changes that characterize development. The child’s perception of and response to pain and developmental trajectory is influenced both by parental characteristics, such as parent emotional functioning, parent behaviors, and medical history (e.g., parent chronic pain), via parent-child interactions and by family factors, such as family functioning, environment, and life cycle stage. In addition, parental and family factors are influenced by the child’s perception of and response to pain and the child’s developmental stage. For example, a parent or family may function differently in the context of caring for an infant experiencing chronic pain versus an adolescent. Lastly, parental characteristics and family factors interact with one another to indirectly influence the child’s pain experience and development. Thus, the model proposes that developmental processes lead to systematic changes in individual, parental, and familial perceptions of and responses to pain. And, in turn, those developmental processes are also influenced by factors at each of these levels; thus creating a bidirectional relationship. In addition, since this model is specific in focus, it should be applied within the larger context of more comprehensive biopsychosocial and ecological models of pediatric pain.
Figure 1.
A developmental perspective on family and parent influences on pediatric chronic pain and disability
Family and Parent Intervention Approaches in Developmental Context
Given the important role of parent and family influences on pediatric chronic pain and disability, parent intervention strategies have been incorporated into many cognitive-behavioral treatments (CBT) for children with chronic pain conditions (Palermo, 2012). However, developmental stage of the child has often been overlooked in this work. Most of the psychosocial pain treatment literature has focused on children with headache (e.g., Trautmann, Lackschewitz, & Kroner-Herwig, 2006); however, effective treatments have also been developed for children with abdominal, musculoskeletal, and disease-related pain (Palermo, Eccleston, Lewandowski, Williams, & Morley, 2010; Walco, Sterling, Conte, & Engel, 1999). In general, parent strategies have focused on operant techniques (e.g., minimizing response to pain complaints, encouraging adaptive behavior) taught to parents in brief individual or group sessions. For example, seven of 25 randomized controlled trials (RCTs) included in a recent meta-analysis of psychological therapies for the treatment of pediatric chronic pain (Palermo, et al., 2010) incorporated intervention strategies directed to parents and they primarily did so by training parents in operant strategies. Notably, these trials were efficacious in reducing children’s pain with similar magnitude of effects as other individually focused cognitive and behavioral treatments. However, the efficacious nature of the treatments has not been examined based upon developmental stage of the child.
Several investigations have also examined whether child-focused CBT can be enhanced with the addition of parent operant strategies, and findings from these trials provide some support for this when directly comparing CBT with and without parental intervention (Allen & Matthews, 1998; Sanders, Shepherd, Cleghorn, & Woolford, 1994). More recently, specific therapeutic change processes have been examined in parents receiving training in operant interventions. In a large RCT of children with functional abdominal pain, Levy and colleagues (2010) found that parents in the CBT condition had greater decreases in solicitous responses to their children’s symptoms compared with parents in an attention control condition.
Beyond operant strategies, several other parental intervention strategies drawn from the childhood chronic illness literature have been tested. For example, parental communication training (e.g., Palermo, Wilson, Peters, Lewandowski, & Somhegyi, 2009) and acceptance strategies (Wicksell, Melin, Lekander, & Olsson, 2009) have been taught to parents in recent RCTs focused on adolescents with chronic pain. Another recent innovative RCT (Barakat, Schwartz, Salamon, & Radcliffe, 2010) used a family support person (e.g., extended family member) to help adolescents with SCD learn brief pain interventions.
To date, there has been limited development of intervention content directed at other aspects of the family environment such as parent modeling or family conflict. For example, if parental pain status is considered influential in children’s pain response (e.g., Hoftun, et al., 2013), then development and testing of interventions that provide instruction to parents in modifying their own response to their chronic pain (e.g., modeling) may be important. In a recent systematic review of parent interventions for children with chronic health conditions (Eccleston, et al., 2012), problem solving therapy was found to significantly improve parent behavior and parent mental health immediately post-treatment; this may be a relevant intervention to explore in youth with chronic pain. While there is emerging support for the combined effect of child and parent interventions to result in improved child pain and functional outcomes, it is unclear how to make these strategies developmentally relevant or to what extent the addition of parental intervention strategies may enhance pain outcomes during different developmental stages. Overall, more research focused on family and parent intervention approaches to child chronic pain in the context of development is called for.
Summary and Future Directions
In this review, we present a conceptual model for applying a developmental perspective on family and parent influences on pediatric chronic pain and disability. There are major research gaps in understanding basic developmental processes and their relationship to the perception and response to chronic pain across the pediatric age span. For example, in regards to the early childhood years, more research is needed identifying common temperamental and attachment patterns in relation to family and parental level influences on early emotional development of children with chronic pain. In the school age period, studies are needed to better understand the influence of parents and families in assisting children with chronic pain in succeeding academically and socially with their peers. Given the high incidence of chronic pain in adolescence, more research is needed that focuses on transitional care and self-management in adolescents and young adults with chronic pain. Additionally, there is currently very limited outcome data for late adolescents and young adults with chronic pain, despite this developmental stage being potentially critical for long-term financial, employment, and social outcomes (Kashikar-Zuck, Parkins, et al., 2010).
Given the lack of research on basic developmental processes in children with chronic pain, there has, in turn, been very limited consideration of children’s developmental processes in perceiving and responding to pain that could inform treatment approaches. To date, CBT has been delivered in a fairly uniform manner across the pediatric age span and little guidance exists for adapting intervention strategies to be developmentally appropriate. There are also no data available on which parent interventions are effective for children at each developmental stage. Thus it is not known, for example, whether operant strategies are equally effective in early childhood and adolescence, nor how to integrate relevant developmental processes into psychosocial intervention. With a greater understanding of these processes, appropriate treatment modifications can be made by clinicians and incorporated into existing protocols. Another priority is to incorporate developmental goals into psychosocial treatment. For example, a developmental goal for a late adolescent may be to achieve independence around using pain medication. Finally, although family therapy approaches have been successfully applied to other pediatric health conditions, such as diabetes and cancer (Kazak et al., 2004; Wysocki et al., 2007), interventions using family therapy approaches have yet to be examined in pediatric chronic pain populations, and may be important to explore.
Although there is emerging research on specific family and parent processes that may influence and be influenced by chronic pain, there are also a number of gaps in the literature regarding our understanding of parent and family influences on child chronic pain, particularly as it relates to children in different developmental stages. Two notable limitations of the current research on functioning in families of children with chronic pain are the lack of longitudinal data on family relationships and individual adjustment, and the overreliance on self-report data on family relationships. Most studies have focused on the relation between parental emotions and behaviors and children’s chronic pain experience at one point in time, and thus are unable to establish causality or temporal ordering of relationships. No data are currently available on how and whether changes occur in parenting over the course of caring for the child with chronic pain or the life cycle of the family. Additionally, the majority of studies on family functioning in pediatric pain populations have used only self-report assessments of the family and have not utilized observational assessments, which could expand knowledge of the impact of specific family processes on children’s coping with chronic pain (Palermo & Chambers, 2005). These topics are important avenues for future research.
In sum, childhood chronic pain represents a common pediatric developmental health issue. As our review highlights, attention to child developmental processes would significantly enhance this area of research and practice, and enhance understanding of the role of parent and family influences on childhood chronic pain. In particular, it is important to understand the dynamic and bi-directional relationships between the child’s perception of and response to pain as shaped by development and parent and family factors. We encourage applications of our proposed model and suggest researchers frame their inquiries concerning pediatric chronic pain, parent, and family influences with consideration of the relevant developmental processes. Greater attention to developmental processes in research on childhood chronic pain may lead to innovations in clinical practice such as applying family therapy approaches with this population, may aid clinicians in incorporating developmentally-relevant goals in treatment of the child or adolescent with chronic pain, and may lead to delivery of developmentally-informed parent interventions for particular age groups.
Acknowledgments
Funding: Preparation of this manuscript was partially supported by K01HL103155 (CRV) and K24HD060068 (TMP)
Contributor Information
Tonya M. Palermo, University of Washington, Seattle Children’s Research Institute
Cecelia R. Valrie, East Carolina University
Cynthia W. Karlson, University of Mississippi Medical Center
References
- Alderfer MA, Fiese BH, Gold JI, Cutuli JJ, Holmbeck GN, Goldbeck L, Patterson J. Evidence-based assessment in pediatric psychology: family measures. Journal of Pediatric Psychology. 2008;33(9):1046–1061. doi: 10.1093/jpepsy/jsm083. discussion 1062-1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen K, Matthews J. Behavioral management of recurrent pain in children. In: Watson T, Gresham F, editors. Handbook of child behavior therapy. NY: Plenum Press; 1998. [Google Scholar]
- American Pain Society Task Force on Pediatric Chronic Pain Management. Assessment and management of children with chronic pain. 2012 Retrieved on March 18, 2013, from http://www.ampainsoc.org/advocacy/downloads/aps12-pcp.pdf.
- Anttila P, Metsahonkala L, Helenius H, Sillanpaa M. Predisposing and provoking factors in childhood headache. Headache. 2000;40(5):351–356. doi: 10.1046/j.15264610.2000.00053.x. [DOI] [PubMed] [Google Scholar]
- Azize PM, Humphreys A, Cattani A. The impact of language on the expression and assessment of pain in children. Intensive and Critical Care Nursing. 2011;27(5):235–243. doi: 10.1016/j.iccn.2011.07.002. [DOI] [PubMed] [Google Scholar]
- Barakat LP, Schwartz LA, Salamon KS, Radcliffe J. A family-based randomized controlled trial of pain intervention for adolescents with sickle cell disease. Journal of Pediatric Hematology Oncology. 2010;32(7):540–547. doi: 10.1097/MPH.0b013e3181e793f9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyer JE, Simmons LE. Home treatment of pain for children and adolescents with sickle cell disease. Pain Management Nursing. 2004;5(3):126–135. doi: 10.1016/j.pmn.2004.03.001. [DOI] [PubMed] [Google Scholar]
- Boey CC, Goh KL. Predictors of recurrent abdominal pain among 9 to 15-year-old urban school-children in Malaysia. Acta Paediatrica. 2001;90(3):353–355. doi: 10.1111/j.1651-2227.2001.tb00318.x. [DOI] [PubMed] [Google Scholar]
- Brace MJ, Scott Smith M, McCauley E, Sherry DD. Family reinforcement of illness behavior: a comparison of adolescents with chronic fatigue syndrome, juvenile arthritis, and healthy controls. Journal of Developmental & Behavioral Pediatrics. 2000;21(5):332–339. doi: 10.1097/00004703-200010000-00003. [DOI] [PubMed] [Google Scholar]
- Bronfenbrenner U. The ecology of human development: Experiments by nature and design. Cambridge, MA: Harvard University Press; 1979. [Google Scholar]
- Caes L, Vervoort T, Eccleston C, Vandenhende M, Goubert L. Parental catastrophizing about child’s pain and its relationship with activity restriction: the mediating role of parental distress. Pain. 2011;152(1):212–222. doi: 10.1016/j.pain.2010.10.037. [DOI] [PubMed] [Google Scholar]
- Campo JV, Bridge J, Ehmann M, Altman S, Lucas A, Birmaher B, Brent DA. Recurrent abdominal pain, anxiety, and depression in primary care. Pediatrics. 2004;113(4):817–824. doi: 10.1542/peds.113.4.817. [DOI] [PubMed] [Google Scholar]
- Campo JV, Bridge J, Lucas A, Savorelli S, Walker L, Di Lorenzo C, Brent DA. Physical and emotional health of mothers of youth with functional abdominal pain. Archives of Pediatrics and Adolescent Medicine. 2007;161(2):131–137. doi: 10.1001/archpedi.161.2.131. [DOI] [PubMed] [Google Scholar]
- Claar RL, Simons LE, Logan DE. Parental response to children’s pain: the moderating impact of children’s emotional distress on symptoms and disability. Pain. 2008;138(1):172–179. doi: 10.1016/j.pain.2007.12.005. [DOI] [PubMed] [Google Scholar]
- Cohen LL, Vowles KE, Eccleston C. Parenting an adolescent with chronic pain: an investigation of how a taxonomy of adolescent functioning relates to parent distress. Journal of Pediatric Psychology. 2010;35(7):748–757. doi: 10.1093/jpepsy/jsp103. [DOI] [PubMed] [Google Scholar]
- Conte PM, Walco GA, Kimura Y. Temperament and stress response in children with juvenile primary fibromyalgia syndrome. Arthritis & Rheumatism. 2003;48(10):2923–2930. doi: 10.1002/art.11244. [DOI] [PubMed] [Google Scholar]
- Daniels D, Moos RH, Billings AG, Miller JJ., 3rd Psychosocial risk and resistance factors among children with chronic illness, healthy siblings, and healthy controls. Journal of Abnormal Child Psychology. 1987;15(2):295–308. doi: 10.1007/BF00916356. [DOI] [PubMed] [Google Scholar]
- DeBaun MR, Telfair J. Transition and sickle cell disease. Pediatrics. 2012;130(5):926–35. doi: 10.1542/peds.2011-3049. [DOI] [PubMed] [Google Scholar]
- Dick BD, Pillai Riddell R. Cognitive and school functioning in children and adolescetns with chronic pain: A critical review. Pain Research & Management. 2010;15(4):238–244. doi: 10.1155/2010/354812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccleston C, Crombez G, Scotford A, Clinch J, Connell H. Adolescent chronic pain: Patterns and predictors of emotional distress in adolescents with chronic pain and their parents. Pain. 2004;108(3):221–229. doi: 10.1016/j.pain.2003.11.008. [DOI] [PubMed] [Google Scholar]
- Eccleston C, Palermo TM, Fisher E, Law E. Psychological interventions for parents of children and adolescents with chronic illness. Cochrane Database of Systematic Reviews. 2012;(2):Art No. CD009660. doi: 10.1002/14651858.CD009660. [DOI] [PMC free article] [PubMed]
- Evans S, Meldrum M, Tsao JC, Fraynt R, Zeltzer LK. Associations between parent and child pain and functioning in a pediatric chronic pain sample: A mixed methods approach. International Journal on Disability and Human Development. 2010;9(1):11–21. doi: 10.1515/IJDHD.2010.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fichtel A, Larsson B. Psychosocial impact of headache and comorbidity with other pains among Swedish school adolescents. Headache. 2002;42(8):766–775. doi: 10.1046/j.1526-4610.2002.02178.x. [DOI] [PubMed] [Google Scholar]
- Fordyce WE, Fowler RS, Jr, Lehmann JF, Delateur BJ, Sand PL, Trieschmann RB. Operant conditioning in the treatment of chronic pain. Archives of Physical Medicine and Rehabilitation. 1973;54(9):399–408. [PubMed] [Google Scholar]
- Forgeron PA, King S, Stinson JN, McGrath PJ, MacDonald AJ, Chambers CT. Social functioning and peer relationships in children and adolescents with chronic pain: A systematic review. Pain Research & Management. 2010;15(1):27–41. doi: 10.1155/2010/820407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldenberg H, Goldenberg I, editors. Family Therapy: An Overview, Seventh Addition. Belmont, CA: Thomson Higher Education; 2008. [Google Scholar]
- Goodman JE, McGrath PJ, Forward SP. Aggregation of pain complaints and pain-related disability and handicap in a community sample of families. Pain Research and Management. In: Jensen TS, Turner JA, Wiesenfeld-Halleb Z, editors. Progress in pain research and management: Vol. 8. Proceedings of the 8th World Congress on Pain. Seattle, WA: IASP; 1997. pp. 1–10. [Google Scholar]
- Grunau RE, Whitfield MF, Petrie-Thomas J, Synnes AR, Cepeda IL, Keidar A, Johannesen D. Neonatal pain, parenting stress and interaction, in relation to cognitive and motor development at 8 and 18 months in preterm infants. Pain. 2009;143(1–2):138–146. doi: 10.1016/j.pain.2009.02.014. doi:0.1016/j.pain.2009.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadjistavropoulos T, Craig KD, Duck S, Cano A, Goubert L, Jackson PL, Mogil JS, Fitzgerald TD. A biopsychosocial formulation of pain communication. Psychological Bulletin. 2011;137(6):910–939. doi: 10.1037/a0023876. [DOI] [PubMed] [Google Scholar]
- Harper FW, Penner LA, Peterson A, Albrecht TL, Taub J. Children’s positive dispositional attributes, parents’ empathic responses, and children’s responses to painful pediatric oncology treatment procedures. Journal of Psychosocial Oncology. 2012;30(5):593–613. doi: 10.1080/07347332.2012.703771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen EH, Holstein BE, Due P, Currie CE. International survey of self-reported medicine use among adolescents. The Annals of Pharmacotherapy. 2003;37(3):361–366. doi: 10.1345/aph.1C111. [DOI] [PubMed] [Google Scholar]
- Hatchette JE, McGrath PJ, Murray M, Finley GA. The role of peer communication in the socialization of adolescents’ pain experiences: a qualitative investigation. BMC Pediatrics. 2008;8:2. doi: 10.1186/1471-2431-8-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hechler T, Vervoort T, Hamann M, Tietze AL, Vocks S, Goubert L, Zernikow B. Parental catastrophizing about their child’s chronic pain: are mothers and fathers different? European Journal of Pain. 2011;15(5):515.e1–9. doi: 10.1016/j.ejpain.2010.09.015. [DOI] [PubMed] [Google Scholar]
- Ho GH, Bennett SM, Cox D, Poole G. Brief report: cognitive functioning and academic achievement in children and adolescents with chronic pain. Journal of Pediatric Psychology. 2009;34(3):311–316. doi: 10.1093/jpepsy/jsn077. [DOI] [PubMed] [Google Scholar]
- Hoftun GB, Romundstad PR, Rygg M. Association of parental chronic pain with chronic pain in the adolescent and young adult: family linkage data from the HUNT study. JAMA Pediatrics. 2013;167(1):61–69. doi: 10.1001/jamapediatrics.2013.422. [DOI] [PubMed] [Google Scholar]
- Huguet A, Miro J. The severity of chronic pediatric pain: an epidemiological study. Journal of Pain. 2008;9:226–236. doi: 10.1016/j.jpain.2007.10.015. [DOI] [PubMed] [Google Scholar]
- Kashikar-Zuck S, Johnston M, Ting TV, Graham BT, Lynch-Jordan AM, Verkamp E, Lovell D. Relationship between school absenteeism and depressive symptoms among adolescents with juvenile fibromyalgia. Journal of Pediatric Psychology. 2010;35(9):996–1004. doi: 10.1093/jpepsy/jsq020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashikar-Zuck S, Parkins IS, Ting TV, Verkamp E, Lynch-Jordan A, Passo M, Graham TB. Controlled follow-up study of physical and psychosocial functioning of adolescents with juvenile primary fibromyalgia syndrome. Rheumatology (Oxford) 2010;49(11):2204–2209. doi: 10.1093/rheumatology/keq254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazak AE. Families of chronically ill children: a systems and social-ecological model of adaptation and challenge. Journal of Consulting and Clinical Psychology. 1989;57(1):25–30. doi: 10.1037/0022-006X.57.1.25. [DOI] [PubMed] [Google Scholar]
- Kazak AE, Alderfer MA, Streisand R, Simms S, Rourke MT, Barakat LP, Cnaan A. Treatment of posttraumatic stress symptoms in adolescent survivors of childhood cancer and their families: a randomized clinical trial. Journal of Family Psychology. 2004;18(3):493–504. doi: 10.1037/0893-3200.18.3.493. [DOI] [PubMed] [Google Scholar]
- King S, Chambers CT, Huguet A, MacNevin RC, McGrath PJ, Parker L, MacDonald AJ. The epidemiology of chronic pain in children and adolescents revisited: A systematic review. Pain. 2011;152(12):2729–2738. doi: 10.1016/j.pain.2011.07.016. [DOI] [PubMed] [Google Scholar]
- Konijnenberg AY, Uiterwaal CS, Kimpen JL, van der Hoeven J, Buitelaar JK, de Graeff-Meeder ER. Children with unexplained chronic pain: substantial impairment in everyday life. Archives of Disease in Childhood. 2005;90(7):680–686. doi: 10.1136/adc.2004.056820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lateef TM, Merikangas KR, He J, Kalaydjian A, Khoromi S, Knight E, Nelson KB. Headache in a national sample of American children: prevalence and comorbidity. Journal of Child Neurology. 2009;24(5):536–543. doi: 10.1177/0883073808327831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lester N, Lefebvre JC, Keefe FJ. Pain in young adults: I. Relationship to gender and family pain history. The Clinical Journal of Pain. 1994;10(4):282–289. doi: 10.1097/00002508-199412000-00007. [DOI] [PubMed] [Google Scholar]
- Levy RL, Langer SL, Walker LS, Romano JM, Christie DL, Youssef N, Whitehead WE. Cognitive-behavioral therapy for children with functional abdominal pain and their parents decreases pain and other symptoms. American Journal of Gastroenterology. 2010;105(4):946–956. doi: 10.1038/ajg.2010.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewandowski AS, Palermo TM. Parent-teen interactions as predictors of depressive symptoms in adolescents with headache. Journal of Clinical Psychology in Medical Settings. 2009;16(4):331–338. doi: 10.1007/s10880-009-9173-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewandowski AS, Palermo TM, Stinson J, Handley S, Chambers CT. Systematic review of family functioning in families of children and adolescents with chronic pain. The Journal of Pain. 2010;11(11):1027–1038. doi: 10.1016/j.jpain.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipani TA, Walker LS. Children’s appraisal and coping with pain: relation to maternal ratings of worry and restriction in family activities. Journal of Pediatric Psychology. 2006;31(7):667–673. doi: 10.1093/jpepsy/jsj038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan DE, Engle LB, Feinstein AB, Sieberg CB, Sparling P, Cohen LL, Masuda A. Ecological system influences in the treatment of pediatric chronic pain. Pain Research and Management. 2012;17(6):407–411. doi: 10.1155/2012/289504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan DE, Simons LE, Stein MJ, Chastain L. School impairment in adolescents with chronic pain. Journal of Pain. 2008;9(5):407–416. doi: 10.1016/j.jpain.2007.12.003. [DOI] [PubMed] [Google Scholar]
- McGoldrick M, Carter B, Garcia-Preto N, editors. The expanded family life cycle: Individual, family, and social perspectives. 4. Needham Heights, MA: Allyn & Bacon; 2011. [Google Scholar]
- Oates J, Wood C, Grayson A, editors. Psychological development and early childhood. Malden, MA: Blackwell Publishing Ltd; 2005. [Google Scholar]
- Oliver-Carpenter G, Barach I, Crosby LE, Valenzuela J, Mitchell MJ. Disease management, coping, and functional disability in pediatric sickle cell disease. Journal of the National Medical Association. 2011;103(2):131–137. doi: 10.1016/s0027-9684(15)30262-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palermo TM. Cognitive-behavioral therapy for chronic pain in children and adolescents. New York, NY: Oxford University Press; 2012. [Google Scholar]
- Palermo TM, Chambers CT. Parent and family factors in pediatric chronic pain and disability: An integrative approach. Pain. 2005;119(1–3):1–4. doi: 10.1016/j.pain.2005.10.027. [DOI] [PubMed] [Google Scholar]
- Palermo TM, Eccleston C. Parents of children and adolescents with chronic pain. Pain. 2009;146(1–2):15–17. doi: 10.1016/j.pain.2009.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palermo TM, Eccleston C, Lewandowski AS, Williams AC, Morley S. Randomized controlled trials of psychological therapies for management of chronic pain in children and adolescents: An updated meta-analytic review. Pain. 2010;148(3):387–397. doi: 10.1016/j.pain.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palermo TM, Putnam J, Armstrong G, Daily S. Adolescent autonomy and family functioning are associated with headache-related disability. Clinical Journal of Pain. 2007;23(5):458–465. doi: 10.1097/AJP.0b013e31805f70e2. [DOI] [PubMed] [Google Scholar]
- Palermo TM, Wilson AC, Peters M, Lewandowski A, Somhegyi H. Randomized controlled trial of an Internet-delivered family cognitive-behavioral therapy intervention for children and adolescents with chronic pain. Pain. 2009;146(1–2):205–213. doi: 10.1016/j.pain.2009.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perquin CW, Hazebroek-Kampschreur AA, Hunfeld JA, Bohnen AM, van Suijlekom-Smit LW, Passchier J, van der Wouden JC. Pain in children and adolescents: A common experience. Pain. 2000;87(1):51–58. doi: 10.1016/S0304-3959(00)00269-4. [DOI] [PubMed] [Google Scholar]
- Pillai Riddell RR, Stevens BJ, McKeever P, Gibbins S, Asztalos L, Katz J, Din L. Chronic pain in hospitalized infants: health professionals’ perspectives. The Journal of Pain. 2009;10(12):1217–1225. doi: 10.1016/j.jpain.2009.04.013. [DOI] [PubMed] [Google Scholar]
- Ramchandani PG, Stein A, Hotopf M, Wiles NJ. Early parental and child predictors of recurrent abdominal pain at school age: results of a large population-based study. Journal of the American Academy of Child & Adolescent Psychiatry. 2006;45(6):729–736. doi: 10.1097/01.chi.0000215329.35928. [DOI] [PubMed] [Google Scholar]
- Rhee H. Physical symptoms in children and adolescents. Annual Review of Nursing Research. 2003;21:95–121. [PubMed] [Google Scholar]
- Romano LJ, Hubbard JA, McAuliffe MD, Morrow MT. Connections between parents’ friendships and childrens’ peer relationships. Journal of Social and Personal Relationships. 2009;26(2–3):315–325. doi: 10.1177/0265407509106720. [DOI] [Google Scholar]
- Roth-Isigkeit A, Thyen U, Stoven H, Schwarzenberger J, Schmucker P. Pain among children and adolescents: Restrictions in daily living and triggering factors. Pediatrics. 2005;115(2):152–162. doi: 10.1542/peds.2004-0682. [DOI] [PubMed] [Google Scholar]
- Rubin KH, Bukowski WM, Laursen B, editors. Handbook of peer interactions, relationships, and groups. New York, NY: Guilford Press; 2009. [Google Scholar]
- Sanders MR, Shepherd RW, Cleghorn G, Woolford H. The treatment of recurrent abdominal pain in children: a controlled comparison of cognitive-behavioral family intervention and standard pediatric care. Journal of Consulting and Clinical Psychology. 1994;62(2):306–314. doi: 10.1037/0022-006X.62.2.306. [DOI] [PubMed] [Google Scholar]
- Schatz J, Finke RL, Kellett JM, Kramer JH. Cognitive functioning in children with sickle cell disease: a meta-analysis. Journal of Pediatric Psychology. 2002;27(8):739–748. doi: 10.1093/jpepsy/27.8.739. [DOI] [PubMed] [Google Scholar]
- Schechter N, Palermo TM, Walco G, Berde C. Persistent pain in children. In: Ii Ballantyne JC, Rathmell JP, Fishman SM, editors. Bonica’s Management of Pain. 4. Philadelphia: Lippincott Williams & Wilkins; 2009. pp. 767–782. [Google Scholar]
- Sleed M, Eccleston C, Beecham J, Knapp M, Jordan A. The economic impact of chronic pain in adolescence: Methodological considerations and a preliminary costs-of-illness study. Pain. 2005;119(1–3):183–190. doi: 10.1016/j.pain.2005.09.028. [DOI] [PubMed] [Google Scholar]
- Stanford EA, Chambers CT, Biesanz JC, Chen E. The frequency, trajectories and predictors of adolescent recurrent pain: A population-based approach. Pain. 2008;138(1):11–21. doi: 10.1016/j.pain.2007.10.032. [DOI] [PubMed] [Google Scholar]
- Telfair J, Myers J, Drezner S. Transfer as a component of the transition of adolescents with sickle cell disease to adult care: adolescent, adult, and parent perspectives. Journal of Adolescent Health. 1994;15(7):558–565. doi: 10.1016/1054-139X(94)90139-T. [DOI] [PubMed] [Google Scholar]
- Trautmann E, Lackschewitz H, Kroner-Herwig B. Psychological treatment of recurrent headache in children and adolescents--a meta-analysis. Cephalalgia. 2006;26(12):1411–1426. doi: 10.1111/j.1468-2982.2006.01226.x. [DOI] [PubMed] [Google Scholar]
- van den Boom DC. The influence of temperament and mothering on attachment and exploration: an experimental manipulation of sensitive responsiveness among lower-class mothers with irritable infants. Child Development. 1994;65(5):1457–1477. doi: 10.1111/j.1467-8624.1994.tb00829.x. [DOI] [PubMed] [Google Scholar]
- van der Net J, van der Torre P, Engelbert RH, Engelen V, van Zon F, Takken T, Helders PJ. Motor performance and functional ability in preschool- and early school-aged children with Juvenile Idiopathic Arthritis: a cross-sectional study. Pediatric Rheumatology. 2008;6:2. doi: 10.1186/1546-0096-6-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Staa AL, Jedeloo S, van Meeteren J, Latour JM. Crossing the transition chasm: experiences and recommendations for improving transitional care of young adults, parents and providers. Child Care Health and Development. 2011;37(6):821–32. doi: 10.1111/j.1365-2214.2011.01261.x. [DOI] [PubMed] [Google Scholar]
- Walco GA, Sterling CM, Conte PM, Engel RG. Empirically supported treatments in pediatric psychology: disease-related pain. Journal of Pediatric Psychology. 1999;24(2):155–167. doi: 10.1093/jpepsy/24.2.155. discussion 168–171. [DOI] [PubMed] [Google Scholar]
- Walker LS, Baber KF, Garber J, Smith CA. A typology of pain coping strategies in pediatric patients with chronic abdominal pain. Pain. 2008;137(2):266–275. doi: 10.1016/j.pain.2007.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker LS, Greene JW. Children with recurrent abdominal pain and their parents: more somatic complaints, anxiety, and depression than other patient families? Journal of Pediatric Psychology. 1989;14(2):231–243. doi: 10.1093/jpepsy/14.2.231. [DOI] [PubMed] [Google Scholar]
- Walker LS, Zeman JL. Parental response to child illness behavior. Journal of Pediatric Psychology. 1992;17(1):49–71. doi: 10.1093/jpepsy/17.1.49. [DOI] [PubMed] [Google Scholar]
- Walsh TM, McGrath PJ, Symons DK. Attachment dimensions and young children’s response to pain. Pain Research and Management. 2008;13(1):33–40. doi: 10.1155/2008/235329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicksell RK, Melin L, Lekander M, Olsson GL. Evaluating the effectiveness of exposure and acceptance strategies to improve functioning and quality of life in longstanding pediatric pain--a randomized controlled trial. Pain. 2009;141(3):248–257. doi: 10.1016/j.pain.2008.11.006. [DOI] [PubMed] [Google Scholar]
- Williamson GM, Walters AS, Shaffer DR. Caregiver models of self and others, coping, and depression: predictors of depression in children with chronic pain. Health Psychology. 2002;21(4):405–410. doi: 10.1037/0278-6133.21.4.405. [DOI] [PubMed] [Google Scholar]
- Wolff N, Darlington AS, Hunfeld J, Verhulst F, Jaddoe V, Hofman A, Tiemeier H. Determinants of somatic complaints in 18-month-old children: the generation R study. Journal of Pediatric Psychology. 2010;35(3):306–316. doi: 10.1093/jpepsy/jsp058. [DOI] [PubMed] [Google Scholar]
- Wysocki T, Harris MA, Buckloh LM, Mertlich D, Lochrie AS, Mauras N, White NH. Randomized trial of behavioral family systems therapy for diabetes: maintenance of effects on diabetes outcomes in adolescents. Diabetes Care. 2007;30(3):555–560. doi: 10.1093/jpepsy/25.1.23. [DOI] [PubMed] [Google Scholar]