Abstract
The traditional view that gene and environment interactions control disease susceptibility can now be expanded to include epigenetic reprogramming as a key determinant of origins of human disease. Currently, epigenetics is defined as heritable changes in gene expression that do not alter DNA sequence but are mitotically and trans-generationally inheritable. Epigenetic reprogramming is the process by which an organism’s genotype interacts with the environment to produce its phenotype and provides a framework for explaining individual variations and the uniqueness of cells, tissues, or organs despite identical genetic information. The main epigenetic mediators are histone modification, DNA methylation, and non-coding RNAs. They regulate crucial cellular functions such as genome stability, X-chromosome inactivation, gene imprinting, and reprogramming of non-imprinting genes, and work on developmental plasticity such that exposures to endogenous or exogenous factors during critical periods permanently alter the structure or function of specific organ systems. Developmental epigenetics is believed to establish “adaptive” phenotypes to meet the demands of the later-life environment. Resulting phenotypes that match predicted later-life demands will promote health, while a high degree of mismatch will impede adaptability to later-life challenges and elevate disease risk. The rapid introduction of synthetic chemicals, medical interventions, environmental pollutants, and lifestyle choices, may result in conflict with the programmed adaptive changes made during early development, and explain the alarming increases in some diseases. The recent identification of a significant number of epigenetically regulated genes in various model systems has prepared the field to take on the challenge of characterizing distinct epigenomes related to various diseases. Improvements in human health could then be redirected from curative care to personalized, preventive medicine based, in part, on epigenetic markings etched in the “margins” of one’s genetic make-up.
Keywords: DNA methylation, Histone modification, Chromatin remodeling, Nongenomic heritage, Developmental plasticity, Relaxation of imprinting
1 Epigenetics meets genetics in disease susceptibility
In the past, susceptibility of disease was believed to be determined solely by inheritable information carried on the primary sequence of the DNA. Individuals are endowed with different genotypes that dictate how they respond to endogenous factors such as development cues, hormones, and cytokines or to exogenous influences, including nutrient availability, infection, physical activities, social behavior, and other environmental factors. Over time, these responses form the basis of genetic variability to disease susceptibility. Aberrant changes in linear DNA sequence result in mutations, deletions, gene fusion, tandem duplications, or gene amplifications causing dysregulation of gene expression that underlies the genesis of disease [1–7]. Recently, however, it has become clear that epigenetic disruption of gene expression plays an equally important role in the development of disease [8–10] and, arguably, that this process is more susceptible than the former to environmental modulation.
The term epigenetics means outside conventional genetics [11] and was coined by the developmental biologist Conrad H. Waddington (1905–1975) [12]. Waddington treated Drosophila pupae with heat and observed altered wing-vein patterns [13]. The altered phenotype persisted in the population long after the stimulus was removed, suggesting that exposure to an environmental factor during a critical developmental window could produce a phenotype-change that lasted a lifetime and was manifested in subsequent generations. He referred to this phenomenon as “genetic assimilation,” or “epigenetics” in modern terminology. According to this paradigm, epigenetics is the process by which the genotype of an organism interacts with the environment to produce its phenotype. It provides a framework to explain the source of variations in individual organisms [14] and also explains what makes cells, tissues, and organs different albeit the identical nature of the genetic information in every cell in the body, since different sets of genes are expressed in different cells in a distinct temporal sequence. The concept has thus expanded the “gene-centric” view of inheritance biology by introducing the possibility of “nongenomic inheritance” as an adaptive mechanism for coping with environmental changes [8, 11].
Currently, epigenetics is defined as heritable changes in gene expression that occur without alterations in DNA sequence. Epigenetic modifications are mitotically and transgenerationally inheritable [15–17]. Three distinct and intertwined mechanisms are now known to regulate the “epigenome”: small-interfering RNAs, DNA methylation, and histone modifications [18–20]. These processes affect transcript stability, DNA folding, nucleosome positioning, chromatin compaction, and ultimately nuclear organization. Synergistically and cooperatively they determine whether a gene is silenced or activated and when and in what tissue it will be expressed. Thus, epigenetics has greatly expanded our understanding of the context of gene expression, which previously was believed to be dictated by the primary nucleotide sequence of a gene. Disruption of the epigenome or induction of “epimutations” [21] certainly underlies disease development [8–10]. Therefore, disease susceptibility is clearly a result of a complex interplay between one’s genetic endowment and epigenetic marks “imprinted” on one’s genome by endogenous or exogenous factors [11].
2 Developmental plasticity, adaptive developmental reprogramming, and early origins of human diseases
Epidemiologic studies now support an early origin of adult human diseases. Classic examples include association between low birth weight and a greater risk of coronary heart disease, hypertension, stroke, depression, type 2 diabetes, and osteoporosis in later life [8, 10, 22–27]. The paradigm of early origins of adult disease is rooted in the process of developmental plasticity [8, 28, 29]. Most human organ systems begin to develop early in gestation and do not become fully mature until weeks, months, or years after birth. A relatively long gestation and a period of postnatal and perhaps prepubertal maturation allow for prolonged interactions with the environment. These include episodes of hypoxia; hypo- or hypernourishment; infection; and hormonal, drug, or toxin exposures. Developmental plasticity occurs when such exposures, during critical periods of maturation, result in permanent alterations in the structure or function of specific organ systems. This process, commonly referred to as “developmental reprogramming or imprinting” [8, 23, 24], is a so-called adaptive trait since it is an attempt to establish phenotypes that meet the demands of later-life environment [8, 10, 11, 28, 29]. When the resulting phenotypes match the predicted later-life demands, the individual will remain healthy. When there is a high degree of mismatch, however, one’s adaptability to adult life challenges will be impeded and disease risk will be elevated. The latter scenario is more frequent today than in past decades since contemporary human life is greatly influenced by lifestyle choices, which often are in conflict with the programmed adaptive changes made during early development. In addition, synthetic chemicals that mimic internal cues and artificial reproductive technologies are introduced into daily life at alarming rates. These can induce developmental reprogramming with no apparent late-life adaptive values. Collectively, these factors have increased the odds of a mismatch between early developmental programming and later-life demands that have caused various human diseases in recent decades.
Mechanistically, epigenetics underpins developmental reprogramming or imprinting. Thus, understanding how environmental factors influence various epigenetic processes during developmental reprogramming should provide new insights into early diagnosis, prevention, and treatment of these diseases.
3 Epigenetics as a mechanism of developmental reprogramming
The two most studied epigenetic mechanisms recognized as having a role in adaptive developmental programming are histone modifications [30] and DNA methylation [31, 32]. In simple terms, histone modifications refer to post-translational modifications of histone tails and DNA methylation involves the methylation of cytosine at the carbon-5 position in CpG dinucleotides. These processes work together to affect chromatin packaging, which in turn determines which gene or gene set is transcribed. Changes mediated by either process are heritable; not only are they transmittable to the daughter cells but to subsequent generations. More recently, the roles of small/micro antisense RNA transcripts in gene regulation have come under intense scrutiny [33, 34]. Disruption of gene expression, via one or more of these mechanisms, likely underpins early origins of adult diseases.
DNA methylation refers to the covalent addition of a methyl group derived from S-adenosyl-L-methionine to the fifth carbon of the cytosine ring to form the fifth base, 5-methyl cytosine (5meC) [31, 32, 35]. The reaction is catalyzed by DNA methyltransferases and accessory proteins (Dnmt1, Dnmt3a, Dnmt 3b, Dnmt2, and Dnmt 3L). Across eukaryotic species, methylation occurs predominantly in cytosines located 5′ of guanines, known as CpG dinucleotides (CpGs). In the mammalian genome, the distribution of CpG dinucleotides is nonrandom [36]. CpG dinucleotides are greatly underrepresented in the genome because of evolutionary loss of 5meCs through deamination to thymine. However, clusters of CpGs known as CpG islands (CGIs) are preserved in 1–2% of the genome. Typically, they range in length from 200 bp to 5 kb. Most are unmethylated (in which occur near the transcription start sites) under normal circumstances except those associated with imprinted genes, genes subjected to X-chromosome inactivation, and transposable elements [31, 32, 35, 36]. In this regard, DNA methylation is thought to repress inappropriate expression of endogenous transposons that may disrupt the genome and are involved in parental-specific silencing of one allele of imprinted genes [31, 35, 37]. In addition, about 70% of CGIs are associated with DNA sequences 200 bp to 2 kb long located in the promoter, the first and second exons, and the first intron regions of all genes (5′ CGIs), suggesting that CGIs are important for gene regulation [31, 35, 36]. There is usually an inverse relationship between the extent of methylation of a regulatory CGI and gene transcription. Two mechanisms have been proposed to explain how cytosine methylation leads to repression of gene transcription [31]. First, the methyl group of the 5meC extends into the major groove of DNA and inhibits binding of transcription factors (TFs) to their CpG-containing recognition sites. Second, a class of proteins known as methyl cytosine binding proteins (MeCPs) specifically binds methylated CGIs and create steric hindrance to the access by TFs to their regulatory elements. Both mechanisms will suppress gene transcription. Furthermore, upon binding to methylated CGIs, MeCPs recruit histone deacetylases (HDACs) and histone methyl-transferases (HMTs). These enzymes mediate complex histone modifications (discussed below) and result in the establishment of repressive chromatin structures that permanently silent gene transcription [31, 35].
DNA methylation patterns are established through defined phases during the development of an organism. With the exception of imprinted genes, gamete methylation patterns are erased by a genome-wide demethylation at around the eight-cell stage of blastocyst formation. During the implantation stage, methylation patterns are established via de novo methylation. During adulthood, the amount and pattern of methylation are tissue- and cell-type-specific. Disruption of these preset patterns of DNA methylation in adult life has been linked to aging and disease development [38–40]. Furthermore, dysregulation of developmental programming by maternal factors or environmental mimics is now believed to induce abnormal DNA methylation of specific genes that permit them to undergo inappropriate expression in adult life, leading to disease development. Recent evidence strongly supports DNA methylation is a key mechanism of the developmental basis of adult diseases. Contemporary methodologies needed to identify these target genes have been reviewed [41].
Linear DNA is wrapped by an octameric complex composed of two molecules of each of the four histones H2A, H2B, H3, and H4 to form an array of nucleosomes. The amino termini of histones contain a diversity of post-translational modifications [42]. Among them, acetylation and methylation of lysine residues in the amino termini of histones H3 and H4 are most highly correlated with transcriptional activities. Histone acetylation is usually associated with transcriptional activation due to the lower affinity of the acetylated histone for DNA, therefore allowing relaxation of the chromatin. Conversely, deacetylation of histone correlates with transcriptional silencing and the heterochromatic state. In addition to histone acetylation, methylation of histone H3 lysine 9 (H3 Lys9) correlates with a repressed state and heterochromatin assembly. Each lysine residue may be methylated in the form of mono-, di-, or trimethylation, adding to the complexity of the “histone code” [42]. Methylation of the histone tails increases the basicity and hydrophobicity of the histones, as well as its affinity for anionic molecules such as DNA. Thus, histones with methylated tails are found in heterochromatin harboring transcriptionally inactive genes. Acetylation is catalyzed by histone acetyl-transferases (HATs) and is removed by opposing histone deacetylases (HDACs). Methyl substitution is catalyzed by the histone methyltransferases (HMTs) and may be removed by yet-unidentified histone demethylases (HDMs). It is now known that histone modifications work hand in hand with DNA methylation to regulate chromatin structure and gene expression. However, it remains tenuous whether early life or environmental factors influence the “histone code” in a manner similar to their influence on DNA methylation.
Evidence is rapidly emerging of a role of noncoding RNA transcripts (ncRNAs) in gene regulation [33, 34]. These ncRNAs have been shown to modulate stability and translation of mRNAs by modulating polysome functions and chromatin structure. However, little to no information is available on whether early life and/or environmental factors could have an impact on these molecules. This will be a very fertile field of future investigation.
4 Early life and environmental factors in epigenetic reprogramming
Table 1 lists the studies demonstrating the epigenetic modifications induced by early-life and environment factors and their associated diseases/disorders. We choose to focus heavily on experimental models that provide more-mechanistic insights, while highlighting relevant epidemiologic studies. Several reviews have provided extensive accounts on this topic [8, 43–47].
Table 1.
Epigenetic effects of diet and environment in endocrine and metabolic disorders
| Dietary Factors | Epigenetic Effects Found in endocrine and metabolic disorders | References |
|---|---|---|
| Dietary Methyl Donors and Cofactors | N/A | [48], [49], [50], [51] |
| Fat intake | Mammary glands tumor | [52] |
| Glucose intake | Type 2 Diabetes | [53], [54] |
| Phytoestrogens | ||
| Coumestrol | Hypermethylation of oncogen, c-H-ras in rat pancreas | [58] |
| Genistein | Prostate disease, obesity, female reproductive tract disorders | [59], [60], [61] |
| (−)-epigallocatechin-3-O-gallate (EGCG) | N/A | [62], [63] |
| Environmental Factors | ||
| Heavy Metal | ||
| Chromium | Sperm cells development | [64], [65] |
| Cadmium | N/A | [66], [67] |
| Arsenic | Ovarian, adrenal glands tumor, cardiovascular disease | [68], [69] |
| Lead | N/A | [71] |
| Nickel | N/A | [72], [73] |
| Xenochemicals | ||
| Diethylstilbestrol (DES) | Uterine cancer, testicular cancer | [75], [76], [77], [78], [79], [80], [81] |
| Bisphenol A (BPA) | Prostate cancer, mammary cancer | [82], [83], [84] |
| Vinclozolin and methoxychlor | Infertility; abnormalities in prostate, testis, mammary gland; and mate preference | [17], [85], [86] |
| 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) | N/A | [87], [88] |
| Phthalate esters | N/A | [90] |
| Polychlorinated biphenyls (PCBs) | Reproductive system disruption | [91], [92] |
| Chlorine disinfection by-products (DBPs) | N/A | [93] |
N/A represents that particular factor that shows epigenetic effect on the genome but not directly associates with endocrine and metabolic disorders.
4.1 Dietary factors
Maternal nutritional status and dietary factors are important developmental cues for fetal reprogramming that impact adult diseases. In animal studies we can pinpoint the exact developmental stages of exposure and the mechanism of reprogramming. However, population-based studies often provide only correlation data for extrapolations.
4.1.1 Dietary methyl donors and cofactors
Folate, methionine, choline, and vitamin B12 are dietary methyl donors and cofactors involved in S-adenosylmethione (SAM)-substrated methylation. Waterland and Jirtle first demonstrated that supplementation of the diets of female mice with methyl donors before and after pregnancy permanently increased DNA methylation at the viable yellow agouti (Avy) metastable epiallele in their offspring [48]. These investigators further showed epigenetic plasticity to methyl-donor diets at another metastable epiallele, axin fused (AxinFu). The window of susceptibility was not restricted to early embryos but extended to mid-gestation [49]. Several population studies have demonstrated a strong correlation between folate status and coronary artery disease. Patients with the atherosclerotic vascular disease often exhibit higher homocysteine and S-adenosylhomocysteine (SAHC) and lower genomic DNA methylation status [50]. However, no specific genes have been identified as the target of reduced methylation in these patients. Another intriguing example of disease susceptibility related to methyl donor diet was illustrated in a population study reporting an association between folate status and a mutation in the gene encoding methylenetetrahydrofolate reductase, a key enzyme in determining genomic DNA methylation. This study suggests an interplay between nutritional epigenetics and genetic susceptibility in the modulation of gene expression [51]. Collectively, these findings offer valuable insights into the pathophysiology of diseases associated with nutritional epigenetics. This knowledge may also inspire novel prevention strategies by modifying the nutritional status of at-risk populations.
4.1.2 High fat or glucose intake
Typical western diets are high in fat and refined sugar (glucose). It is now known that a high-fat diet during pregnancy is associated with an elevated fetal estrogenic environment and a higher incidence of mammary cancer in the offspring. In Sprague–Dawley rats, during normal aging, mammary estrogen receptor (ER) undergoes progressive hypermethylation-mediated transcriptional silencing, which may offer a protective mechanism against cell growth and tumorigenesis. However, in utero exposure to a high-fat diet resulted in hypomethylation of the receptor’s promoter and overexpression of ER in rat mammary glands, which is associated with a higher incidence of tumorigenesis in the exposed offspring [52]. Thus, early exposure to a high-fat diet has permanently disrupted a natural developmental program that might afford protection against mammary tumorigenesis.
Prenatal glucose levels influence the risk of developing type 2 diabetes in later life, suggesting the presence of epigenetic memory that may persist in insulin/glucose target tissues [53]. Furthermore, the development of obesity or type 2 diabetes is associated with glucose-induced, persistent changes in gene expression. Among the affected genes is glucose transporter 4 (GLUT4), which is the major glucose transporter in adipose tissue, and skeletal and cardiac muscles. With 3T3-L1 used as a cell model, it was shown that both methylation of specific CpG sites and a methylation-sensitive transcription factor contribute to GLUT4 gene regulation during preadipocytes to adipocytes differentiation [53]. Additionally, differential DNA methylation was observed in promoters of genes involved in glucose metabolism. These include the facilitative glucose transporter 4 [54] and the uncoupling protein 2 [55]. Both are major targets involved in the development of type 2 diabetes.
4.1.3 Bioflavonoids and Tea catechins
Recently, there have been grave concerns about the health impacts of the isoflavones, a class of phytoestrogens readily found in the diet, particularly in soy products. They are biologically active and can signal via ER- and non-ER-mediated pathways [56, 57]. Moreover, early developmental exposure to the phytoestrogens equol and coumestrol induced hypermethylation of the c-H-ras promoter and gene silencing in the rat pancreas [58]. Adult dietary supplement with genistein, a major isoflavone in soy, was shown to hypermethylate a set of genes in rat prostate [59]. In contrast, maternal supplementation with genistein was shown to shift the coat color and protect Avy mouse offspring from obesity by modifying the fetal epigenome [60]. Additionally, genistein treatment of neonatal mice caused significant abnormalities in the reproductive systems of the female mice [61]. Collectively, these animal studies raise concerns about the safety of developmental exposure to genistein. Questions related to the susceptibility of developmental plasticity to phytoestrogens and long-term epigenetic memories remain unanswered. Beside bioflavonids, the tea polyphenol (−)-epigallocatechin-3-O-gallate (EGCG), is another natural compounds found to have modulating effects on DNA methylation [62]. EGCG was found to inhibit DNA methyltransferases and reactivate specific methylation-silenced genes in human colon and prostate cancer cell lines [63].
4.2 Environmental factors
4.2.1 Heavy metals
The adverse health effects of occupational or environmental exposure to heavy metals can be mediated by epigenetic mechanisms. Heavy metals such as chromium (Cr), cadmium (Cd), arsenic (As), lead (Pb), and nickel (Ni) have been reported to exert epigenetic effects on the genome. Paternal exposure to Cr(III) was shown to modify the epigenome/genome and to induce transgenerational carcinogenesis [64]. Paternal exposure to Cr(III) increases tumor risk in offspring. One allele of the 45S rRNA spacer promoter was found to be hypomethylated in sperm germ cells after exposure to the heavy metal, leading to speculation that this epimutation may increase tumor risk in the offspring [65]. Short-term exposure of rat liver cells to cadmium inhibited DNA methyltransferase activity, but prolonged exposure to this metal ion caused neoplastic transformation and attended increases in DNA methylation and DNA methyltransferase activity [66]. The pathologies of heavy metal (Ni, As, and Cd) toxicity in rodents resemble those seen in animals fed a methyl-deficient diet (lacking choline and folate) [67]. The metal ions, as well as the diet, significantly inhibited DNA methyltransferase activity, perhaps leading to hypomethylation of disease-causing genes. Epidemiologic data corroborate experimental findings and consistently show a broad range of disorders associated with arsenic exposure in humans. These include cancer, atherosclerosis, neurologic disturbance, and cardiovascular diseases [68]. Moreover, exposure to arsenic in utero induces cancer of the liver, lungs, urinary bladder, and kidneys [69]. In this regard, arsenic is classified as a “complete” transplacental carcinogen in mice. In addition to its potent mutagenic action, arsenic can act through epigenetic mechanisms that modify DNA methylation patterns. The metal ion has been shown to deplete SAM and repress DNMT1 and DNMT3A [70]. Other metals, such as lead and nickel, have also been reported to have relevance in transplacental exposures and later carcinogenic effects by acting through epigenetic mechanisms [71, 72]. More recent studies have also reported heavy metal-associated histone modifications and chromatin organization [72, 73]. Specifically, nickel ions induce the silencing of the gpt transgene in G12 Chinese hamster cells by increasing histone H3K9 dimethylation via inhibition of H3K9 demethylation [73].
4.2.2 Xenochemicals and endocrine disruptors
The link between in utero exposure to the synthetic estrogen diethylstilbestrol (DES) and increased incidence of reproductive tract cancers in “DES daughters” has been a difficult lesson learned by the health-care community [74]. DES was prescribed during 1938 to 1971 to women to prevent miscarriages It is a long-acting estrogen and hence a potent endocrine disruptor. In humans and experimental animals, exposure to DES during critical windows in early development disrupts the differentiation of the reproductive tract and results in a high incidence of structural and functional abnormalities, as well as of cancers in hormone-sensitive organs. Neonatal exposure of mice to DES induced demethylation of a single CpG site in the promoter of the lactoferrin gene [75] and exon-4 demethylation in the c-fos gene [76] in the uteri of the exposed animals. Prenatal exposure of mice to this xenoestrogen increased both liver weight and ribosomal DNA hypermethylation [77]. Recently, with the use of restriction landmark genomic scanning (RLGS), which can analyze genome-wide DNA methylation, seven loci of the genomic DNA were found to be demethylated and one locus methylated in the epididymis of fetal and neonatal DES-treated mice [78]. In collaboration with Newbold and associates, we recently reported that neonatal treatment of mice with DES or genistein prevents the ovarian steroid-induced silencing of NSBP1 gene in the uteri of intact mice after the onset of puberty, via DNA hypermethylation [79]. The developmentally reprogrammable gene was identified by methylation-sensitive restriction fingerprinting (MSRF) [41]. In this model, neonatal exposure to DES or genistein induces high incidences of endometrial cancer in intact mice approaching 18 months of age. Therefore, we hypothesize that the normal silencing of NSBP1 by ovarian steroids is disrupted by neonatal estrogen exposure. In this regard, epigenetic reprogramming by ovarian steroids during adult life may serve as a protective mechanism against endometrial cancer development. Early life disruption of this program could be the culprit of DES/genistein-induced endometrial cancer. Most important, it has been proposed that the adverse effects of DES can be transmitted across generations [80, 81]. The molecular targets that mediate this process are yet to be discovered.
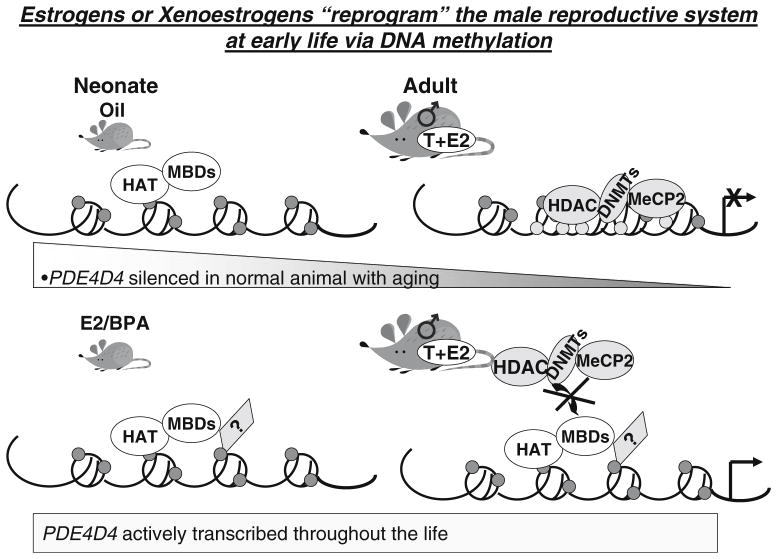
Bisphenol A (BPA) was first synthesized as an estrogen mimic. At present, it is commonly used in the manufacture of polycarbonate plastics and epoxy resins. Developmental exposure to BPA results in morphologic and functional alterations of the male and female genital tracts and mammary glands and subsequently in an increase in susceptibility to infertility and the development of malignancies of the breast and prostate [82, 83]. In collaboration with Prins and associates, we first reported that neonatal exposure to environmentally relevant doses of BPA alters the prostate epigenome [84]. Using MSRF as an unbiased screening platform, we identified prostate phosphodiesterase type 4 variant 4 (PDE4D4) to be a target of epigenetic reprogramming (Fig. 1). PDE4D4 is destined to undergo age-dependent transcriptional silencing via progressive hypermethylation of its promoter. However, neonatal exposure of mice to BPA or estrogen disrupts this developmental program and leaves the promoter of PDE4D4 resistance to a predetermined “shut-off’ program. This aberration allows persistent overexpression of the gene throughout adult life. Since we recently found that PDE4D4 promotes prostate cell growth (Tang, unpublished data), we therefore hypothesize that chronic aberrant overexpression of PDE4D4 leads to unscheduled growth of the prostate that may predispose it to neoplastic transformation. Indeed, our data [84] showed an increased incidence of precancerous lesions in the prostates of mice neonatally treated with estrogen/BPA.
Fig. 1.
Schematic diagram of our hypothesis on how neonatal estrogen (E2) and bisphenol A (BPA) alter the prostate genome via DNA methylation and histone modifications with phosphodiesterase type 4 variant 4 (PDE4D4) used as an example. Without exposure to E2 and BPA, PDE4D4 is silenced with aging. A group of enzymes, including histone deacetylases (HDACs), DNA methyltransferases (DNMTs), and methylated DNA-binding protein (MeCP2), may be involved in gene silencing. Nevertheless, after exposure to E2 and BPA, PDE4D4 fails to “shut-off” and is actively transcribed throughout life. We propose that chromatin structure is remodeled by opening the chromatin in the presence of histone acetyltransferase (HAT), methylation binding domains (MBDs) and other unknown factors and that this remodeling further prevents HDACs, DNMTs, and MeCP2 from binding to silence the gene. Persistent elevation of PDE4D4 expression is associated with the increase in incidence of prostate cancer later in life
Anway, Skinner, and co-workers [17, 85, 86] have provided the first evidence that in utero exposure of male mice to vinclozolin, a pesticide and an antiandrogen, induced epigenetic changes that could be transmitted across four generations. The epigenetic modification in specific DNA sequences is associated with adult phenotypes such as decreased spermatogenic capacity and increased incidence of infertility [17]; abnormalities in the prostate, breast, kidney, testis, and immune system [85]; and aberrant mating behavior [86]. The latter phenotype has significant ramifications in evolutionary biology as it suggests that epigenetic transgenerational inheritance of endocrine disruptor action impacts sexual selection and natural selection. In this regard, epigenetics could play a determinant role in evolution and natural selection of species [86].
2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD) is an environmental contaminant that has been reported to disrupt the normal development of experimental animals [87]. Exposure of mouse pre-implantation embryos to TCDD inhibited fetal growth, a response strongly associated with the changes in the methylation status of the imprinted genes H19 and IGF2 [88]. TCDD also induced histone modifications in normal human mammary epithelial cells [89]. Other environmental contaminants such as phthalate esters [90], polychlorinated biphenyls [91, 92], and chlorine disinfection by-products [93] also affect the reproductive system or induce tumor development by altering DNA methylation and/or steroid hormone metabolism and signaling.
5 Conclusions
Although this review has focused heavily on the interaction between environmental factors and epigenetics, early origins of disease can easily be determined by endogenous factors such as steroid hormones. In particular, estrogens and androgens play crucial roles in development, as well as in the genesis of diseases and cancer. They are now known to have profound epigenetic influences by acting upon developmental plasticity [17, 41, 85], although their traditional actions are still mediated via classical nuclear receptor signaling pathways. The fact that the epigenetic “memory” can be transmitted to daughter cells and across several generations, influence sexual behavior, and thus impact Darwinian evolution makes this a field of investigation that should be highly valuable for generating new insights into the basis of disease development. The pioneer research examining a few epigenetic targets at a time are the proof-of-principle studies (McLachan [75, 76], New-bold [61, 80], Prins [94], Ho [41, 79], Soto [82], Skinner [17, 85] and Hilakivi-Clarke [46, 52]). To attain the next level of excellence, research in this area should focus on high-throughput comprehensive characterization of an entire epigenome susceptible to specific or multifaceted developmental reprogramming. Large-scale projects such as those defining an estrogen-sensitive epigenome are in order. Yet these investigations can progress only with adequate support from high-throughput modern technology platforms such as promoter tiling arrays, pyrosequencing, mass spectrometry, and bioinformatics analyses. The relatively long duration of developmental plasticity in humans (months to years) will allow the environment to have a significant impact on reprogramming. Furthermore, some of the phenotypes will take many years to be manifested. The challenges to fully understand the basis of a disease could thus be overwhelming. Optimistically, however, since the epigenetic processes are long term and potentially reversible, once the origin of the disease is understood, intervention and prevention strategies could be devised and applied to reverse the course. These may include dietary interventions, lifestyle changes, and drug treatment. Future clinical practices to improve human health thus should shift from curative/ palliative care to personalized preventive medicine which could be based, in part, on the epigenetic marks engraved along the “book-margins” of one’s genetic make-up.
6 Key unanswered questions
Q1: How long or how many generations does it take to reverse an epigenetic imprint? How plastic is the system for reversal? What are the therapeutic or lifestyle opportunities? Are there non-invasive remedies?
Q2: Does each class of environmental factor work on a unique set of reprogrammable genes? How much overlap between two unrelated environmental factors? Can one identify a distinct set of epigenetic marks for each stimulus and could these be developed into early predictors of diseases?
Q3: What are the mechanisms responsible for integrating the various epigenomic changes induced by the vast number of environmental stimuli?
Q4: What are the critical windows for epigenetic reprogramming? How long will it take for the ultimate phenotype to emerge? Could surrogate markers be used for prediction of disease and prescription of intervention?
Acknowledgments
Grant support: NIH grants to S-M Ho: ES12281, ES13071, ES15905, and ES15584 and the Department of Defense award to W-Y Tang: DAMD W81XWH-06-1-0373 (W-Y Tang).
Contributor Information
Wan-yee Tang, Email: tangwy@uc.edu, Department of Environmental Health, College of Medicine, University of Cincinnati, Cincinnati, OH, USA.
Shuk-mei Ho, Email: shuk-mei.ho@uc.edu, Department of Environmental Health and Cancer Center, College of Medicine, University of Cincinnati, Cincinnati, OH, USA.
References
- 1.Liu Y, Freedman BI. Genetics of progressive renal failure in diabetic kidney disease. Kidney Int. 2005;(Suppl):S94–7. doi: 10.1111/j.1523-1755.2005.09917.x. [DOI] [PubMed] [Google Scholar]
- 2.Kroll TG. Molecular events in follicular thyroid tumors. Cancer Treat Res. 2004;122:85–105. doi: 10.1007/1-4020-8107-3_4. [DOI] [PubMed] [Google Scholar]
- 3.Moore MA. Converging pathways in leukemogenesis and stem cell self-renewal. Exp Hematol. 2005;33:719–37. doi: 10.1016/j.exphem.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 4.Scher HI, Sawyers CL. Biology of progressive, castration-resistant prostate cancer: directed therapies targeting the androgen-receptor signaling axis. J Clin Oncol. 2005;23:8253–61. doi: 10.1200/JCO.2005.03.4777. [DOI] [PubMed] [Google Scholar]
- 5.Tusie Luna MT. Genes and type 2 diabetes mellitus. Arch Med Res. 2005;36:210–22. doi: 10.1016/j.arcmed.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 6.Soussi T, Ishioka C, Claustres M, Beroud C. Locus-specific mutation databases: pitfalls and good practice based on the p53 experience. Nat Rev Cancer. 2006;6:83–90. doi: 10.1038/nrc1783. [DOI] [PubMed] [Google Scholar]
- 7.Garg V. Insights into the genetic basis of congenital heart disease. Cell Mol Life Sci. 2006;63:1141–8. doi: 10.1007/s00018-005-5532-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Godfrey KM, Lillycrop KA, Burdge GC, Gluckman PD, Hanson MA. Epigenetic mechanisms and the mismatch concept of the developmental origins of health and disease. Pediatr Res. 2007;61:5R–10R. doi: 10.1203/pdr.0b013e318045bedb. [DOI] [PubMed] [Google Scholar]
- 9.Jiang YH, Bressler J, Beaudet AL. Epigenetics and human disease. Annu Rev Genomics Hum Genet. 2004;5:479–510. doi: 10.1146/annurev.genom.5.061903.180014. [DOI] [PubMed] [Google Scholar]
- 10.Dolinoy DC, Weidman JR, Jirtle RL. Epigenetic gene regulation: linking early developmental environment to adult disease. Reprod Toxicol. 2007;23:297–307. doi: 10.1016/j.reprotox.2006.08.012. [DOI] [PubMed] [Google Scholar]
- 11.Jaenisch R, Bird A. Epigenetic regulation of gene expression: how the genome integrates intrinsic and environmental signals. Nat Genet. 2003;33 (Suppl):245–54. doi: 10.1038/ng1089. [DOI] [PubMed] [Google Scholar]
- 12.Van Speybroeck L. From epigenesis to epigenetics: the case of C. H. Waddington. Ann NY Acad Sci. 2002;981:61–81. [PubMed] [Google Scholar]
- 13.Waddington CH. The stragegy of the genes: a discussion of some aspects of theoretical biology. New York: Macmillan; 1957. [Google Scholar]
- 14.Akhtar A, Cavalli G. The epigenome network of excellence. PLoS Biol. 2005;3:e177. doi: 10.1371/journal.pbio.0030177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rakyan VK, Blewitt ME, Druker R, Preis JI, Whitelaw E. Metastable epialleles in mammals. Trends Genet. 2002;18:348–51. doi: 10.1016/s0168-9525(02)02709-9. [DOI] [PubMed] [Google Scholar]
- 16.Rakyan VK, Chong S, Champ ME, Cuthbert PC, Morgan HD, Luu KV, Whitelaw E. Transgenerational inheritance of epigenetic states at the murine Axin(Fu) allele occurs after maternal and paternal transmission. Proc Natl Acad Sci USA. 2003;100:2538–43. doi: 10.1073/pnas.0436776100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Anway MD, Cupp AS, Uzumcu M, Skinner MK. Epigenetic transgenerational actions of endocrine disruptors and male fertility. Science. 2005;308:1466–9. doi: 10.1126/science.1108190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morris KV. siRNA-mediated transcriptional gene silencing: the potential mechanism and a possible role in the histone code. Cell Mol Life Sci. 2005;62:3057–66. doi: 10.1007/s00018-005-5182-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cheung P, Lau P. Epigenetic regulation by histone methylation and histone variants. Mol Endocrinol. 2005;19:563–73. doi: 10.1210/me.2004-0496. [DOI] [PubMed] [Google Scholar]
- 20.Esteller M. Aberrant DNA methylation as a cancer-inducing mechanism. Annu Rev Pharmacol Toxicol. 2005;45:629–56. doi: 10.1146/annurev.pharmtox.45.120403.095832. [DOI] [PubMed] [Google Scholar]
- 21.Holliday R. Mutations and epimutations in mammalian cells. Mutat Res. 1991;250:351–63. doi: 10.1016/0027-5107(91)90192-q. [DOI] [PubMed] [Google Scholar]
- 22.Fernandez-Twinn DS, Ozanne SE. Mechanisms by which poor early growth programs type-2 diabetes, obesity and the metabolic syndrome. Physiol Behav. 2006;88:234–43. doi: 10.1016/j.physbeh.2006.05.039. [DOI] [PubMed] [Google Scholar]
- 23.Barker DJ, Osmond C, Simmonds SJ, Wield GA. The relation of small head circumference and thinness at birth to death from cardiovascular disease in adult life. BMJ. 1993;306:422–6. doi: 10.1136/bmj.306.6875.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gluckman PD, Hanson MA. The developmental origins of the metabolic syndrome. Trends Endocrinol Metab. 2004;15:183–7. doi: 10.1016/j.tem.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 25.Ravelli AC, Van Der Meulen JH, Michels RP, Osmond C, Barker DJ, Hales CN, Bleker OP. Glucose tolerance in adults after prenatal exposure to famine. Lancet. 1998;351:173–7. doi: 10.1016/s0140-6736(97)07244-9. [DOI] [PubMed] [Google Scholar]
- 26.Dennison EM, Arden NK, Keen RW, Syddall H, Day IN, Spector TD, Cooper C. Birthweight, vitamin D receptor genotype and the programming of osteoporosis. Paediatr Perinat Epidemiol. 2001;15:211–9. doi: 10.1046/j.1365-3016.2001.00350.x. [DOI] [PubMed] [Google Scholar]
- 27.Thompson C, Syddall H, Rodin I, Osmond C, Barker DJ. Birth weight and the risk of depressive disorder in late life. Br J Psychiatry. 2001;179:450–5. doi: 10.1192/bjp.179.5.450. [DOI] [PubMed] [Google Scholar]
- 28.Bateson P, Barker D, Clutton-Brock T, Deb D, D’Udine B, Foley RA, Gluckman P, Godfrey K, Kirkwood T, Lahr MM, McNamara J, Metcalfe NB, Monaghan P, Spencer HG, Sultan SE. Developmental plasticity and human health. Nature. 2004;430:419–21. doi: 10.1038/nature02725. [DOI] [PubMed] [Google Scholar]
- 29.Gluckman PD, Hanson MA. Living with the past: evolution, development, and patterns of disease. Science. 2004;305:1733–6. doi: 10.1126/science.1095292. [DOI] [PubMed] [Google Scholar]
- 30.Saha A, Wittmeyer J, Cairns BR. Chromatin remodelling: the industrial revolution of DNA around histones. Nat Rev Mol Cell Biol. 2006;7:437–47. doi: 10.1038/nrm1945. [DOI] [PubMed] [Google Scholar]
- 31.Singal R, Ginder GD. DNA methylation. Blood. 1999;93:4059–70. [PubMed] [Google Scholar]
- 32.Dolinoy DC, Das R, Weidman JR, Jirtle RL. Metastable epialleles, imprinting, and the fetal origins of adult diseases. Pediatr Res. 2007;61:30R–7R. doi: 10.1203/pdr.0b013e31804575f7. [DOI] [PubMed] [Google Scholar]
- 33.Verdel A, Jia S, Gerber S, Sugiyama T, Gygi S, Grewal SI, Moazed D. RNAi-mediated targeting of heterochromatin by the RITS complex. Science. 2004;303:672–6. doi: 10.1126/science.1093686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Matzke MA, Birchler JA. RNAi-mediated pathways in the nucleus. Nat Rev Genet. 2005;6:24–35. doi: 10.1038/nrg1500. [DOI] [PubMed] [Google Scholar]
- 35.Costello JF, Plass C. Methylation matters. J Med Genet. 2001;38:285–303. doi: 10.1136/jmg.38.5.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Antequera F, Bird A. Number of CpG islands and genes in human and mouse. Proc Natl Acad Sci USA. 1993;90:11995–9. doi: 10.1073/pnas.90.24.11995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Murphy SK, Jirtle RL. Imprinted genes as potential genetic and epigenetic toxicologic targets. Environ Health Perspect. 2000;108 (Suppl 1):5–11. doi: 10.1289/ehp.00108s15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Monk M, Boubelik M, Lehnert S. Temporal and regional changes in DNA methylation in the embryonic, extraembryonic and germ cell lineages during mouse embryo development. Development. 1987;99:371–82. doi: 10.1242/dev.99.3.371. [DOI] [PubMed] [Google Scholar]
- 39.Kafri T, Ariel M, Brandeis M, Shemer R, Urven L, McCarrey J, Cedar H, Razin A. Developmental pattern of gene-specific DNA methylation in the mouse embryo and germ line. Genes Dev. 1992;6:705–14. doi: 10.1101/gad.6.5.705. [DOI] [PubMed] [Google Scholar]
- 40.Issa JP. CpG-island methylation in aging and cancer. Curr Top Microbiol Immunol. 2000;249:101–18. doi: 10.1007/978-3-642-59696-4_7. [DOI] [PubMed] [Google Scholar]
- 41.Ho SM, Tang WY. Techniques used in studies of epigenome dysregulation due to aberrant DNA methylation: an emphasis on fetal-based adult diseases. Reprod Toxicol. 2007;23:267–82. doi: 10.1016/j.reprotox.2007.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jenuwein T, Allis CD. Translating the histone code. Science. 2001;293:1074–80. doi: 10.1126/science.1063127. [DOI] [PubMed] [Google Scholar]
- 43.Crews D, McLachlan JA. Epigenetics, evolution, endocrine disruption, health, and disease. Endocrinology. 2006;147:S4–10. doi: 10.1210/en.2005-1122. [DOI] [PubMed] [Google Scholar]
- 44.Gatz M, Prescott CA, Pedersen NL. Lifestyle risk and delaying factors. Alzheimer Dis Assoc Disord. 2006;20:S84–8. doi: 10.1097/00002093-200607001-00013. [DOI] [PubMed] [Google Scholar]
- 45.Heindel JJ. Role of exposure to environmental chemicals in the developmental basis of reproductive disease and dysfunction. Semin Reprod Med. 2006;24:168–77. doi: 10.1055/s-2006-944423. [DOI] [PubMed] [Google Scholar]
- 46.Hilakivi-Clarke L, De Assis S. Fetal origins of breast cancer. Trends Endocrinol Metab. 2006;17:340–48. doi: 10.1016/j.tem.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 47.Phillips DI. Programming of the stress response: a fundamental mechanism underlying the long-term effects of the fetal environment? J Intern Med. 2007;261:453–60. doi: 10.1111/j.1365-2796.2007.01801.x. [DOI] [PubMed] [Google Scholar]
- 48.Waterland RA, Jirtle RL. Transposable elements: targets for early nutritional effects on epigenetic gene regulation. Mol Cell Biol. 2003;23:5293–300. doi: 10.1128/MCB.23.15.5293-5300.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Waterland RA, Dolinoy DC, Lin JR, Smith CA, Shi X, Tahiliani KG. Maternal methyl supplements increase offspring DNA methylation at Axin Fused. Genesis. 2006;44:401–6. doi: 10.1002/dvg.20230. [DOI] [PubMed] [Google Scholar]
- 50.Castro R, Rivera I, Struys EA, Jansen EE, Ravasco P, Camilo ME, Blom HJ, Jakobs C, Tavares dAI. Increased homocysteine and S-adenosylhomocysteine concentrations and DNA hypomethylation in vascular disease. Clin Chem. 2003;49:1292–6. doi: 10.1373/49.8.1292. [DOI] [PubMed] [Google Scholar]
- 51.Friso S, Choi SW, Dolnikowski GG, Selhub J. A method to assess genomic DNA methylation using high-performance liquid chromatography/electrospray ionization mass spectrometry. Anal Chem. 2002;74:4526–31. doi: 10.1021/ac020050h. [DOI] [PubMed] [Google Scholar]
- 52.Yenbutr P, Hilakivi-Clarke L, Passaniti A. Hypomethylation of an exon I estrogen receptor CpG island in spontaneous and carcinogen-induced mammary tumorigenesis in the rat. Mech Ageing Dev. 1998;106:93–102. doi: 10.1016/s0047-6374(98)00093-1. [DOI] [PubMed] [Google Scholar]
- 53.Dabelea D, Hanson RL, Lindsay RS, Pettitt DJ, Imperatore G, Gabir MM, Roumain J, Bennett PH, Knowler WC. Intrauterine exposure to diabetes conveys risks for type 2 diabetes and obesity: a study of discordant sibships. Diabetes. 2000;49:2208–11. doi: 10.2337/diabetes.49.12.2208. [DOI] [PubMed] [Google Scholar]
- 54.Yokomori N, Tawata M, Onaya T. DNA demethylation during the differentiation of 3T3-L1 cells affects the expression of the mouse GLUT4 gene. Diabetes. 1999;48:685–90. doi: 10.2337/diabetes.48.4.685. [DOI] [PubMed] [Google Scholar]
- 55.Carretero MV, Torres L, Latasa U, Garcia-Trevijano ER, Prieto J, Mato JM, Avila MA. Transformed but not normal hepatocytes express UCP2. FEBS Lett. 1998;439:55–8. doi: 10.1016/s0014-5793(98)01335-0. [DOI] [PubMed] [Google Scholar]
- 56.Lamartiniere CA, Cotroneo MS, Fritz WA, Wang J, Mentor-Marcel R, Elgavish A. Genistein chemoprevention: timing and mechanisms of action in murine mammary and prostate. J Nutr. 2002;132:552S–8S. doi: 10.1093/jn/132.3.552S. [DOI] [PubMed] [Google Scholar]
- 57.Valachovicova T, Slivova V, Bergman H, Shuherk J, Sliva D. Soy isoflavones suppress invasiveness of breast cancer cells by the inhibition of NF-kappaB/AP-1-dependent and -independent pathways. Int J Oncol. 2004;25:1389–95. [PubMed] [Google Scholar]
- 58.Lyn-Cook BD, Blann E, Payne PW, Bo J, Sheehan D, Medlock K. Methylation profile and amplification of proto-oncogenes in rat pancreas induced with phytoestrogens. Proc Soc Exp Biol Med. 1995;208:116–9. doi: 10.3181/00379727-208-43842. [DOI] [PubMed] [Google Scholar]
- 59.Day JK, Bauer AM, DesBordes C, Zhuang Y, Kim BE, Newton LG, Nehra V, Forsee KM, MacDonald RS, Besch-Williford C, Huang TH, Lubahn DB. Genistein alters methylation patterns in mice. J Nutr. 2002;132:2419S–23S. doi: 10.1093/jn/132.8.2419S. [DOI] [PubMed] [Google Scholar]
- 60.Dolinoy DC, Weidman JR, Waterland RA, Jirtle RL. Maternal genistein alters coat color and protects Avy mouse offspring from obesity by modifying the fetal epigenome. Environ Health Perspect. 2006;114:567–72. doi: 10.1289/ehp.8700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jefferson WN, Padilla-Banks E, Newbold RR. Disruption of the female reproductive system by the phytoestrogen genistein. Reprod Toxicol. 2007;23:308–16. doi: 10.1016/j.reprotox.2006.11.012. [DOI] [PubMed] [Google Scholar]
- 62.Lee WJ, Shim JY, Zhu BT. Mechanisms for the inhibition of DNA methyltransferases by tea catechins and bioflavonoids. Mol Pharmacol. 2005;68:1018–30. doi: 10.1124/mol.104.008367. [DOI] [PubMed] [Google Scholar]
- 63.Fang MZ, Wang Y, Ai N, Hou Z, Sun Y, Lu H, Welsh W, Yang CS. Tea polyphenol (−)-epigallocatechin-3-gallate inhibits DNA methyltransferase and reactivates methylation-silenced genes in cancer cell lines. Cancer Res. 2003;63:7563–70. [PubMed] [Google Scholar]
- 64.Cheng RY, Hockman T, Crawford E, Anderson LM, Shiao YH. Epigenetic and gene expression changes related to transgenerational carcinogenesis. Mol Carcinog. 2004;40:1–11. doi: 10.1002/mc.20022. [DOI] [PubMed] [Google Scholar]
- 65.Shiao YH, Crawford EB, Anderson LM, Patel P, Ko K. Allele-specific germ cell epimutation in the spacer promoter of the 45S ribosomal RNA gene after Cr(III) exposure. Toxicol Appl Pharmacol. 2005;205:290–96. doi: 10.1016/j.taap.2004.10.017. [DOI] [PubMed] [Google Scholar]
- 66.Takiguchi M, Achanzar WE, Qu W, Li G, Waalkes MP. Effects of cadmium on DNA-(Cytosine-5) methyltransferase activity and DNA methylation status during cadmium-induced cellular transformation. Exp Cell Res. 2003;286:355–65. doi: 10.1016/s0014-4827(03)00062-4. [DOI] [PubMed] [Google Scholar]
- 67.Poirier LA, Vlasova TI. The prospective role of abnormal methyl metabolism in cadmium toxicity. Environ Health Perspect. 2002;110 (Suppl 5):793–5. doi: 10.1289/ehp.02110s5793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Waalkes MP, Liu J, Ward JM, Diwan BA. Mechanisms underlying arsenic carcinogenesis: hypersensitivity of mice exposed to inorganic arsenic during gestation. Toxicology. 2004;198:31–8. doi: 10.1016/j.tox.2004.01.017. [DOI] [PubMed] [Google Scholar]
- 69.Waalkes MP, Ward JM, Diwan BA. Induction of tumors of the liver, lung, ovary and adrenal in adult mice after brief maternal gestational exposure to inorganic arsenic: promotional effects of postnatal phorbol ester exposure on hepatic and pulmonary, but not dermal cancers. Carcinogenesis. 2004;25:133–41. doi: 10.1093/carcin/bgg181. [DOI] [PubMed] [Google Scholar]
- 70.Reichard JF, Schnekenburger M, Puga A. Long term low-dose arsenic exposure induces loss of DNA methylation. Biochem Biophys Res Commun. 2007;352:188–92. doi: 10.1016/j.bbrc.2006.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Silbergeld EK, Waalkes M, Rice JM. Lead as a carcinogen: experimental evidence and mechanisms of action. Am J Ind Med. 2000;38:316–23. doi: 10.1002/1097-0274(200009)38:3<316::aid-ajim11>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 72.Salnikow K, Costa M. Epigenetic mechanisms of nickel carcinogenesis. J Environ Pathol Toxicol Oncol. 2000;19:307–18. [PubMed] [Google Scholar]
- 73.Chen H, Ke Q, Kluz T, Yan Y, Costa M. Nickel ions increase histone H3 lysine 9 dimethylation and induce transgene silencing. Mol Cell Biol. 2006;26:3728–37. doi: 10.1128/MCB.26.10.3728-3737.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Veurink M, Koster M, Berg LT. The history of DES, lessons to be learned. Pharm World Sci. 2005;27:139–43. doi: 10.1007/s11096-005-3663-z. [DOI] [PubMed] [Google Scholar]
- 75.Li S, Washburn KA, Moore R, Uno T, Teng C, Newbold RR, McLachlan JA, Negishi M. Developmental exposure to diethylstilbestrol elicits demethylation of estrogen-responsive lactoferrin gene in mouse uterus. Cancer Res. 1997;57:4356–9. [PubMed] [Google Scholar]
- 76.Li S, Hansman R, Newbold R, Davis B, McLachlan JA, Barrett JC. Neonatal diethylstilbestrol exposure induces persistent elevation of c-fos expression and hypomethylation in its exon-4 in mouse uterus. Mol Carcinog. 2003;38:78–84. doi: 10.1002/mc.10147. [DOI] [PubMed] [Google Scholar]
- 77.Alworth LC, Howdeshell KL, Ruhlen RL, Day JK, Lubahn DB, Huang TH, Besch-Williford CL, vom Saal FS. Uterine responsiveness to estradiol and DNA methylation are altered by fetal exposure to diethylstilbestrol and methoxychlor in CD-1 mice: effects of low versus high doses. Toxicol Appl Pharmacol. 2002;183:10–22. doi: 10.1006/taap.2002.9459. [DOI] [PubMed] [Google Scholar]
- 78.Sato K, Fukata H, Kogo Y, Ohgane J, Shiota K, Mori C. Neonatal exposure to diethylstilbestrol alters the expression of DNA methyltransferases and methylation of genomic DNA in the epididymis of mice. Endocr J. 2006;53:331–7. doi: 10.1507/endocrj.k06-009. [DOI] [PubMed] [Google Scholar]
- 79.Tang W, Barker J, Jefferson WN, Newbold RR, Ho S. Early exposure to diethylstilbestrol or genistein and uterine cancer risk: investigating nucleosomal binding protein 1 (Nsbp1) as a gene susceptible to estrogen reprogramming in the mouse uterus. Endocrine Society’s 89th Annual Meeting Proceeding; 2007. p. 95. [Google Scholar]
- 80.Newbold RR, Padilla-Banks E, Jefferson WN. Adverse effects of the model environmental estrogen diethylstilbestrol are transmitted to subsequent generations. Endocrinology. 2006;147:S11–7. doi: 10.1210/en.2005-1164. [DOI] [PubMed] [Google Scholar]
- 81.Ruden DM, Xiao L, Garfinkel MD, Lu X. Hsp90 and environmental impacts on epigenetic states: a model for the trans-generational effects of diethylstibesterol on uterine development and cancer. Hum Mol Genet. 2005;14(Spec No. 1):R149–55. doi: 10.1093/hmg/ddi103. [DOI] [PubMed] [Google Scholar]
- 82.Maffini MV, Rubin BS, Sonnenschein C, Soto AM. Endocrine disruptors and reproductive health: the case of bisphenol-A. Mol Cell Endocrinol. 2006;254–255:179–86. doi: 10.1016/j.mce.2006.04.033. [DOI] [PubMed] [Google Scholar]
- 83.Welshons WV, Nagel SC, vom Saal FS. Large effects from small exposures. III. Endocrine mechanisms mediating effects of bisphenol A at levels of human exposure. Endocrinology. 2006;147:S56–69. doi: 10.1210/en.2005-1159. [DOI] [PubMed] [Google Scholar]
- 84.Ho SM, Tang WY, Belmonte dF, Prins GS. Developmental exposure to estradiol and bisphenol A increases susceptibility to prostate carcinogenesis and epigenetically regulates phosphodiesterase type 4 variant 4. Cancer Res. 2006;66:5624–32. doi: 10.1158/0008-5472.CAN-06-0516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Anway MD, Leathers C, Skinner MK. Endocrine disruptor vinclozolin induced epigenetic transgenerational adult onset disease. Endocrinology. 2006;147:5515–23. doi: 10.1210/en.2006-0640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Crews D, Gore AC, Hsu TS, Dangleben NL, Spinetta M, Schallert T, Anway MD, Skinner MK. Transgenerational epigenetic imprints on mate preference. Proc Natl Acad Sci USA. 2007;104:5942–6. doi: 10.1073/pnas.0610410104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Couture LA, Abbott BD, Birnbaum LS. A critical review of the developmental toxicity and teratogenicity of 2,3,7,8-tetrachloro-dibenzo-p-dioxin: recent advances toward understanding the mechanism. Teratology. 1990;42:619–27. doi: 10.1002/tera.1420420606. [DOI] [PubMed] [Google Scholar]
- 88.Wu Q, Ohsako S, Ishimura R, Suzuki JS, Tohyama C. Exposure of mouse preimplantation embryos to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) alters the methylation status of imprinted genes H19 and Igf2. Biol Reprod. 2004;70:1790–7. doi: 10.1095/biolreprod.103.025387. [DOI] [PubMed] [Google Scholar]
- 89.Bradley C, vander MR, Roodi N, Yan H, Chandrasekharan MB, Sun ZW, Mernaugh RL, Parl FF. Carcinogen-induced histone alteration in normal humanmammaryepithelialcellscarcinogenesis. 2007. [DOI] [PubMed] [Google Scholar]
- 90.Foster PM. Disruption of reproductive development in male rat offspring following in utero exposure to phthalate esters. Int J Androl. 2006;29:140–7. doi: 10.1111/j.1365-2605.2005.00563.x. [DOI] [PubMed] [Google Scholar]
- 91.Desaulniers D, Xiao GH, Leingartner K, Chu I, Musicki B, Tsang BK. Comparisons of brain, uterus, and liver mRNA expression for cytochrome p450s, DNA methyltransferase-1, and catechol-o-methyltransferase in prepubertal female Sprague–Dawley rats exposed to a mixture of aryl hydrocarbon receptor agonists. Toxicol Sci. 2005;86:175–84. doi: 10.1093/toxsci/kfi178. [DOI] [PubMed] [Google Scholar]
- 92.McLachlan JA, Simpson E, Martin M. Endocrine disrupters and female reproductive health. Best Pract Res Clin Endocrino Metab. 2006;20:63–75. doi: 10.1016/j.beem.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 93.Tao L, Wang W, Li L, Kramer PK, Pereira MA. DNA hypomethylation induced by drinking water disinfection by-products in mouse and rat kidney. Toxicol Sci. 2005;87:344–52. doi: 10.1093/toxsci/kfi257. [DOI] [PubMed] [Google Scholar]
- 94.Prins GS, Birch L, Tang WY, Ho SM. Developmental estrogen exposures predispose to prostate carcinogenesis with aging. Reprod Toxicol. 2007;23:374–82. doi: 10.1016/j.reprotox.2006.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]