Abstract
Background
Many programs aim to alleviate vitamin A deficiency. Biofortification is an approach to improve provitamin A carotenoid concentrations of staple crops in some developing countries. In rural Zambia, maize accounts for the majority of energy intake. Provitamin A–biofortified (orange) maize has been released in Zambia.
Objective
This study quantified food intake of Zambian children from records collected in a feeding trial in 2012 in order to compare adoption of orange maize and a new vegetable (green beans) with white maize and traditional foods.
Methods
One hundred thirty-six children with a mean age of 71.5 ± 6.9 months were fed three meals a day for 6 days a week for 15 weeks at four feeding centers. Breakfast consisted of maize porridge, and lunch and dinner were stiff porridge (nshima) with various side dishes (relishes). There were three treatment groups, which received orange maize and placebo oil, white maize and placebo oil, or white maize and a daily vitamin A supplement. Food was weighed before and after consumption. Nutritionists were trained to interview the children’s caregivers about the previous day’s intake using dietary recalls. Nine dietary recalls for each child were recorded and analyzed.
Results
Total food intake did not differ among the groups (p = .31) and energy intakes on Sundays (<880 kcal) were below recommendations. Nshima intake was lower in the orange-maize group (p = .008), largely due to a genotype effect. Intakes of relish, green bean, and porridge did not differ among the groups (p > .19). Dietary recalls revealed that children living in sites closer to the main road consumed more on Sundays than children living about 8 km from the main road, but less in the evenings when children were off site.
Conclusions
The intakes of energy of these Zambian children suggest inadequacy. Implementation and adoption of new and biofortified foods is possible with promotion.
Keywords: Dietary recall, green beans, maize, nontraditional
Introduction
Vitamin A deficiency affects millions of children and adults in the developing world and is one of the most prevalent public health concerns [1]. Vitamin A deficiency is associated with xerophthalmia, poor growth and reproduction, and suppression of the immune system [2]. Lack of access to provitamin A carotenoid–rich food is one of the underlying causes of vitamin A deficiency [2]. Continuing efforts have been in place to alleviate vitamin A deficiency, including high-dose supplements of retinyl ester every 6 months for young children [3]. However, biannual supplementation does not prevent marginal vitamin A status from occurring between supplements [4–6].
Several interventions are addressing undernutrition worldwide. Zambia has fortified maize with iron and sugar with vitamin A [7]. Despite high-dose vitamin A supplementation to eligible children and fortification of sugar with vitamin A, the prevalence of low serum retinol concentration (< 0.7 µmol/L) among children is over 50% [8], which is above the World Health Organization criterion of 20% for a public health problem [9]. Recently, staple foods have been developed by plant breeders through conventional breeding techniques in consultation with nutritionists to increase the concentrations of certain macro- and micronutrients that may be lacking in a population’s diet, such as iron, essential amino acids, and vitamin A, in a process called biofortification. Quality protein maize with increased amounts of lysine and tryptophan [10] improved the nutritional status of African children [11, 12]. Maize with high provitamin A carotenoids has been bred in Mexico and Zambia, where maize is a culturally important staple crop [13]. β-Carotene from biofortified maize cooked as porridge has good bioavailability and approximately 75% retention when cooked [14, 15].
In Zambia, traditional stiff maize porridge, called nshima, is extensively consumed. Nshima and other widely consumed foods, such as breakfast porridge and fermented drinks, are made with white maize meal, which lacks provitamin A carotenoids [13, 16]. Maize with high levels of β-carotene is yellow to orange, colors that are sometimes associated with animal feed and poor taste and may not be accepted by consumers and farmers accustomed to eating white maize [16]. The efficacy of biofortified orange maize to alleviate vitamin A deficiency has not been thoroughly researched in human populations, but promising results have been found in vitro [17], in animal models [18–20], and in small human trials [15, 21]. Fortunately, acceptance of orange maize is likely in areas where white maize is consumed [16, 22, 23], especially if nutrition information is included [24].
A controlled feeding trial tested the efficacy of orange maize to improve vitamin A status in children 5 to 7 years old in rural Zambia. The objective of this paper is to examine the intake patterns of biofortified maize and other foods in these children during the 15-week intervention. In addition, the children were fed a nontraditional relish (side dish) made with green beans once per week to determine their adaptation to a “new” vegetable and break up the monotony of the study diet. Eating patterns were captured during nine dietary recalls for all children, including two Sundays when the children were not fed study food and each weekday at designated times. We report this unique data set of weighed food records and dietary recalls for Zambian preschool children who were older (5 to 7 years) than the 3- to 5-year-olds in prior studies [8, 16]. Intake data are important for evaluating target levels of provitamin A in biofortified maize.
Materials and methods
Subjects
All procedures involving human subjects were approved by the Tropical Diseases Research Center Ethics Review Committee in Zambia and the University of Wisconsin Health Sciences Human Subjects Institutional Review Board. This is NIH Clinical Trial registry number NCT01814891. Written informed consent was obtained from the parents or caregivers. The trial was conducted in 2012 in Nyimba District of the Eastern Province of Zambia on children 5 to 7 years of age (n = 136 active enrollment, aged 71.5 ± 6.9 months). Nyimba District was selected because local communities had expressed high interest in participating in the study following community sensitization programs related to orange maize performed by the National Food and Nutrition Commission, Lusaka, in previous years. Four feeding sites were chosen: sites A and B were adjacent to the main highway and sites C and D were further from the road in the bush.
Before enrollment, the children were treated with anthelmintics and screened for various exclusion factors (e.g., positive malaria test, fever, chronic illness). If the requisite blood sample was obtained, the child was eligible for entrance into the central study. Children were randomized by picking a card from opaque envelopes with an orange, white, or blue sticker to assign them to one of three treatment groups, which received orange maize and placebo oil, white maize and placebo oil, or white maize and a daily vitamin A supplement. A 2-week white maize washout period occurred before switching to orange maize or continuing on white maize. Field notes were taken during the entire trial by the researchers.
Project menu and feeding structure
Three meals were provided at each of the feeding centers, consisting of breakfast at 0800, lunch at 1200, and dinner at 1700. The feeding sites were located at a church, two clinics, and a former health post. During meal times, attendance was taken and the children sat at numbered placemats in a designated “white/blue” or “orange” area. During the treatment period (15 weeks), the orange group received orange maize sweet porridge and nshima (stiff porridge) produced with a noncommercial line of maize supplied by the International Maize and Wheat Improvement Center (CIMMYT, Mexico) for its high provitamin A content. The blue and white groups received white maize porridge and nshima made with a popular local variety. All groups received the same relishes (table 1). The study followed a weekly rotating menu with standardized recipes and weighed food so that each child received the same amount. Every day before lunch was served, children in the blue group received a dose of retinyl palmitate in oil (400 µg retinol equivalents in 214 µL of oil delivered with a positive displacement pipet onto a spoon), while the orange and white groups received a dose of placebo oil. The same spoon was used to feed the child a spoonful of relish to ensure the entire dose was consumed. The children were then allowed to sit down to eat their meals. Food items were served in separate dishes to record food-specific intakes. Child-care supervisors encouraged the children to finish their meals. When the children had finished eating, their bowls were left on their placemats and weighed on a 7-kg maximum scale with a precision of 1 g, and waste records were completed. All of these activities were standardized across all four villages in designated areas on site.
TABLE 1.
Weekly rotating menu items and weekly serving sizes for a 15-week intervention in rural Zambian children. The nutrients were provided by the 6-day rotating menua
| Menu item | Food (g) |
Calories (kcal) |
Carbohydrate (g) |
Protein (g) |
Fat (g) |
Vitamin A (RAE) |
Vitamin A (IU) |
Iron (mg) |
Zinc (mg) |
|---|---|---|---|---|---|---|---|---|---|
| Porridge/groundnuts (3/wk)b | 750 | 650 | 138 | 12.6 | 7.5 | 0.18 | 3.6 | 3.4 | 3 |
| Porridge/milk (3/wk) | 750 | 621 | 135 | 9.9 | 6.3 | 30 | 108 | 3 | 2.4 |
| Nshima (12/wk) | 3,600 | 3,600 | 768 | 69.6 | 38.4 | 1.5 | 30 | 24 | 18 |
| Kapenta relish (2/wk) | 100 | 294 | 2.4 | 4.8 | 30 | 22 | 316 | 0.4 | 0.4 |
| White bean relish (2/wk) | 100 | 50 | 10.4 | 2 | 0.4 | 0.6 | 12.4 | 0.6 | 0.4 |
| Cabbage relish (3/wk) | 150 | 84 | 7.2 | 1.5 | 6 | 19.8 | 396 | 0.6 | 0.3 |
| Cabbage groundnut relish (1/wk) | 50 | 72 | 8.2 | 5.1 | 2.8 | 13.4 | 264 | 1.0 | 1.0 |
| Cowpea relish (2/wk) | 100 | 70 | 8 | 1.2 | 4 | 24 | 464 | 0.4 | 0.4 |
| Brown bean relish (1/wk) | 50 | 33 | 4 | 1.4 | 1.2 | 2.5 | 49 | 0.3 | 0.2 |
| Cabbage/groundnut relish (2/wk) | 150 | 86 | 10 | 6 | 3.4 | 16 | 312 | 1.0 | 1.0 |
| Cabbage relish (1/wk) | 75 | 42 | 3.5 | 0.7 | 3 | 10 | 197 | 0.2 | 0.1 |
| Brown bean relish (1/wk) | 75 | 50 | 6.7 | 2 | 1.7 | 3.7 | 74 | 0.5 | 0.3 |
| Green bean relish (1/wk) | 75 | 43 | 3.4 | 0.8 | 3.3 | 17 | 343 | 0.4 | 0.12 |
| Chicken relish (1/wk) | 75 | 108 | 1 | 15 | 4.5 | 13 | 129 | 0.6 | 1.1 |
| Study menu total/wk | 6,100 | 5,800 | 1,110 | 133 | 113 | 174 | 2,700 | 36.4 | 28.7 |
RAE, Retinol Activity Equivalents
Vitamin A was that provided by dishes made with white maize only. Groundnuts are Zambian peanuts and kapenta are small dried fish.
24-Hour dietary recalls
Nine 24-hour dietary recalls were recorded throughout the treatment period at designated times during the study across sites, which included two Sundays and every weekday [25]. The mothers or caregivers were asked to stay on site to talk to trained nutritionists about what the child consumed off site. The nutritionists were instructed how to ask questions in the local language and how to estimate the amount of food that the children were eating by showing the mothers bowls or cups of different sizes and filling them with dry rice. Careful documentation was acquired on seven different weekdays throughout the feeding trial (i.e., what the child ate from 1700 the previous day until 0800 on the day of the recall) and on two Sundays when the children were fed off site. Dietary recalls were entered into Nutritionist Pro Software for each child. The data were exported into Microsoft Excel files and compiled. Zeros were entered for dietary recalls in which children did not eat any additional food, and blanks were entered for dietary recalls that were not recorded. Means were calculated per dietary recall per child and then per site.
Weekly intake patterns
For weekly intake patterns of menu items, days of feeding were translated into weeks of feeding to account for the 6-day rotating menu combinations; thus one “week” is 6 days of project feeding. Food intakes were calculated for each feeding group (orange, white, and blue) during the 15-week treatment period and categorized according to menu item (porridge, nshima, relish, green beans). Green bean relish was analyzed separately because this was considered a new vegetable for the children. Nutrient analysis for the week was also conducted with Nutritionist Pro Software (table 1).
Statistical analysis
The data were analyzed by two-way analysis of variance (ANOVA) followed by Tukey–Kramer testing using the SAS statistical package, version 9.2, to determine significant differences among groups. For all comparisons, individual intakes were averaged per menu item and used to calculate feeding group means for the time period being considered. All tests were two-sided, and p < .05 was considered to indicate statistical significance. Meal absences were excluded from the data analyses. Mean values (± SD) were rounded to the nearest gram to reflect the precision of the field scales.
Results
Among- and within-group comparison of weekly intakes
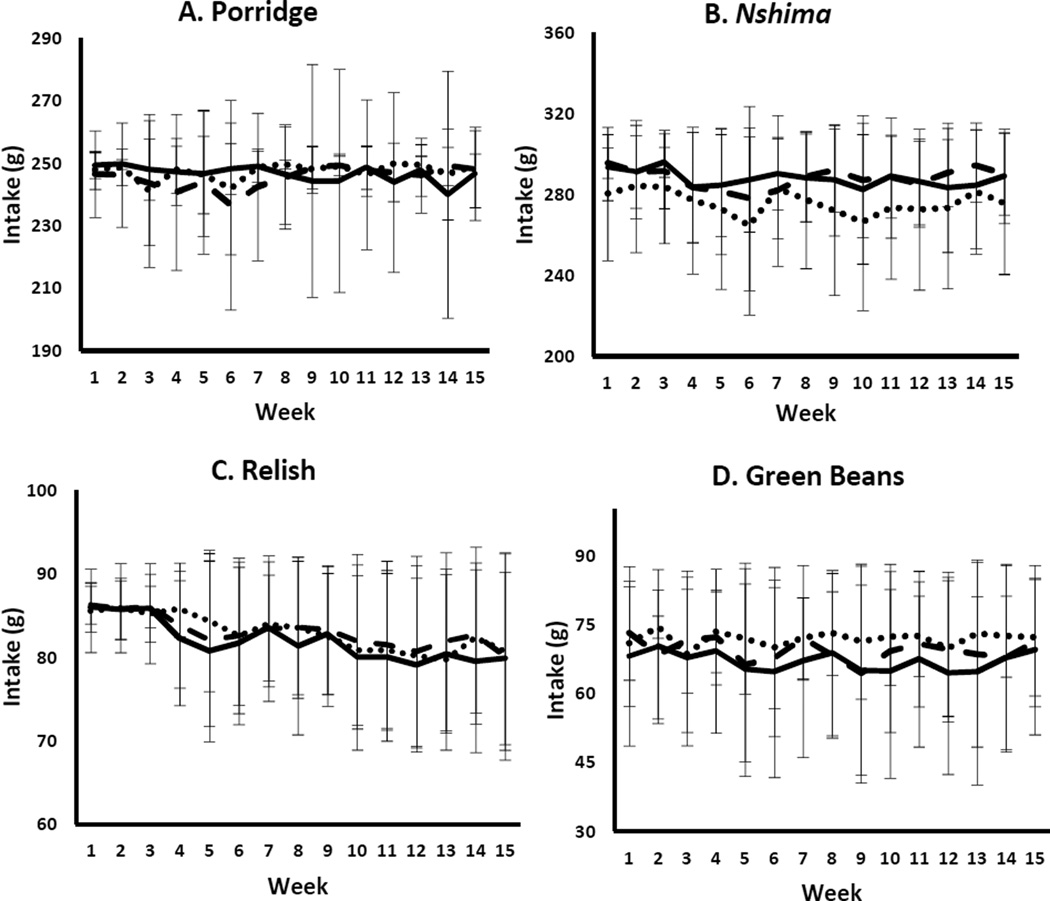
Baseline anthropometric data were recorded and did not differ among the groups (table 2). A total of 136 children completed the intervention period. Eight children (5.9%) had a height-for-age z-score < − 3 and therefore were considered stunted. Only one child had a body mass index (BMI)-for-age z-score < − 2; this child’s BMI was 12.7 kg/m2. The daily recording of each child’s food consumption allowed comparisons of menu item intakes over time and among feeding groups. The rate of meal absences (670 absences out of 36,720 meals served [1.8%]) was not related to treatment group. The mean weekly intakes of menu items generally did not differ among the groups (fig. 1), with variance staying fairly consistent but increasing over time, potentially due to observed increasing illnesses. Although overall food intake (i.e., total intake of nshima, porridge, and relishes) was not significantly different among groups throughout the study (611 ± 53 g, p = .31), significantly less food was eaten during week 6 than during weeks 1 and 2 in all groups (p = .002). There were no interactions between treatment group and time (p = .38).
TABLE 2.
Anthropometric data collected at baseline screening by treatment group (mean ± SD) for all children enrolled
| Measurement | Orange (n = 44) |
White (n = 46) |
Blue (n = 46) |
p | All children (n = 136) |
|---|---|---|---|---|---|
| Weight (kg) | 17.4 ± 2.1 | 17.3 ± 2.1 | 17.1 ± 1.8 | .78 | 17.3 ± 2.0 |
| Height (cm) | 108.2 ± 5.4 | 107.2 ± 6.3 | 107.6 ± 4.6 | .68 | 107.6 ± 5.4 |
| Age (mo) | 71.5 ± 7.2 | 70.8 ± 6.7 | 71.3 ± 7.0 | .89 | 71.5 ± 6.9 |
| BMI (kg/m2) | 14.8 ± 0.9 | 15.0 ± 1.1 | 14.8 ± 1.0 | .44 | 14.9 ± 1.0 |
| HAZ | − 1.40 ± 0.92 | − 1.54 ± 1.19 | − 1.53 ± 0.89 | .77 | − 1.49 ± 1.01 |
| WAZ | − 1.16 ± 0.81 | − 1.18 ± 0.94 | − 1.30 ± 0.81 | .71 | − 1.21 ± 0.85 |
| BAZ | − 0.36 ± 0.64 | − 0.23 ± 0.79 | − 0.42 ± 0.71 | .44 | − 0.34 ± 0.72 |
BMI, body mass index; BAZ, BMI-for-age z-score; HAZ, height-for-age z-score; WAZ, weight-for-age z-score
FIG. 1.
Weekly intakes (mean ± SD) of maize-based foods (porridge and nshima), relish, and green beans by treatment group (n = 136). Nshima intake was lower in the orange group than in the white and blue groups (p = .008). The intakes of the other three food items did not differ among groups (p > .05). Overall intakes (the sum of all four items in composite analysis) did not differ among groups (p = .31)
There were differences among groups in the amounts of some food items eaten. The orange group ate significantly less nshima by total weight (276 ± 36 g) than the blue and white groups (288 ± 26 g and 288 ± 25 g, respectively, p = .008). An effect of elapsed time (p < .0001) and a trend for an interaction occurred (p = .079). The lower intake of orange maize is probably due to a maize genotype difference, because orange maize took up less water during cooking. Food allocation was based on cooked serving weight and not dry weight of the maize served to the children. Food intake did not differ between the white and blue groups throughout the study. A significant time-dependent effect for nshima intake revealed that the children consumed the most nshima in week 3 (290 ± 20 g) and the least in weeks 4, 5, 6, 10, and 12. When the interaction was evaluated, no clear pattern emerged. Porridge intake did not differ (p = .55), but there was a slight time effect (p = .050) and an interaction of treatment by elapsed time (p = .028). However, no differences were noted in the post hoc analysis.
Relish intake did not differ overall (p = .66), but there was a time effect, with lower intakes occurring toward the end of the trial (weeks 10–15) than during the earlier weeks (weeks 1–4) (p < .0001). No interaction occurred (p = .59). The children may have been less active toward the end of the study due to the hot weather and therefore consumed less food than in the first 2 months. Intake of green bean relish, a new vegetable for the children, did not differ among groups (p = .19). A time-dependent effect occurred for green beans (p = .012), but no clear difference with time emerged in the post hoc analysis, and no interaction was noted (p = .76).
Dietary recalls
The total number of dietary records analyzed to capture off-site food intake was 241 on Sundays and 807 on weekdays. Vast differences in the children’s intakes (table 3) occurred among sites. Site A reported the most kilocalories consumed on Sundays, followed by sites D, C, and B. Children at site B consumed more kilocalories off site during the week, followed by sites D, C, and A. Vitamin A intake differed on Sundays but not during the week. Intake of all other nutrients analyzed differed among the sites on Sundays.
TABLE 3.
| A. Sunday 24-hour dietary recalls (n = 241) when children were fed off site | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Site | Energy (kcal) |
Protein (g) |
Carbohydrate (g) |
Fat (g) |
Sodium (mg) |
Vitamin A (IU) |
Vitamin C (mg) |
Calcium (mg) |
Fiber (g) |
Iron (mg) |
Zinc (mg) |
| A | 880 ± 360a | 20 ± 9.7a | 160 ± 66a | 18 ± 15a | 1,400 ± 1,500bc | 3,000 ± 7,800a | 19 ± 20a | 100 ± 90ab | 11 ± 7.1a | 5.0 ± 3.5a | 2.6 ± 2.8a |
| B | 570 ± 280c | 14 ± 8.1c | 100 ± 52c | 12 ± 9.4b | 930 ± 1,100c | 460 ± 1,500b | 6.6 ± 12b | 73 ± 71b | 5.3 ± 2.6c | 2.7 ± 1.6b | 1.3 ± 0.7b |
| C | 600 ± 260bc | 14 ± 6.2bc | 99 ± 54bc | 16 ± 9.1ab | 2,200 ± 1,600a | 1,100 ± 3,500ab | 20 ± 20a | 130 ± 130a | 7.2 ± 4.2b | 4.3 ± 3.0a | 1.2 ± 0.6b |
| D | 660 ± 230b | 16 ± 5.8ab | 120 ± 50b | 15 ± 11ab | 1,700 ± 1,300ab | 890 ± 2,300ab | 8.4 ± 9.1b | 60 ± 56b | 6.8 ± 3.3b | 3.0 ± 1.5b | 1.7 ± 0.8b |
| B. Monday–Saturday dietary recall analyses (n = 807) to capture what the children ate from 1700 to 0800 off site | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Site | Energy (kcal) |
Protein (g) |
Carbohydrate (g) |
Fat (g) |
Sodium (mg) |
Vitamin A (IU) |
Vitamin C (mg) |
Calcium (mg) |
Fiber (g) |
Iron (mg) |
Zinc (mg) |
| A | 90 ± 150c | 2.1 ± 3.5c | 16 ± 28c | 2.2 ± 5.2b | 140 ± 470b | 170 ± 1,100 | 2.4 ± 8.2b | 10 ± 23b | 0.9 ± 1.6c | 0.51 ± 1.1b | 0.29 ± 0.94c |
| B | 280 ± 230a | 7.2 ± 5.5a | 49 ± 37a | 6.6 ± 9.4a | 600 ± 630a | 50 ± 120 | 2.4 ± 4.3b | 31 ± 43a | 2.8 ± 2.2a | 1.4 ± 1.5a | 0.70 ± 0.55a |
| C | 120 ± 140c | 3.2 ± 4.1c | 18 ± 25c | 4.4 ± 7.4ab | 450 ± 550a | 120 ± 490 | 4.1 ± 9.5a | 25 ± 55a | 1.3 ± 1.7c | 0.96 ± 1.5a | 0.39 ± 0.84bc |
| D | 220 ± 130b | 5.8 ± 3.6b | 36 ± 26b | 5.4 ± 5.7a | 480 ± 360a | 120 ± 690 | 1.8 ± 2.8b | 22 ± 33ab | 2.1 ± 1.3b | 1.1 ± 0.8a | 0.57 ± 0.45ab |
All numbers are mean intakes ± SD. Means without a common superscript are different between sites for each nutrient listed (p < .05).
Discussion
Biofortification of staple crops using traditional plant breeding is a promising approach to increase the intake of provitamin A carotenoids in certain populations to alleviate vitamin A deficiency. Currently, a targeted micronutrient density, such as 17 µg of provitamin A carotenoids per gram of dry maize [26], acts as a guide for breeders. The use of biofortification could sustainably alleviate macro- and micronutrient deficiencies in malnourished populations by the foods they regularly eat instead of relying on supplementation programs or fortification. Furthermore, biofortification efforts can be used in conjunction with these programs and interventions because they do not pose toxicity issues [6]. For example, quality protein maize could be biofortified with provitamin A carotenoids and perhaps zinc to meet the requirements for macronutrients and some micronutrients [10, 11, 13]. The bioefficacy of carotenoid-biofortified maize to improve or maintain liver stores has not been documented in humans, but analyses are under way. Point estimates for bioconversion factors of β-carotene equivalents to retinol for provitamin A–biofortified maize have been favorable in two small human studies [15, 21]. Furthermore, a variety of rice that is biofortified with β-carotene had the same bioconversion factor as a β-carotene supplement in children [27]. Biofortifying maize with provitamin A carotenoids has great potential to alleviate vitamin A deficiency in countries where maize is a widely consumed staple crop. The overriding issue is to provide evidence that biofortified staple foods alleviate or measurably impact micronutrient deficiencies, and then to have these foods accepted and widely consumed.
The 5- to 7-year-old children enrolled in this study were apparently healthy. None of the children had a weight-for-age z-score < − 3 at enrollment. Only 5.9% would be consider severely stunted by international standards (height-for-age z-score < − 3), and only one child had a BMI < 13 kg/m2. They enthusiastically consumed all foods they were given. Overall, the orange-maize group consumed less nshima than the groups eating white maize; however, there was no specific week in which the children eating orange-maize nshima consumed less than those in the white-maize groups. The children eating orange-maize nshima did not have an apparent acclimation period, as did the 3- to 5-year old children in the first and second weeks of the Zambia 2010 feeding trial [16]. This is likely due to the prior exposure of this small community to orange maize.
The staff and children indicated that orange-maize nshima had a softer texture and sweeter flavor than the white maize that is traditionally eaten. Because of its texture it was difficult to use orange-maize nshima to scoop up relishes. This is probably a genotypic effect, which maize breeders should address in the future development of orange maize lines that are to be commercially released. Based on our outcomes, the post hoc calculated sample size needed to attain 80% power for detecting a difference in nshima intake was 190 subjects. The actual power was 65% for this analysis. This means that caution needs to be exercised in concluding that the nshima intake difference is totally robust, given the shortfall in sample size to test the null hypothesis. Nonetheless, staff members and mothers preferred the orange maize over the white maize porridge because of its sweet flavor and soft texture when prepared as thin porridge. According to the staff, the children in the orange-maize group were happy when presented with white-maize nshima during the washout period at the end of the study. The orange group subjects did say they missed the orange porridge, and the children receiving white maize felt it was their turn to try orange maize. The eagerness of the children to try a new food and their acceptance of new foods are promising for the future of acceptance of orange maize in Zambia.
The variation in the amount of relish that the children consumed increased over time. The reason for this is unknown, but it could be due to the observed illnesses in addition to the change in season. A documented chickenpox outbreak occurred in the district, particularly at site C where all children fell ill during weeks 5 and 6 of the intervention, which accounts for the low intakes during that time because of appetite loss. Intake of green bean relish was examined separately because green beans are not widely available, nor are they a traditional relish consumed in rural Zambia, and therefore they were considered a new vegetable. The green beans were sourced from Lusaka, the capital city, and delivered biweekly to Nyimba. The children took no documented time to acclimate to the taste or sight of the green beans and readily consumed them, even though their mothers informed us otherwise. We analyzed the diet records specifically because of recorded comments from the mothers and caregivers on site saying that the children did not enjoy eating the green bean relish and that they wanted something different. The preliminary examination of the food records in the field did not confirm these comments, and therefore we happily continued to serve the green bean relish. The mothers were trying to persuade the researchers to serve more chicken during the trial, which is a highly regarded food but not commonly consumed due to its expense. To appease the community pleas to change the menu, a live chicken was promised to each household, which was delivered at the end of the study.
The Sunday dietary recalls indicated low food intake by the children. Children 5 to 7 years old who are moderately active have an average recommended daily calorie intake of about 1,400 kcal [28]. These children were growing and getting lots of physical activity because of their rural environment. However, their Sunday 24-hour intakes were only 570 to 880 kcal (table 3). It is unknown what effect the intervention may have had on off-site eating during the evenings and on Sundays. Nonetheless, children from sites nearest to the road consumed higher amounts of energy on Sundays than those from sites that were further in the bush. However, children from sites A and B consumed less off site during the feeding week than did children from the sites in the bush. Comparison of macronutrient intakes with dietary reference intakes [29] revealed that the intakes of carbohydrate, protein, and fat met the requirements both on Sundays and during the week. Dietary recalls are not always the best measure of long-term intake and may be inaccurate because of recall bias; however, they are generally better predictors of macronutrient intake than other methods, such as food frequency questionnaires [30–32].
Although no variation in vitamin A consumption existed during the week, high variation occurred on Sundays, and some children were consuming double the Recommended Dietary Allowance (RDA) for vitamin A, which is 400 µg of retinol activity equivalents for children 4 to 8 years of age [29]. This was mainly due to the intake of rape, a dark-green leafy vegetable, which is widely available. Twenty-four-hour vitamin A dietary recalls do not reflect long-term intake because of the day-to-day differences in intake of complementary foods. On Sundays, vitamin C intake met the Estimated Average Requirement (EAR) at two sites but not the others. Sodium intake was around the Adequate Intake (AI) across groups. Calcium intake was well below the EAR and fiber intake was below the AI for this age group. Iron and zinc intakes on Sundays were below the EAR, but during the week iron intake was above the EAR and zinc intake met the EAR.
This study emphasizes the importance of education when incorporating new foods into a very traditional culture that is deeply connected to their food. Targeting young children and educating parents about why biofortified foods are important for sustainable health is imperative. Nutrition campaigns will likely improve acceptance and willingness to pay [24]. In addition, nutrition education should emphasize the importance of general nutrition in the light of low caloric intakes among the children in the studied areas. The fact that the children readily ate the green beans despite the mothers’ complaints implies that new foods are best introduced to people when they are young. School feeding programs could be one such venue for the introduction of biofortified foods to children.
Acknowledgments
We thank Kevin Pixley, International Maize and Wheat Improvement Center (CIMMYT), Texcoco, Mexico, for producing the synthetic maize that was used in this trial. We thank Peter Crump, Senior Information Processing Consultant at the University of Wisconsin-Madison College of Agriculture and Life Sciences Statistical Consulting Service, for providing statistical assistance. We also thank Fabiana Moura, HarvestPlus, for assisting in the coordination of this intervention trial. Financial support for this study was provided by HarvestPlus 8256, an endowment to SAT entitled “Friday Chair for Vegetable Processing Research”, and NIH grant T32-DK007665 (BG). HarvestPlus (www.harvestplus.org) is a global alliance of agriculture and nutrition research institutions working to increase the micronutrient density of staple food crops through biofortification. The views expressed do not necessarily reflect those of HarvestPlus.
Footnotes
Authors’ contributions
Samantha Schmaelzle, Chisela Kaliwile, Sara A. Arscott, Bryan Gannon, and Sherry A. Tanumihardjo conducted the intervention. Samantha Schmaelzle wrote the initial draft of the manuscript as part of the master’s in science degree, and all authors commented on the manuscript. Cassim Masi coordinated communication with the local district officials for conduct of the trial. Sherry A. Tanumihardjo was responsible for finalizing the manuscript and designing the study.
Contributor Information
Samantha Schmaelzle, Interdepartmental Graduate Program in Nutritional Sciences, University of Wisconsin, Madison, Wisconsin, USA.
Chisela Kaliwile, National Food and Nutrition Commission, Lusaka, Zambia.
Sara A. Arscott, Interdepartmental Graduate Program in Nutritional Sciences, University of Wisconsin, Madison, Wisconsin, USA
Bryan Gannon, Interdepartmental Graduate Program in Nutritional Sciences, University of Wisconsin, Madison, Wisconsin, USA.
Cassim Masi, National Food and Nutrition Commission, Lusaka, Zambia.
Sherry A. Tanumihardjo, Interdepartmental Graduate Program in Nutritional Sciences, University of Wisconsin, Madison, Wisconsin, USA
References
- 1.Tanumihardjo SA, Palacios N, Pixley KV. Provitamin A carotenoid bioavailability: What really matters? Int J Vitam Nutr Res. 2010;80:336–350. doi: 10.1024/0300-9831/a000042. [DOI] [PubMed] [Google Scholar]
- 2.Sherwin JC, Reacher MH, Dean WH, Ngondi J. Epidemiology of vitamin A deficiency and xerophthalmia in at-risk populations. Trans R Soc Trop Med Hyg. 2012;106:205–214. doi: 10.1016/j.trstmh.2012.01.004. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization. Geneva: WHO; 2011. Guideline: Vitamin A supplementation in infants and children 6–59 months of age. [PubMed] [Google Scholar]
- 4.Tanumihardjo SA, Permaesih D, Muherdiyantiningsih, Rustan E, Rusmil K, Fatah AC, Wilbur S, Muhilal, Karyadi D, Olson JA. Vitamin A status of Indonesian children infected with Ascaris lumbricoides after dosing with vitamin A supplements and albendazole. J Nutr. 1996;126:451–457. doi: 10.1093/jn/126.2.451. [DOI] [PubMed] [Google Scholar]
- 5.Tanumihardjo SA, Permaesih D, Muhilal Vitamin A status and hemoglobin concentrations are improved in Indonesian children with vitamin A and deworming interventions. Eur J Clin Nutr. 2004;58:1223–1230. doi: 10.1038/sj.ejcn.1601953. [DOI] [PubMed] [Google Scholar]
- 6.Tanumihardjo SA. Food-based approaches for ensuring adequate vitamin A nutrition. Compr Rev Food Sci Food Saf. 2008;7:373–381. [Google Scholar]
- 7.van den Briel T, Cheung E, Zewari J, Khan R. Fortifying food in the field to boost nutrition: Case studies from Afghanistan, Angola, and Zambia. Food Nutr Bull. 2007;28:353–364. doi: 10.1177/156482650702800312. [DOI] [PubMed] [Google Scholar]
- 8.Hotz C, Chileshe J, Siamusantu W, Palaniappan W, Kafwembe E. Vitamin A intake and infection are associated with plasma retinol among pre-school children in rural Zambia. Public Health Nutr. 2012;23:1–9. doi: 10.1017/S1368980012000924. [DOI] [PubMed] [Google Scholar]
- 9.World Health Organization. Geneva: WHO; 1996. Indicators for assessing vitamin A deficiency and their application in monitoring and evaluating intervention programs. [Google Scholar]
- 10.Nuss ET, Tanumihardjo SA. Quality protein maize for Africa: Closing the protein inadequacy gap in vulnerable populations. Adv Nutr. 2011;2:217–224. doi: 10.3945/an.110.000182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gunaratna NS, De Groote H, Nestel P, Pixley KV, McCabe CP. A meta-analysis of community-based studies on quality protein maize. Food Policy. 2010;35:202–210. [Google Scholar]
- 12.Akalu G, Taffesse S, Gunaratna NS, De Groote H. The effectiveness of quality protein maize in improving the nutritional status of young children in the Ethiopian highlands. Food Nutr Bull. 2010;31:418–430. doi: 10.1177/156482651003100304. [DOI] [PubMed] [Google Scholar]
- 13.Nuss ET, Tanumihardjo SA. Maize: A paramount staple crop in the context of global nutrition. Compr Rev Food Sci Food Saf. 2010;9:417–436. doi: 10.1111/j.1541-4337.2010.00117.x. [DOI] [PubMed] [Google Scholar]
- 14.Li S, Tayie FAK, Young MF, Rocheford T, White WS. Retention of provitamin A carotenoids in high-β-carotene maize (Zea mays) during traditional African household processing. J Agric Food Chem. 2007;55:10744–10750. doi: 10.1021/jf071815v. [DOI] [PubMed] [Google Scholar]
- 15.Li S, Nugroho A, Rocheford T, White WS. Vitamin A equivalence of the β-carotene in β-carotene-biofortified maize porridge consumed by women. Am J Clin Nutr. 2010;92:1105–1112. doi: 10.3945/ajcn.2010.29802. [DOI] [PubMed] [Google Scholar]
- 16.Nuss ET, Arscott SA, Bresnahan K, Pixley KV, Rocheford T, Hotz C, Siamusantu W, Chileshe J, Tanumihardjo SA. Comparative intake of white-versus orange-colored maize by Zambian children in the context of promotion of biofortified maize. Food Nutr Bull. 2012;33:63–71. doi: 10.1177/156482651203300106. [DOI] [PubMed] [Google Scholar]
- 17.Thakkar SK, Failla ML. Bioaccessibility of pro-vitamin A carotenoids is minimally affected by non pro-vitamin A xanthophylls in maize (Zea mays sp.) J Agric Food Chem. 2008;56:11441–11446. doi: 10.1021/jf802430u. [DOI] [PubMed] [Google Scholar]
- 18.Howe JA, Tanumihardjo SA. Carotenoid-biofortified maize maintains adequate vitamin A status in Mongolian gerbils. J Nutr. 2006;136:2562–2567. doi: 10.1093/jn/136.10.2562. [DOI] [PubMed] [Google Scholar]
- 19.Davis C, Jing H, Howe JA, Rocheford T, Tanumihardjo SA. β-Cryptoxanthin from supplements or carotenoid-enhanced maize maintains liver vitamin A in Mongolian gerbils (Meriones unguiculatus) better than or equal to β-carotene supplements. Br J Nutr. 2008;100:786–793. doi: 10.1017/S0007114508944123. [DOI] [PubMed] [Google Scholar]
- 20.Davis CR, Howe JA, Rocheford TR, Tanumihardjo SA. The xanthophyll composition of biofortified maize (Zea mays Sp.) does not influence the bioefficacy of provitamin A carotenoids in Mongolian gerbils (Meriones unguiculatus) J Agric Food Chem. 2008;56:6745–6750. doi: 10.1021/jf800816q. [DOI] [PubMed] [Google Scholar]
- 21.Muzhingi T, Gadaga TH, Siwela AH, Grusak MA, Russell RM, Tang G. Yellow maize with high β-carotene is an effective source of vitamin A in healthy Zimbabwean men. Am J Clin Nutr. 2011;94:510–519. doi: 10.3945/ajcn.110.006486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.De Groote H, Chege Kimenju S. Comparing consumer preferences for color and nutritional quality in maize: Application of a semidouble-bound logistic model on urban consumers in Kenya. Food Policy. 2008;33:362–370. [Google Scholar]
- 23.Muzhingi T, Langyintuo AS, Malaba LC, Banziger M. Consumer acceptability of yellow maize products in Zimbabwe. Food Policy. 2008;33:352–361. [Google Scholar]
- 24.Meenakshi JV, Banerji A, Manyong V, Tomlins K, Mittal N, Hamukwala P. Using a discrete choice experiment to elicit the demand for a nutritious food: Willingness-to-pay for orange maize in rural Zambia. J Health Econ. 2012;31:62–71. doi: 10.1016/j.jhealeco.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 25.Hammond K. Assessment: Dietary and clinical data. In: Escott-Stump S, Krause MV, Mahan LK, editors. Krause's food & nutrition therapy. St. Louis, Mo, USA: Elsevier Saunders; 2008. pp. 383–410. [Google Scholar]
- 26.Bouis HE, Hotz C, McClafferty B, Meenakshi JV, Pfeiffer WH. Biofortification: A new tool to reduce micronutrient malnutrition. Food Nutr Bull. 2011;32:S31–S40. doi: 10.1177/15648265110321S105. [DOI] [PubMed] [Google Scholar]
- 27.Tang G, Hu Y, Yin SA, Wang Y, Dallal GE, Grusak MA, Russell RM. β-Carotene in Golden Rice is as good as β-carotene in oil at providing vitamin A to children. Am J Clin Nutr. 2012;96:658–664. doi: 10.3945/ajcn.111.030775. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 28.US Department of Agriculture. Estimated calorie needs per day. [Accessed on April 29, 2013];2002 http://www.cnpp.usda.gov/Publications/USDAFoodPatterns/EstimatedCalorieNeedsPerDayTable.pdf.
- 29.Otten JJ, Hellwig JP, Meyer LD, editors. Institute of Medicine. Dietary Reference Intakes: The essential guide to nutrient requirements. Washington, DC: National Academies of Sciences; 2006. [Google Scholar]
- 30.Thomson CA, Giuliano A, Rock CL, Ritenbaugh CK, Flatt SW, Faerber S, Newman V, Caan B, Graver E, Hartz V, Whitacre R, Parker F, Pierce JP, Marshall JR. Measuring dietary change in a diet intervention trial: Comparing food frequency questionnaire and dietary recalls. Am J Epidemiol. 2003;157:754–762. doi: 10.1093/aje/kwg025. [DOI] [PubMed] [Google Scholar]
- 31.Schatzkin A, Kipnis V, Carroll RJ, Midthune D, Subar AF, Bingham S, Schoeller DA, Troiano RP, Freedman LS. A comparison of a food frequency questionnaire with a 24-hour recall for use in an epidemiological cohort study: Results from the biomarker-based Observing Protein and Energy Nutrition (OPEN) study. Int J Epidemiol. 2003;32:1054–1062. doi: 10.1093/ije/dyg264. [DOI] [PubMed] [Google Scholar]
- 32.Stein AD, Shea S, Basch CE, Contento IR, Zybert P. Consistency of the Willett semiquantitative food frequency questionnaire and 24-hour dietary recalls in estimating nutrient intakes of preschool children. Am J Epidemiol. 1992;135:667–677. doi: 10.1093/oxfordjournals.aje.a116346. [DOI] [PubMed] [Google Scholar]