Abstract
High mobility group box 1 (HMGB1) is an evolutionarily conserved protein, and constitutively expressed in virtually all types of cells. Infection and injury converge on common inflammatory responses that are mediated by HMGB1 secreted from immunologically activated immune cells or passively released from pathologically damaged cells. Herein we review the emerging molecular mechanisms underlying the regulation of pathogen-associated molecular patterns (PAMPs)-induced HMGB1 secretion, and summarize many HMGB1-targeting therapeutic strategies for the treatment of infection- and injury-elicited inflammatory diseases. It may well be possible to develop strategies that specifically attenuate damage-associated molecular patterns (DAMPs)-mediated inflammatory responses without compromising the PAMPs-mediated innate immunity for the clinical management of infection- and injury-elicited inflammatory diseases.
Keywords: PAMPs, DAMPs, Infection, Injury, HMGB1, signaling, herbal components
1.INTRODUCTION
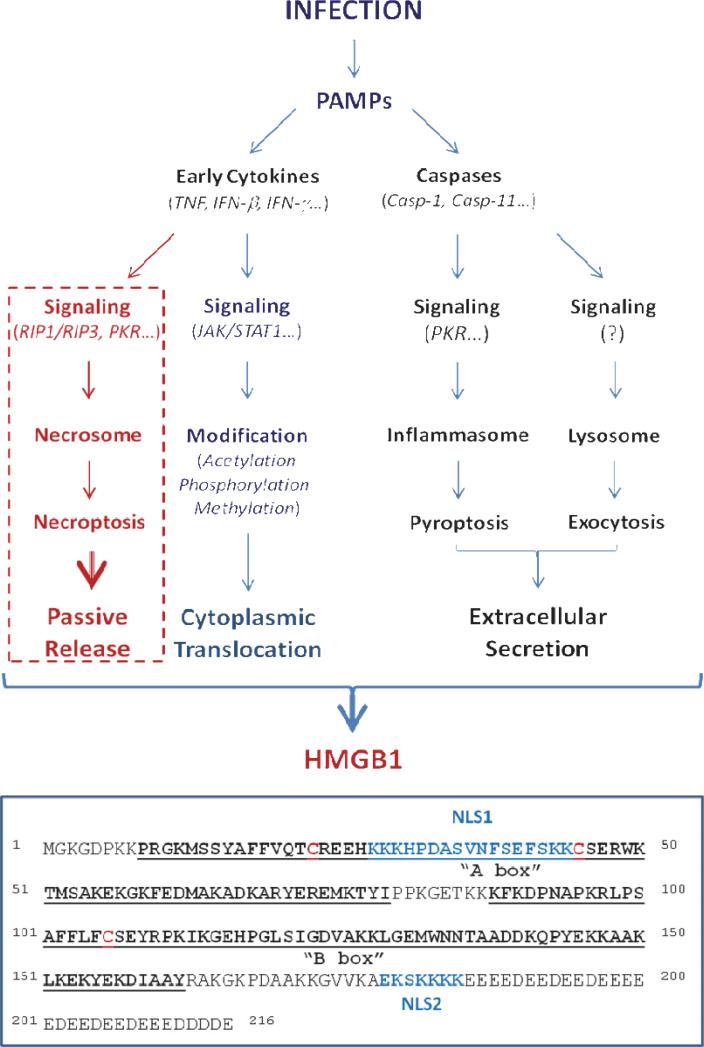
High mobility group box 1 (HMGB1), an evolutionarily conserved 30 kDa DNA-binding protein, is constitutively expressed in virtually all types of cells. Bearing two nuclear-localization sequences (NLS), HMGB1 is transported into the nucleus by the nuclear import complexes, thereby maintaining a large nuclear “pool” of pre-formed protein [1]. It carries two internal repeats of positively charged domains (“HMG boxes” known as “A box” and “B box”) in the N-terminus, and a continuous stretch of negatively charged (aspartic and glutamic acid) residues in the C-terminus (Figure 1). These HMG boxes enable HMGB1 to bind chromosomal DNA, and fulfill nuclear functions in stabilizing nucleosomal structure and regulating gene expression [1]. Disrupted local expression of HMGB1 renders animals susceptible to infectious [2] or injurious insults [3,4], suggesting an overall beneficial role of intracellular HMGB1. In response to infections and injuries, however, HMGB1 is secreted from activated immune cells or passively released from stressed cells. If dysregulated, excessive HMGB1 release adversely contributes to the pathogenesis of both infection- and injury-elicited inflammatory diseases. In this review, we summarize the novel mechanisms underlying the regulation of active HMGB1 secretion by innate immune cells, and highlight potential HMGB1-targeting therapeutic strategies for the treatment of infection- and injury-elicited inflammatory diseases.
Figure 1. Pathogen-associated molecular patterns (PAMPs) induced active HMGB1 secretion and possibly passive release.
The functional domains of human HMGB1 are noted.
2. HMGB1 ACTIVE SECRETION AND PASSIVE RELEASE
2.1. Active HMGB1 Secretion
In response to pathogen-associated molecular patterns (PAMPs, e.g., ds-RNA, CpGDNA and endotoxins) [5,6], macrophages/monocytes sequentially secrete early proinflammatory cytokines (e.g., TNF, IL-1, IFN-β and IFN-γ) and late mediators (e.g., HMGB1, Figure 1). Some early cytokines, including IFN-β and IFN-γ, can also stimulate immune cells to secrete HMGB1 in a time- and dose-dependent fashion [7- 9]. Lacking a leader peptide sequence, HMGB1 cannot be actively secreted through classical endoplasmic reticulum - Golgi exocytotic pathways [5]. Instead, macrophages/monocytes activated by exposure to products of infection or injury translocate nuclear HMGB1 into cytoplasmic vesicles which are destined for secreting into the extracellular environment. Recent evidence reveals that the initial HMGB1 nuclear to cytoplasmic translocation is regulated by the JAK/STAT1-mediated acetylation, while the subsequent extracellular release is partly controlled by the double-stranded RNA-activated protein kinase R (PKR)/inflammasome-mediated pyroptosis (Figure 1).
2.1.1. Role of JAK/STAT1 in the regulation of nuclear-cytoplasmic translocation
The nucleus-to-cytoplasm protein shuttle is regulated by posttranslational modifications (e.g., acetylation, phosphorylation, methylation) of the transported protein's nuclear localization or export sequences (NLS or NES), which interact specifically with nuclear import and export complexes on the nuclear membrane [10]. In addition to two NLS sites (Figure 1), HMGB1 also contains two non-classical NES, and therefore shuttles continually between the nucleus and the cytoplasm. In quiescent cells, however, the equilibrium is tilted towards nuclear accumulation [11].
In response to exogenous PAMPs or endogenous cytokines (e.g., IFNs), innate immune cells acetylate lysine residues 28, 29, 42, 43, 179, 181, 183 within the NLS sites, leading to sequestration of HMGB1 into cytoplasmic vesicles (Figure 1) [7,9,11,12]. The acetylation is controlled both by histone acetylases (HATs) and histone deacetylases (HDACs), and pharmacological inhibition of HDACs also leads to HMGB1 hyperacetylation and nuclear-cytoplasmic translocation [11]. Following hypoxia or ischemia, non-immune cells (e.g., hepatocytes) also acetylate HMGB1 to trigger cytoplasmic translocation [13]. Unlike immune cells which actively secrete HMGB1 to modulate inflammatory responses, hepatocytes mobilize HMGB1 to the cytoplasm for a different purpose: binding to beclin-1 to induce autophagy - a cellular degradation process to remove damaged and reactive oxygen species (ROS)-producing mitochondria [14]. As aforementioned, intracellular HMGB1 is required for the normal physiological cellular response to stress [2,4], and conditional knock-out of liver HMGB1 indeed leads to significant enhancement of hepatic ischemia/reperfusion injury [3].
JAK/STAT1 signaling is critically important for LPS- or IFN-induced HMGB1 hyperacetylation within the NLS sites, thereby functioning as the underlying mechanism that regulates HMGB1 nuclear-cytoplasmic translocation [9] (Figure 1). Indeed, pharmacological inhibition or genetic interference with JAK/STAT1 signaling uniformly inhibits HMGB1 secretion induced by IFN-β, IFN-γ or LPS. Given the critical involvement of the JAK/STAT1 signaling in type 1 and type 2 IFNs action, as well as the essential roles of IFNs in the innate immunity against virus and bacteria [15], it is plausible that IFNs-induced cytoplasmic HMGB1 translocation participates in the beneficial host protection against viral or intracellular bacterial invasion. This is likely, as cytoplasmic or extracellular HMGB1 serves a sentinel function that facilitates innate recognition of microbial nucleic acids, an essential step in innate immunity against pathogen infection [6,16].
Phosphorylation of serine residues within the HMGB1 NLS sites may also contribute to the regulation of HMGB1 cytoplasmic translocation [17] (Figure 1). Although the upstream signaling pathway remains poorly elucidated, the calcium/calmodulin-dependent protein kinase (CaMK) IV has been implicated in the regulation of LPS-induced HMGB1 phosphorylation and release [18]. Unlike macrophages/monocytes, quiescent neutrophils carry HMGB1 predominantly in the cytoplasm, possibly because the methylation of lysine 42 weakens HMGB1/DNA interaction and forces nuclear HMGB1 to passively diffuse into the cytoplasm [19]. Thus, neutrophils likely provide another important source of extracellular HMGB1 during infection or injury.
2.1.2. Role of PKR in the regulation of HMGB1 secretion
Following cytoplasmic translocation, HMGB1 is secreted into the extracellular space following signal transduction through several pathways, including the Caspase-1/Caspase-11-mediated pyroptosis and the lysosome-mediated exocytosis (Figure 1). Indeed, pharmacological inhibition with a broad-spectrum caspase inhibitor (Z-VADFMK), or genetic deletion of Caspase-1/Caspase-11, uniformly reduces HMGB1 secretion from activated macrophages [20,21]. Notably, these caspases function not only as key regulators of the proinflammatory programmed cell death – pyroptosis [21], but also as important modulators of the canonical and non-canonical inflammasomes - key components of innate inflammatory protein complexes. Specifically, Pro-caspase-1 forms a heteromeric protein complex with several other proteins, including an adaptor protein (termed apoptosis-associated speck-like protein containing a CARD, ASC), a NOD-Like Receptor (NLR, e . g . , NLRP1, NLRP3, NLRC4) or a member of the PYHIN family. The assembly of this protein complex, termed the “inflammasome,” cleaves pro-caspase-1 to generate caspase-1, which triggers pyroptosis [22]. Caspase-11, on the other hand, mediates pyroptosis and HMGB1 release in the absence of caspase-1, a process termed the “non-canonical inflammasome pathway” [23]. Although the components of the non-canonical inflammasome remain largely unknown, it has been suggested that an intracellular LPS receptor-like molecule may participate in the mechanism, because intracellular LPS induces caspase-11 activation even in the absence of TLR4 [24]. In contrast to the ultra-pure LPS that fails to trigger HMGB1 release in the absence of other stimulus (e.g., ATP) [21,25], crude LPS (containing trace amounts of bacterial proteins and nucleic acids) significantly up-regulates PKR expression (> 2-fold) and phosphorylation (> 8-fold), and markedly stimulates HMGB1 secretion [5,26].
Inflammasome activation is the regulatory mechanism of LPS/ATP-induced HMGB1 release [21,25], because genetic disruption of key inflammasome components (e.g., caspase 1 or Nalp3) completely impairs LPS/ATP-induced HMGB1 release. We recently reported a novel role for the double-stranded RNA-activated PKR in the regulation of inflammasome activation and HMGB1 release (Figure 1) [25]. In addition to dsRNA, other molecules that activate inflammasome assembly and signaling, ATP and anthrax lethal toxin, also induce PKR autophosphorylation. Genetic disruption of PKR expression or pharmacological inhibition of PKR phosphorylation [with 2-aminopurine (2-AP) or 7-desacetoxy-6,7-dehydrogedunin (7DG)] markedly reduces NLRP3 or NLRP1 agonists-induced inflammasome activation [25,27], pyroptosis [25,27], and HMGB1 release [25]. In agreement with the two-step control of HMGB1 nuclear translocation and extracellular release, pharmacological inhibition of PKR (with 2-AP) abrogates LPS-induced HMGB1 release, but does not prevent HMGB1 cytoplasmic translocation. Thus, the mechanisms of LPS-induced HMGB1 cellular secretion is differentially controlled at two stages: 1) JAK/STAT-mediated nuclear-cytoplasmic translocation; and 2) PKR/inflammasome-dependent pyroptosis and secretion (Figure 1).
In addition to pyroptosis, activated innate immune cells may also secrete HMGB1 via other non-classical vesicle-mediated secretory pathways including exocytosis of secretory lysosomes [12] (Figure 1). Indeed, a fraction of cytoplasmic HMGB1 has been localized in secretory lysosomes [12], where the key regulator of unconventional protein secretion, caspase-1 [28], co-resides with its enzymatic substrate pro-IL-1β. It is likely that these secretory lysosomes deliver their cargo (e.g., IL-1 and HMGB1) into the extracellular space via exocytosis, but this hypothesis is as yet unproved, and other mechanisms may be involved.
2.2. Passive HMGB1 Release from Necrotic Cells
HMGB1 can be passively released from damaged cells [29] following sterile tissue injury due to ischemia/reperfusion [30,31], non-penetrating trauma [32,33], or chemical toxemia [34-36]. As a DAMP (damage-associated molecular pattern molecule), extracellular HMGB1 stimulates innate immune cells to respond to sterile injury [1,37], triggering an injury-elicited systemic inflammatory response syndrome (SIRS) that is indistinguishable from microbial infection-induced responses [38]. Necrosis can also be induced by viral infection or proinflammatory cytokines (e.g. TNF, IFNs) (Figure 1) [39,40], and HMGB1 is passively released by cells infected by various viruses (e.g., West Nile, Salmon anemia, Dengue, and influenza viruses) [41- 43]. This implicates HMGB1 as a pathogenic mediator of viral infection-elicited inflammatory diseases [44]. Notably, the cytokine-induced necrosis is also a highly regulated programmed process, termed necroptosis or programmed necrosis (Figure 1). Several signaling molecules such as the protein kinase receptor-interacting protein 3 (RIP3) and PKR are involved in the assembly of a “necrosome” protein complex [39,40,45], which contributes to passive HMGB1 release following infection (Figure 1). Thus, the innate response mechanisms of infection and injury converge on a common process - inflammation [1], which is orchestrated by HMGB1 and other proinflammatory mediators (e.g., mitochondrial DNA, cold-inducible RNA-binding protein, CIRP) released both by activated immune cells and by damaged tissues [46,47].
2.3. Extracellular HMGB1 as a DAMP Molecule
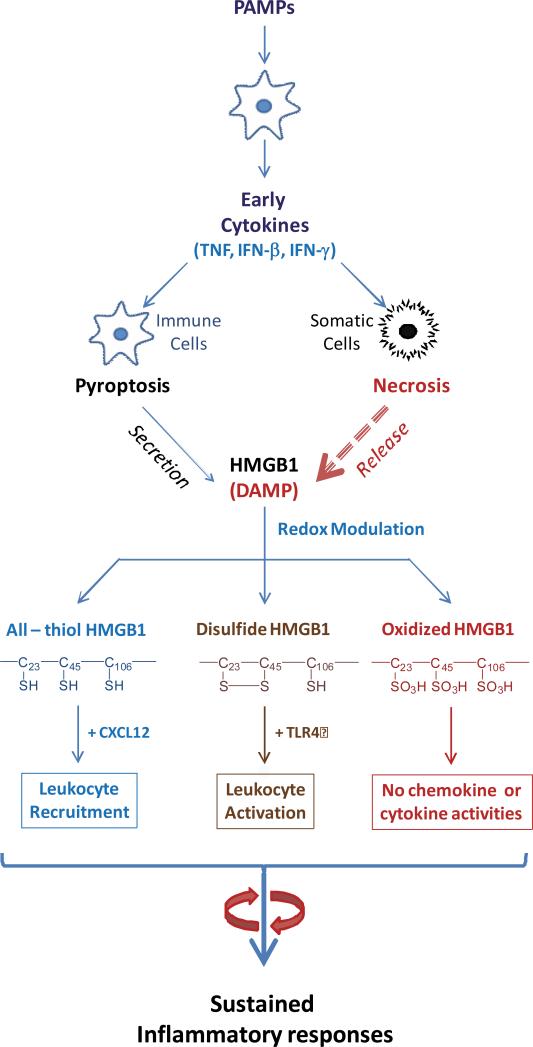
Once released, extracellular HMGB1 functions as a DAMP to alert, recruit, and activate immune cells. For instance, HMGB1 binds to various PAMPs (e.g., CpGDNA or LPS), thereby facilitating their recognition by respective receptors [48], and consequently augmenting the PAMPs-induced inflammatory responses [48]. Furthermore, HMGB1 can stimulate the migration of monocytes, dendritic cells [49,50] and neutrophils [51], functioning as a chemokine to facilitate leukocyte recruitment to the sites of infection or injury [52] (Figure 2). HMGB1 interacts with a family of cell surface receptors and binding proteins including RAGE [48], TLR4 [53], TLR9 [6,48], cluster of differentiation 24 (CD24)/Siglec-10 [54], Mac-1 [51], thrombomodulin [55], as well as single transmembrane domain proteins (e.g., syndecans) [56]. Consequently, it can activate macrophages [57] and endothelial cells [58] to produce proinflammatory cytokines, chemokines, and adhesion molecules (Figure 2).
Figure 2. A microbial infection triggers a systemic inflammatory response mediated by HMGB1 secreted by immunologically activated innate immune cells and pathologically damaged cells.
Microbial invasion leads to the libration of PAMPs, which trigger active HMGB1 secretion and passive release. Extracellular HMGB1 then amplifies the rigorous inflammatory responses by facilitating leukocyte recruitment and activation, resulting in cytokine storm and organ dysfunction. Note the immunological activities are modulated by the redox status in a divergent fashion.
HMGB1 contains three cysteine residues at highly conserved positions: 23, 45 and 106 (C23, C45 and C106, Figure 1). They are redox-sensitive and their atomic structure is modified by redox reactions to produce three HMGB1 isoforms (Figure 2) [59-61]. The redox status of HMGB1 dictates its chemokine or cytokine-inducing properties. A recent consensus conference proposed nomenclature of the isoforms [62], termed “HMGB1” (which refers to the all thiol form), “disulfide HMGB1” (which is partially oxidized); and oxidized HMGB1. Specifically, the fully reduced (“all-thiol”) HMGB1 binds to other chemokines (e.g., CXCL12) and stimulates leukocyte recruitment via the CXCR4 receptor [63]. On the other hand, the partially oxidized HMGB1 bearing Cys23-Cys45 disulfide can activate immune cells to produce cytokines/chemokines via the TLR4 or other receptors. Once fully oxidized, the HMGB1 is devoid of either chemokine or cytokine activities (Figure 2) [60,64]. Thus, the release of redox-modulated HMGB1 from immunologically activated or pathologically injured cells contributes to the sequential leukocyte recruitment, activation, and eventual resolution of inflammation.
The posttranslational modifications of HMGB1 are not limited to the production of redox sensitive isoforms, because HMGB1 secreted via pyroptosis is also hyperacetylated at the NLS sites. This is consistent with the understanding that inflammasome-mediated HMGB1 release is a highly regulated process. Passively released HMGB1, as occurs in necrotic cells, is predominantly the “all-thiol” isoform. Furthermore, different types of inflammasome activation induce distinct HMGB1 post-translational modifications. For instance, the NLRP3 inflammasome stimuli, such as ATP, monosodium uric acids, and adjuvant aluminum, induce the secretion of “disulfide” HMGB1 [25]; whereas the activation of the NLRC4 inflammasome results in the secretion of fully-reduced HMGB1 [65]. One possible explanation is that activation of the NLRP3 inflammasome, but not the NLRC4 inflammasome, is associated with mitochondrial ROS production, which promotes HMGB1 oxidation and formation of the C23 - C45 disulphide bond [66]. Although both the NLRP3 and the NLRC4 inflammasome mediate maturation of IL-1β and IL-18, their distinct impact on the redox status of HMGB1 might enable fine-tuning of the immune response against different pathogens. For example, the NLRP3 inflammasome can be activated by several RNA virus [67], and extracellular release of disulfide HMGB1 initiates anti-viral inflammatory responses. On the other hand, as an intracellular sentinel against Salmonella infection [68], the NLRC4 inflammasome enables the release of reduced HMGB1, which facilitates leukocyte recruitment to eliminate invading bacteria.
3. HMGB1 AS A MEDIATOR OF INFECTION- AND INJURY-ELICITED INFLAMMATION
In response to infection and injury, the host's innate immune system mounts an immediate inflammatory response to eliminate the invading pathogens and to heal the wounds [69]. To accomplish this, the innate immune cells (e.g., macrophages/monocytes) are equipped with receptors (e.g., CD14, MD-2 and TLR4) that can efficiently recognize both PAMPs (e.g., LPS) [70,71] and DAMPs (e.g., HMGB1 or CIRP) [47,72]. The underlying recognition mechanisms for PAMPs and DAMPs utilize numerous pathways, and extensive evidence reveals an essential role for HMGB1 in both infection- and injury-elicited inflammatory diseases.
3.1. HMGB1 as a Late Mediator of Sepsis
Sepsis refers to the host's deleterious and non-resolving systemic inflammatory response to microbial infection [38], and represents the leading cause of death in the intensive care unit. Substantial evidence has supported the necessity to preserve the early PAMPs-mediated innate immune response to fight against microbial infection. For instance, the impairment of the early inflammatory responses leads to severe immune deficiency during bacterial infection [73]. Although early proinflammatory cytokines (e.g., TNF, IFN-γ) might be protective against infection, the sustained accumulation of late proinflammatory mediators (e.g., HMGB1) contributes to the pathogenesis of lethal infection (Figure 2). These scenarios cannot be replicated in the clinic, because by the time patients develop these early cytokine responses, there is no opportunity to intervene. In animal models of lethal infection induced by endotoxemia or cecal ligation and puncture (CLP), HMGB1 is first detected in the circulation eight hours after the disease onset, and subsequently increased to plateau levels from 16 to 32 hours [5,74]. This late appearance of circulating HMGB1 parallels the onset of animal lethality from endotoxemia or sepsis, and distinguishes itself from TNF and other early proinflammatory cytokines [75]. The pathogenic role of HMGB1 in endotoxemia is inferred from studies that HMGB1-neutralizing antibodies confer a dose-dependent protection against endotoxin-induced lethality [5]. In a more clinically relevant animal model of sepsis (induced by CLP), delayed administration of HMGB1-specific neutralizing antibodies, beginning 24 h after CLP, dose-dependently rescue rodents from lethal sepsis [20,74]. Moreover, targeted inhibition of HMGB1 expression in innate immune cells (e.g., macrophages and dendritic cells) reduces systemic HMGB1 accumulation, and similarly rescues mice from sepsis [76]. Taken together, these experimental data establish extracellular HMGB1 as a critical late mediator of experimental sepsis, which can be therapeutically targeted within wider therapeutic windows than other early cytokines.
3.2. HMGB1 as an Early Mediator of Injury
As a ubiquitous nuclear protein, HMGB1 can also be passively released from necrotic cells [29], and functions as a DAMP to elicit inflammatory responses. Following primary tissue injury, HMGB1 can be passively released from damaged cells, and released into the surrounding periphery, where it accumulates and amplifies inflammatory responses by inducing various cytokines, chemokines, tissue factor and adhesion molecules (Figure 2). Indeed, accumulative evidence has suggested a pathogenic role of HMGB1 in injury-elicited inflammatory diseases, as HMGB1-neutralizing antibodies are protective in animal models of ischemia/reperfusion [30,77,78], trauma [79,80], chemical toxemia [34,81,82], atherosclerosis [83], gastric ulcer [84] and hyperoxia [85].
3.3. HMGB1 as a mediator of autoimmune diseases
Extensive evidence has also implicated HMGB1 in the pathogenesis of autoimmune diseases such as the systemic lupus erythematosus (SLE) [86-88] and rheumatoid arthritis [86,89]. The pathogenesis of lupus is partly attributable to the impaired clearance of apoptotic cells, which may gradually enter secondary necrosis to passively release HMGB1 into the extracellular space [86,87]. Extracellular HMGB1, however, may also impair the elimination of apoptotic cells [90], and further exacerbate these vicious inflammatory cascades [91]. In lupus patients with higher photosensitivity, ultraviolet (UV) irradiation induces more pronounced keratinocyte apoptosis and HMGB1 release [92-94], which triggers elevated leukocyte influx to the irradiated skin [95]. Furthermore, emerging evidence suggests that some HMGB1-neutralizing antibodies may reduce systemic accumulation of proinflammatory cytokines and associated glomerulonephritis in a murine model of lupus [96]. As a chronic synovitis, rheumatoid arthritis is characterized by persistent immune cell activation and joint tissue damage [86], which are similarly mediated by extracellular accumulation of HMGB1 and other proinflammatory cytokines [97]. In animal models of rheumatoid arthritis, HMGB1-neutralizing antibodies confer significant protection against joint tissue edema and structural damages [97-99], supporting a pathogenic role for HMGB1 in the pathogenesis of autoimmune diseases.
4. THERAPEUTIC POTENTIAL OF HMGB1-INHIBITING AGENTS
The discovery of HMGB1 as a mediator of lethal infection and injury has prompted to the search of endogenous and exogenous agents that can inhibit HMGB1 release and protect animals against infection or injury.
4.1. Endogenous HMGB1 Inhibitors
Evolution has selected for counter-regulatory or anti-inflammatory mechanisms that suppress the damage to host tissues. For instance, the central nervous system can directly and rapidly respond to PAMPs and DAMPs, and down-regulates production of inflammatory mediators by transmitting efferent vagus nerve signals to tissue-resident T cells [100] and macrophages [101]. This cytokine suppressing mechanism is dependent on the release of acetylcholine by specific T cells, as well as the presence of the alpha-7 nAChR on targeted immune cells [101-103]. At the sites of infection or injury, another ubiquitous biogenic molecule, spermine, can also be passively released by injured cells, and functions as a local counter-regulatory mechanism for PAMPs- and DAMPs-induced inflammatory responses [104-107]. In response to infection and injury, the liver strategically re-prioritizes the synthesis and systemic release of a group of proteins collectively termed “acute phase proteins” (APPs), which may also function as counter-regulatory mechanisms against infection or injury. For instance, the hepatic expression of fetuin-A is negatively regulated by TNF, IL-1, IL-6 and IFN-γ [108], but positively regulated by HMGB1 [108]. Consistently, fetuin-A functions as a negative APP during infection, but serves as a positive APP in injury. Regardless, supplementation with exogenous fetuin-A confers protection against both injury- [109] and infection-elicited inflammatory responses [108] (Table 1). The integral role of fetuin-A in host defense against lethal systemic inflammation was supported by the observations that fetuin-A-deficient C57BL/6J mice were more susceptible to lethal endotoxemic or septic insult than sex- and body-matched (male, 27-29 g) wild-type C57BL/6J mice [109].
Table 1.
Endogenous HMGB1-inhibiting agents.
| Agents | Infection Models | Injury Models |
|---|---|---|
| Neutralizing antibodies | LPS/CLP | Atherosclerosis |
| Crush | ||
| Chemical toxemia | ||
| Liver I/R | ||
| Brain I/R | ||
| Heart I/R | ||
| Hyperoxia | ||
| Hemorrhagic | ||
| Trauma | ||
| Ulcer | ||
| Anti-coagulant agents | ||
| Anti-thrombin III | LPS | Ischemia |
| Thrombomodulin | LPS | I/R |
| Heatstroke | ||
| Acute phase proteins | ||
| Fetuin-A | LPS/CLP | Cerebral Ischemia |
| Burn | ||
| Endogenous hormones | ||
| Vasoactive intestinal peptide | CLP | Ischemia |
| Hemorrhagic injury | ||
| Ghrelin | CLP | Intestinal I/R |
| Hypoxia | ||
| Radiation | ||
| Intravenous immunoglobulin | CLP | Cerebral Ischemia |
Note: LPS, lipopolysaccharide; CLP, cecal ligation and puncture; I/R, ischemia/reperfusion
In addition, a number of other endogenous molecules (Table 1), including the intravenous immunoglobulin (IVIG) [110], anti-coagulant agents (anti-thrombin III, thrombomodulin) [55,111], and endogenous hormones (e.g., vasoactive intestinal peptide and ghrelin) [112,113], have proven protective in animal models of infection through HMGB1-inhibiting mechanisms. Notably, these endogenous molecules are also protective in animal models of ischemia/reperfusion injury [114-118], crush injury [119], burn injury [120], chemical toxemia [121,122], hypoxic injury [123], radiation [124] (Table 1). It is thus important to investigate whether the protective effects are associated with similar inhibition of HMGB1 release or activities.
4.2. Exogenous HMGB1-inhibiting Agents
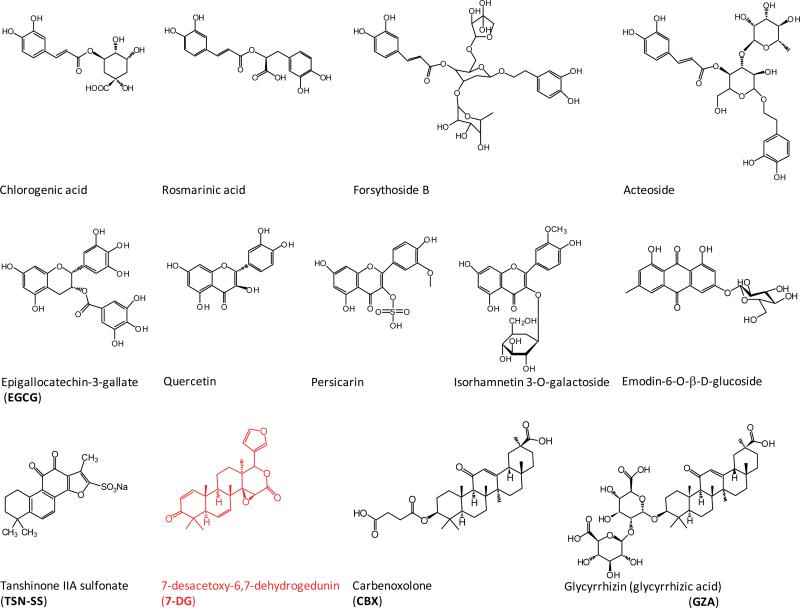
A number of herbal extracts (e.g., Danggui, Mung bean, and Prunella vulgaris) confer significant protection against lethal endotoxemia or sepsis (Table 2) [125-127]. Similarly, these herbs are also protective in animal models of radiation injury [128] and chemical toxemia [129], although it is not yet known whether the protective mechanism is dependent on the HMGB1 suppression. In addition, an increasing number of herbal components (e.g., nicotine, EGCG, tanshinone, glycyrrhizin, chlorogenic acid, Emodin-6-O-β-D-glucoside, Rosmarinic acid, isorhamnetin-3-O-galactoside, Persicarin, Forsythoside B, chloroquine, acteroside) [103,130-140] have been proven effective in inhibiting endotoxin-induced HMGB1 release (Figure 3, Table 2).
Table 2.
Exogenous HMGB1-inhibiting agents.
| Agents | Infection Models | Injury |
|---|---|---|
| Herbal extract | ||
| Danggui | LPS/CLP | Radiation |
| Mung bean | LPS/CLP | Chemical toxemia |
| Prunella vulgaris | CLP | - |
| Herbal components | ||
| Nicotine | LPS/CLP | - |
| TSN-SS | LPS/CLP | Cerebral Ischemia |
| EGCG | LPS/CLP | Pain |
| Crush i | ||
| I/R | ||
| Carbenoxolone | CLP | I/R |
| Trauma | ||
| Glycyrrhizin | LPS | Vascular Injury |
| Chloroquine | LPS/CLP | I/R |
| Radiation | ||
| Acteoside | CLP | Chemical toxemia |
| Chlorogenic acid | LPS/CLP | Pain |
| I/R | ||
| Emodin-6-O-β-D-glucoside | CLP | - |
| Rosmarinic acid (RA) | LPS/CLP | Radiation |
| Oxidative | ||
| Chemical toxemia | ||
| Isorhamnetin-3-O-galactoside | LPS/CLP | - |
| Persicarin | LPS/CLP | I/R |
| Forsythoside B | CLP | |
| Hypoxic | ||
| Higenamine | - |
Figure 3. Chemical structures of HMGB1-inhibiting herbal components.
Note the chemical structural similarly between two PKR inhibiting agents: CBX and 7DG.
Interestingly, different herbal components appear to utilize distinct mechanisms to prevent HMGB1 release by activated macrophages/monocytes. For instance, a major green tea component, EGCG, prevents the LPS-induced HMGB1 release strategically by destroying it in the cytoplasm via a cellular degradation process – autophagy [141]. Autophagy, literally meaning “self-eating”, refers to an evolutionarily conserved process that maintains cellular homeostasis by degrading cytoplasmic macromolecules. The relationship between autophagy and HMGB1 remains a subject of on-going investigation. In 2009, Thorburn et al proposed a potential role for autophagy in the regulation of HMGB1 release in neoplastic cells, because an agent [i.e., epidermal growth factor receptor (EGFR)-targeted diphtheria toxin] simultaneously induced autophagy and HMGB1 release in glioblastoma tumor cells [142]. Subsequently, Tang et al suggested HMGB1 as an important regulator of autophagy based on the findings that the knock-down of HMGB1 expression led to a reduction of stress-induced autophagy in cancer cells [143,144]. In contrast, Huebener et al recently reported that HMGB1 is dispensable for autophagy regulation in non-transformed cells, because conditional knockout of HMGB1 in hepatocytes or cardiomyocytes completely failed to impair glucagon- or rotenone-induced autophagy [145]. Together with our observations that some agents capable of stimulating autophagy (e.g., EGCG) surprisingly inhibited HMGB1 release in primary macrophage cultures [141], these new findings are calling for further investigation of the seemingly complex relationship between autophagy and HMGB1 in primary and transformed cells.
In contrast, a derivative of tanshinone IIA, TSN-SS selectively inhibits HMGB1 release by facilitating endocytosis of exogenous HMGB1, leading to subsequent degradation via a lysosome-dependent pathway [146]. A pannexin-1 channel blocker, carbenoxolone (CBX), attenuates LPS-induced HMGB1 release by preventing the expression and phosphorylation of PKR [25]. Given the similarity in the chemical structure between CBX and a newly identified PKR inhibitor (7DG, Figure 3), it is important to investigate whether CBX directly binds and inhibits PKR activation.
In light of the capacity of herbal ingredients in preventing endotoxin-induced HMGB1 release, we explored the efficacy of several compounds in animal models of CLP-induced sepsis. Considering the late and prolonged kinetics of HMGB1 accumulation in experimental sepsis [74], the first dose of HMGB1 inhibitors was given in a delayed fashion - 24 h after the onset of sepsis. Repetitive intraperitoneal administration of EGCG [130], TSN IIA-SS [131], or CBX [26], at 24, 48, and 72 h post CLP, significantly increased animal survival rates. Even when given orally, EGCG still rescued mice from lethal sepsis, significantly increasing animal survival rates from 16% to 44% [141]. Intriguingly, we found that EGCG facilitated bacterial elimination in selective organs (e.g., the liver and lung) in an animal model of sepsis [147]. It is not yet known whether these antibacterial properties are attributable to the possibilities that EGCG directly kill microbes by altering microbial protein conformations and functions, or indirectly by modulating macrophage-associated innate immune responses. A number of other herbal components have been proven protective against lethal infection or injury by attenuating systemic HMGB1 release or action (Table 2), raising further interest in future clinical studies. Importantly, these herbal components have also been proven beneficial in animal models of ischemia [148-155], trauma [156,157], crush injury [158], hemorrhage [159], radiation [160,161], chemical toxemia [162,163]. Nevertheless, it remains unknown whether the protective effects are associated with inhibition of HMGB1 release or chemokine/cytokine activities during injury.
Notably, agents capable of inhibiting HMGB1 release [103,130,131] or action [5,74] confer protection against sepsis, particularly if given in a delay fashion to strategically preserve the PAMPs-mediated early inflammatory response. At a late stage of infection, the PAMPs-mediated inflammatory response may be accompanied by unintended cell injury and DAMPs release that further amplifies the cytokine storm to precipitate organ dysfunction [164] (Figure 1 and Figure 2). This possibility is supported by recent findings that HMGB1 is persistently elevated during a late stage of sepsis despite the cessation of initial infection [165], and contributes to the long-term pathological consequence of sepsis. Although the microbial infection-induced sepsis is similar to the sterile injury-elicited systemic inflammatory response syndrome (SIRS) [38,166], it may be more advantageous to develop strategies that specifically attenuate DAMPs-mediated inflammatory responses without compromising the PAMPs-mediated innate immunity.
5. EXPERT COMMENTARY & FIVE-YEAR VIEW
Therapeutic strategies targeting PAMPs (e.g., endotoxin) [167] or PAMP signaling (e.g., Eritoran) [168] fail to improve survival in clinical trials of human sepsis, raising questions about the feasibility of PAMPs-blocking agents in the treatment of infectious diseases. The investigation of pathogenic cytokines in animal models of diseases has led to the development of successful cytokine-targeting therapeutic strategies (e.g., chimeric anti-TNF monoclonal antibody, infliximab; and a soluble TNF receptors-Fc fusion protein, sTNF-R-Fc, etanercept) for autoimmune diseases such as rheumatoid arthritis (RA) [169]. However, neutralizing antibodies for early cytokines (e.g., TNF) also did not show efficacy in sepsis clinical trials [170], as in the clinic, the early cytokine responses are established or completed prior to the ability to administer these reagents. Thus, it remains highly important to identify feasible therapeutic targets for management of inflammatory diseases.
In contrast to early proinflammatory cytokines, HMGB1 is secreted from immunologically activated innate immune cells and released from pathologically damaged cells, and functions as a critically important mediator in lethal infection and injury. In animal model of sepsis, HMGB1-neutralizing antibodies or inhibitors can rescue mice from the lethality particularly if given in a delayed manner to preserve the potentially beneficial early PAMPs-mediated inflammatory responses. It may be possible to develop novel strategies to specifically modulate DAMP-elicited injurious inflammatory response without impairing the PAMP-mediated beneficial innate immunity against infection. Therefore, it is important to investigate whether HMGB1 can ever be a clinically feasible therapeutic target for human sepsis or other autoimmune diseases.
Future clinical studies are anticipated to test the efficacy of HMGB1-neutralizing antibodies in the clinical management of human inflammatory diseases. Of course, humanized monoclonal antibodies (mAb) are manufactured in low-yield and time-consuming mammalian cells, and are thus tremendously more expensive than small molecule chemical drugs [164]. For example, the recommended dose for frequent injections of Humira (TNF mAb) to treat rheumatoid arthritis is 40 mg every two weeks, totaling > 1 gram (> $16,000) per year. It is thus essential to develop cost-effective small molecule drugs for the clinical management of human sepsis. One of the most selective HMGB1 inhibitor, TSN-SS, has already been used in China as a medicine for patients with cardiovascular disorders. The dual effects of TSN-SS in attenuating late inflammatory response and improving cardiovascular function make it a promising therapeutic agent for sepsis. The capacity to facilitate endocytic HMGB1 uptake by professional phagocytes may provide basis for the treatment of both infection- and injury-elicited inflammatory diseases [164]. It is not yet known whether a better protection could be achieved by combinational therapy with several anti-HMGB1 agents, which makes it important to further explore the therapeutic potential of these HMGB1-inhibiting agents in future studies.
6. KEY ISSUES.
5.1. PAMPs stimulate immune cells to sequentially release early proinflammatory cytokines and late proinflammatory mediators (e.g., HMGB1).
5.2. The secretion of HMGB1 from immunologically activated immune cells is regulated by specific molecular mechanisms: JAK/STAT1 regulates cytoplasmic translocation, and PKR/inflammasomes-dependent pyroptosis regulates cellular secretion.
5.2. HMGB1 can be passively released from necrotic cells following ischemiareperfusion, trauma, and injury; in this context it is a DMAP which orchestrates injury-elicited inflammatory responses by interacting with a family of receptors.
5.3. Early proinflammatory cytokines (e.g. TNF, IFN-β or IFN-γ) also induce a highly regulated cell death process, termed necroptosis, which may contribute to HMGB1 release.
5.4. Extracellular HMGB1 functions as a DAMP molecule to alert, recruit, and activate immune cells, thereby serving as a mediator of lethal infection and injury.
5.5. A number of endogenous macromolecules (e.g., intravenous immunoglobulin, anti-coagulants, acute phase proteins, and hormones) have proven effective in inhibiting HMGB1 release, and protecting against lethal infection and injury.
5.6. Many herbal extracts and components have been proven effective in inhibiting HMGB1 release and protective against lethal infection and injury.
5.7. Different herbal components (e.g., EGCG, TSN-SS, and CBX) inhibit active HMGB1 secretion through divergently distinct mechanisms, ranging from inducing autophagic degradation, stimulating endocytic uptake, to preventing PKR activation.
5.8. Many agents capable of inhibiting HMGB1 secretion are also proven protective in various animal models of injury, but the protective mechanisms remains poorly elucidated.
Acknowledgments and funding
We are grateful to the peer reviewers for their critical and constructive comments. Work in authors’ laboratory was supported by grants from the National Center of Complementary and Alternative Medicine (NCCAM, R01AT005076) and the National Institute of General Medical Sciences (NIGMS, R01GM063075).
References
Papers of special note have been highlighted as:
* of interest
** of considerable interests.
- 1.Andersson U, Tracey KJ. HMGB1 is a therapeutic target for sterile inflammation and infection. Annu Rev Immunol. 2011;29:139–62. doi: 10.1146/annurev-immunol-030409-101323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yanai H, Matsuda A, An J, et al. Conditional ablation of HMGB1 in mice reveals its protective function against endotoxemia and bacterial infection. Proc Natl Acad Sci U S A. 2013;110:20699–20704. doi: 10.1073/pnas.1320808110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang H, Nace GW, McDonald KA, et al. Hepatocyte specific HMGB1 deletion worsens the injury in liver ischemia/reperfusion: A role for intracellular HMGB1 in cellular protection. Hepatology. 2014 doi: 10.1002/hep.26976. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kang R, Zhang Q, Hou W, et al. Intracellular Hmgb1 Inhibits Inflammatory Nucleosome Release and Limits Acute Pancreatitis in Mice. Gastroenterology. 2014 doi: 10.1053/j.gastro.2013.12.015. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5**.Wang H, Bloom O, Zhang M, et al. HMG-1 as a late mediator of endotoxin lethality in mice. Science. 1999;285:248–251. doi: 10.1126/science.285.5425.248. [These authors made the seminal discovery on the extracellular role of HMG-1 (now called HMGB1) as a late mediator of lethal systemic inflammation.] [DOI] [PubMed] [Google Scholar]
- 6.Ivanov S, Dragoi AM, Wang X, et al. A novel role for HMGB1 in TLR9-mediated inflammatory responses to CpG-DNA. Blood. 2007;110:1970–1981. doi: 10.1182/blood-2006-09-044776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7*.Rendon-Mitchell B, Ochani M, Li J, et al. IFN-gamma Induces High Mobility Group Box 1 Protein Release Partly Through a TNF-Dependent Mechanism. J Immunol. 2003;170:3890–3897. doi: 10.4049/jimmunol.170.7.3890. [These authors first reported the important role of early cytokines in stimulating HMGB1 release.] [DOI] [PubMed] [Google Scholar]
- 8.Kim JH, Kim SJ, Lee IS, et al. Bacterial endotoxin induces the release of high mobility group box 1 via the IFN-beta signaling pathway. J Immunol. 2009;182:2458–2466. doi: 10.4049/jimmunol.0801364. [DOI] [PubMed] [Google Scholar]
- 9.Lu B, Antoine DJ, Kwan K, et al. JAK/STAT1 signaling promotes HMGB1 hyperacetylation and nuclear translocation. Proc Natl Acad Sci U S A. 2014 doi: 10.1073/pnas.1316925111. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hao N, Budnik BA, Gunawardena J, et al. Tunable signal processing through modular control of transcription factor translocation. Science. 2013;339:460–464. doi: 10.1126/science.1227299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11*.Bonaldi T, Talamo F, Scaffidi P, et al. Monocytic cells hyperacetylate chromatin protein HMGB1 to redirect it towards secretion. EMBO J. 2003;22:5551–5560. doi: 10.1093/emboj/cdg516. [These authors first reported the regulatory role of acetylation in the regulation of HMGB1 nuclear-cytoplasmic translocation.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12*.Gardella S, Andrei C, Ferrera D, et al. The nuclear protein HMGB1 is secreted by monocytes via a non-classical, vesicle-mediated secretory pathway. EMBO Rep. 2002;3:955–1001. doi: 10.1093/embo-reports/kvf198. [These authors first showed that HMGB1 is secreted by activated monocytes via a non-classical secretory pathway.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Evankovich J, Cho SW, Zhang R, et al. High mobility group box 1 release from hepatocytes during ischemia and reperfusion injury is mediated by decreased histone deacetylase activity. J Biol Chem. 2010;285:39888–39897. doi: 10.1074/jbc.M110.128348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kang R, Livesey KM, Zeh HJ, et al. HMGB1: A novel Beclin 1-binding protein active in autophagy. Autophagy. 2010;6:1209–1211. doi: 10.4161/auto.6.8.13651. [DOI] [PubMed] [Google Scholar]
- 15.O'Shea JJ, Plenge R. JAK and STAT signaling molecules in immunoregulation and immune-mediated disease. Immunity. 2012;36:542–550. doi: 10.1016/j.immuni.2012.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yanai H, Ban T, Wang Z, et al. HMGB proteins function as universal sentinels for nucleic-acid-mediated innate immune responses. Nature. 2009;462:99–103. doi: 10.1038/nature08512. [DOI] [PubMed] [Google Scholar]
- 17.Youn JH, Shin JS. Nucleocytoplasmic shuttling of HMGB1 is regulated by phosphorylation that redirects it toward secretion. J Immunol. 2006;177:7889–7897. doi: 10.4049/jimmunol.177.11.7889. [DOI] [PubMed] [Google Scholar]
- 18.Zhang X, Wheeler D, Tang Y, et al. Calcium/calmodulin-dependent protein kinase (CaMK) IV mediates nucleocytoplasmic shuttling and release of HMGB1 during lipopolysaccharide stimulation of macrophages. J Immunol. 2008;181:5015–5023. doi: 10.4049/jimmunol.181.7.5015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ito I, Fukazawa J, Yoshida M. Post-translational methylation of high mobility group box 1 (HMGB1) causes its cytoplasmic localization in neutrophils. J Biol Chem. 2007;282:16336–16344. doi: 10.1074/jbc.M608467200. [DOI] [PubMed] [Google Scholar]
- 20.Qin S, Wang H, Yuan R, et al. Role of HMGB1 in apoptosis-mediated sepsis lethality. J Exp Med. 2006;203:1637–1642. doi: 10.1084/jem.20052203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21*.Lamkanfi M, Sarkar A, Vande WL, et al. Inflammasome-dependent release of the alarmin HMGB1 in endotoxemia. J Immunol. 2010;185:4385–4392. doi: 10.4049/jimmunol.1000803. [These authors first reported the role of inflammasome in the regulation of endotoxin-induced HMGB1 release.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Strowig T, Henao-Mejia J, Elinav E, et al. Inflammasomes in health and disease. Nature. 2012;481:278–286. doi: 10.1038/nature10759. [DOI] [PubMed] [Google Scholar]
- 23.Kayagaki N, Warming S, Lamkanfi M, et al. Non-canonical inflammasome activation targets caspase-11. Nature. 2011;479:117–121. doi: 10.1038/nature10558. [DOI] [PubMed] [Google Scholar]
- 24.Kayagaki N, Wong MT, Stowe IB, et al. Noncanonical inflammasome activation by intracellular LPS independent of TLR4. Science. 2013;341:1246–1249. doi: 10.1126/science.1240248. [DOI] [PubMed] [Google Scholar]
- 25**.Lu B, Nakamura T, Inouye K, et al. Novel role of PKR in inflammasome activation and HMGB1 release. Nature. 2012;488:670–674. doi: 10.1038/nature11290. [These authors made the seminal discovery on the important role of PKR in the regulation of inflammasome activation and HMGB1 release.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li W, Li J, Sama AE, et al. Carbenoxolone Blocks Endotoxin-Induced Protein Kinase R (PKR) Activation and High Mobility Group Box 1 (HMGB1) Release. Mol Med. 2013;19:203–211. doi: 10.2119/molmed.2013.00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27*.Hett EC, Slater LH, Mark KG, et al. Chemical genetics reveals a kinase-independent role for protein kinase R in pyroptosis. Nat Chem Biol. 2013;9:398–405. doi: 10.1038/nchembio.1236. [These authors first reported the important role of PKR activation in pyroptosis.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Keller M, Ruegg A, Werner S, et al. Active caspase-1 is a regulator of unconventional protein secretion. Cell. 2008;132:818–831. doi: 10.1016/j.cell.2007.12.040. [DOI] [PubMed] [Google Scholar]
- 29**.Scaffidi P, Misteli T, Bianchi ME. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature. 2002;418:191–195. doi: 10.1038/nature00858. [These authors first reported that HMGB1 can be passively released by necrotic cells, and induces cytokine production.] [DOI] [PubMed] [Google Scholar]
- 30*.Tsung A, Sahai R, Tanaka H, et al. The nuclear factor HMGB1 mediates hepatic injury after murine liver ischemia-reperfusion. J Exp Med. 2005;201:1135–1143. doi: 10.1084/jem.20042614. [These authors first reported the extracellular role of HMGB1 as a damage-associated molecular pattern (DAMP) molecule.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Andrassy M, Volz HC, Igwe JC, et al. High-mobility group box-1 in ischemia reperfusion injury of the heart. Circulation. 2008;117:3216–3226. doi: 10.1161/CIRCULATIONAHA.108.769331. [DOI] [PubMed] [Google Scholar]
- 32.Cohen MJ, Brohi K, Calfee CS, et al. Early release of high mobility group box nuclear protein 1 after severe trauma in humans: role of injury severity and tissue hypoperfusion. Crit Care. 2009;13:R174. doi: 10.1186/cc8152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peltz ED, Moore EE, Eckels PC, et al. HMGB1 is markedly elevated within 6 hours of mechanical trauma in humans. Shock. 2009;32:17–22. doi: 10.1097/shk.0b013e3181997173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhou RR, Liu HB, Peng JP, et al. High mobility group box chromosomal protein 1 in acute-on-chronic liver failure patients and mice with ConA-induced acute liver injury. Exp Mol Pathol. 2012;93:213–219. doi: 10.1016/j.yexmp.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 35.Antoine DJ, Dear JW, Lewis PS, et al. Mechanistic biomarkers provide early and sensitive detection of acetaminophen-induced acute liver injury at first presentation to hospital. Hepatology. 2013;58:777–787. doi: 10.1002/hep.26294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Seo YS, Kwon JH, Yaqoob U, et al. HMGB1 recruits hepatic stellate cells and liver endothelial cells to sites of ethanol induced parenchymal cell injury. Am J Physiol Gastrointest Liver Physiol. 2013;305(11):G838–G848. doi: 10.1152/ajpgi.00151.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhu S, Li W, Ward MF, et al. High mobility group box 1 protein as a potential drug target for infection- and injury-elicited inflammation. Inflamm Allergy Drug Targets. 2010;9:60–72. doi: 10.2174/187152810791292872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vincent JL, Opal SM, Marshall JC, et al. Sepsis definitions: time for change. Lancet. 2013;381:774–775. doi: 10.1016/S0140-6736(12)61815-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cho YS, Challa S, Moquin D, et al. Phosphorylation-driven assembly of the RIP1-RIP3 complex regulates programmed necrosis and virus-induced inflammation. Cell. 2009;137:1112–1123. doi: 10.1016/j.cell.2009.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thapa RJ, Nogusa S, Chen P, et al. Interferon-induced RIP1/RIP3-mediated necrosis requires PKR and is licensed by FADD and caspases. Proc Natl Acad Sci U S A. 2013;110:E3109–E3118. doi: 10.1073/pnas.1301218110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen LC, Yeh TM, Wu HN, et al. Dengue virus infection induces passive release of high mobility group box 1 protein by epithelial cells. J Infect. 2008;56:143–150. doi: 10.1016/j.jinf.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 42.Alleva LM, Budd AC, Clark IA. Systemic release of high mobility group box 1 protein during severe murine influenza. J Immunol. 2008;181:1454–1459. doi: 10.4049/jimmunol.181.2.1454. [DOI] [PubMed] [Google Scholar]
- 43.Whilding LM, Archibald KM, Kulbe H, et al. Vaccinia Virus Induces Programmed Necrosis in Ovarian Cancer Cells. Mol Ther. 2013;21(11):2074–2086. doi: 10.1038/mt.2013.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang H, Ward MF, Fan XG, et al. Potential role of high mobility group box 1 in viral infectious diseases. Viral Immunol. 2006;19:3–9. doi: 10.1089/vim.2006.19.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gunther C, Martini E, Wittkopf N, et al. Caspase-8 regulates TNF-alpha-induced epithelial necroptosis and terminal ileitis. Nature. 2011;477:335–339. doi: 10.1038/nature10400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang Q, Raoof M, Chen Y, et al. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature. 2010;464:104–107. doi: 10.1038/nature08780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Qiang X, Yang WL, Wu R, et al. Cold-inducible RNA-binding protein (CIRP) triggers inflammatory responses in hemorrhagic shock and sepsis. Nat Med. 2013;19:1489–1495. doi: 10.1038/nm.3368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tian J, Avalos AM, Mao SY, et al. Toll-like receptor 9-dependent activation by DNA-containing immune complexes is mediated by HMGB1 and RAGE. Nat Immunol. 2007;8:487–496. doi: 10.1038/ni1457. [DOI] [PubMed] [Google Scholar]
- 49.Yang D, Chen Q, Yang H, et al. High mobility group box-1 protein induces the migration and activation of human dendritic cells and acts as an alarmin. J Leukoc Biol. 2007;81:59–66. doi: 10.1189/jlb.0306180. [DOI] [PubMed] [Google Scholar]
- 50.Dumitriu IE, Bianchi ME, Bacci M, et al. The secretion of HMGB1 is required for the migration of maturing dendritic cells. J Leukoc Biol. 2007;81:84–91. doi: 10.1189/jlb.0306171. [DOI] [PubMed] [Google Scholar]
- 51.Orlova VV, Choi EY, Xie C, et al. A novel pathway of HMGB1-mediated inflammatory cell recruitment that requires Mac-1-integrin. EMBO J. 2007;26:1129–1139. doi: 10.1038/sj.emboj.7601552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Degryse B, Bonaldi T, Scaffidi P, et al. The high mobility group (HMG) boxes of the nuclear protein HMG1 induce chemotaxis and cytoskeleton reorganization in rat smooth muscle cells. J Cell Biol. 2001;152:1197–1206. doi: 10.1083/jcb.152.6.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yu M, Wang H, Ding A, et al. HMGB1 SIGNALS THROUGH TOLL-LIKE RECEPTOR (TLR) 4 AND TLR2. Shock. 2006;26:174–179. doi: 10.1097/01.shk.0000225404.51320.82. [DOI] [PubMed] [Google Scholar]
- 54.Chen GY, Tang J, Zheng P, et al. CD24 and Siglec-10 selectively repress tissue damage-induced immune responses. Science. 2009;323:1722–1725. doi: 10.1126/science.1168988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Abeyama K, Stern DM, Ito Y, et al. The N-terminal domain of thrombomodulin sequesters high-mobility group-B1 protein, a novel antiinflammatory mechanism. J Clin Invest. 2005;115:1267–1274. doi: 10.1172/JCI22782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Salmivirta M, Rauvala H, Elenius K, et al. Neurite growth-promoting protein (amphoterin, p30) binds syndecan. Exp Cell Res. 1992;200:444–451. doi: 10.1016/0014-4827(92)90194-d. [DOI] [PubMed] [Google Scholar]
- 57.Zhu S, Ashok M, Li J, et al. Spermine protects mice against lethal sepsis partly by attenuating surrogate inflammatory markers. Mol Med. 2009;15:275–282. doi: 10.2119/molmed.2009.00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fiuza C, Bustin M, Talwar S, et al. Inflammation-promoting activity of HMGB1 on human microvascular endothelial cells. Blood. 2003;101:2652–2660. doi: 10.1182/blood-2002-05-1300. [DOI] [PubMed] [Google Scholar]
- 59.Kazama H, Ricci JE, Herndon JM, et al. Induction of immunological tolerance by apoptotic cells requires caspase-dependent oxidation of high-mobility group box-1 protein. Immunity. 2008;29:21–32. doi: 10.1016/j.immuni.2008.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Venereau E, Casalgrandi M, Schiraldi M, et al. Mutually exclusive redox forms of HMGB1 promote cell recruitment or proinflammatory cytokine release. J Exp Med. 2012;209:1519–1528. doi: 10.1084/jem.20120189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Liu A, Fang H, Dirsch O, et al. Oxidation of HMGB1 causes attenuation of its pro-inflammatory activity and occurs during liver ischemia and reperfusion. PLoS One. 2012;7:e35379. doi: 10.1371/journal.pone.0035379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Antoine DJ, Harris HE, Andersson U, Tracey KJ, Bianchi ME. A systematic nomenclature for the redox states of high mobility group box (HMGB) proteins. Mol. Med. 2014 doi: 10.2119/molmed.2014.00022. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Schiraldi M, Raucci A, Munoz LM, et al. HMGB1 promotes recruitment of inflammatory cells to damaged tissues by forming a complex with CXCL12 and signaling via CXCR4. J Exp Med. 2012;209:551–563. doi: 10.1084/jem.20111739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yang H, Lundback P, Ottosson L, et al. Redox modification of cysteine residues regulates the cytokine activity of high mobility group box-1 (HMGB1). Mol Med. 2012;18:250–259. doi: 10.2119/molmed.2011.00389. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 65.Nystrom S, Antoine DJ, Lundback P, et al. TLR activation regulates damage-associated molecular pattern isoforms released during pyroptosis. EMBO J. 2013;32:86–99. doi: 10.1038/emboj.2012.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhou R, Yazdi AS, Menu P, et al. A role for mitochondria in NLRP3 inflammasome activation. Nature. 2011;469:221–225. doi: 10.1038/nature09663. [DOI] [PubMed] [Google Scholar]
- 67.Allen IC, Scull MA, Moore CB, et al. The NLRP3 inflammasome mediates in vivo innate immunity to influenza A virus through recognition of viral RNA. Immunity. 2009;30:556–565. doi: 10.1016/j.immuni.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Miao EA, Leaf IA, Treuting PM, et al. Caspase-1-induced pyroptosis is an innate immune effector mechanism against intracellular bacteria. Nat Immunol. 2010;11:1136–1142. doi: 10.1038/ni.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang H, Zhu S, Zhou R, et al. Therapeutic potential of HMGB1-targeting agents in sepsis. Expert Rev Mol Med. 2008;10:e32. doi: 10.1017/S1462399408000884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Poltorak A, He X, Smirnova I, et al. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 1998;282:2085–2088. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- 71.Nagai Y, Akashi S, Nagafuku M, et al. Essential role of MD-2 in LPS responsiveness and TLR4 distribution. Nat Immunol. 2002;3:667–672. doi: 10.1038/ni809. [DOI] [PubMed] [Google Scholar]
- 72.Kim S, Kim SY, Pribis JP, et al. Signaling of high mobility group box 1 (HMGB1) through toll-like receptor 4 in macrophages requires CD14. Mol Med. 2013;19:88–98. doi: 10.2119/molmed.2012.00306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hsu LC, Enzler T, Seita J, et al. IL-1beta-driven neutrophilia preserves antibacterial defense in the absence of the kinase IKKbeta. Nat Immunol. 2011;12:144–150. doi: 10.1038/ni.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yang H, Ochani M, Li J, et al. Reversing established sepsis with antagonists of endogenous high-mobility group box 1. Proc Natl Acad Sci U S A. 2004;101:296–301. doi: 10.1073/pnas.2434651100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang H, Yang H, Czura CJ, et al. HMGB1 as a Late Mediator of Lethal Systemic Inflammation. Am J Respir Crit Care Med. 2001;164:1768–1773. doi: 10.1164/ajrccm.164.10.2106117. [DOI] [PubMed] [Google Scholar]
- 76.Ye C, Choi JG, Abraham S, et al. Human macrophage and dendritic cell-specific silencing of high-mobility group protein B1 ameliorates sepsis in a humanized mouse model. Proc Natl Acad Sci U S A. 2012;109:21052–21057. doi: 10.1073/pnas.1216195109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wu H, Ma J, Wang P, et al. HMGB1 contributes to kidney ischemia reperfusion injury. J Am Soc Nephrol. 2010;21:1878–1890. doi: 10.1681/ASN.2009101048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Qiu J, Nishimura M, Wang Y, et al. Early release of HMGB-1 from neurons after the onset of brain ischemia. J Cereb Blood Flow Metab. 2008;28:927–938. doi: 10.1038/sj.jcbfm.9600582. [DOI] [PubMed] [Google Scholar]
- 79.Okuma Y, Liu K, Wake H, et al. Anti-high mobility group box-1 antibody therapy for traumatic brain injury. Ann Neurol. 2012;72:373–384. doi: 10.1002/ana.23602. [DOI] [PubMed] [Google Scholar]
- 80.Shimazaki J, Matsumoto N, Ogura H, et al. Systemic involvement of high-mobility group box 1 protein and therapeutic effect of anti-high-mobility group box 1 protein antibody in a rat model of crush injury. Shock. 2012;37:634–638. doi: 10.1097/SHK.0b013e31824ed6b7. [DOI] [PubMed] [Google Scholar]
- 81.Yang R, Zhang S, Cotoia A, et al. High mobility group B1 impairs hepatocyte regeneration in acetaminophen hepatotoxicity. BMC Gastroenterol. 2012;12:45. doi: 10.1186/1471-230X-12-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Nadatani Y, Watanabe T, Tanigawa T, et al. High mobility group box 1 promotes small intestinal damage induced by nonsteroidal anti-inflammatory drugs through Toll-like receptor 4. Am J Pathol. 2012;181:98–110. doi: 10.1016/j.ajpath.2012.03.039. [DOI] [PubMed] [Google Scholar]
- 83.Hirata Y, Kurobe H, Higashida M, et al. HMGB1 plays a critical role in vascular inflammation and lesion formation via toll-like receptor 9. Atherosclerosis. 2013;231:227–233. doi: 10.1016/j.atherosclerosis.2013.09.010. [DOI] [PubMed] [Google Scholar]
- 84.Nadatani Y, Watanabe T, Tanigawa T, et al. High-Mobility Group Box 1 Inhibits Gastric Ulcer Healing through Toll-Like Receptor 4 and Receptor for Advanced Glycation End Products. PLoS One. 2013;8:e80130. doi: 10.1371/journal.pone.0080130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Patel VS, Sitapara RA, Gore A, et al. High Mobility Group Box-1 mediates hyperoxia-induced impairment of Pseudomonas aeruginosa clearance and inflammatory lung injury in mice. Am J Respir Cell Mol Biol. 2013;48:280–287. doi: 10.1165/rcmb.2012-0279OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Harris HE, Andersson U, Pisetsky DS. HMGB1: a multifunctional alarmin driving autoimmune and inflammatory disease. Nat Rev Rheumatol. 2012;8:195–202. doi: 10.1038/nrrheum.2011.222. [DOI] [PubMed] [Google Scholar]
- 87.Schaper F, Westra J, Bijl M. Recent developments on the role of High Mobility Group Box 1 in Systemic Lupus Erythematosus. Mol Med. 2014;10 doi: 10.2119/molmed.2014.00019. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yu SL, Wong CK, Tam LS. The alarmin functions of high-mobility group box-1 and IL-33 in the pathogenesis of systemic lupus erythematosus. Expert Rev Clin Immunol. 2013;9:739–749. doi: 10.1586/1744666X.2013.814428. [DOI] [PubMed] [Google Scholar]
- 89.Andersson U, Harris HE. The role of HMGB1 in the pathogenesis of rheumatic disease. Biochim Biophys Acta. 2010;1799:141–148. doi: 10.1016/j.bbagrm.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 90.Liu G, Wang J, Park YJ, et al. High Mobility Group Protein-1 Inhibits Phagocytosis of Apoptotic Neutrophils through Binding to Phosphatidylserine. J Immunol. 2008;181:4240–4246. doi: 10.4049/jimmunol.181.6.4240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Urbonaviciute V, Furnrohr BG, Meister S, et al. Induction of inflammatory and immune responses by HMGB1-nucleosome complexes: implications for the pathogenesis of SLE. J Exp Med. 2008;205:3007–3018. doi: 10.1084/jem.20081165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Popovic K, Ek M, Espinosa A, et al. Increased expression of the novel proinflammatory cytokine high mobility group box chromosomal protein 1 in skin lesions of patients with lupus erythematosus. Arthritis Rheum. 2005;52:3639–3645. doi: 10.1002/art.21398. [DOI] [PubMed] [Google Scholar]
- 93.Barkauskaite V, Ek M, Popovic K, et al. Translocation of the novel cytokine HMGB1 to the cytoplasm and extracellular space coincides with the peak of clinical activity in experimentally UV-induced lesions of cutaneous lupus erythematosus. Lupus. 2007;16:794–802. doi: 10.1177/0961203307081895. [DOI] [PubMed] [Google Scholar]
- 94.Abdulahad DA, Westra J, Reefman E, et al. High mobility group box1 (HMGB1) in relation to cutaneous inflammation in systemic lupus erythematosus (SLE). Lupus. 2013;22:597–606. doi: 10.1177/0961203313483377. [DOI] [PubMed] [Google Scholar]
- 95.Bald T, Quast T, Landsberg J, et al. Ultraviolet-radiation-induced inflammation promotes angiotropism and metastasis in melanoma. Nature. 2014;507:109–113. doi: 10.1038/nature13111. [DOI] [PubMed] [Google Scholar]
- 96.Zhang C, Li C, Jia S, et al. High mobility group box 1 inhibition alleviates lupus-like disease in BXSB mice. Scand J Immunol. 2014;10 doi: 10.1111/sji.12165. in press. [DOI] [PubMed] [Google Scholar]
- 97.Kokkola R, Li J, Sundberg E, et al. Successful treatment of collagen-induced arthritis in mice and rats by targeting extracellular high mobility group box chromosomal protein 1 activity. Arthritis Rheum. 2003;48:2052–2058. doi: 10.1002/art.11161. [DOI] [PubMed] [Google Scholar]
- 98.Schierbeck H, Lundback P, Palmblad K, et al. Monoclonal anti-HMGB1 (high mobility group box chromosomal protein 1) antibody protection in two experimental arthritis models. Mol Med. 2011;17:1039–1044. doi: 10.2119/molmed.2010.00264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ostberg T, Kawane K, Nagata S, et al. Protective targeting of high mobility group box chromosomal protein 1 in a spontaneous arthritis model. Arthritis Rheum. 2010;62:2963–2972. doi: 10.1002/art.27590. [DOI] [PubMed] [Google Scholar]
- 100.Rosas-Ballina M, Olofsson PS, Ochani M, et al. Acetylcholine-synthesizing T cells relay neural signals in a vagus nerve circuit. Science. 2011;334:98–101. doi: 10.1126/science.1209985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Borovikova LV, Ivanova S, Zhang M, et al. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature. 2000;405:458–462. doi: 10.1038/35013070. [DOI] [PubMed] [Google Scholar]
- 102.Wang H, Yu M, Ochani M, et al. Nicotinic acetylcholine receptor alpha7 subunit is an essential regulator of inflammation. Nature. 2000;421:384–388. doi: 10.1038/nature01339. [DOI] [PubMed] [Google Scholar]
- 103.Wang H, Liao H, Ochani M, et al. Cholinergic agonists inhibit HMGB1 release and improve survival in experimental sepsis. Nat Med. 2004;10:1216–1221. doi: 10.1038/nm1124. [DOI] [PubMed] [Google Scholar]
- 104.Zhang M, Caragine T, Wang H, et al. Spermine inhibits proinflammatory cytokine synthesis in human mononuclear cells: a counterregulatory mechanism that restrains the immune response. J Exp Med. 1997;185:1759–1768. doi: 10.1084/jem.185.10.1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zhang M, Borovikova LV, Wang H, et al. Spermine inhibition of monocyte activation and inflammation. Mol Med. 1999;5:595–605. [PMC free article] [PubMed] [Google Scholar]
- 106.Wang H, Zhang M, Bianchi M, et al. Fetuin (alpha2-HS-glycoprotein) opsonizes cationic macrophagedeactivating molecules. Proc Natl Acad Sci U S A. 1998;95:14429–14434. doi: 10.1073/pnas.95.24.14429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zhang M, Wang H, Tracey KJ. Regulation of macrophage activation and inflammation by spermine: a new chapter in an old story. Crit Care Med. 2000;28:N60–N66. doi: 10.1097/00003246-200004001-00007. [DOI] [PubMed] [Google Scholar]
- 108.Li W, Zhu S, Li J, et al. A hepatic protein, fetuin-A, occupies a protective role in lethal systemic inflammation. PLoS ONE. 2011;6:e16945. doi: 10.1371/journal.pone.0016945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wang H, Li W, Zhu S, et al. Peripheral administration of fetuin-A attenuates early cerebral ischemic injury in rats. J Cereb Blood Flow Metab. 2010;30:493–504. doi: 10.1038/jcbfm.2009.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hagiwara S, Iwasaka H, Hasegawa A, et al. High-dose intravenous immunoglobulin G improves systemic inflammation in a rat model of CLP-induced sepsis. Intensive Care Med. 2008;34:1812–1819. doi: 10.1007/s00134-008-1161-1. [DOI] [PubMed] [Google Scholar]
- 111.Hagiwara S, Iwasaka H, Matsumoto S, et al. High dose antithrombin III inhibits HMGB1 and improves endotoxin-induced acute lung injury in rats. Intensive Care Med. 2008;34:361–367. doi: 10.1007/s00134-007-0887-5. [DOI] [PubMed] [Google Scholar]
- 112.Chorny A, Delgado M. Neuropeptides rescue mice from lethal sepsis by down-regulating secretion of the late-acting inflammatory mediator high mobility group box 1. Am J Pathol. 2008;172:1297–1307. doi: 10.2353/ajpath.2008.070969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Chorny A, Anderson P, Gonzalez-Rey E, et al. Ghrelin Protects against Experimental Sepsis by Inhibiting High-Mobility Group Box 1 Release and by Killing Bacteria. J Immunol. 2008;180:8369–8377. doi: 10.4049/jimmunol.180.12.8369. [DOI] [PubMed] [Google Scholar]
- 114.Favreau F, Thuillier R, Cau J, et al. Anti-thrombin therapy during warm ischemia and cold preservation prevents chronic kidney graft fibrosis in a DCD model. Am J Transplant. 2010;10:30–39. doi: 10.1111/j.1600-6143.2009.02924.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Herzog C, Lorenz A, Gillmann HJ, et al. Thrombomodulin's lectin-like domain reduces myocardial damage by interfering with HMGB1-mediated TLR2 signalling. Cardiovasc Res. 2014 doi: 10.1093/cvr/cvt275. in press. [DOI] [PubMed] [Google Scholar]
- 116.Jiang W, Tang W, Geng Q, et al. Inhibition of Toll-like receptor 4 with vasoactive intestinal peptide attenuates liver ischemia-reperfusion injury. Transplant Proc. 2011;43:1462–1467. doi: 10.1016/j.transproceed.2011.01.191. [DOI] [PubMed] [Google Scholar]
- 117.Zhang H, Cui Z, Luo G, et al. Ghrelin attenuates intestinal ischemia/reperfusion injury in mice by activating the mTOR signaling pathway. Int J Mol Med. 2013;32:851–859. doi: 10.3892/ijmm.2013.1452. [DOI] [PubMed] [Google Scholar]
- 118.Fann DY, Lee SY, Manzanero S, et al. Intravenous immunoglobulin suppresses NLRP1 and NLRP3 inflammasome-mediated neuronal death in ischemic stroke. Cell Death Dis. 2013;4:e790. doi: 10.1038/cddis.2013.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Mohri T, Tanaka H, Tajima G, et al. Synergistic effects of recombinant human soluble thrombomodulin and fluid-volume resuscitation in a rat lethal crush injury model. Shock. 2006;26:581–586. doi: 10.1097/01.shk.0000233198.48612.6b. [DOI] [PubMed] [Google Scholar]
- 120.Wang XQ, Hayes MT, Kempf M, et al. Fetuin-A: a major fetal serum protein that promotes “wound closure” and scarless healing. J Invest Dermatol. 2008;128:753–757. doi: 10.1038/sj.jid.5701119. [DOI] [PubMed] [Google Scholar]
- 121.Luo Q, Wang Y, Feng D, et al. Vasoactive intestinal peptide attenuates concanavalin A-mediated liver injury. Eur J Pharmacol. 2009;607:226–233. doi: 10.1016/j.ejphar.2009.02.012. [DOI] [PubMed] [Google Scholar]
- 122.Imazu Y, Yanagi S, Miyoshi K, et al. Ghrelin ameliorates bleomycin-induced acute lung injury by protecting alveolar epithelial cells and suppressing lung inflammation. Eur J Pharmacol. 2011;672:153–158. doi: 10.1016/j.ejphar.2011.09.183. [DOI] [PubMed] [Google Scholar]
- 123.Yang D, Liu Z, Zhang H, et al. Ghrelin protects human pulmonary artery endothelial cells against hypoxia-induced injury via PI3-kinase/Akt. Peptides. 2013;42:112–117. doi: 10.1016/j.peptides.2013.01.012. [DOI] [PubMed] [Google Scholar]
- 124.Jacob A, Shah KG, Wu R, et al. Ghrelin as a novel therapy for radiation combined injury. Mol Med. 2010;16:137–143. doi: 10.2119/molmed.2009.00154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wang H, Li W, Li J, et al. The aqueous extract of a popular herbal nutrient supplement, Angelica sinensis, protects mice against lethal endotoxemia and sepsis. J Nutr. 2006;136:360–365. doi: 10.1093/jn/136.2.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Zhu S, Li W, Li J, et al. It Is Not Just Folklore: The Aqueous Extract of Mung Bean Coat Is Protective against Sepsis. Evid Based Complement Alternat Med. 2012;2012:498467. doi: 10.1155/2012/498467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Jun MS, Kim HS, Kim YM, et al. Ethanol extract of Prunella vulgaris var. lilacina inhibits HMGB1 release by induction of heme oxygenase-1 in LPS-activated RAW 264.7 cells and CLP-induced septic mice. Phytother Res. 2012;26:605–612. doi: 10.1002/ptr.3613. [DOI] [PubMed] [Google Scholar]
- 128.Xie CH, Zhang MS, Zhou YF, et al. Chinese medicine Angelica sinensis suppresses radiation-induced expression of TNF-alpha and TGF-beta1 in mice. Oncol Rep. 2006;15:1429–1436. [PubMed] [Google Scholar]
- 129.Mohd AN, Mohd YH, Long K, et al. Antioxidant and hepatoprotective effect of aqueous extract of germinated and fermented mung bean on ethanol-mediated liver damage. Biomed Res Int. 2013;2013:693613. doi: 10.1155/2013/693613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Li W, Ashok M, Li J, et al. A Major Ingredient of Green Tea Rescues Mice from Lethal Sepsis Partly by Inhibiting HMGB1. PLoS ONE. 2007;2:e1153. doi: 10.1371/journal.pone.0001153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Li W, Li J, Ashok M, et al. A cardiovascular drug rescues mice from lethal sepsis by selectively attenuating a late-acting proinflammatory mediator, high mobility group box 1. J Immunol. 2007;178:3856–3864. doi: 10.4049/jimmunol.178.6.3856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Wang W, Zhao F, Fang Y, et al. Glycyrrhizin protects against porcine endotoxemia through modulation of systemic inflammatory response. Crit Care. 2013;17:R44. doi: 10.1186/cc12558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Lee CH, Yoon SJ, Lee SM. Chlorogenic acid attenuates high mobility group box 1 (HMGB1) and enhances host defense mechanisms in murine sepsis. Mol Med. 2013;18:1437–1448. doi: 10.2119/molmed.2012.00279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Lee W, Ku SK, Kim TH, et al. Emodin-6-O-beta-D-glucoside inhibits HMGB1-induced inflammatory responses in vitro and in vivo. Food Chem Toxicol. 2013;52:97–104. doi: 10.1016/j.fct.2012.10.061. [DOI] [PubMed] [Google Scholar]
- 135.Yang EJ, Ku SK, Lee W, et al. Barrier protective effects of rosmarinic acid on HMGB1-induced inflammatory responses in vitro and in vivo. J Cell Physiol. 2013;228:975–982. doi: 10.1002/jcp.24243. [DOI] [PubMed] [Google Scholar]
- 136.Kim TH, Ku SK, Bae JS. Anti-inflammatory activities of isorhamnetin-3-O-galactoside against HMGB1-induced inflammatory responses in both HUVECs and CLP-induced septic mice. J Cell Biochem. 2013;114:336–345. doi: 10.1002/jcb.24361. [DOI] [PubMed] [Google Scholar]
- 137.Kim TH, Ku SK, Bae JS. Persicarin is anti-inflammatory mediator against HMGB1-induced inflammatory responses in HUVECs and in CLP-induced sepsis mice. J Cell Physiol. 2013;228:696–703. doi: 10.1002/jcp.24214. [DOI] [PubMed] [Google Scholar]
- 138.Jiang WL, Yong X, Zhang SP, et al. Forsythoside B protects against experimental sepsis by modulating inflammatory factors. Phytother Res. 2012;26:981–987. doi: 10.1002/ptr.3668. [DOI] [PubMed] [Google Scholar]
- 139.Yang M, Cao L, Xie M, et al. Chloroquine inhibits HMGB1 inflammatory signaling and protects mice from lethal sepsis. Biochem Pharmacol. 2013;86(3):410–418. doi: 10.1016/j.bcp.2013.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Seo ES, Oh BK, Pak JH, et al. Acteoside improves survival in cecal ligation and puncture-induced septic mice via blocking of high mobility group box 1 release. Mol Cells. 2013;35:348–354. doi: 10.1007/s10059-013-0021-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Li W, Zhu S, Li J, et al. EGCG stimulates autophagy and reduces cytoplasmic HMGB1 levels in endotoxin-stimulated macrophages. Biochem Pharmacol. 2011;81:1152–1163. doi: 10.1016/j.bcp.2011.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Thorburn J, Horita H, Redzic J, et al. Autophagy regulates selective HMGB1 release in tumor cells that are destined to die. Cell Death Differ. 2009;16:175–183. doi: 10.1038/cdd.2008.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Tang D, Kang R, Livesey KM, et al. Endogenous HMGB1 regulates autophagy. J Cell Biol. 2010;190:881–892. doi: 10.1083/jcb.200911078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Tang D, Kang R, Cheh CW, et al. HMGB1 release and redox regulates autophagy and apoptosis in cancer cells. Oncogene. 2010;29:5299–5310. doi: 10.1038/onc.2010.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Huebener P, Gwak GY, Pradere JP, et al. High-mobility group box 1 is dispensable for autophagy, mitochondrial quality control, and organ function in vivo. Cell Metab. 2014;19:539–547. doi: 10.1016/j.cmet.2014.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Zhang Y, Li W, Zhu S, et al. Tanshinone IIA sodium sulfonate facilitates endocytic HMGB1 uptake. Biochem Pharmacol. 2012;84:1492–1500. doi: 10.1016/j.bcp.2012.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Zhao L, Li W, Zhu S, et al. Green tea catechins quench the fluorescence of bacteria-conjugated Alexa fluor dyes. Inflamm Allergy Drug Targets. 2013;12:308–314. doi: 10.2174/18715281113129990057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Wang JG, Bondy SC, Zhou L, et al. Protective Effect of Tanshinone IIA Against Infarct Size and Increased HMGB1, NFkappaB, GFAP and Apoptosis Consequent to Transient Middle Cerebral Artery Occlusion. Neurochem Res. 2014;39:295–304. doi: 10.1007/s11064-013-1221-y. [DOI] [PubMed] [Google Scholar]
- 149.Giakoustidis AE, Giakoustidis DE, Iliadis S, et al. Attenuation of intestinal ischemia/reperfusion induced liver and lung injury by intraperitoneal administration of (−)-epigallocatechin-3-gallate. Free Radic Res. 2006;40:103–110. doi: 10.1080/10715760500133479. [DOI] [PubMed] [Google Scholar]
- 150.Tamura K, Alessandri B, Heimann A, et al. The effect of a gap-junction blocker, carbenoxolone, on ischemic brain injury and cortical spreading depression. Neuroscience. 2011;194:262–71. doi: 10.1016/j.neuroscience.2011.07.043. [DOI] [PubMed] [Google Scholar]
- 151.Zhai CL, Zhang MQ, Zhang Y, et al. Glycyrrhizin protects rat heart against ischemia-reperfusion injury through blockade of HMGB1-dependent phospho-JNK/Bax pathway. Acta Pharmacol Sin. 2012;33:1477–1487. doi: 10.1038/aps.2012.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Ogiku M, Kono H, Hara M, et al. Glycyrrhizin prevents liver injury by inhibition of high-mobility group box 1 production by Kupffer cells after ischemia reperfusion in rats. J Pharmacol Exp Ther. 2011;339:93–98. doi: 10.1124/jpet.111.182592. [DOI] [PubMed] [Google Scholar]
- 153.Fang H, Liu A, Dahmen U, et al. Dual role of chloroquine in liver ischemia reperfusion injury: reduction of liver damage in early phase, but aggravation in late phase. Cell Death Dis. 2013;4:e694. doi: 10.1038/cddis.2013.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Yun N, Kang JW, Lee SM. Protective effects of chlorogenic acid against ischemia/reperfusion injury in rat liver: molecular evidence of its antioxidant and anti-inflammatory properties. J Nutr Biochem. 2012;23:1249–1255. doi: 10.1016/j.jnutbio.2011.06.018. [DOI] [PubMed] [Google Scholar]
- 155.Jiang WL, Tian JW, Fu FH, et al. Neuroprotective efficacy and therapeutic window of Forsythoside B: in a rat model of cerebral ischemia and reperfusion injury. Eur J Pharmacol. 2010;640:75–81. doi: 10.1016/j.ejphar.2010.04.055. [DOI] [PubMed] [Google Scholar]
- 156.Yin X, Yin Y, Cao FL, et al. Tanshinone IIA attenuates the inflammatory response and apoptosis after traumatic injury of the spinal cord in adult rats. PLoS One. 2012;7:e38381. doi: 10.1371/journal.pone.0038381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Hellmich HL, Rojo DR, Micci MA, et al. Pathway analysis reveals common pro-survival mechanisms of metyrapone and carbenoxolone after traumatic brain injury. PLoS One. 2013;8:e53230. doi: 10.1371/journal.pone.0053230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Renno WM, Al Maghrebi M, Alshammari A, et al. (−)-Epigallocatechin-3-gallate (EGCG) attenuates peripheral nerve degeneration in rat sciatic nerve crush injury. Neurochem Int. 2013;62:221–231. doi: 10.1016/j.neuint.2012.12.018. [DOI] [PubMed] [Google Scholar]
- 159.Ohnishi M, Katsuki H, Fukutomi C, et al. HMGB1 inhibitor glycyrrhizin attenuates intracerebral hemorrhage-induced injury in rats. Neuropharmacology. 2011;61:975–980. doi: 10.1016/j.neuropharm.2011.06.026. [DOI] [PubMed] [Google Scholar]
- 160.Lim Y, Hedayati M, Merchant AA, et al. Chloroquine improves survival and hematopoietic recovery after lethal low-dose-rate radiation. Int J Radiat Oncol Biol Phys. 2012;84:800–806. doi: 10.1016/j.ijrobp.2012.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Sanchez-Campillo M, Gabaldon JA, Castillo J, et al. Rosmarinic acid, a photo-protective agent against UV and other ionizing radiations. Food Chem Toxicol. 2009;7:386–392. doi: 10.1016/j.fct.2008.11.026. [DOI] [PubMed] [Google Scholar]
- 162.Lee KJ, Woo ER, Choi CY, et al. Protective effect of acteoside on carbon tetrachloride-induced hepatotoxicity. Life Sci. 2004;74:1051–1064. doi: 10.1016/j.lfs.2003.07.020. [DOI] [PubMed] [Google Scholar]
- 163.Kim DW, Cho HI, Kim KM, et al. Isorhamnetin-3-O-galactoside Protects against CCl4-Induced Hepatic Injury in Mice. Biomol Ther (Seoul ) 2012;20:406–412. doi: 10.4062/biomolther.2012.20.4.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Wang H, Ward MF, Sama AE. Targeting HMGB1 in the treatment of sepsis. Expert Opin Ther Targets. 2014 doi: 10.1517/14728222.2014.863876. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Valdes-Ferrer SI, Rosas-Ballina M, Olofsson PS, et al. High-mobility group box 1 mediates persistent splenocyte priming in sepsis survivors: evidence from a murine model. Shock. 2013;40:492–495. doi: 10.1097/SHK.0000000000000050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Sursal T, Stearns-Kurosawa DJ, Itagaki K, et al. Plasma bacterial and mitochondrial DNA distinguish bacterial sepsis from sterile systemic inflammatory response syndrome and quantify inflammatory tissue injury in nonhuman primates. Shock. 2013;39:55–62. doi: 10.1097/SHK.0b013e318276f4ca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Ziegler EJ, Fisher CJ, Jr., Sprung CL, et al. Treatment of gram-negative bacteremia and septic shock with HA-1A human monoclonal antibody against endotoxin. A randomized, double-blind, placebo-controlled trial. The HA-1A Sepsis Study Group. N Engl J Med. 1991;324:429–436. doi: 10.1056/NEJM199102143240701. [DOI] [PubMed] [Google Scholar]
- 168.Opal SM, Laterre PF, Francois B, et al. Effect of eritoran, an antagonist of MD2-TLR4, on mortality in patients with severe sepsis: the ACCESS randomized trial. JAMA. 2013;309:1154–1162. doi: 10.1001/jama.2013.2194. [DOI] [PubMed] [Google Scholar]
- 169.Feldmann M, Maini RN. Anti-TNF alpha therapy of rheumatoid arthritis: what have we learned? Annu Rev Immunol. 2001;19:163–96. doi: 10.1146/annurev.immunol.19.1.163. [DOI] [PubMed] [Google Scholar]
- 170.Abraham E, Wunderink R, Silverman H, et al. Efficacy and safety of monoclonal antibody to human tumor necrosis factor alpha in patients with sepsis syndrome. A randomized, controlled, double-blind, multicenter clinical trial. TNF-alpha MAb Sepsis Study Group. JAMA. 1995;273:934–941. [PubMed] [Google Scholar]