Abstract
Cocaine exposure in utero causes severe alterations in the development of the central nervous system. To study the basis of these teratogenic effects in vitro, we have used cocultures of neurons and glial cells from mouse embryonic brain. Cocaine selectively affected embryonic neuronal cells, causing first a dramatic reduction of both number and length of neurites and then extensive neuronal death. Scanning electron microscopy demonstrated a shift from a multipolar neuronal pattern towards bi- and unipolarity prior to the rounding up and eventual disappearance of the neurons. Selective toxicity of cocaine on neurons was paralleled by a concomitant decrease of the culture content in microtubule-associated protein 2 (MAP2), a neuronal marker measured by solid-phase immunoassay. These effects on neurons were reversible when cocaine was removed from the culture medium. In contrast, cocaine did not affect astroglial cells and their glial fibrillary acidic protein (GFAP) content. Thus, in embryonic neuronal-glial cell cocultures, cocaine induces major neurite perturbations followed by neuronal death without affecting the survival of glial cells. Provided similar neuronal alterations are produced in the developing human brain, they could account for the qualitative or quantitative defects in neuronal pathways that cause a major handicap in brain function following in utero exposure to cocaine.
Full text
PDF
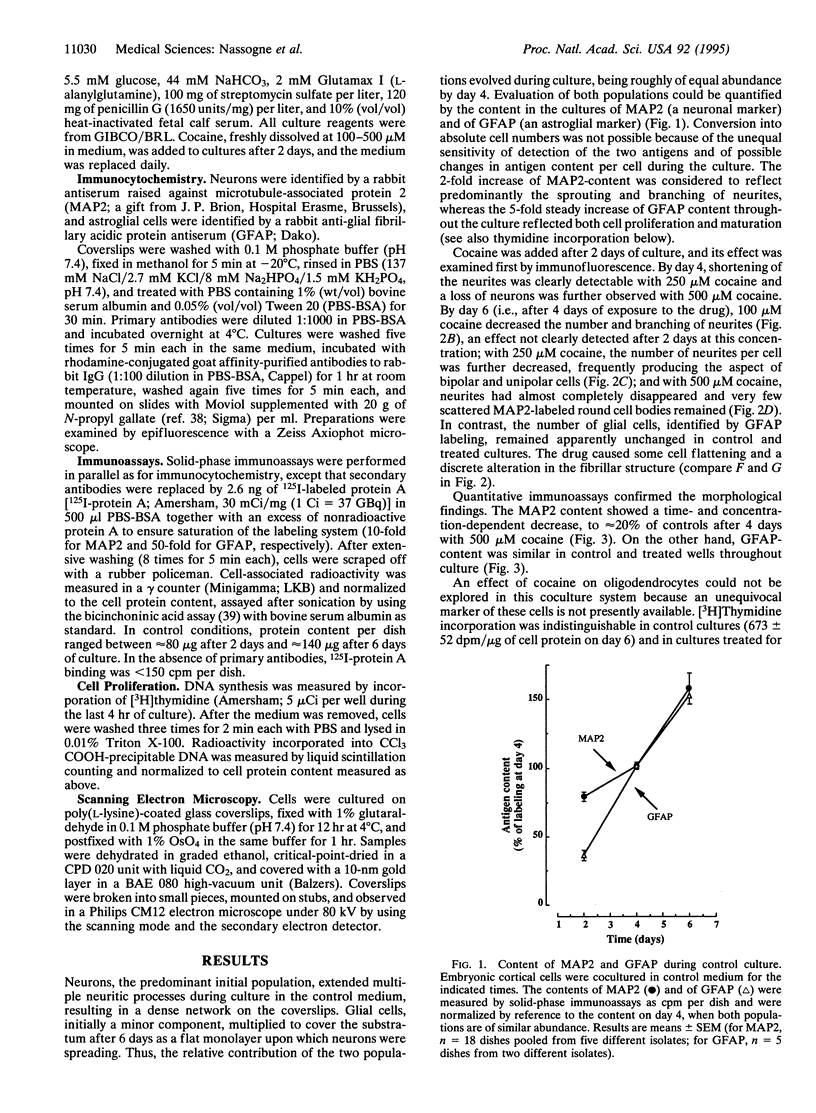

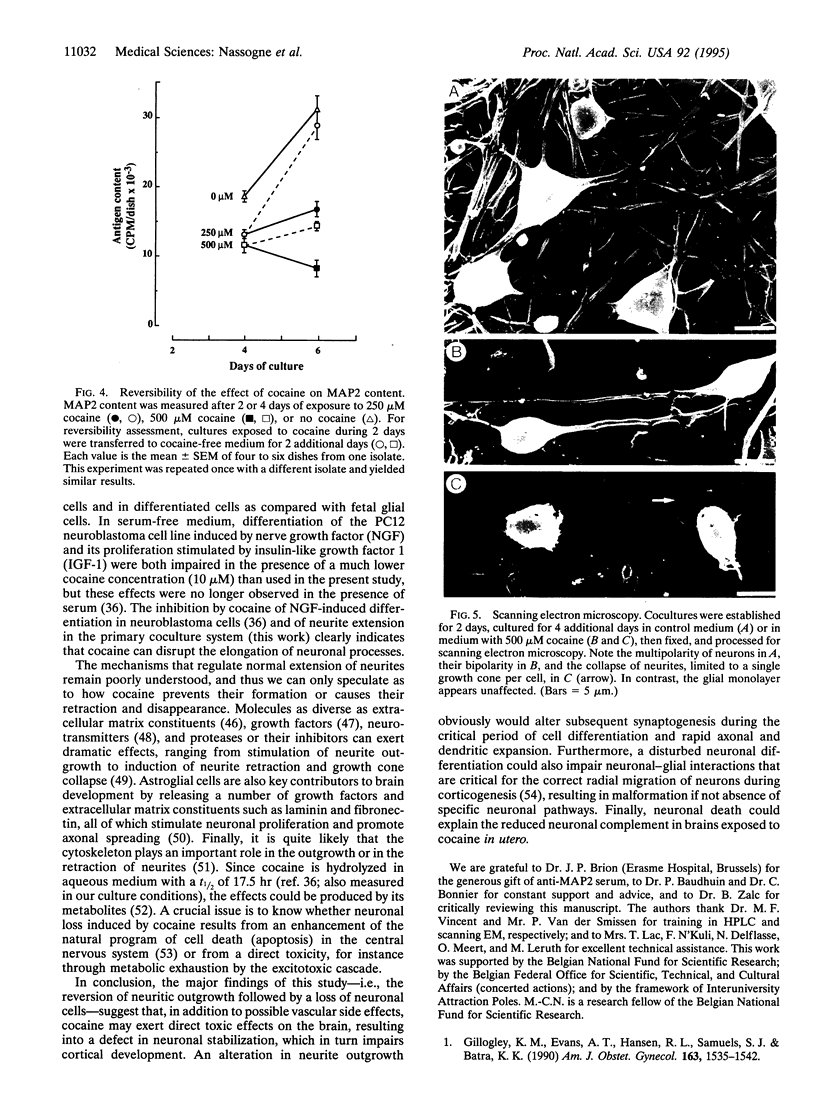

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akbari H. M., Kramer H. K., Whitaker-Azmitia P. M., Spear L. P., Azmitia E. C. Prenatal cocaine exposure disrupts the development of the serotonergic system. Brain Res. 1992 Feb 14;572(1-2):57–63. doi: 10.1016/0006-8993(92)90450-n. [DOI] [PubMed] [Google Scholar]
- Albuquerque M. L., Kurth C. D. Cocaine constricts immature cerebral arterioles by a local anesthetic mechanism. Eur J Pharmacol. 1993 Nov 9;249(2):215–220. doi: 10.1016/0014-2999(93)90435-k. [DOI] [PubMed] [Google Scholar]
- Anderson-Brown T., Slotkin T. A., Seidler F. J. Cocaine acutely inhibits DNA synthesis in developing rat brain regions: evidence for direct actions. Brain Res. 1990 Dec 24;537(1-2):197–202. doi: 10.1016/0006-8993(90)90358-i. [DOI] [PubMed] [Google Scholar]
- Azuma S. D., Chasnoff I. J. Outcome of children prenatally exposed to cocaine and other drugs: a path analysis of three-year data. Pediatrics. 1993 Sep;92(3):396–402. [PubMed] [Google Scholar]
- Barron S., Kaiser D. H., Hansen L. S. Neonatal cocaine exposure, activity, and responsivity to cocaine in a rodent model. Neurotoxicol Teratol. 1994 Jul-Aug;16(4):401–409. doi: 10.1016/0892-0362(94)90029-9. [DOI] [PubMed] [Google Scholar]
- Bingol N., Fuchs M., Diaz V., Stone R. K., Gromisch D. S. Teratogenicity of cocaine in humans. J Pediatr. 1987 Jan;110(1):93–96. doi: 10.1016/s0022-3476(87)80297-4. [DOI] [PubMed] [Google Scholar]
- Bixby J. L., Jhabvala P. Extracellular matrix molecules and cell adhesion molecules induce neurites through different mechanisms. J Cell Biol. 1990 Dec;111(6 Pt 1):2725–2732. doi: 10.1083/jcb.111.6.2725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burchfield D. J., Abrams R. M. Cocaine depresses cerebral glucose utilization in fetal sheep. Brain Res Dev Brain Res. 1993 Jun 8;73(2):283–288. doi: 10.1016/0165-3806(93)90148-4. [DOI] [PubMed] [Google Scholar]
- Cherukuri R., Minkoff H., Feldman J., Parekh A., Glass L. A cohort study of alkaloidal cocaine ("crack") in pregnancy. Obstet Gynecol. 1988 Aug;72(2):147–151. [PubMed] [Google Scholar]
- Chow M. J., Ambre J. J., Ruo T. I., Atkinson A. J., Jr, Bowsher D. J., Fischman M. W. Kinetics of cocaine distribution, elimination, and chronotropic effects. Clin Pharmacol Ther. 1985 Sep;38(3):318–324. doi: 10.1038/clpt.1985.179. [DOI] [PubMed] [Google Scholar]
- Church M. W., Overbeck G. W. Prenatal cocaine exposure in the Long-Evans rat: II. Dose-dependent effects on offspring behavior. Neurotoxicol Teratol. 1990 Jul-Aug;12(4):335–343. doi: 10.1016/0892-0362(90)90052-e. [DOI] [PubMed] [Google Scholar]
- Covert R. F., Schreiber M. D., Tebbett I. R., Torgerson L. J. Hemodynamic and cerebral blood flow effects of cocaine, cocaethylene and benzoylecgonine in conscious and anesthetized fetal lambs. J Pharmacol Exp Ther. 1994 Jul;270(1):118–126. [PubMed] [Google Scholar]
- Culican S. M., Baumrind N. L., Yamamoto M., Pearlman A. L. Cortical radial glia: identification in tissue culture and evidence for their transformation to astrocytes. J Neurosci. 1990 Feb;10(2):684–692. doi: 10.1523/JNEUROSCI.10-02-00684.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doberczak T. M., Shanzer S., Senie R. T., Kandall S. R. Neonatal neurologic and electroencephalographic effects of intrauterine cocaine exposure. J Pediatr. 1988 Aug;113(2):354–358. doi: 10.1016/s0022-3476(88)80283-x. [DOI] [PubMed] [Google Scholar]
- Dominguez R., Aguirre Vila-Coro A., Slopis J. M., Bohan T. P. Brain and ocular abnormalities in infants with in utero exposure to cocaine and other street drugs. Am J Dis Child. 1991 Jun;145(6):688–695. doi: 10.1001/archpedi.1991.02160060106030. [DOI] [PubMed] [Google Scholar]
- Dow-Edwards D. L., Freed L. A., Milhorat T. H. Stimulation of brain metabolism by perinatal cocaine exposure. Brain Res. 1988 Jul 1;470(1):137–141. doi: 10.1016/0165-3806(88)90209-x. [DOI] [PubMed] [Google Scholar]
- Eisen L. N., Field T. M., Bandstra E. S., Roberts J. P., Morrow C., Larson S. K., Steele B. M. Perinatal cocaine effects on neonatal stress behavior and performance on the Brazelton Scale. Pediatrics. 1991 Sep;88(3):477–480. [PubMed] [Google Scholar]
- Emerit M. B., Riad M., Hamon M. Trophic effects of neurotransmitters during brain maturation. Biol Neonate. 1992;62(4):193–201. doi: 10.1159/000243872. [DOI] [PubMed] [Google Scholar]
- Fantel A. G., Person R. E., Burroughs-Gleim C. J., Mackler B. Direct embryotoxicity of cocaine in rats: effects on mitochondrial activity, cardiac function, and growth and development in vitro. Teratology. 1990 Jul;42(1):35–43. doi: 10.1002/tera.1420420106. [DOI] [PubMed] [Google Scholar]
- Farrar H. C., Kearns G. L. Cocaine: clinical pharmacology and toxicology. J Pediatr. 1989 Nov;115(5 Pt 1):665–675. doi: 10.1016/s0022-3476(89)80640-7. [DOI] [PubMed] [Google Scholar]
- Frank D. A., Bresnahan K., Zuckerman B. S. Maternal cocaine use: impact on child health and development. Adv Pediatr. 1993;40:65–99. [PubMed] [Google Scholar]
- Garg U. C., Turndorf H., Bansinath M. Effect of cocaine on macromolecular syntheses and cell proliferation in cultured glial cells. Neuroscience. 1993 Nov;57(2):467–472. doi: 10.1016/0306-4522(93)90079-u. [DOI] [PubMed] [Google Scholar]
- Gillogley K. M., Evans A. T., Hansen R. L., Samuels S. J., Batra K. K. The perinatal impact of cocaine, amphetamine, and opiate use detected by universal intrapartum screening. Am J Obstet Gynecol. 1990 Nov;163(5 Pt 1):1535–1542. doi: 10.1016/0002-9378(90)90621-d. [DOI] [PubMed] [Google Scholar]
- Gingras J. L., Weese-Mayer D. E., Hume R. F., Jr, O'Donnell K. J. Cocaine and development: mechanisms of fetal toxicity and neonatal consequences of prenatal cocaine exposure. Early Hum Dev. 1992 Nov;31(1):1–24. doi: 10.1016/0378-3782(92)90011-5. [DOI] [PubMed] [Google Scholar]
- Gressens P., Evrard P. The glial fascicle: an ontogenic and phylogenic unit guiding, supplying and distributing mammalian cortical neurons. Brain Res Dev Brain Res. 1993 Dec 17;76(2):272–277. doi: 10.1016/0165-3806(93)90218-y. [DOI] [PubMed] [Google Scholar]
- Gressens P., Kosofsky B. E., Evrard P. Cocaine-induced disturbances of corticogenesis in the developing murine brain. Neurosci Lett. 1992 Jun 8;140(1):113–116. doi: 10.1016/0304-3940(92)90694-3. [DOI] [PubMed] [Google Scholar]
- Henderson M. G., McMillen B. A. Changes in dopamine, serotonin and their metabolites in discrete brain areas of rat offspring after in utero exposure to cocaine or related drugs. Teratology. 1993 Nov;48(5):421–430. doi: 10.1002/tera.1420480506. [DOI] [PubMed] [Google Scholar]
- Inaba T., Stewart D. J., Kalow W. Metabolism of cocaine in man. Clin Pharmacol Ther. 1978 May;23(5):547–552. doi: 10.1002/cpt1978235547. [DOI] [PubMed] [Google Scholar]
- Joshi H. C., Baas P. W. A new perspective on microtubules and axon growth. J Cell Biol. 1993 Jun;121(6):1191–1196. doi: 10.1083/jcb.121.6.1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jover R., Ponsoda X., Gómez-Lechón M. J., Herrero C., del Pino J., Castell J. V. Potentiation of cocaine hepatotoxicity by ethanol in human hepatocytes. Toxicol Appl Pharmacol. 1991 Mar 1;107(3):526–534. doi: 10.1016/0041-008x(91)90315-6. [DOI] [PubMed] [Google Scholar]
- Kandall S. R., Gaines J., Habel L., Davidson G., Jessop D. Relationship of maternal substance abuse to subsequent sudden infant death syndrome in offspring. J Pediatr. 1993 Jul;123(1):120–126. doi: 10.1016/s0022-3476(05)81554-9. [DOI] [PubMed] [Google Scholar]
- Kapfhammer J. P., Schwab M. E. Modulators of neuronal migration and neurite growth. Curr Opin Cell Biol. 1992 Oct;4(5):863–868. doi: 10.1016/0955-0674(92)90112-p. [DOI] [PubMed] [Google Scholar]
- Kapur R. P., Shaw C. M., Shepard T. H. Brain hemorrhages in cocaine-exposed human fetuses. Teratology. 1991 Jul;44(1):11–18. doi: 10.1002/tera.1420440104. [DOI] [PubMed] [Google Scholar]
- Keller R. W., Jr, Maisonneuve I. M., Nuccio D. M., Carlson J. N., Glick S. D. Effects of prenatal cocaine exposure on the nigrostriatal dopamine system: an in vivo microdialysis study in the rat. Brain Res. 1994 Jan 21;634(2):266–274. doi: 10.1016/0006-8993(94)91929-1. [DOI] [PubMed] [Google Scholar]
- Kosofsky B. E., Wilkins A. S., Gressens P., Evrard P. Transplacental cocaine exposure: a mouse model demonstrating neuroanatomic and behavioral abnormalities. J Child Neurol. 1994 Jul;9(3):234–241. doi: 10.1177/088307389400900303. [DOI] [PubMed] [Google Scholar]
- LeBlanc P. E., Parekh A. J., Naso B., Glass L. Effects of intrauterine exposure to alkaloidal cocaine ('crack') Am J Dis Child. 1987 Sep;141(9):937–938. doi: 10.1001/archpedi.1987.04460090014001. [DOI] [PubMed] [Google Scholar]
- Lin Y., Leskawa K. C. Cytotoxicity of the cocaine metabolite benzoylecgonine. Brain Res. 1994 Apr 18;643(1-2):108–114. doi: 10.1016/0006-8993(94)90015-9. [DOI] [PubMed] [Google Scholar]
- Lindsay R. M., Wiegand S. J., Altar C. A., DiStefano P. S. Neurotrophic factors: from molecule to man. Trends Neurosci. 1994 May;17(5):182–190. doi: 10.1016/0166-2236(94)90099-x. [DOI] [PubMed] [Google Scholar]
- Longin A., Souchier C., Ffrench M., Bryon P. A. Comparison of anti-fading agents used in fluorescence microscopy: image analysis and laser confocal microscopy study. J Histochem Cytochem. 1993 Dec;41(12):1833–1840. doi: 10.1177/41.12.8245431. [DOI] [PubMed] [Google Scholar]
- Martin D. L. Synthesis and release of neuroactive substances by glial cells. Glia. 1992;5(2):81–94. doi: 10.1002/glia.440050202. [DOI] [PubMed] [Google Scholar]
- Mittleman R. E., Cofino J. C., Hearn W. L. Tissue distribution of cocaine in a pregnant woman. J Forensic Sci. 1989 Mar;34(2):481–486. [PubMed] [Google Scholar]
- Oppenheim R. W. Cell death during development of the nervous system. Annu Rev Neurosci. 1991;14:453–501. doi: 10.1146/annurev.ne.14.030191.002321. [DOI] [PubMed] [Google Scholar]
- Oro A. S., Dixon S. D. Perinatal cocaine and methamphetamine exposure: maternal and neonatal correlates. J Pediatr. 1987 Oct;111(4):571–578. doi: 10.1016/s0022-3476(87)80125-7. [DOI] [PubMed] [Google Scholar]
- Potter S., Klein J., Valiante G., Stack D. M., Papageorgiou A., Stott W., Lewis D., Koren G., Zelazo P. R. Maternal cocaine use without evidence of fetal exposure. J Pediatr. 1994 Oct;125(4):652–654. doi: 10.1016/s0022-3476(94)70029-x. [DOI] [PubMed] [Google Scholar]
- Schenker S., Yang Y., Johnson R. F., Downing J. W., Schenken R. S., Henderson G. I., King T. S. The transfer of cocaine and its metabolites across the term human placenta. Clin Pharmacol Ther. 1993 Mar;53(3):329–339. doi: 10.1038/clpt.1993.29. [DOI] [PubMed] [Google Scholar]
- Singer L. T., Garber R., Kliegman R. Neurobehavioral sequelae of fetal cocaine exposure. J Pediatr. 1991 Oct;119(4):667–672. doi: 10.1016/s0022-3476(05)82426-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith P. K., Krohn R. I., Hermanson G. T., Mallia A. K., Gartner F. H., Provenzano M. D., Fujimoto E. K., Goeke N. M., Olson B. J., Klenk D. C. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985 Oct;150(1):76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Tyrala E. E., Mathews S. V., Rao G. S. Effect of intrauterine exposure to cocaine on acetylcholinesterase in primary cultures of fetal mouse brain cells. Neurotoxicol Teratol. 1992 Jul-Aug;14(4):229–233. doi: 10.1016/0892-0362(92)90001-q. [DOI] [PubMed] [Google Scholar]
- Vega W. A., Kolody B., Hwang J., Noble A. Prevalence and magnitude of perinatal substance exposures in California. N Engl J Med. 1993 Sep 16;329(12):850–854. doi: 10.1056/NEJM199309163291207. [DOI] [PubMed] [Google Scholar]
- Volpe J. J. Effect of cocaine use on the fetus. N Engl J Med. 1992 Aug 6;327(6):399–407. doi: 10.1056/NEJM199208063270607. [DOI] [PubMed] [Google Scholar]
- Weese-Mayer D. E., Silvestri J. M., Lin D., Buhrfiend C. M., Lo E. S., Carvey P. M. Effect of cocaine in early gestation on striatal dopamine and neurotrophic activity. Pediatr Res. 1993 Sep;34(3):389–392. doi: 10.1203/00006450-199309000-00029. [DOI] [PubMed] [Google Scholar]
- Wiggins R. C., Ruiz B. Development under the influence of cocaine. II. Comparison of the effects of maternal cocaine and associated undernutrition on brain myelin development in the offspring. Metab Brain Dis. 1990 Jun;5(2):101–109. doi: 10.1007/BF01001050. [DOI] [PubMed] [Google Scholar]
- Zachor D., Cherkes J. K., Fay C. T., Ocrant I. Cocaine differentially inhibits neuronal differentiation and proliferation in vitro. J Clin Invest. 1994 Mar;93(3):1179–1185. doi: 10.1172/JCI117071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuckerman B., Frank D. A., Hingson R., Amaro H., Levenson S. M., Kayne H., Parker S., Vinci R., Aboagye K., Fried L. E. Effects of maternal marijuana and cocaine use on fetal growth. N Engl J Med. 1989 Mar 23;320(12):762–768. doi: 10.1056/NEJM198903233201203. [DOI] [PubMed] [Google Scholar]





