Abstract
The earliest recorded application of therapeutic hypothermia in medicine spans about 5000 years; however, its use has become widespread since 2002, following the demonstration of both safety and efficacy of regimens requiring only a mild (32°C-35°C) degree of cooling after cardiac arrest. We review the mechanisms by which hypothermia confers neuroprotection as well as its physiological effects by body system and its associated risks. With regard to clinical applications, we present evidence on the role of hypothermia in traumatic brain injury, intracranial pressure elevation, stroke, subarachnoid hemorrhage, spinal cord injury, hepatic encephalopathy, and neonatal peripartum encephalopathy. Based on the current knowledge and areas undergoing or in need of further exploration, we feel that therapeutic hypothermia holds promise in the treatment of patients with various forms of neurologic injury; however, additional quality studies are needed before its true role is fully known.
Keywords: neurocritical care, clinical specialty, trauma, nervous system, clinical, neurophysiology, techniques
Historical Landmarks of Therapeutic Hypothermia Over 5000 years: Nihil Novi Sub Sole
Interest in hypothermia as a therapeutic agent dates back to several millennia when it was first recommended in the Edwin Smith Papyrus, an ancient Egyptian treatise on medicine and surgery written 5000 years ago.1 Hippocrates advised snow and ice packing to reduce hemorrhage in the wounded,2 and total body cooling was used for tetanus treatment in the fourth- and fifth-century BCE.1 Centuries later, the 22-year-old Anne Greene was hung on a cold day in December 1650; when brought down half an hour later, she eventually demonstrated signs of life and progressed to full recovery; she was pardoned and lived a normal life thereafter, marrying and having children.3 In the late 1700s, Dr James Currie (Figure 1), a Scottish physician, carried out the first systematic experiments on humans to determine the effects of various methods of cooling upon body temperature, pulse, and respiration. He successfully used body cooling via application of cold water (hydrotherapy) for the treatment of several clinical disorders and documented the first records of human temperatures in health, disease, and experimental conditions.1 Around the same time, a French physician described the case of a lunatic who escaped from an asylum and wandered naked in a forest during the winter; subsequently, the person was reported to have been cured of his mania.1 Russians have applied hypothermia therapeutically since 1803 by covering people with snow in an attempt to resuscitate them.4 Baron de Larrey, Napoleon’s chief surgeon during the 1812 campaign, packed limbs in ice prior to amputations to render the procedure painless; he also observed that soldiers who were hypothermic and placed closer to a fire died faster than those who remained hypothermic.1 In 1892, at Johns Hopkins, Sir William Osler experimented with hypothermia on patients with typhoid fever and reported a decline in mortality from 24.2% to 7.1%.1
Figure 1.

Dr James Currie. Reprinted from Cosby CB. James Currie and hydrotherapy. J Hist Med Allied Sci. 1950;5(3):280-288, with permission from Oxford University Press.
Dr Temple Fay (Figure 2) is credited with reintroducing therapeutic hypothermia to modern day medicine in a famous experiment in 1938 in which he cooled (“refrigeration” as he described in his publications) a female patient with intractable pain from metastatic breast cancer to 32°C for 24 hours.5,6 This was followed by the application of hypothermia for pain relief in additional patients with metastatic malignancy; a reduction in pain symptoms was reported in 95.7% of such patients.7 Dr Temple Fay is noted to have invented one of the earliest “cooling blankets,” as shown in Figure 3, which is a special insulated mattress in between the bed and a “zipper” blanket containing rubber tubing for continuous circulation of chilled fluids. The addition of a hood allows full application of cold to the head.1,6 Dr Fay successfully implanted a metal capsule intracranially to deliver localized hypothermia to a tumor bed and went on to develop a program to manage various forms of brain injury.1 His data collection, however, was intercepted by the Nazis around the time of World War II, setting hypothermia research back by a couple of decades due to negative associations with Nazi experiments.
Figure 2.

Dr Temple Fay. Reprinted from Alzaga AG, Salazar GA, Varon J,6 with permission from Elsevier.
Figure 3.
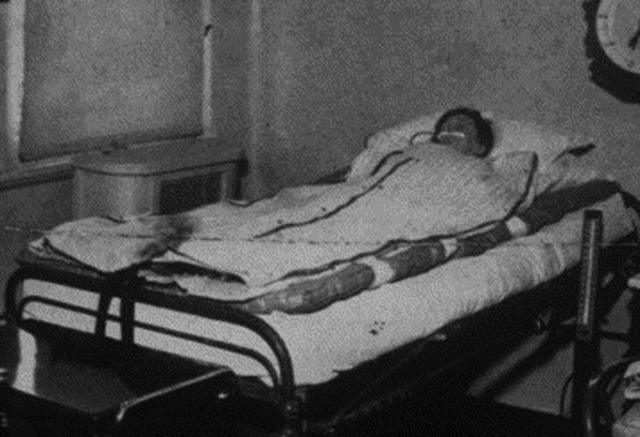
Special insulated mattress between the bed and a “zipper” blanket containing rubber tubing for continuous circulation of chilled fluids. Reprinted from Alzaga AG, Salazar GA, Varon J,6 with permission from Elsevier.
Hypothermia was again “rediscovered” in the 1950s by Bigelow and colleagues who documented its positive effects on the brain during cardiac surgery in animals.8 Rosomoff and others demonstrated that hypothermia reduced cerebral blood flow and oxygen consumption (Figure 4) and had a beneficial effect on intracranial pressure (ICP) in dogs in experimental traumatic brain injury (TBI).9 Around the same time, hypothermia was successfully applied during intracranial aneurysm repair.10 By the end of the decade, the use of therapeutic hypothermia in neurosurgery and for neurological indications became increasingly common11 and continued into the 1960s.12,13 The first clinical trial of hypothermia in the treatment of comatose patients following cardiac arrest was published in 1958, reporting a 50% survival for patients (6 of 12) managed with hypothermia at 33°C compared to 14% (1 of 7) of patients in the normothermic group.14 In 1964, hypothermia even became a part of the first published algorithm on heart–lung resuscitation by Dr Peter Safar who advocated cooling patients within 30 minutes of the return of spontaneous circulation if there were no signs of central nervous system recovery.15 Interest in hypothermia then declined due to the difficulty in managing side effects such as arrhythmias, coagulopathy, and infection since moderate hypothermia (28°C-32°C) was used, as such temperature was thought to be the most beneficial at the time.2
Figure 4.
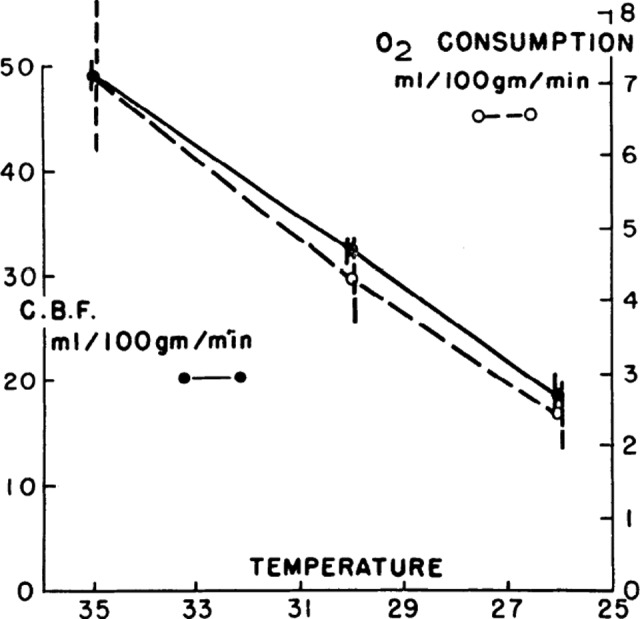
Solid circles joined by solid lines represent mean cerebral blood flows of 4 animals at 35°C, 30°C, and 26°C. Open circles joined by broken lines represent mean cerebral O2 consumption of the same animals. Vertical lines represent standard error of each mean. Reprinted from Rosomoff HL, Holaday DA,9 with permission from the American Physiological Society.
Some continued exploring the neuroprotective use of moderate hypothermia throughout the 1970s and demonstrated promising results16,17; however, true resurgence of interest in neurologic applications of therapeutic hypothermia did not take place until the late 1980s, following the reports of its benefits even with a mild degree of cooling.18–20 Thus, in the 1990s, extensive research on the use of mild hypothermia in animal models ensued: it was shown to confer neuroprotection following cardiac arrest,21 cerebral ischemia,22 and even bacterial meningitis.23
Mild hypothermia was successfully implemented in the 1990s in patients with severe brain injury24 and as a treatment modality to control traumatic intracranial hypertension25; others demonstrated its benefits following successful cardiopulmonary resuscitation.26 This lead to the 2 prospective randomized controlled trials of patients with anoxic brain injury following out-of-hospital cardiac arrest, which were published in 2002.27,28 Bernard et al demonstrated that 49% of hypothermia-treated patients survived versus 26% of control patients, with odds ratio (OR) = 5.25 in benefit of hypothermia.27 The hypothermia after cardiac arrest study group28 trial demonstrated better neurological outcomes in the treated group (55% vs 39% in the control) as well as lower 6-month mortality (41% vs 55%). Following these reports, therapeutic hypothermia finally took its due place in postresuscitation guidelines.29 These 2 randomized studies have reinvigorated numerous basic science and clinical trials in hypothermia. However, before venturing into the current use of hypothermia for neuroprotection and its present day indications, it is important to understand the mechanisms of its beneficial effects; these vary based on the interval from the initial insult.30 We therefore will review the effects of therapeutic hypothermia in the context of time: acutely (minutes to hours), subacutely (hours to days), and chronically (weeks to months).
Mechanisms of Neuroprotection
Acute Phase
Cerebral metabolic rate is a principal determinant of cerebral perfusion, whereas ischemia is a state characterized by a deficiency in oxygen, adenosine triphosphate (ATP), and glucose.31 Cerebral metabolic rate decreases by about 6% to 7% for every 1°C drop in body temperature,31 thereby reducing oxygen demand,32 preserving phosphate compounds and energy stores, and preventing lactate production and development of acidosis.33 As shown in Figure 4, Rosomoff and Holaday9 described a linear correlation and “coupling” of brain temperature with cerebral metabolism and cerebral blood flow. Hypothermia is also thought to improve brain glucose utilization as shown by a lower lactate–glucose and lactate–pyruvate ratio in cooled patients with TBI compared to controls.34 Cerebral blood flow decreases in parallel with cerebral oxygen consumption during hypothermia, suggesting the preservation of autoregulation.32 The major protective effect of hypothermia on cerebral blood flow appears to be a decrease in hyperemia following reperfusion.32
Cerebral ischemia and brain injury trigger a release in excitatory amino acids and glutamate, thereby causing excitotoxicity.30 Additionally, ATP deficit in ischemia results in the disruption of ion gradients with calcium influx, leading to mitochondrial dysfunction and depolarization of neuronal cell membranes causing the release of glutamate into extracellular space; prolonged glutamate exposure leads to a permanent state of neuronal hyperexcitability followed by neuronal injury and death.35 The extent of glutamate concentration depends on the severity of trauma and follows a positive feedback loop.30 Neuron exposure to excessive levels of excitatory amino acids leads to the stimulation of non–N-methyl-D-aspartic acid (NMDA) subtype glutamate receptors, resulting in a toxic level of extracellular acidosis.35 Excess glutamate also leads to acidosis in addition to increased intracellular calcium, potassium, protease activation, and the synthesis of nitric oxide (NO) and reactive oxygen species (ROS).35 Hypothermia is known to reduce the release of excitatory amino acids.36 It also appears to downregulate astrocytic glutamate transporter 1, which mediates the reverse transport of glutamate and attenuate glutamate receptor expression.37 Moreover, hypothermia prevents glutamate-induced increase in NO synthesis31 and suppresses NMDA receptor phosphorylation.38
Subacute Phase
During this time, secondary injury mechanisms such as reperfusion with ROS generation, inflammation, and cellular apoptosis take place, leading to the disruption of the blood–brain barrier and edema formation.30 Ischemia–reperfusion is known to trigger ROS release, whereby hypothermia appears to blunt this response via attenuation of oxidative and nitrosative stress markers with up to a 50-fold decrease in hydrogen peroxide concentrations, allowing neurons to retain viability.35 With regard to the effect of timing of cooling on ischemia–reperfusion injury, animal data showed better outcomes with intra-arrest head cooling compared to postarrest surface cooling.39 In the 2 landmark 2002 trials, when hypothermia was achieved within 2 versus 8 hours, the outcome data were similar.27,28 A subsequent study examining the effect of time to initiation of cooling (interquartile range [IQR] 1-1.8 hours) and time to achieving target temperature (IQR 3-6.7 hours) did not show an association of earlier cooling with improved neurological outcome.40 Others investigated the effect of prehospital cooling as a means to decrease the time to therapeutic hypothermia; rapid infusion of ice-cold intravenous fluid did not improve outcomes at hospital discharge compared with cooling that commenced in the hospital in patients with cardiac arrest due to ventricular fibrillation.41 However, a study of patients with initial rhythm of asystole or pulseless electrical activity showed that 17% of those who received prehospital cooling had a favorable outcome at hospital discharge compared with 7% in the hospital-cooled group.42
Inflammation following brain injury is a physiologic response with the goal of repairing the damaged tissue and defending it from pathogens; however, this response has both beneficial and deleterious effects, with a predominance of the latter, especially in subacute and chronic stages.43 Inflammatory response following brain injury is comprised of a cellular component with the activation of glial cells, microglia, and astrocytes as well as blood leukocyte infiltration.44 Overall, inflammation exacerbates acute brain injury with the release of such proinflammatory cytokines as interleukin (IL) 1, IL-6, IL-18, and tumor necrosis factor α as well as the complement activation additionally stimulating neutrophil pathways.45 Hypothermia has been shown to mitigate the inflammatory response by reducing astrocyte and microglial activation and decreasing expression of inflammatory cytokines, endothelial molecules, and neutrophils as well as monocyte infiltration.31,46 However, the effect of hypothermia on inflammation is more complex and appears to involve inhibition of not only proinflammatory but also anti-inflammatory factors as has been shown by decreased levels of IL-10 and transforming growth factor β.47 Overall, the effect of hypothermia on inflammation is predominantly suppressive.
In addition to ischemia–reperfusion injury and ensuing inflammation following a brain insult, cells may recover, become necrotic, or enter a pathway of programmed cell death or apoptosis.35 Immediately following insult, neurons die by necrosis due to membrane disruption and excitotoxicity, whereas a subsequent wave of neuronal death occurs via an apoptotic pathway.48 This process is mediated by mitochondrial dysfunction and the release of regulatory proteins that can either initiate (B-cell lymphoma 2 [Bcl-2] associated X protein, Fas, caspase 3, cytochrome C) or inhibit (Bcl-2) apoptotic reactions.48,49 Hypothermia attenuates the release of proapoptotic mediators and activates antiapoptotic pathways and increases the expression of p53 to promote subsequent repair.48
Perhaps one of the most important effects of hypothermia is the preservation of the blood–brain barrier following the disruptive effects of ischemia–reperfusion, traumatic injury, or even mannitol administration.31 Hypothermia primarily prevents the activation of metalloproteinases that degrade the extracellular matrix and augments the expression of endogenous metalloproteinase inhibitors.30 Additionally, increased vascular permeability of brain endothelial cells that occurs via the release of NO is also attenuated by hypothermia, which decreases neuronal NO synthase recruitment31,38 and suppresses aquaporin 4 expression.30 Hypothermia therefore acts via several mechanisms that have a protective effect against brain edema due to the loss of blood–brain barrier integrity, thereby limiting ICP increase.30
Chronic Phase
Following initial brain insult and variable recovery, evidence supporting hypothermia appears less defined. Hypothermia of 4 to 24 hours’ duration has been shown to play a role in postischemic neurogenesis50,51 although the same effect has not been observed when hypothermia was used for 45 minutes only.52 Reports on the role of hypothermia in gliogenesis and angiogenesis have been inconclusive, and it remains an active area of investigation.30
Overall, hypothermia exerts a protective effect over a variety of injurious mechanisms occurring in the brain following ischemia–reperfusion or trauma; earlier, these effects involve a decrease in cerebral metabolism, mitochondrial injury, ion pump dysfunction, and excitotoxicity.31 Subsequently, hypothermia attenuates reperfusion injury, ROS production, inflammation, apoptosis, blood–brain barrier permeability, and edema formation31; hypothermia might also play a role in neuronal cell regeneration and neuronal circuit repair.30 However, besides the neuroprotective effects, it is equally important to understand the overall physiological effects of this therapeutic modality and the potential risks of its implementation.
Physiologic Effects of Hypothermia by Organ Systems and Associated Risks
Temperature, substrate concentration, and pH determine the rate of all biochemical reactions in nature; reduction in body temperature therefore affects all biological processes in the body. Therapeutic hypothermia consists of 3 phases: induction, maintenance, and rewarming; each is associated with its own risks31; however, a detailed description of the physiology of cooling is beyond the scope of this article. Shivering occurs with a decrease in core temperature less than 35.5°C and ceases at temperatures less than 30°C; it increases metabolic rate and oxygen consumption.48 However, since shiver control and prevention is a part of the hypothermia care protocol, we will not review it here. Instead, we will focus on the effects of hypothermia by organ systems—cardiac, pulmonary, hematologic, renal, liver, endocrine, gastrointestinal—as well as infectious complications and its influence on drug metabolism.
Cardiovascular Effects
A decrease in heart rate parallels that of temperature with average heart rate of about 40 to 45 beats/min at 32°C.31 This is a normal physiologic response and does not require an intervention. Hypothermia exerts an overall positive inotropic effect as a reduction in heart rate improves left ventricular filling. Augmentation of heart rate during hypothermia is not recommended as it impairs contractility, increases oxygen uptake, and promotes arrhythmias.53 The risk of arrhythmias generally does not increase at temperatures more than 30°C31,48; a landmark trial by Bernard et al did not show a significant effect on arrhythmias in the group treated with 33°C.27 Physiologically, hypothermia decreases spontaneous repolarization of cardiac myocytes and prolongs the duration of action potential and impulse conduction; most classic electrocardiographic (ECG) abnormalities are J (Osborn) waves as well as prolonged PR, QRS, and QT intervals; J waves are rarely seen in mild (32°C-34°C) hypothermia.31 However, interval prolongation can also be seen in normothermic individuals with various forms of brain injury and is common in patients with aneurysmal subarachnoid hemorrhage (aSAH),54 whereas J waves have also been associated with various ischemic conditions, brain injury, and aSAH.55 Notably, Takotsubo cardiomyopathy is an increasingly recognized complication of a variety of neurologic conditions, with aSAH being the most common,56 which can also present with arrhythmias, ECG, and hemodynamic changes, so it is important to keep in mind the potential alternative explanations for any cardiac derangements observed during hypothermia. Additional cardiovascular changes seen with lowering of body temperature include an increase in mean arterial pressure despite a drop in heart rate and therefore cardiac output due to an increase in systemic vascular resistance from hypothermia-induced peripheral vasoconstriction.31,53 Resultant increase in venous return leads to atrial natriuretic peptide activation and decreased levels of antidiuretic hormone leading to “cold diuresis.”53 This effect is even more pronounced in patients receiving mannitol; therefore, if hypotension develops during the induction phase of hypothermia, a fluid challenge may be reasonable.31
Pulmonary Effects and Infectious Complications
Just like with cardiovascular manifestations, separating the effects of hypothermia from those triggered by the underlying neurologic insult might be challenging. As many as one-third of intubated neurologic patients have been reported to develop acute respiratory distress syndrome (ARDS),57 and neurologic pulmonary edema is also common.56 Interestingly, the reported rate of ARDS in patients undergoing even prolonged hypothermia is half of that for normothermic population.58 This is not necessarily surprising, as reduction in metabolic rate with hypothermia decreases oxygen consumption and CO2 production.31 Recently, Aslami et al59 compared the respiratory parameters in patients with cardiac arrest during hypothermia and after rewarming and demonstrated a reduction in PaCO2 that was still present following rewarming as well as an increase in the PaO2-FiO2 ratio upon completion of hypothermia. There has been much controversy in the literature between pH-stat and α-stat measurement and in the reporting of blood gas data in patients undergoing hypothermia. The most recent meta-analysis comparing trials using either protocol concluded that the choice of the technique appears to be age related: pH-stat produces better results in pediatric patients and α-stat in the adults.60 Additionally, due to the inhibition of inflammatory response by hypothermia, there has been much debate whether it can increase the rate of infectious complications, most notably pneumonia. Nonetheless, most studies using hypothermia of 24-hour duration reported either no or only a small difference in the rates of infection.31 Studies reviewing outcomes of patients with severe head injury treated with prolonged hypothermia compared to controls reported no difference in the rates of infections.61,62 Recently, an investigation of patients undergoing prolonged hypothermia who underwent selective digestive tract decontamination actually demonstrated a significantly lower rate of infection in the hypothermia group compared to controls.63 Nonetheless, hypothermia may increase the risk of wound infections; therefore, extra care to prevent bedsores and close monitoring of any catheter insertion sites is paramount.31
Hematologic Function
Perhaps one of the most important concerns regarding the effects of hypothermia in patients with aSAH, TBI, and stroke is its effect on coagulation. Hypothermia may affect platelet count,58 although hemoglobin levels appear unchanged.62,64 The coagulation cascade is affected at temperatures less than 33°C, whereas platelet function may be decreased to less than 35°C.31 Some reported significant prolongation of activated partial thromboplastin time (aPTT),64 while others saw no difference.63 Since these effects may be masked if analyses are performed on rewarmed blood,48 close aPTT monitoring with concomitant subcutaneous heparin administration is advisable. Reassuringly, large clinical trials in patients with aSAH, TBI, and stroke did not report an increased risk of bleeding with hypothermia, although persons with heritable bleeding disorders may not have been studied and application of hypothermia in patients with active bleeding or at high risk of bleeding would have to be done with caution, with cooling to no lower than 35°C.31
Renal, Endocrine, and Gastrointestinal Effects
Electrolyte disorders are common, especially in the induction phase and therefore require close protocolized monitoring; magnesium depletion in particular can worsen brain injury.31 Potassium levels decline with hypothermia62 and are one of the reasons why slow rewarming is advised, since the converse can occur if a patient is rewarmed rapidly. Myocardium sensitivity to potassium is increased in hypothermia; therefore, hypokalemia may have a protective effect.62 Serum sodium levels do not appear to be affected.62,64 Hypothermia can also decrease insulin sensitivity and lead to a reduction in insulin secretion, resulting in hyperglycemia, particularly during the induction stage.31 Overall, with close monitoring, no significant differences in blood glucose levels or insulin requirements were reported in trials comparing patients undergoing hypothermia to controls.62–64 With regard to bowel function, hypothermia may promote ileus and delayed gastric emptying.48
Liver Function and Drug Metabolism
Although no difference in albumin levels has been observed among patients requiring hypothermia over 48 hours’ duration compared to controls,62 the effect of hypothermia on drug metabolism depends on the route of elimination.65 Hypothermia affects the rates of tubular secretion and reabsorption with overall reductions in enzymatic activity; cytochrome p450 functional activity is also reduced.65 Reductions in drug clearance have been demonstrated in commonly used medications such as vasopressors, opiates, sedatives, volatile anesthetics, neuromuscular blocking drugs, and phenytoin.31 Since this can increase drug potency, adding a bolus as opposed to increasing the drip rate may be advised in cases that require more sedation.31
Clinical Applications of Therapeutic Hypothermia
In 2011, 5 international critical care societies issued a joint report regarding evidence-based recommendations for the use of therapeutic hypothermia; they recommended replacing the term “therapeutic hypothermia” with “targeted temperature management (TTM)” to emphasize the importance of defining a complete temperature profile.48 The jury strongly recommended the use of TTM for out-of-hospital cardiac arrest survivors with ECG rhythm of ventricular tachycardia or fibrillation, who remain unconscious following the return of spontaneous circulation, and weakly recommended its use in newborns following sustained asphyxia with acidosis and/or encephalopathy.48 The jury did not recommend either for or against the use of TTM in other cardiac rhythms or in-hospital cardiac arrest as well as in the management of TBI, ICP, acute ischemic stroke, aSAH, spinal cord injury (SCI), and acute liver failure encephalopathy due to insufficient evidence of its benefit.48 Previously published guidelines for the management of TBI in 2007 contained a level III recommendation stating “greater decrease in mortality risk is observed when target temperatures are maintained for more than 48 hours. Prophylactic hypothermia is associated with significantly higher Glasgow Outcome Scale scores when compared to scores for normothermic controls.”66 Although a detailed description of the use of TTM in cardiac arrest survivors is beyond the scope of this article, we will review the evidence of its implementation in various forms of neurologic injury with emphasis on most recent works and ongoing trials.
Traumatic Brain Injury and ICP Elevation
Intracranial pressure elevation is common following TBI due to either mechanical forces or a blood–brain barrier disruption and inflammation leading to interstitial fluid volume expansion and cellular swelling.48 Therefore, ICP management is an integral part of TBI therapies. Since the publication of available evidence by the TTM study group, researchers in Europe started recruitment for the largest study of its kind (1800 patients) aimed at evaluating the effect of therapeutic hypothermia (32°C-35°C) on ICP reduction following TBI, that is, the Eurotherm3235Trial.67 Authors reviewed evidence from 29 clinical studies on hypothermia in TBI; of those, 17 had control groups but only 1 study reported outcome data at 3 months, where there was no significant difference.67 The major difficulty in interpretation of that trial data lied in the variability of injuries and treatment protocols, prompting the much needed randomized controlled trial across 70 hospitals. In parallel, investigators in Australia and New Zealand started the “POLAR-RCT: Prophylactic Hypothermia Trial to Lessen Traumatic Brain Injury—Randomized Controlled Trial.”68
At the same time, results of the National Acute Brain Injury Study: Hypothermia II (NABIS: H II) trial were published.64 In that randomized controlled study of 97 patients, authors instituted hypothermia of 33°C for 48 hours within 4 hours of severe brain injury. No improvement in primary outcome defined as Glasgow outcome scale score at 6 months was observed, and the study was terminated prematurely. Contrasting previous studies, authors reported higher ICP in the intervention group, attributing it to aggressive methods of blood pressure control. Notably, subgroup analyses showed worse results in cases of diffuse brain injury but significantly better outcomes among patients with surgically removed hematomas managed with hypothermia.64 The NABIS: H II trial investigators only provided measurements of ICP for the first 4 days of study in reply to an inquiry of whether ICPs documented during rewarming might have influenced obtained values in the hypothermia group69; it is therefore unclear what the ICP values were over the remainder of the hospital course and how it might have influenced the outcome. Stein et al prospectively examined ICP in 191 patients with TBI over 7 days (180 hours) and reported that the median ICP was significantly higher in the 84- to 180-hour than the 0 to 84-hour time period.70 Although there was no significant effect of ICP on functional outcomes in the first 84 hours, every 5% increase in ICP over time in the subsequent period was independently associated with a 21% higher chance of having a poor functional outcome (adjusted OR = 1.21, 95% confidence interval [CI] 1.02-1.42; P = .03).70
Therefore, although there does not appear to be sufficient data to support the use of hypothermia for neuroprotective indications in early phases of TBI at present, it remains unclear whether hypothermia might improve outcomes if used in later stages of a hospital course for ICP management. Eurotherm3235Trial protocol involves the use of hypothermia for at least 48 hours, which was continued for as long as necessary to maintain ICP less than 20 mm Hg67; the obtained results will help to advance our understanding on the role and consequences of hypothermia in the management of TBI. In the meantime, the most recent review of 18 publications using hypothermia for ICP management in TBI, including 13 randomized controlled trials, concluded that, pending results from multicenter studies, hypothermia should be included as a therapeutic option for ICP management in patients with severe TBI.71
Stroke
The application of hypothermia in patients with stroke can be challenging, as most are awake and not intubated. Despite the widespread use of alteplase, the narrow treatment window limits patient eligibility, and only a third of patients are free from disability following recovery.72 Several small clinical trials investigating the use of hypothermia in ischemic stroke have been published.72 Most recently, researchers in the Intravenous Thrombolysis Plus Hypothermia for Acute Treatment of Ischemic Stroke (ICTuS-L) trial randomized 58 patients with acute stroke to 24 hours of hypothermia versus normothermia in addition to standard therapy; no differences in mortality or 90-day outcomes were apparent.73 Following these results, a new trial commenced: Intravascular Cooling in the Treatment of Stroke 2/3 Trial (ICTuS2/3) with a planned enrollment of 1600 patients with ischemic stroke eligible to receive alteplase followed by randomization to standard therapy versus hypothermia of 33°C across 10 centers in the United States and 1 in Austria.72,74 Another trial is currently underway in Europe: “EuroHYP-1: A European, multicentre, randomised, phase III, clinical trial of hypothermia plus medical treatment versus best medical treatment alone for acute ischaemic stroke.”75 Investigators plan to enroll 1500 awake patients presenting within 6 hours of stroke and cooled to 34°C to 35°C for 24 hours across over 60 hospitals.75 It therefore remains to be seen whether those trials will validate the use of hypothermia in this group of patients.
Aneurysmal Subarachnoid Hemorrhage and Intracerebral Hemorrhage
Current aSAH management guidelines do not address the use of hypothermia, but that it may be reasonable during aneurysm surgery (Class III; Level of Evidence B).76 Nonetheless, these patients may develop subsequent global cerebral edema that portends a poor outcome and is associated with 50% to 60% of 30-day mortality.77 Gasser et al evaluated 21 patients with aSAH having severe brain edema with ICP > 15 mm Hg and reported good functional outcomes in 48% of the patients treated with a combination of prolonged hypothermia and barbiturate coma.78 A study of 100 patients with intracranial hypertension or cerebral vasospasm reported favorable treatment outcomes with prolonged hypothermia alone (n = 13) or a combination of hypothermia and barbiturate coma (n = 87); however, of the patients undergoing hypothermia for refractory ICP, the reported 1-year mortality was 61% compared to 29% among patients receiving therapy for vasospasm.58 Most recently, Staykov et al reported no increase in cerebral edema in the prolonged hypothermia as opposed to the historical control group of patients with intracerebral hemorrhage (ICH) selected on a basis of hemorrhage volume >25 mL; moreover, there was no ICP increase in the treatment group compared to an increase in 44% of controls; mortality was half of that for controls.79 Given that no bleeding complications were reported in those trials, in populations with aSAH and ICH having refractory ICP, hypothermia might be a reasonable adjunct to conventional therapy and decompressive hemicraniectomy.
Spinal Cord Injury
Spinal cord injury is a devastating neurological event with less than 1% of discharged patients being neurologically normal.80 The first case series on the use of systemic hypothermia of 33°C in SCI consisted of a retrospective analysis of American Spinal Injury Association and International Medical Society of Paraplegia Impairment Scale (AIS) scores and complications in 14 patients with a complete cervical SCI (AIS A) compared to 14 age- and injury-matched patients treated at the same institution.81 There was no increase in complications during hospital stay and no statistically significant difference between the final AIS grade in the control and the hypothermia groups, but more patients converted to AIS B and C in the hypothermia group (5 patients) when compared with the control group (2 patients).81 The same authors had just reported their extended experience with 35 patients with cervical SCI receiving hypothermia for 48 hours, with improvement in 43% by at least 1 grade at follow-up82; this was better than the previously reported rate of spontaneous recovery.80 At present, no randomized controlled trials investigated hypothermia for SCI. The Department of Neurological Surgery at the University of Miami Miller School of Medicine in collaboration with the Neurological Emergency Treatment Trials Group is in the process of finalizing a randomized 17-center trial of 200 patients to provide more definitive evidence on the usefulness of modest hypothermia in acute SCI (www.themiamiproject.org).80
Hepatic Encephalopathy/Acute Liver Failure
Acute liver failure (ALF) with hepatic encephalopathy frequently leads to the development of cerebral edema and intracranial hypertension: high ICP was observed in 80% to 95% of patients with stage III-IV hepatic encephalopathy and is a major contributor to mortality and neurocognitive complications in survivors.83 The main mechanisms are alterations of brain glucose metabolism, leading to glucolysis and synthesis of lactate and hyperammonemia with an increase in intracellular osmolality of cortical astrocytes and accumulation of glutamine, which in addition to osmotic effect leads to further mitochondrial dysfunction affecting oxidative metabolism and lactate accumulation.83 Decrease in body temperature lowers brain ammonia uptake and concentration with a larger reduction in cerebral metabolic glucose than oxygen, suggesting improvement in oxidative metabolism; it also attenuates liver injury.83 Case reports and series suggest a very favorable effect.84–87 Most recent work evaluated the outcomes of 14 patients with ALF having refractory ICP awaiting orthotopic liver transplantation, who were successfully bridged to transplantation following initiation of 32°C to 33°C hypothermia without significant cooling-relating complications at any time and had a complete neurologic recovery.87 Authors reported significant increase in mean arterial pressure and cerebral perfusion pressure with decrease in the need for inotropes; hypothermia resulted in a significant reduction in arterial ammonia concentration and its brain metabolism, cerebral blood flow, brain cytokine production, and markers of oxidative stress.87 Nonetheless, no recommendations regarding the use of hypothermia for this indication exist, primarily due to the lack of randomized controlled trials.48
Neonatal Encephalopathy Due to Intrapartum Asphyxia
The 2010 guidelines in neonatal resuscitation include a recommendation to induce hypothermia (33.5°C-34.5°C) within 6 hours after birth for birth asphyxia in term or near-term infants.88 The 2011 joint report regarding evidence-based recommendations for the use of therapeutic hypothermia issued a weak recommendation for the use of hypothermia in management of perinatal asphyxia, with evidence suggesting a mortality reduction in infants with objective evidence of encephalopathy and signs of perinatal distress, although the ideal temperature profile remains to be determined.48 Most recently, a trial of 116 neonates randomized to 72 hours of hypothermia (33°C to 34°C) reported a lower risk of subsequent developmental deficit in the treatment group.89 Overall, compared to less than a decade ago, hypothermia has become the standard of practice for management of neonatal encephalopathy in the United States.90
Conclusions
Despite several millennia of reported sporadic use, the “dark ages” of hypothermia appear to have ended, and it has now entered a period of renaissance where recognition of its medical benefits and applications is expanding rapidly, once mild cooling was shown to be beneficial without many of the feared side effects. Nonetheless, body cooling requires an intensive care unit setting with protocolized implementation and close monitoring. Understanding the mechanisms by which hypothermia affects body systems, particularly the brain, is paramount to the advancement of its application into the promising novel areas especially in areas those where treatment options are limited. Therapeutic hypothermia holds promise in the treatment of patients with various forms of neurologic injury; however, additional quality studies are needed before its true role is fully known.
Footnotes
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Wang H, Olivero W, Wang D, Lanzino G. Cold as a therapeutic agent. Acta Neurochir (Wien). 2006; 148(5):565–570 [DOI] [PubMed] [Google Scholar]
- 2. Polderman KH. Application of therapeutic hypothermia in the ICU: opportunities and pitfalls of a promising treatment modality. Part 1: Indications and evidence. Intensive Care Med. 2004;30(4):556–575 [DOI] [PubMed] [Google Scholar]
- 3. Breathnach CS, Moynihan JB. Intensive care 1650: the revival of Anne Greene (c. 1628-59). J Med Biogr. 2009;17(1):35–38 [DOI] [PubMed] [Google Scholar]
- 4. Varon J, Acosta P. Therapeutic hypothermia: past, present, and future. Chest. 2008;133(5):1267–1274 [DOI] [PubMed] [Google Scholar]
- 5. Fay T. Observations on generalized refrigeration in cases of severe cerebral trauma. Res Publ Assos Res Nerv Dis. 1945;4:611–619 [Google Scholar]
- 6. Alzaga AG, Salazar GA, Varon J. Resuscitation great. breaking the thermal barrier: Dr. Temple Fay. Resuscitation. 2006;69(3):359–364 [DOI] [PubMed] [Google Scholar]
- 7. Fay T. Early experiences with local and generalized refrigeration of the human brain. J Neurosurg. 1959;16(3):239–260 [DOI] [PubMed] [Google Scholar]
- 8. Bigelow WG, Mcbirnie JE. Further experiences with hypothermia for intracardiac surgery in monkeys and groundhogs. Ann Surg. 1953;137(3):361–365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rosomoff HL, Holaday DA. Cerebral blood flow and cerebral oxygen consumption during hypothermia. Am J Physiol. 1954;179(1):85–88 [DOI] [PubMed] [Google Scholar]
- 10. Botterell EH, Lougheed WM, Scott JW, Vandewater SL. Hypothermia, and interruption of carotid, or carotid and vertebral circulation, in the surgical management of intracranial aneurysms. J Neurosurg. 1956;13(1):1–42 [DOI] [PubMed] [Google Scholar]
- 11. Lazorthes G, Campan L. Hypothermia in the treatment of craniocerebral traumatism. J Neurosurg. 1958;15(2):162–167 [DOI] [PubMed] [Google Scholar]
- 12. Drake CG, Barr HWK, Coles JC. The use of extracorporeal circulation and profound hypothermia in the treatment of ruptured intracranial aneurysms. J Neurosurg. 1964;21:575–581 [DOI] [PubMed] [Google Scholar]
- 13. Rosomoff HL, Safar P. Management of the comatose patient. Clin Anesth. 1965;1:244–258 [PubMed] [Google Scholar]
- 14. Williams GR, Jr, Spencer FC. The clinical use of hypothermia following cardiac arrest. Ann Surg. 1958;148(3):462–468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Safar P. Community-wide cardiopulmonary resuscitation. J Iowa Med Soc. 1964;54:629–635 [PubMed] [Google Scholar]
- 16. Symington GR, Mackay IR, Currie TT. Improvement in multiple sclerosis during prolonged induced hypothermia. Neurology. 1977;27(3):302–303 [DOI] [PubMed] [Google Scholar]
- 17. Norwood WI, Norwood CR, Castaneda AR. Cerebral anoxia: effect of deep hypothermia and pH. Surgery. 1979;86(2):203–209 [PubMed] [Google Scholar]
- 18. Gilston A. Another chance for therapeutic hypothermia? Crit Care Med. 1985;13(9):775. [DOI] [PubMed] [Google Scholar]
- 19. Busto R, Dietrich WD, Globus MY, Valdés I, Scheinberg P, Ginsberg MD. Small differences in intraischemic brain temperature critically determine the extent of ischemic neuronal injury. J Cereb Blood Flow Metab. 1987;7(6):729–738 [DOI] [PubMed] [Google Scholar]
- 20. Safar P. Resuscitation from clinical death: pathophysiologic limits and therapeutic potentials. Crit Care Med. 1988;16(10):923–941 [DOI] [PubMed] [Google Scholar]
- 21. Leonov Y, Sterz F, Safar P, et al. Mild cerebral hypothermia during and after cardiac arrest improves neurologic outcome in dogs. J Cereb Blood Flow Metab. 1990;10(1):57–70 [DOI] [PubMed] [Google Scholar]
- 22. Ridenour TR, Warner DS, Todd MM, McAllister AC. Mild hypothermia reduces infarct size resulting from temporary but not permanent focal ischemia in rats. Stroke. 1992;23(5):733–738 [DOI] [PubMed] [Google Scholar]
- 23. Irazuzta JE, Pretzlaff R, Rowin M, Milam K, Zemlan FP, Zingarelli B. Hypothermia as an adjunctive treatment for severe bacterial meningitis. Brain Res. 2000;881(1):88–97 [DOI] [PubMed] [Google Scholar]
- 24. Marion DW, Penrod LE, Kelsey SF, et al. Treatment of traumatic brain injury with moderate hypothermia. N Engl J Med. 1997;336(8):540–546 [DOI] [PubMed] [Google Scholar]
- 25. Shiozaki T, Sugimoto H, Taneda M, et al. Effect of mild hypothermia on uncontrollable intracranial hypertension after severe head injury. J Neurosurg. 1993;79(3):363–368 [DOI] [PubMed] [Google Scholar]
- 26. Yamashita C, Nakagiri K, Yamashita T, et al. Mild hypothermia for temporary brain ischemia during cardiopulmonary support systems: report of three cases. Surg Today. 1999;29(2):182–185 [DOI] [PubMed] [Google Scholar]
- 27. Bernard SA, Gray TW, Buist MD, et al. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N Engl J Med. 2002;346(8):557–563 [DOI] [PubMed] [Google Scholar]
- 28. Hypothermia after Cardiac Arrest Study Group. Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N Engl J Med. 2002;346(8):549–556 [DOI] [PubMed] [Google Scholar]
- 29. Peberdy MA, Callaway CW, Neumar RW, et al. Part 9: post-cardiac arrest care: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2010;122(18 suppl 3):S768–S786 [DOI] [PubMed] [Google Scholar]
- 30. Yenari MA, Han HS. Neuroprotective mechanisms of hypothermia in brain ischaemia. Nat Rev Neurosci. 2012;13(4):267–278 [DOI] [PubMed] [Google Scholar]
- 31. Polderman KH. Mechanisms of action, physiological effects, and complications of hypothermia. Crit Care Med. 2009;37(7 suppl):S186–S202 [DOI] [PubMed] [Google Scholar]
- 32. Erecinska M, Thoresen M, Silver IA. Effects of hypothermia on energy metabolism in Mammalian central nervous system. J Cereb Blood Flow Metab. 2003;23(5):513–530 [DOI] [PubMed] [Google Scholar]
- 33. Zhao QJ, Zhang XG, Wang LX. Mild hypothermia therapy reduces blood glucose and lactate and improves neurologic outcomes in patients with severe traumatic brain injury. J Crit Care. 2011;26(3):311–315 [DOI] [PubMed] [Google Scholar]
- 34. Wang Q, Li AL, Zhi DS, Huang HL. Effect of mild hypothermia on glucose metabolism and glycerol of brain tissue in patients with severe traumatic brain injury. Chin J Traumatol. 2007;10(4):246–249 [PubMed] [Google Scholar]
- 35. Kuffler DP. Maximizing neuroprotection: where do we stand? Ther Clin Risk Manag. 2012;8:185–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Nakashima K, Todd MM. Effects of hypothermia on the rate of excitatory amino acid release after ischemic depolarization. Stroke. 1996;27(5):913–918 [DOI] [PubMed] [Google Scholar]
- 37. Wang D, Zhao Y, Zhang Y, et al. Hypothermia protects against oxygen-glucose deprivation-induced neuronal injury by down-regulating the reverse transport of glutamate by astrocytes as mediated by neurons. Neuroscience. 2013;237:130–138 [DOI] [PubMed] [Google Scholar]
- 38. Mueller Burke D, Koehler RC, Martin LJ. Rapid NMDA receptor phosphorylation and oxidative stress precede striatal neurodegeneration after hypoxic ischemia in newborn piglets and are attenuated with hypothermia. Int J Dev Neurosci. 2008;26(1):67–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tsai MS, Barbut D, Wang H, et al. Intra-arrest rapid head cooling improves postresuscitation myocardial function in comparison with delayed postresuscitation surface cooling. Crit Care Med. 2008;36(11 suppl):S434–S439 [DOI] [PubMed] [Google Scholar]
- 40. Nielsen N, Hovdenes J, Nilsson F, et al. Outcome, timing and adverse events in therapeutic hypothermia after out-of-hospital cardiac arrest. Acta Anaesthesiol Scand. 2009;53(7):926–934 [DOI] [PubMed] [Google Scholar]
- 41. Bernard SA, Smith K, Cameron P, et al. Rapid Infusion of Cold Hartmanns (RICH) Investigators. Induction of therapeutic hypothermia by paramedics after resuscitation from out-of-hospital ventricular fibrillation cardiac arrest: a randomized controlled trial. Circulation. 2010;122(7):737–742 [DOI] [PubMed] [Google Scholar]
- 42. Bernard SA, Smith K, Cameron P, et al. Rapid infusion of cold hartmanns investigators. induction of prehospital therapeutic hypothermia after resuscitation from nonventricular fibrillation cardiac arrest. Crit Care Med. 2012;40(3):747–753 [DOI] [PubMed] [Google Scholar]
- 43. Schmidt OI, Heyde CE, Ertel W, Stahel PF. Closed head injury--an inflammatory disease? Brain Res Brain Res Rev. 2005;48(2):388–399 [DOI] [PubMed] [Google Scholar]
- 44. Wang Q, Tang XN, Yenari MA. The inflammatory response in stroke. J Neuroimmunol. 2007;184(1-2):53–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lin Y, Wen L. Inflammatory response following diffuse axonal injury. Int J Med Sci. 2013;10(5):515–521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Xiong M, Yang Y, Chen GQ, Zhou WH. Post-ischemic hypothermia for 24 h in P7 rats rescues hippocampal neuron: association with decreased astrocyte activation and inflammatory cytokine expression. Brain Res Bull. 2009;79(6):351–357 [DOI] [PubMed] [Google Scholar]
- 47. Matsui T, Kakeda T. IL-10 production is reduced by hypothermia but augmented by hyperthermia in rat microglia. J Neurotrauma. 2008;25(6):709–715 [DOI] [PubMed] [Google Scholar]
- 48. Nunnally ME, Jaeschke R, Bellingan GJ, et al. Targeted temperature management in critical care: a report and recommendations from five professional societies. Crit Care Med. 2011;39(5):1113–1125 [DOI] [PubMed] [Google Scholar]
- 49. Pastuszko P, Pirzadeh A, Reade E, et al. The effect of hypothermia on neuronal viability following cardiopulmonary bypass and circulatory arrest in newborn piglets. Eur J Cardiothorac Surg. 2009;35(4):577–581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Bregy A, Nixon R, Lotocki G, et al. Posttraumatic hypothermia increases doublecortin expressing neurons in the dentate gyrus after traumatic brain injury in the rat. Exp Neurol. 2012;233(2):821–828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Silasi G, Colbourne F. Therapeutic hypothermia influences cell genesis and survival in the rat hippocampus following global ischemia. J Cereb Blood Flow Metab. 2011;31(8):1725–1735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Lasarzik I, Winkelheide U, Thal SC, et al. Mild hypothermia has no long-term impact on postischemic neurogenesis in rats. Anesth Analg. 2009;109(5):1632–1639 [DOI] [PubMed] [Google Scholar]
- 53. Giraud R, Siegenthaler N, Bendjelid K. Cardiac index during therapeutic hypothermia: which target value is optimal? Crit Care. 2013;17(2):214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Chatterjee S. ECG changes in subarachnoid haemorrhage: a synopsis. Neth Heart J. 2011;19(1):31–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Shinde R, Shinde S, Makhale C, et al. Occurrence of “J waves” in 12-lead ECG as a marker of acute ischemia and their cellular basis. Pacing Clin Electrophysiol. 2007;30(6):817–819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Inamasu J, Nakatsukasa M, Mayanagi K, et al. Subarachnoid hemorrhage complicated with neurogenic pulmonary edema and takotsubo-like cardiomyopathy. Neurol Med Chir (Tokyo). 2012;52(2):49–55 [DOI] [PubMed] [Google Scholar]
- 57. Hoesch RE, Lin E, Young M, et al. Acute lung injury in critical neurological illness. Crit Care Med. 2012;40(2):587–593 [DOI] [PubMed] [Google Scholar]
- 58. Seule MA, Muroi C, Mink S, Yonekawa Y, Keller E. Therapeutic hypothermia in patients with aneurysmal subarachnoid hemorrhage, refractory intracranial hypertension, or cerebral vasospasm. Neurosurgery. 2009;64(1):86–92 [DOI] [PubMed] [Google Scholar]
- 59. Aslami H, Binnekade JM, Horn J, Huissoon S, Juffermans NP. The effect of induced hypothermia on respiratory parameters in mechanically ventilated patients. Resuscitation. 2010;81(12):1723–1725 [DOI] [PubMed] [Google Scholar]
- 60. Abdul Aziz KA, Meduoye A. Is pH-stat or alpha-stat the best technique to follow in patients undergoing deep hypothermic circulatory arrest? Interact Cardiovasc Thorac Surg. 2010;10(2):271–282 [DOI] [PubMed] [Google Scholar]
- 61. Polderman KH, Tjong Tjin Joe R, Peerdeman SM, Vandertop WP, Girbes AR. Effects of therapeutic hypothermia on intracranial pressure and outcome in patients with severe head injury. Intensive Care Med. 2002;28(11):1563–1573 [DOI] [PubMed] [Google Scholar]
- 62. Tokutomi T, Miyagi T, Morimoto K, Karukaya T, Shigemori M. Effect of hypothermia on serum electrolyte, inflammation, coagulation, and nutritional parameters in patients with severe traumatic brain injury. Neurocrit Care. 2004;1(2):171–182 [DOI] [PubMed] [Google Scholar]
- 63. Kamps M, Bisschops LA, van der Hoeven JG, Hoedemaekers CW. Hypothermia does not increase the risk of infection: a case control study. Crit Care. 2011;15(1):R48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Clifton GL, Valadka A, Zygun D, et al. Very early hypothermia induction in patients with severe brain injury (the National Acute Brain Injury Study: Hypothermia II): a randomised trial. Lancet Neurol. 2011;10(2):131–139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Zhou J, Poloyac SM. The effect of therapeutic hypothermia on drug metabolism and response: cellular mechanisms to organ function. Expert Opin Drug Metab Toxicol. 2011;7(7):803–816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Brain Trauma Foundation, American Association of Neurological Surgeons, Congress of Neurological Surgeons, et al. Guidelines for the management of severe traumatic brain injury. III. Prophylactic hypothermia. J Neurotrauma. 2007;24 suppl 1:S21–S25 [DOI] [PubMed] [Google Scholar]
- 67. Andrews PJ, Sinclair HL, Battison CG, et al. European society of intensive care medicine study of therapeutic hypothermia (32-35°C) for intracranial pressure reduction after traumatic brain injury (the Eurotherm3235Trial). Trials. 2011;12:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. The Prophylactic Hypothermia Trial to Lessen Traumatic Brain Injury (POLAR-RCT) ClinicalTrials.gov Identifier: NCT00987688. http://www.anzicrc.monash.org/polar-rct.html accessed January 4, 2014.
- 69. Clifton GL on behalf of all NABIS: H II investigators. Hypothermia in patients with brain injury: the way forward? – Author's reply. Lancet Neurol. 2011;10(5):406–407 [DOI] [PubMed] [Google Scholar]
- 70. Stein DM, Brenner M, Hu PF, et al. Timing of intracranial hypertension following severe traumatic brain injury. Neurocrit Care. 2013;18(3):332–340 [DOI] [PubMed] [Google Scholar]
- 71. Sadaka F, Veremakis C. Therapeutic hypothermia for the management of intracranial hypertension in severe traumatic brain injury: a systematic review. Brain Inj. 2012;26(7-8):899–908 [DOI] [PubMed] [Google Scholar]
- 72. Wu TC, Grotta JC. Hypothermia for acute ischaemic stroke. Lancet Neurol. 2013;12(3):275–284 [DOI] [PubMed] [Google Scholar]
- 73. Hemmen TM, Raman R, Guluma KZ, et al. Intravenous thrombolysis plus hypothermia for acute treatment of ischemic stroke (ICTuS-L): final results. Stroke. 2010;41(10):2265–2270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. The Intravascular Cooling in the Treatment of Stroke 2/3 Trial (ICTuS2/3): ClinicalTrials.gov Identifier: NCT01123161; http://clinicaltrials.gov/show/NCT01123161 accessed January 4, 2014
- 75. Kollmar R, Gebhardt B, Schwab S. EuroHYP-1 trial: EU-funded therapy study on the effectiveness of mild therapeutic hypothermia for acute ischemic stroke. Nervenarzt. 2012;83(10):1252–1259 [DOI] [PubMed] [Google Scholar]
- 76. Connolly ES, Jr, Rabinstein AA, Carhuapoma JR, et al. Guidelines for the management of aneurysmal subarachnoid hemorrhage: a guideline for healthcare professionals from the American Heart Association/american Stroke Association. Stroke. 2012;43(6):1711–1737 [DOI] [PubMed] [Google Scholar]
- 77. Le Roux AA, Wallace MC. Outcome and cost of aneurysmal subarachnoid hemorrhage. Neurosurg Clin N Am. 2010;21(2):235–246 [DOI] [PubMed] [Google Scholar]
- 78. Gasser S, Khan N, Yonekawa Y, Imhof HG, Keller E. Long-term hypothermia in patients with severe brain edema after poor-grade subarachnoid hemorrhage: feasibility and intensive care complications. J Neurosurg Anesthesiol. 2003;15(3):240–248 [DOI] [PubMed] [Google Scholar]
- 79. Staykov D, Wagner I, Volbers B, Doerfler A, Schwab S, Kollmar R. Mild prolonged hypothermia for large intracerebral hemorrhage. Neurocrit Care. 2013;18(2):178–183 [DOI] [PubMed] [Google Scholar]
- 80. Ahmad F, Wang MY, Levi AD. Hypothermia for acute spinal cord injury-a review [published online January 5, 2013.]. World Neurosurg. 2013. pii: S1878-8750(13)00015-6. doi: 10.1016/j.wneu.2013.01.008 [DOI] [PubMed] [Google Scholar]
- 81. Levi AD, Casella G, Green BA, et al. Clinical outcomes using modest intravascular hypothermia after acute cervical spinal cord injury. Neurosurgery. 2010;66(4):670–677 [DOI] [PubMed] [Google Scholar]
- 82. Dididze M, Green BA, Dalton Dietrich W, Vanni S, Wang MY, Levi AD. Systemic hypothermia in acute cervical spinal cord injury: a case-controlled study. Spinal Cord. 2013;51(5):395–400 doi: 10.1038/sc.2012.161 [DOI] [PubMed] [Google Scholar]
- 83. Vaquero J. Therapeutic hypothermia in the management of acute liver failure. Neurochem Int. 2012;60(7):723–735 [DOI] [PubMed] [Google Scholar]
- 84. Jacob S, Khan A, Jacobs ER, Kandiah P, Nanchal R. Prolonged hypothermia as a bridge to recovery for cerebral edema and intracranial hypertension associated with fulminant hepatic failure. Neurocrit Care. 2009;11(2):242–246 [DOI] [PubMed] [Google Scholar]
- 85. Chawla R, Smith D, Marik PE. Near fatal posterior reversible encephalopathy syndrome complicating chronic liver failure and treated by induced hypothermia and dialysis: a case report. J Med Case Rep. 2009;3:6623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Castillo L, Pérez C, Ruiz C, et al. Intravascular hypothermia for the management of Intracranial hypertension in acute liver failure: case report. Rev Med Chil. 2009;137(6):801–806 [PubMed] [Google Scholar]
- 87. Jalan R, Olde Damink SW, Deutz NE, Hayes PC, Lee A. Moderate hypothermia in patients with acute liver failure and uncontrolled intracranial hypertension. Gastroenterology. 2004;127(5):1338–1346 [DOI] [PubMed] [Google Scholar]
- 88. Roehr CC, Hansmann G, Hoehn T, Bührer C. The 2010 Guidelines on Neonatal Resuscitation (AHA, ERC, ILCOR): similarities and differences--what progress has been made since 2005? Klin Padiatr. 2011;223(5):299–307 [DOI] [PubMed] [Google Scholar]
- 89. Joy R, Pournami F, Bethou A, Bhat VB, Bobby Z. Effect of therapeutic hypothermia on oxidative stress and outcome in term neonates with perinatal asphyxia: a randomized controlled trial. J Trop Pediatr. 2013;59(1):17–22 [DOI] [PubMed] [Google Scholar]
- 90. Harris MN, Carey WA, Ellsworth MA, et al. Perceptions and practices of therapeutic hypothermia in American neonatal intensive care units [published online March 1, 2013.]. Am J Perinatol. 2013 [DOI] [PubMed] [Google Scholar]


