Abstract
Thyroid hormones are critical regulators of normal development and physiological functioning in all vertebrates. Radioimmunoassay (RIA) approaches have been the method of choice for measuring circulating levels of thyroid hormones in vertebrates. While sensitive, RIA-based approaches only allow for a single analyte measurement per assay, can lack concordance across platforms and laboratories, and can be prone to analytical interferences especially when used with fish plasma. Ongoing advances in liquid chromatography tandem mass spectrometry (LC/MS/MS) have led to substantial decreases in detection limits for thyroid hormones and other biomolecules in complex matrices, including human plasma. Despite these advances, current analytical approaches do not allow for the measurement of native thyroid hormone in teleost fish plasma by mass spectrometry and continue to rely on immunoassay. In this study, we developed a new method that allows for the rapid extraction and simultaneous measurement of total T4 (TT4) and total T3 (TT3) in low volumes (50 μL) of fish plasma by LC/MS/MS. Methods were optimized initially in plasma from rainbow trout (Oncorhynchus mykiss) and applied to plasma from other teleost fishes, including fathead minnows (Pimephales promelas), mummichogs (Fundulus heteroclitus), sockeye salmon (Oncorhynchus nerka), and coho salmon (Oncorhynchus kisutch). Validation of method performance with T4- and T3-spiked rainbow trout plasma at 2 and 4 ng/mL produced mean recoveries ranging from 82 to 95 % and 97 to 105 %, respectively. Recovery of 13C12-T4 internal standard in plasma extractions was: 99±1.8 % in rainbow trout, 85±11 % in fathead minnow, 73±5.0 % in mummichog, 73±1.7 % in sockeye salmon, and 80±8.4 % in coho salmon. While absolute levels of thyroid hormones measured in identical plasma samples by LC/MS/MS and RIA varied depending on the assay used, T4/T3 ratios were generally consistent across both techniques. Less variability was measured among samples subjected to LC/MS/MS suggesting a more precise estimate of thyroid hormone homeostasis in the species targeted. Overall, a sensitive and reproducible method was established that takes advantage of LC/MS/MS techniques to rapidly measure TT4 and TT3 with negligible interferences in low volumes of plasma across a variety of teleost fishes.
Keywords: Endocrine disruption, Fathead minnow, Mummichog, Radioimmunoassay, Rainbowtrout, Salmon, Thyroid hormone, Thyroxine, Mass spectrometry
Introduction
The thyroid is a large endocrine gland system that is important in normal physiological functioning among all vertebrates. Increasing attention has focused on the potential for internal and external perturbations of the thyroid system linked to disease, genetic factors, radiation, and chemical exposures. Incidences of thyroid cancer have risen sharply all over the world, although questions remain as to the extent this increase is related to more sensitive diagnostic tools [1, 2]. A growing body of evidence, as outlined in several informative reviews, has demonstrated that exposures to an increasing array of chemicals can impair thyroid hormone regulation in humans, fish, and other animals [3–5]. Thyroid hormones play a crucial role in brain development and small changes in maternal or fetal/early life stage thyroid hormone levels can elicit severe motor skill deficiencies and cognitive impairments [6]. In fishes, thyroid hormones are key mediators of many physiological, developmental, and behavioral processes, including growth and metamorphic transitioning [7, 8], osmoregulation [9], olfactory imprinting [10], interrenal regulation [11], otolith formation [12], and reproduction [13, 14], often acting in concert with other hormones. For instance, smoltification among salmonids (parr–smolt transformation to allow for seawater tolerance) is regulated by interactions between thyroid hormones, cortisol, insulin, and prolactin [15, 16]. Flounder and other flatfish undergo a profound post-hatch metamorphosis from larvae to juveniles that is comparable to amphibian metamorphosis in which spikes in plasma thyroxine (T4) induce migration of the eye and other neurological structures to one side of the head [8].
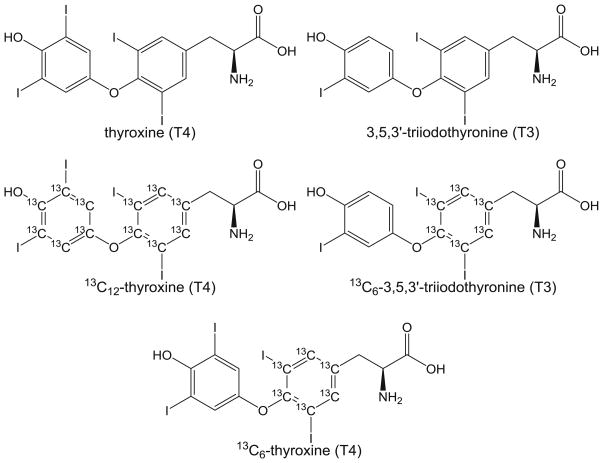
The measurement of thyroid hormone levels in circulation has long served as an important approach for discerning thyroid system dysfunction associated with disease, chemical exposures, and other disturbances. Circulating levels of thyroid hormones are tightly controlled by both a centrally operating hypothalamic–pituitary–thyroid (HPT) axis and in peripheral tissues through the activity of iodothyronine deiodinase enzymes, among other dynamically operating regulatory processes. The functional unit of the central HPT is the thyroid follicle where the thyroid hormones T4 and 3,5,3′-triiodothyronine (T3) are synthesized and secreted into circulation (Fig. 1; chemical structures). In teleosts, thyroid follicles are found dispersed predominantly in the ventral pharyngeal region, rather than being organized in a compact lobular gland as seen in higher vertebrates. T4 is also thought to be the primary, possibly only, thyroid hormone released from thyroid follicles of fishes, making T3 production in the periphery an important aspect of overall thyroid hormone homeostasis [17]. Most thyroid hormone in circulation is bound to protein, including thyroid-binding globulin, transthyretin, and albumin, with only a small amount (<1 %) free and available for uptake into cells.
Fig. 1.
Chemical structure of thyroxine (T4) and 3,5,3′-triiodothyronine (T3) the isotopically labeled thyroid hormones 13C12-T4 and 13C6-T3 used as internal standards and 13C6-T4 used as a recovery standard
Circulating levels of thyroid hormone in plasma and serum are often measured by radioimmunoassay (RIA) methods with many clinical laboratories routinely measuring free and total T4 and T3 in human sera using automated immunoassay approaches. RIA methods have also been adopted to measure circulating and tissue levels of thyroid hormones in some fish species, including several species of salmonids and cyprinids [15, 18, 19]. While sensitive, immunoassay approaches have several weaknesses, including that they can be compromised by a lack of specificity and accuracy due to analytical interferences. Biomolecules may vary across species, cross-react with antibodies, and interfere with assay performance [20–22]. Moreover, RIA methods measure thyroid hormones individually thereby limiting thorough evaluations of interactive thyroid hormone homeostasis. While a number of commercial RIA kits are available to measure thyroid hormone levels in human sera, these kits have not been well-validated for use in nonhuman animals and so their use in fish can be problematic. Commercial RIA kits also have been validated over a narrow range of standards that are usually too high for the comparatively lower levels of circulating thyroid hormones in fish.
Therefore, there is a need to develop approaches that allow for more direct and reliable measurements of circulating thyroid hormones in fishes. The use of liquid chromatography tandem mass spectrometry (LC/MS/MS) is increasingly the preferred tool for overcoming some of the analytical difficulties raised by immunoassay approaches [23]. For instance, reference measurement procedures have been published for total T3 [24] and total T4 [25] in human sera using isotope dilution coupled with LC/MS/MS. Our laboratory and others have also developed analytical methods using LC/MS/MS to measure spiked and native thyroid hormones in mammalian sera and tissues [26–29] and some fish tissues [30, 31]. While these are important advances, no LC/MS/MS based methods are available to permit measurement of circulating levels of native thyroid hormone in fish plasma. This limitation has made it difficult to evaluate thyroid system perturbations associated with disease, environmental impacts, and chemical exposures among wild and laboratory fishes, which also are being used increasingly as models to examine underlying mechanisms of disease and chemical toxicity in humans. Therefore, this study has developed a new method that allows for the rapid extraction and measurement of native thyroid hormones, both bound to plasma proteins and unbound (i.e., total T3 (TT3) and total T4 (TT4)), in teleost fish plasma using LC-ESI/MS/MS analytical approaches. In addition to being the first method that allows for the reliable measurement of native thyroid hormones in fish circulation by LC/MS/MS, it has been optimized to work in low volumes (50 μL) of plasma across several species, making it advantageous as blood volumes are usually limited in fishes. The following species were targeted for this study: rainbow trout (Oncorhynchus mykiss), fathead minnow (Pimephales promelas), mummichog (Fundulus heteroclitus), sockeye salmon (Oncorhynchus nerka), and coho salmon (Oncorhynchus kisutch). These species were selected based on their importance as research models and because they occupy different ecological niches (freshwater, estuarine, seawater) across a range of teleost taxa (Salmonidae, Cyprinidae, Fundulidae).
Materials and methods
Chemicals and materials
Unlabeled thyroid hormones (T4, >98 %; T3, >97 %), citric acid, ascorbic acid, and dithiothreitol (DTT) were purchased from Sigma-Aldrich (St. Louis, MO). The stable isotope internal standards used were 13C12-T4 (>98 %) and 13C6-T3 (>97 %) (Fig. 1; Cambridge Isotope Laboratories, Andover, MA). The solid phase extractions used SampliQ OPT polymer cartridges (60 mg/3 mL; Agilent). A standard stock solution of T4 and T3 was prepared in an amber glass bottle in methanol (MeOH) and deionized water (DI; 1:1, v/v) at 10 ng/mL. An internal standard stock solution of 13C12-T4 and 13C6-T3 was also prepared in MeOH at 10 ng/mL, whereas a recovery standard stock solution of 13C6-T4 (>97 %; Isotec, Canton, GA) was prepared at 50 ng/mL. Standard stock solutions were stored at 4 °C and all solvents used were high-performance liquid chromatography grade.
Fish plasma collection
Three pools of plasma were collected from targeted fish species as follows: 8–12 male fathead minnows/sample pool purchased from Aquatic BioSystems (Fort Collins, CO, USA), 4–6 male mummichogs/sample pool collected from the Kings Creek, VA, USA, and 3–5 adult rainbow trout/ sample pool donated by the Armstrong Hatchery, Marion, NC, USA. Sockeye salmon plasma was donated by Dr. Andrew Dittman’s laboratory at the Northwest Fisheries Science Center, Seattle, WA, USA, and coho salmon plasma was donated by Dr. Evan Gallagher’s laboratory at the Department of Environmental and Occupational Health Sciences, University of Washington, Seattle, WA, USA. The salmon plasma was not pooled and represents individual fish (n =3). Fish were euthanized using MS-222 and blood samples were taken from the caudal vasculature using either heparin-coated 75-mm capillary tubes (fathead minnow and mummichog) or heparin-coated syringes (trout and salmon). Plasma fractions were isolated by centrifugation at 3,000×g for 5 min and stored at −80 °C until thyroid hormone analysis.
Thyroid hormone extractions
Thyroid hormones were extracted from plasma by initially incubating 50 μL of plasma with 50 μL of 13C12-T4 and 13C6-T3 (0.5 ng; 10 ng/mL in MeOH) in 15-mL sterile polypropylene conical test tubes (Sigma-Aldrich) for one hour covered on ice to allow for equilibration of endogenous and labeled thyroid hormones with plasma proteins [25]. The incubation medium also contained 100 μL of an antioxidant/reducing solution containing 25 g/L of ascorbic acid, citric acid, and DTT to prevent deiodination of thyroid hormones in the incubation medium. Plasma samples were vortexed before and after adding standards and antioxidant solutions. After this equilibration step, a 1-mL volume of hydrochloric acid (6 M; Sigma-Aldrich) was added, vortexed, and samples incubated covered for 60 min in a 50 °C water bath oscillating at 180 rpm to allow for denaturation of plasma proteins and release of protein-bound hormones.
Thyroid hormones were isolated from extracts using a solid-phase extraction (SPE) procedure with SampliQ OPT polymer cartridges (60 mg/3 mL; Agilent). Specifically, polymer cartridges were conditioned with 3 mL of MeOH followed by 3 mL of water. Samples were added to filter cartridges using graduated Pasteur pipettes. Cartridges were further washed of protein and lipid matrix using 2 mL of water followed by 0.5 mL of 30 % MeOH (in water) and dried gently under vacuum for ~1 min. Thyroid hormones were then eluted with 4 mL of MeOH into polypropylene test tubes. Extracts were evaporated in a heated manifold block at 40 °C under carbon-filtered nitrogen gas to approximately 50 μL volumes and reconstituted with 400 μL (1:1 (v/v) MeOH/water). Reconstituted extracts were vortexed gently, transferred to a Mini-Uniprep Syringeless Filter (PTFE, 0.2 μm; Whatman/GE Healthcare, Picataway, NJ), and analyzed by LC/MS/MS. Each pool of plasma was extracted in triplicate.
Instrumental analysis by LC/MS/MS
Levels of TT4 and TT3 in plasma were determined using our published LC/MS/MS method [26, 29, 30] with some modification. Mobile phases used were LCMS-grade acetonitrile (ACN) and water (Honeywell, Burdick & Jacksonboth buffered with 10 mM of formic acid. Analyte separation was performed using a Synergi Polar RP column (50×2.0 mm, 2.5 μm particle size; Phenomenex, Torrence, CA) and a SecurityGuard Polar-RP (4×2.0 mm) guard cartridge. The injection volume was 20 μL and the flow rate was 400 μL/ min. Initial conditions were 70:30 water/ACN held for 3.1 min, ramped to a 50:50 mixture of water/ACN for 0.4 min followed by a second ramp from 3.5 to 8.5 min of 1:99 water/ACN, which was held for 5 min before returning to baseline conditions of 70:30 water/ACN for 3.5 min. MeOH blanks were run periodically between samples (approximately every 6 samples) to monitor carryover, which was not observed during the course of this study. To maintain sensitivity during longer runs (>30 samples), an additional column cleaning method was integrated between groups of samples under the following conditions: 1:99 water:ACN for 7.0 min and 70:30 water:ACN for 0.5 min to return to baseline conditions. Table 1 provides the MS/MS conditions for detecting and quantifying thyroid hormones in ESI(+) using multiple reaction monitoring (MRM) transitions.
Table 1.
Tandem mass spectrometer (MS/MS) parameters and multiple reaction monitoring (MRM) transitions operated in ESI-positive mode
| Compound | MRM transitions (m/z) | Fragmenter (V) | Collision energy (V) |
|---|---|---|---|
| 13C12-T4 | 789.7>743.6 (Q) | 150 | 24 |
| 13C6-T4 | 783.8>737.8 (Q) | 150 | 24 |
| T4 | 777.7>731.7 (Q) | 150 | 24 |
| 777.7>633.7 (q) | |||
| 13C6-T3 | 657.8>611.3 (Q) | 135 | 20 |
| T3 | 651.8>605.8 (Q) | 135 | 20 |
| 651.8>478.7 (q) |
Q transitions for quantifier ions, q transitions for qualifier ions
Radioimmunoassay analysis
Circulating levels of TT4 and TT3 were also measured by RIA in plasma of the target species to compare with levels of thyroid hormones measured by LC/MS/MS. The RIA methods have been described in full detail previously [15] and have been used for physiological studies in a variety of salmonids (e.g., O. kisutch, O. mykiss, and O. nerka) and other fish species [10, 16, 32, 33]. Unextracted plasma (10 μL) was incubated for 2 hrs at 37 °C in a 250 μL solution comprised of 50 μL of 125I-labeled T4 or T3 (approx. 22,000 cpm; Perkin-Elmer, Waltham, MA) in 0.11 M sodium barbital (SB) buffer (pH 8.6), anti-T4 antiserum (50 μL; 1:4,000; Accurate Chemical & Scientific Corp., Westbury, NY) or anti-T3 antiserum (50 μL; 1:10,000; Accurate Chemical & Scientific Corp., Westbury, NY) in 0.11 M SB, 150 μL of 0.11 M SB buffer containing 0.25 % bovine gamma globulin (BGG) and 0.01 M 8-anilino-1-naphthalene-sufonic acid to inhibit hormone binding to thyroglobulin. After the 2 hr incubation, samples were placed on ice for 15 min to stop additional binding reactions. Ice-cold 0.11 M SB buffer (300 μL) containing 20 % polyethelyene glycol was then added to each sample, and samples were centrifuged (3,000×g) for 20 min at 4 °C. The supernatant was then removed to separate free and bound hormone, and the remaining pellet was assayed for radioactivity (Cobra II gamma counter, Packard). T4 standards from 1.25 to 60 ng/mL and T3 standards from 0.625 to 60 ng/mL dissolved in 0.11 M SB buffer with 0.25 % BGG defined the sensitivity of the RIAs. The anti-T4 antiserum used in the RIA has a reported cross-reactivity of <5 % with T3 and < 0.01 % with both diiodotyrosine and monoiodotyrosine, whereas the anti-T3 antiserum has a cross-reactivity of 0.14 % with T4, 0.5 % with, and <0.001 % with diiodotyrosine and monoiodotyrosine. All samples were run in a single assay with samples from rainbow trout, sockeye sample, and fathead minnow run in triplicate, but samples from mummichog were run in duplicate due to limited plasma volumes. The intra-assay CV was 6.95 % for the T3 assay, and 7.09 % for the T4 assay, as calculated from a single rainbow trout sample run as 13 replicates. These CV values fall within the typical CV range for this RIA in other fishes [10, 16, 32, 33]. Parallelism curves were run previously in sockeye salmon, rainbow trout plasma samples to confirm the parallel cross-reactivity of samples and standards [15] and are provided in the Electronic supplementary material (Fig. S1) for fathead minnows [32], but were not run for mummichog due to the small volume of plasma obtained from this species. Insufficient plasma was available from coho salmon for measurement by RIA.
Method performance/quality assurance
In addition to the RIA analysis, a number of tests were undertaken to examine the performance of the developed LC/MS/MS method. Seven point calibration curves (0.05–10 ng) were used to measure levels of TT4 and TT3 in all samples. Equal masses of isotopically labeled internal standards 13C12-T4 and 13C6-T3 (0.5 ng of each standard) were spiked into each sample at the start of the extraction and into calibration standards to quantify levels of TT4 and TT3, respectively. Resulting values were normalized to the plasma volume extracted (50 μL). Blank controls run in triplicate that contained 0.5 ng of 13C-T4/-T3 in DI were extracted alongside plasma samples to correct for trace levels (~0.5 %) of unlabeled hormones present as commercial impurities in the 13C-labeled internal standards. Recovery of the 13C12-T4 internal standard was monitored by adding 0.5 ng of 13C6-T4 to each sample and blank prior to LC/MS/MS analysis. During the optimization phase, plasma from rainbow trout was spiked with either 2 or 4 ng of T4 and T3 and the percent recovery of these matrix spikes was measured. Method detection limits (MDLs) were calculated as three times the standard deviation of thyroid hormone detected in blanks containing no plasma, or using the instrument limit of detection that was equivalent to a signal:noise ratio of three. MDLs normalized to the amount of plasma extracted were: T4<0.42 ng/mL and T3< 0.24 ng/mL.
Intra-assay CVs were calculated by performing repeated extractions and LC/MS/MS measurements of each plasma pool in triplicate in a single run and calculating CV values as follows: CV=SD TH levels/mean TH levels (in triplicate)× 100. These intra-assay CVs are included in Tables 2 and 3. Inter-assay CVs were measured in rainbow trout and fathead minnows by performing triplicate thyroid hormone extractions and measurements of a single plasma volume in four independent assays. Insufficient plasma was available to allow for multiple extractions in mummichog and salmon. The ratio of T4/T3, as measured by LC/MS/MS and RIA, was calculated for each sample pool or specimen (mean±SD; triplicate measures/sample). Differences in T4/T3 ratios between LC/ MS/MS and RIA measurements for each sample were examined for statistical significance using unpaired, two-tailed t tests with statistical significance defined at the p <0.05 level (GraphPad Prism 6, La Jolla, CA).
Table 2.
Optimization of deproteinizing conditions and efficiency of thyroid hormone extraction conditions using rainbow trout plasma (50 μL plasma; n =3 (mean±SD); triplicate extractions/sample)
| Extraction condition | TT3 (ng/mL) | CV | TT4 (ng/mL) | CV |
|---|---|---|---|---|
| Variations in acid–base (heating (50 μL plasma; 20 min incubations)) | ||||
| KOH (0.5 M), 25 °C | 2.51±1.06 | 42 | 3.12±1.45 | 46 |
| KOH (0.5 M), 50 °C | 3.12±0.66 | 21 | 3.50±1.18 | 34 |
| HCl (6 M), 25 °C | 2.73±0.79 | 29 | 3.31±1.55 | 47 |
| HCl (6 M), 50 °C | 3.03±0.42 | 14 | 3.12±0.35 | 11 |
| Repeated measures (50 μL plasma; 1 mL of 6 M HCl at 50 °C for 20 min) | ||||
| Assay 1 | 2.13±0.60 | 28 | 3.48±0.96 | 28 |
| Assay 2 | 1.28±0.38 | 29 | 2.41±0.97 | 41 |
| Assay 3 | 2.06±0.83 | 41 | 2.80±0.22 | 7.7 |
| Variation in temperatures and times (1 mL of 6 M HCl) | ||||
| 50 °C, 60 min | 1.95±0.06 | 3.5 | 4.11±0.17 | 4.3 |
| 50 °C, 90 min | 1.50±0.16 | 11 | 1.39±0.81 | 58 |
| 50 °C, 120 min | 1.95±0.70 | 36 | 3.11±1.07 | 34 |
| 50 °C, 180 min | 2.40±0.36 | 15 | 3.58±1.60 | 45 |
| 70 °C, 20 min | 1.29±0.11 | 8.7 | 3.38±0.56 | 17 |
| 70 °C, 60 min | 2.08±0.19 | 9.1 | 3.84±0.70 | 18 |
| Variation in plasma volumes (1 mL of 6 M HCl at 50 °C for 60 min) | ||||
| Pool 1, 50 μL plasma | 1.81±0.07 | 4.0 | 3.99±0.12 | 3.0 |
| Pool 1, 30 μL plasma | 1.33±0.64 | 48 | 4.11±0.40 | 9.8 |
| Pool 2, 50 μL plasma | 1.84±0.11 | 5.9 | 3.69±0.24 | 6.4 |
| Pool 2, 30 μL plasma | 1.63±0.79 | 48 | 3.35±0.51 | 15 |
| Pool 3, 50 μL plasma | 1.82±0.13 | 7.4 | 3.90±0.16 | 4.2 |
| Pool 3, 30 μL plasma | 1.43±0.40 | 28 | 3.31±0.83 | 25 |
Percent recovery of 13C12-T4, 96–103 % across all tests, with % recovery of 13C12-T4 being 99±1.8 under the optimized extraction conditions (6 M HCl incubated at 50 °C for 60 min). Inter-assay CVs (50 μL volumes)=4.6 % (TT3) and 3.4 % (TT4); inter-assay CVs (30 μL volumes)=34 % (TT3) and 15 % (TT4)
Table 3.
Concentrations of circulating total 3,5,3 -triiodothyronine (TT3) and total thyroxine (TT4) measured in plasma (50 μL) from teleost fishes using LC/MS/MS and RIA (n =3; mean±SD; CVs in parenthesis) with RIA-based levels reported in the literature
| Sample | LC/MS/MS
|
RIA
|
Literature | ||||
|---|---|---|---|---|---|---|---|
| TT3 levels | TT4 levels | T4/T3 | TT3 levels | TT4 levels | T4/T3 | ||
| Rainbow trout (Oncorhynchus mykiss) | |||||||
| Pool 1 | 1.81±0.07 (4.0) | 3.99±0.12 (3.0) | 2.2±0.1 | 2.80±0.24 (8.4) | 7.74±0.64 (8.2) | 2.8±0.4 | T3, T4=1–6 ng/mL [34]; 1–4 ng/mL [35]; 2–5 ng/mL [36]; T3=2–3 ng/mL, T4=5–6 ng/mL [37] T3=2 ng/mL, T4=~8 ng/mL [38]; T3, T4=3–5 ng/mL [39, 40]; and 6–14 ng/mL [41] |
| Pool 2 | 1.84±0.11 (5.9) | 3.69±0.24 (6.4) | 2.0±0.1 | 2.91±0.04 (1.4) | 8.10±0.86 (11) | 2.8±0.4 | |
| Pool 3 | 1.82±0.13 (7.4) | 3.90±0.16 (4.2) | 2.2±0.2 | 2.42±0.14 (5.7) | 12.0±2.86 (24) | 4.9±1.0 | |
| Fathead minnow (Pimephales promelas) | |||||||
| Pool 1 | 2.98±0.12 (4.0) | 3.38±0.05 (1.4) | 1.3±0.1 | 9.97±0.52 (5.2) | 13.2±0.23 (1.7) | 1.3±0.1 | T3, T4=3–4 ng/mL [42, 43]; 9–14 ng/mL [32, 44]; and T3=7–10 ng/mL and T4=15–30 ng/mL (males, age dependent) [19, 45] |
| Pool 2 | 3.42±0.15 (4.3) | 3.21±0.29 (8.9) | 1.1±0.1 | 7.97±1.54 (19) | 12.6±0.47 (3.7) | 1.6±0.4 | |
| Pool 3 | 3.31±0.19 (5.9) | 3.29±0.25 (7.6) | 1.0±0.1 | 8.35±0.35 (4.2) | 15.0±0.61 (4.1) | 1.8±0.3 | |
| Mummichog (Fundulus heteroclitus) | |||||||
| Pool 1 | 2.48±0.18 (7.1) | 2.79±0.37 (13) | 1.1±0.2 | 18.9±9.42 (50) | 22.5±5.00 (22) | 1.2±0.6 | T3, T4=~4 ng/mL [46]; T3=~6 ng/mL, T4=~8 ng/mL (males) [47] |
| Pool 2 | 3.41±0.13 (3.7) | 3.31±0.13 (3.9) | 1.0±0.1 | 11.0±4.64 (42) | 19.0±3.00 (16) | 1.7±0.3 | |
| Pool 3 | 3.55±0.22 (6.1) | 3.17±0.13 (4.1) | 1.0±0.2 | 9.77±0.90 (9.2) | 28.4±6.41 (23) | 2.9±0.3 | |
| Sockeye salmon (Oncorhynchus nerka) | |||||||
| Specimen 1 | 2.00±0.14 (7.1) | 3.23±0.31 (9.6) | 1.6±0.1 | 7.48±0.40 (5.3) | 10.6±1.09 (10) | 1.4±0.2 | T3≤5 ng/mL and T4≤4 ng/mL [48] and T3=~2 ng/mL and T4=~12 ng/mL [38] |
| Specimen 2 | 2.01±0.07 (3.6) | 3.51±0.26 (7.5) | 1.8±0.2 | 10.6±0.69 (6.5) | 15.7±0.46 (2.9) | 1.5±0.1 | |
| Specimen 3 | 2.48±0.25 (10) | 3.07±0.18 (5.9) | 1.3±0.2 | 8.58±0.44 (5.1) | 13.2±0.63 (4.8) | 1.5±0.1 | |
| Coho salmon (Oncorhynchus kisutch) | |||||||
| Specimen 1 | 1.27±0.09 (6.9) | 2.68±0.62 (23) | 2.1±0.6 | Not measured: insufficient plasma | T3, T4≤1 ng/mL [49]; T4=1–10 ng/mL [50]; T3=2–6 ng/mL, T4=3–10 ng/mL [16]; T4=2–10 ng/mL [51]; and T3=3–12 ng/mL and T4=6–40 ng/mL [15] | ||
| Specimen 2 | 2.46±0.40 (16) | 3.60±0.29 (8.0) | 1.2±0.1 | ||||
| Specimen 3 | 2.48±0.17 (6.7) | 3.90±0.16 (4.1) | 1.4±0.3 | ||||
RIA measurements in mummichog performed in duplicate/sample with parallelism curves not run because of limited plasma. Plasma pooled from 8–12 male fathead minnows/sample, 4–6 male mummichogs/sample, and 3–5 juvenile rainbow trout/sample; salmon were individual specimens. Percent recovery of 13C12-T4 by LC/MS/MS, 99±1.8 % (rainbow trout), 85±11 % (fathead minnow), 73±5.0 % (mummichog), 73±1.7 % (sockeye salmon), and 80±8.4 % (coho salmon). Inter-assay CVs, rainbow trout=4.6 (TT3) and 3.4 % (TT4); fathead minnow=6.4 (TT3) and 13 % (TT4); insufficient plasma for measurement in mummichog and salmon
Results and discussion
Extraction optimization in rainbow trout plasma
A clean extract was obtained after deproteinization of the plasma samples using HCl (1 mL; 6 M) incubated at 50 °C for 60 min coupled with SPE and matrix filtration (0.2 μm), which produced nicely resolved peaks for the T4 and T3 ions monitored. Figure 2 displays total ion chromatograms of T4 and T3 along with their labeled internal standards from a calibration standard and 50 μL sample of rainbow trout plasma.
Fig. 2.
Examples of LC/MS/MS total ion chromatograms for T3, T4, 13C6-T3, and 13C12-T4 in a standard (A, B) containing 0.52 ng of T3 and 0.56 ng of T4 and a 50 μL plasma sample from rainbow trout (C, D) containing 0.10 ng of T3 and 0.21 ng of T4. Standards and samples were spiked with 0.5 ng each of 13C12-T4 and 13C6-T3 internal standards and the volume of extract analyzed was 450 μL
During method development, several additional parameters were evaluated (Table 2). The effects of pH and temperature were tested using potassium hydroxide (KOH; 0.5 M) or HCl (6 M) and applying heat (50 °C and 70 °C) or maintaining ambient temperatures (25 °C) over several times ranging from 20–180 min. While KOH (0.5 M) and increased incubation temperatures and times appeared to allow for the isolation of TT3, these conditions did not perform well in the extraction of TT4 as indicated by the poor precision (intra-assay CVs=34 and 46 %). Initial optimization conditions using HCl heated to 50 °C for 20 min produced promising results with TT3 and TT4 levels at 3.03±0.42 ng/mL (CV=14 %) and 3.12±0.35 (CV=11 %). However, as shown in Table 2, subsequent repeated analysis of the same trout plasma pools using the same extraction conditions (i.e., 1-mL volume of HCl heated to 50 °C for 20 min) were unable to repeat these initial results, with reduced average levels of TT3 (1.28–2.13 ng/mL) and TT4 (2.41–3.48) coupled with elevated and variable intra-assay CVs (TT3=28–41 %; TT4=7.7–41 %). Moreover, inter-assay CVs were lower in 50 μL plasma volumes (TT3= 4.6 %; TT4=3.4 %) than in 30 μL volumes (TT3=34 %; TT4=15 %).
To address the reduced precision evident with 20 min incubations, additional tests were conducted to examine effects of both extended incubation times (60–180 min) and elevated temperatures (50 and 70 °C) on the extraction performance. This additional testing produced an optimal temperature and incubation time combination that included 60 min incubations at 50 °C, with high sensitivity and reproducibility providing confidence that hormones were released from the protein-hormone complex. Specifically, TT3 and TT4 levels were measured at 1.95±0.06 (CV=3.5 %) and 4.11±0.17 ng/mL (CV=4.3 %), respectively, after incubating 50 μL plasma volumes with 1 mL of HCl (6 M) at 50 °C for 60 min.
In addition to optimizing extraction conditions, lower volumes of plasma were tested in rainbow trout to further maximize efficiency and minimize the volume needed for the assay because fish blood is often limited due to the small size of some fish, tested here (fathead minnow and mummichog). As shown in Table 2, extractions were more repeatable in 50 μL plasma pools than 30 μL plasma pools as demonstrated by the low variability measured in the higher plasma volumes. Unpaired, two-tailed t tests comparing thyroid hormone levels measured in 50 μL versus 30-μL plasma volumes revealed, however, that the thyroid hormone concentrations measured at the two volumes examined were not significantly different from each other (p >0.20). Thus, while there was substantial intra-assay variability in the 30-μL volumes of plasma, especially in TT3 concentrations, these values were not statistically different from the higher extraction volumes (50 μL). However, the high variability observed in 30 μL extractions may prove problematic should there be a need to determine more subtle changes in thyroid hormone status among targeted teleost populations.
The percent recovery of the 13C12-T4 in rainbow trout plasma ranged from 96–103 % regardless of the optimization condition tested. This high percent recovery across a range of optimization conditions was indicative of high extraction efficiency and improved repeatability that was likely attributable to enhanced, consistent deproteinization as opposed to other extraction parameters. For the optimized conditions using 6 M HCl and applying heat at 50 °C for 60 min, percent recovery of the 13C12-T4 was 99±1.8 %. As an additional method performance test, rainbow trout plasma samples (50 μL; n =3; mean±SD) were spiked with either 2 or 4 ng each of T3 and T4 to measure recovery of spiked thyroid hormones using the optimized extraction parameters. In the 2 ng matrix spikes, percent recoveries of T3 and T4 were 90±2 (range, 88–91 %) and 102±4 % (range, 97–105 %), respectively, after subtracting endogenous levels of hormone. Recoveries of T3 and T4 were 87±7 (range, 82–95 %) and 102±1 % (range, 101–103 %), respectively, in 4-ng spikes, after subtracting endogenous levels of hormone. These results provide further support for the high sensitivity and performance of the optimized method parameters.
Thyroid hormone measurements in other teleosts
Table 3 provides levels of thyroid hormones measured in plasma of fathead minnows, mummichogs, sockeye salmon, and coho salmon using the optimized LC/MS/MS methods developed in rainbow trout plasma. The optimized method showed high sensitivity and reproducibility in these other species tested, with slightly more intra-assay variability detected in thyroid hormone measurements in coho salmon plasma. Inter-assay CVs in thyroid hormone measurements in rainbow trout plasma were 3.4 (TT4) and 4.6 % (TT3), and Inter-assay CVs in thyroid hormone measurements in fathead minnow plasma were 13 (TT4) and 6.4 % (TT3). Recovery values of the 13C12-T4 internal standard in these additional species tested were: fathead minnows=85±11 % (mean±SD); mummichogs=73±5.0 %; sockeye salmon=73±1.7 %; and coho salmon=80±8.4 %. The underlying differences in 13C12-T4 recovery across species are likely linked to different characteristics in the levels and types of plasma proteins, lipoproteins, and lipid constituents among the targeted species [52]. While not well understood, these constituents may not only vary across species, but they can also vary substantially across individuals of the same species depending on a variety of factors, including diet, time since the last feeding, temperature, age, and health status [53]. These differing plasma constituents may be expected to differentially bind thyroid hormones and could have altered the extraction efficiency.
This is one of the first studies to use LC/MS/MS-based applications in the measurement of circulating thyroid hormones in fish, which makes direct comparisons with other similar studies difficult. However, this method was recently used in our laboratory to measure circulating thyroid hormone concentrations in a larger population of adult male fathead minnows (~600 fish) from the same hatchery as used here. Levels of TT4 and TT3 measured in negative control groups from this recently published PBDE flame retardant toxicity study ranged from ~3 to 4 ng/mL with intra-assay and inter-assay CVs measured at <10 and <12 %, respectively [42]. This LC/MS/MS method was also recently applied to measure circulating levels of thyroid hormones in hyperthyroid (T3-treated) and hypothyroid (methimazole-treated) fathead minnows as part of a study to identify and deduce tissue distributions and mRNA abundances of thyroid hormone transporters, monocarboxylate transporters and organic anion transporter polypeptides [43]. Results from these other studies are consistent with thyroid hormone levels and CV values measured in adult male fathead minnows in the present work, providing further confidence in the precision and repeatability of the developed method over time.
Comparison with RIA measurements
Published studies to date in fishes employ RIA-based approaches exclusively to measure thyroid hormones in blood circulation and extracted from whole body larvae or specific tissues. Therefore, to enhance evaluation of the LC/MS/MS-derived values measured, this study also measured TT4 and TT3 levels in the same plasma pools using previously published RIA methods [15] to allow for more direct comparisons as has been undertaken in humans and some marine mammals [27]. These values are included in Table 3. Absolute levels of circulating thyroid hormones measured by RIA were generally higher and more variable than those measured by LC/MS/MS.
In rainbow trout plasma, using RIA, TT3, and TT4 concentrations (averaged across the three pools) were 2.71±0.25 and 9.29±2.38 ng/mL, respectively. The optimized LC/MS/ MS method measured TT3 at 1.82±0.01 ng/mL and TT4 at 3.86±0.15 ng/mL. Thus, absolute levels of TT3 measured by LC/MS/MS and RIA were more closely aligned than TT4 values, which were lower when measured by LC/MS/MS. The rainbow trout is a commonly targeted species for thyroid-related research, and as can be seen in Table 3, a great deal of variability in thyroid hormone levels has been observed in published studies that bracket the range of values measured here by LC/MS/MS and RIA. Some of this variation across studies is attributable to physiological (e.g., age, hormones, diet, and reproductive status) and environmental conditions (diurnal/seasonal rhythms, temperature, and salinity) that can markedly influence thyroid hormone homeostasis. In addition, this study employed only one RIA methodology [15], and the variation observed may be associated with differences in RIA methods and performance across laboratories (e.g., antibody interferences and binding protein variation).
Few studies have measured thyroid hormone levels in fathead minnows and mummichogs. RIA analysis conducted here of fathead minnow plasma pools measured TT3 and TT4 levels at 8.76±1.06 and 13.6±1.26 ng/mL, respectively. These values are consistent with levels measured in adult male fathead minnows using the same RIA methods [32, 44], but are higher than levels measured by LC/MS/MS (Table 3). However, plasma TT4 concentrations measured here by RIA are less than those measured in other studies [19, 45], further demonstrating varied outcomes depending on the RIA techniques used. Like with fathead minnows, absolute levels of thyroid hormones measured in the plasma of mummichogs were considerably lower and less variable with the optimized LC/MS/MS method when compared with the RIA-derived values. Previous RIA work has shown circulating levels of TT3 and TT4 in wild-caught male mummichogs from a non-polluted control site at ~6 and ~8 ng/mL, respectively, which falls between the values measured here by LC/MS/MS and RIA [54]. Consistent with findings here with LC/MS/MS, however, other studies have measured absolute levels of thyroid hormone in mummichog plasma circulation at approximately 4 ng/mL [46]. The high variation in thyroid hormone concentrations obtained here by RIA may be indicative of analytical interferences caused by plasma protein content in this species. The RIA method used in the present study was optimized originally for use in salmonid species, and the mummichog (Cyprinodontiform) is evolutionarily distant from salmonids. It is therefore likely that these taxa vary in thyroid hormone binding protein composition. Moreover, the only two previous studies that have quantified plasma thyroid hormone concentrations in mummichogs used very different RIA methods from the method used here. The study by Zhou et al. [47] used a double-antibody RIA method that employed rabbit anti-T4 and rabbit anti-T3 antiserum previously optimized for use with Sceloporus lizards. The second study [46] employed primary anti-serum sources that are no longer commercially available. As such, direct comparisons in absolute thyroid hormone concentrations between these previous mummichog studies and the RIA results here should be made with caution and further demonstrate the variability of RIA methods and results across different laboratories.
For the sockeye salmon measurements, in at least one study of migrant and land-locked sockeye, plasma TT3 levels measured by RIA were <5 ng/mL in migrant and land-locked sockeye, and TT4 levels were <4 and <10 ng/mL, respectively [48]. The sockeye plasma tested here was from migrant stock suggesting some alignment with the LC/MS/MS results. However, other studies have measured TT4 levels that are consistent with RIA levels measured here [38]. While we were unable to measure thyroid hormone levels in coho salmon with RIA due to low plasma volumes, results measured here generally fall within levels measured in other published studies that use RIA-based approaches, although on the low end of the range with considerable variation evident based on the RIA quantification method (e.g., anti-serum source), fish life-stage, and environmental condition [16, 49–51]. Thus, taken together, there is not only a lack of concordance across RIA results but there appear to be inconsistencies in absolute levels of TT3 and TT4 levels depending on whether LC/MS/MS- or RIA-based approaches are applied.
The underlying reasons for differences in absolute levels of thyroid hormones measured by LC/MS/MS versus RIA are not clear. It is possible that the RIA methodology may be overestimating circulating thyroid hormone levels due to interferences with antibodies or other plasma matrices as has been previously reported with thyroid hormone-immunoassay based approaches in clinical settings [20, 22, 55]. For instance, heterophilic antibodies in plasma or serum can promote non-specific binding to anti-T4 and anti-T3 antibodies (and other thyroid-targeted antibodies) producing abnormal values in RIAs. To overcome this problem, most current immunoassay approaches, including the one used in the present study, contain blocking reagents in an effort to overcome heterophilic antibody interferences. However, some tests have shown that these interferences may persist if antibody titres are especially high [21, 55]. While some human antibodies (e.g., human anti-mouse antibody) have been well-characterized and shown to react with mouse monoclonal antibodies, no information is available on the nature and influence of heterophilic antibodies on the performance of fish thyroid immunoassays.
It is also the case that little is known about the diversity and relative abundance of proteins that bind and transport thyroid hormones in the circulation of fishes [56]. Given the extent of evolutionary divergence among fishes, there may be considerable variation among species in serum binding protein composition that could interfere with plasma thyroid hormone measurement. Indeed, species variations in extraction recovery values of 13C12-T4 internal standard using the LC/MS/MS method provide some support for this idea. Thus, the general lack of concordance in RIA-derived thyroid hormone levels measured in fishes across laboratories and methods, as observed here, may be attributable to some of these interfering conditions that continue to be poorly described. It is also possible that the LC/MS/MS extraction method here did not fully deproteinize the plasma sample or potentially some additional matrix was interfering or causing other non-specific binding that was leading to lower detections. However, thyroid hormone levels measured in 30 ul and 50 ul plasma volumes were not significantly different from each other (Table 2), providing some support that the extraction method was deproteinizing the plasma volume efficiently with no apparent bias at the volumes extracted. Moreover, high recovery of the 13C12-T4 internal standard suggested that matrix interferences were minimized and not causing signal inhibition.
Comparison of relative levels of T4/T3
While absolute levels of thyroid hormone measured by RIA were generally higher than those measured by LC/MS/MS, ratios of T4/T3 were relatively consistent across the two methods examined for most species (see Fig. 3). The ratio of T4/T3 serves as an important indicator of overall thyroid health providing insights into the functioning of the entire thyroid system (central HPT axis and extrathyroidal tissues), as opposed to the central thyroid alone [57]. This outcome suggests that both methods were measuring the relative physiological homeostasis of circulating T4 and T3 with some level of consistency. While significant differences in the T4/ T3 ratio were detected for some of the teleost plasma pools examined, there was generally less variability within and across sample pools of all the targeted species with the LC/ MS/MS methods. An advantage of the LC/MS/MS method developed here is that it can measure both TT3 and TT4 in parallel, providing a sensitive measurement of the physiological status of thyroid hormone homeostasis in circulation. Thus, the reduced variability measured with LC/MS/MS may in part be attributable to both hormones being measured simultaneously rather than individually as is required with RIA-based approaches.
Fig. 3.

Comparisons of ratios of circulating T4/T3 measured in teleost fish plasma using LC/MS/MS-based and RIA-based approaches across three separate pools of plasma. Each pool of plasma was measured in triplicate (mean±SD) and differences in T4/T3 ratios were evaluated using unpaired, two-tailed t tests with statistical significance determined at p <0.05
Conclusions
The LC/MS/MS method developed here presents a new rapid analytical procedure that permits the simultaneous measurement of circulating thyroid hormone in low volumes of fish plasma across a range of species with high specificity and reproducibility. It takes advantage of the emerging capabilities of mass spectrometry in endocrine research, and should provide for greater standardization across teleost species, experiments, and laboratories. The method produced a clean extract and achieved high recovery of both thyroid hormones in rainbow trout matrix spikes and the mass-labeled internal standards across a diversity of fish species. Several studies have raised important questions about method variability and antibody interferences in RIA-based assays used in clinical settings. Moreover, little is known about the nature of these interferences in fish plasma. This is the first study to examine the relative performance of LC/MS/MS and RIA methods in the measurement of circulating thyroid hormones in fishes. While absolute levels of thyroid hormone were less than levels measured by RIA, T4/T3 ratios were generally well-aligned providing confidence in the capacity of the method to detect thyroid hormone homeostasis. Given the lack of concordance in RIA methodologies across laboratories and our limited understanding of interfering confounders in these assays, particularly among fish, future work should continue to standardize and advance the use of LC/MS/MS based approaches in thyroid hormone measurements in fish and other vertebrates. It would be especially useful to expand this method in the future to allow for the measurement of circulating levels of 3,3′,5′-triiodothyronine (rT3) and 3,3′-diiodothyronine (T2), which would provide further insights into thyroid hormone regulation and deiodination in fishes.
Supplementary Material
Acknowledgments
This study was supported by a National Institute of Environmental Health Sciences research grant (R01-ES016099) and US EPA STAR graduate fellowship (FP-917145010). Findings and conclusions in this article are those of the authors and do not necessarily represent the views of the NIEHS or EPA. The authors thank Dr. Even Gallagher and Chase Williams, University of Washington for providing coho salmon plasma, and Dr. Andrew Dittman, NOAA Fisheries, Northwest Fisheries Science Center for providing sockeye salmon plasma, and the Armstrong Hatchery for their donation of rainbow trout plasma. We also thank Dr. Penny Swanson and Jon Dickey, NOAA Fisheries, Northwest Fisheries Science Center, for assistance with the RIA methods.
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s00216-013-7528-3) contains supplementary material, which is available to authorized users.
Contributor Information
Pamela D. Noyes, Nicholas School of the Environment, Duke University, Box 90328, Durham, NC 2770, USA
Sean C. Lema, Department of Biological Sciences, California Polytechnic State University, San Luis Obispo, CA 93407, USA
Simon C. Roberts, Nicholas School of the Environment, Duke University, Box 90328, Durham, NC 2770, USA
Ellen M. Cooper, Nicholas School of the Environment, Duke University, Box 90328, Durham, NC 2770, USA
Heather M. Stapleton, Email: heather.stapleton@duke.edu, Nicholas School of the Environment, Duke University, Box 90328, Durham, NC 2770, USA
References
- 1.Davies L, Welch HG. Increasing incidence of thyroid cancer in the United States, 1973–2002. JAMA J Am Med Assoc. 2006;295(18):2164–2167. doi: 10.1001/jama.295.18.2164. [DOI] [PubMed] [Google Scholar]
- 2.Pellegriti G, Frasca F, Regalbuto C, Squatrito S, Vigneri R. Worldwide increasing incidence of thyroid cancer: update on epidemiology and risk factors. J Cancer Epidemiol. 2013;2013:965212–965212. doi: 10.1155/2013/965212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cheek AO, Kow K, Chen J, McLachlan JA. Potential mechanisms of thyroid disruption in humans: interaction of organochlorine compounds with thyroid receptor, transthyretin, and thyroid-binding globulin. Environ Health Perspect. 1999;107(4):273–278. doi: 10.1289/ehp.99107273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gilbert ME, Rovet J, Chen Z, Koibuchi N. Developmental thyroid hormone disruption: prevalence, environmental contaminants and neurodevelopmental consequences. Neurotoxicology. 2012;33(4):842–852. doi: 10.1016/j.neuro.2011.11.005. [DOI] [PubMed] [Google Scholar]
- 5.McNabb FMA, Fox GA. Avian thyroid development in chemically contaminated environments: is there evidence of alterations in thyroid function and development? Evol Dev. 2003;5(1):76–82. doi: 10.1046/j.1525-142x.2003.03012.x. [DOI] [PubMed] [Google Scholar]
- 6.Zhang JS, Lazar MA. The mechanism of action of thyroid hormones. Annu Rev Physiol. 2000;62:439–466. doi: 10.1146/annurev.physiol.62.1.439. [DOI] [PubMed] [Google Scholar]
- 7.Shiao JC, Hwang PP. Thyroid hormones are necessary for the metamorphosis of tarpon Megalops cyprinoides leptocephali. J Exp Mar Biol Ecol. 2006;331(2):121–132. [Google Scholar]
- 8.Schreiber AM, Specker JL. Metamorphosis in the summer flounder (Paralichthys dentatus): stage-specific developmental response to altered thyroid status. Gen Comp Endocrinol. 1998;111(2):156–166. doi: 10.1006/gcen.1998.7095. [DOI] [PubMed] [Google Scholar]
- 9.Klaren PHM, Guzman JM, Mancera JM, Geven EJW, Flik G. The involvement of thyroid hormone metabolism in ilthead sea bream. (Sparus auratus) osmoregulation. In: Vaudry H, Roubos E, Schoofs L, Fiik G, Larhammar D, editors. Trends in comparative endocrinology and neurobiology, vol 1040. Annals of the New York Academy of Sciences. 2005. pp. 360–362. [DOI] [PubMed] [Google Scholar]
- 10.Lema SC, Nevitt GA. Evidence that thyroid hormone induces olfactory cellular proliferation in salmon during a sensitive period for imprinting. J Exp Biol. 2004;207(19):3317–3327. doi: 10.1242/jeb.01143. [DOI] [PubMed] [Google Scholar]
- 11.Peter MCS. The role of thyroid hormones in stress response of fish. Gen Comp Endocrinol. 2011;172(2):198–210. doi: 10.1016/j.ygcen.2011.02.023. [DOI] [PubMed] [Google Scholar]
- 12.Coffin AB, Raine JC, Hawryshyn CW. Exposure to thyroid hormone in ovo affects otolith crystallization in rainbow trout Oncorhynchus mykiss. Exp Biol Fish. 2012;95(3):347–354. [Google Scholar]
- 13.Blanton ML, Specker JL. The hypothalamic-pituitary-thyroid (HPT) axis in fish and its role in fish development and reproduction. Crit Rev Toxicol. 2007;37(1–2):97–115. doi: 10.1080/10408440601123529. [DOI] [PubMed] [Google Scholar]
- 14.Nelson ER, Allan ERO, Pang FY, Habibi HR. Thyroid hormone and reproduction: regulation of estrogen receptors in goldfish gonads. Mol Reprod Dev. 2010;77(9):784–794. doi: 10.1002/mrd.21219. [DOI] [PubMed] [Google Scholar]
- 15.Dickhoff WW, Folmar LC, Mighell JL, Mahnken CVW. Plasma thyroid hormones during smoltification of yearling and underyearling Coho salmon and yearling Chinook salmon and Steelhead trout. Aquaculture. 1982;28(1–2):39–48. [Google Scholar]
- 16.Larsen DA, Swanson P, Dickhoff WW. The pituitary-thyroid axis during the parr-smolt transformation of Coho salmon, Oncorhynchus kisutch: quantification of TSH beta mRNA, TSH, and thyroid hormones. Gen Comp Endocrinol. 2011;171(3):367–372. doi: 10.1016/j.ygcen.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 17.Maclatchy DL, Eales JG. Intracellular and extracellular sources of T3 binding to putative thyroid-hormone receptors in liver, kidney, and gill nuclei of immature rainbow trout, Oncorhynchus mykiss. J Exp Zool. 1992;262(1):22–29. doi: 10.1002/jez.1402620105. [DOI] [PubMed] [Google Scholar]
- 18.Brown S, Eales JG. Measurement of L-thyroxine and 3,5,3′-triiodo-L-thyronine levels in fish plasma by radioimmunoassay. Can J Zool. 1977;55(2):293–299. doi: 10.1139/z77-039. [DOI] [PubMed] [Google Scholar]
- 19.Crane HM, Pickford DB, Hutchinson TH, Brown JA. Developmental changes of thyroid hormones in the fathead minnow, Pimephales promelas. Gen Comp Endocrinol. 2004;139(1):55–60. doi: 10.1016/j.ygcen.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 20.Ghosh S, Howlett M, Boag D, Malik I, Collier A. Interference in free thyroxine immunoassay. Eur J Intern Med. 2008;19(3):221–222. doi: 10.1016/j.ejim.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 21.Hoofnagle AN, Wener MH. The fundamental flaws of immunoassays and potential solutions using tandem mass spectrometry. J Immunol Methods. 2009;347(1–2):3–11. doi: 10.1016/j.jim.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tate J, Ward G. Interferences in immunoassay. Clin Biochem Rev. 2004;25(2):105–120. [PMC free article] [PubMed] [Google Scholar]
- 23.Soldin SJ, Soukhova N, Janicic N, Jonklaas J, Soldin OP. The measurement of free thyroxine by isotope dilution tandem mass spectrometry. Clin Chim Acta. 2005;358(1–2):113–118. doi: 10.1016/j.cccn.2005.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tai SSC, Bunk DM, White EV, Welch MJ. Development and evaluation of a reference measurement procedure for the determination of total 3,3′,5-trilodothyronine in human serum using isotope-dilution liquid chromatography-tandem mass spectrometry. Anal Chem. 2004;76(17):5092–5096. doi: 10.1021/ac049516h. [DOI] [PubMed] [Google Scholar]
- 25.Tai SSC, Sniegoski LT, Welch MJ. Candidate reference method for total thyroxine in human serum: use of isotope-dilution liquid chromatography-mass spectrometry with electrospray ionization. Clin Chem. 2002;48(4):637–642. [PubMed] [Google Scholar]
- 26.Butt CM, Wang DL, Stapleton HM. Halogenated phenolic contaminants inhibit the in vitro activity of the thyroid-regulating deiodinases in human liver. Toxicol Sci. 2011;124(2):339–347. doi: 10.1093/toxsci/kfr117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kunisue T, Eguchi A, Iwata H, Tanabe S, Kannan K. Analysis of thyroid hormones in serum of baikal seals and humans by liquid chromatography-tandem mass spectrometry (LC-MS/MS) and immunoassay methods: application of the LC-MS/MS method to wildlife tissues. Environ Sci Technol. 2011;45(23):10140–10147. doi: 10.1021/es203002a. [DOI] [PubMed] [Google Scholar]
- 28.Kunisue T, Fisher JW, Kannan K. Determination of Six thyroid hormones in the brain and thyroid gland using isotope-dilution liquid chromatography/tandem mass spectrometry. Anal Chem. 2011;83(1):417–424. doi: 10.1021/ac1026995. [DOI] [PubMed] [Google Scholar]
- 29.Wang DL, Stapleton HM. Analysis of thyroid hormones in serum by liquid chromatography-tandem mass spectrometry. Anal Bioanal Chem. 2010;397(5):1831–1839. doi: 10.1007/s00216-010-3705-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Noyes PD, Hinton DE, Stapleton HM. Accumulation and debromination of decabromodiphenyl ether (BDE-209) in juvenile fathead minnows (Pimephales promelas) induces thyroid disruption and liver alterations. Toxicol Sci. 2011;122(2):265–274. doi: 10.1093/toxsci/kfr105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Little AG, Kunisue T, Kannan K, Seebacher F. Thyroid hormone actions are temperature-specific and regulate thermal acclimation in zebrafish (Danio rerio) BMC Biol. 2013:11. doi: 10.1186/1741-7007-11-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lema SC, Dickey JT, Schultz IR, Swanson P. Thyroid hormone regulation of mRNAs encoding thyrotropin beta-subunit, glycoprotein alpha-subunit, and thyroid hormone receptors alpha and beta in brain, pituitary gland, liver, and gonads of an adult teleost, Pimephales promelas. J Endocrinol. 2009;202(1):43–54. doi: 10.1677/JOE-08-0472. [DOI] [PubMed] [Google Scholar]
- 33.Johnson KM, Lema SC. Tissue-specific thyroid hormone regulation of gene transcripts encoding iodothyronine deiodinases and thyroid hormone receptors in striped parrotfish (Scarus iseri) Gen Comp Endocrinol. 2011;172(3):505–517. doi: 10.1016/j.ygcen.2011.04.022. [DOI] [PubMed] [Google Scholar]
- 34.Brown SB, Adams BA, Cyr DG, Eales JG. Contaminant effects on the teleost fish thyroid. Environ Toxicol Chem. 2004;23(7):1680–1701. doi: 10.1897/03-242. [DOI] [PubMed] [Google Scholar]
- 35.Eales JG, Hughes M, Uin L. Effect of food intake on diel variation in plasma thyroid hormone levels in rainbow trout. Gen Comp Endocrinol. 1981;45(2):167–174. doi: 10.1016/0016-6480(81)90101-5. [DOI] [PubMed] [Google Scholar]
- 36.Todd KJ, Eales JG. The effect of handling and blood removal on plasma levels and hepatic deiodination of thyroid hormones in adult male and female rainbow trout, Oncorhynchus mykiss. Can J Zool. 2002;80(2):372–375. [Google Scholar]
- 37.Gomez JM, Boujard T, Boeuf G, Solari A, LeBail PY. Individual diurnal plasma profiles of thyroid hormones in rainbow trout (Oncorhynchus mykiss) in relation to cortisol, growth hormone, and growth rate. Gen Comp Endocrinol. 1997;107(1):74–83. doi: 10.1006/gcen.1997.6897. [DOI] [PubMed] [Google Scholar]
- 38.Plate EM, Adams BA, Allison WT, Martens G, Hawryshyn CW, Eales JG. The effects of thyroxine or a GnRH analogue on thyroid hormone deiodination in the olfactory epithelium and retina of rainbow trout, Oncorhynchus mykiss, and sockeye salmon, Oncorhynchus nerka. Gen Comp Endocrinol. 2002;127(1):59–65. doi: 10.1016/s0016-6480(02)00022-9. [DOI] [PubMed] [Google Scholar]
- 39.Reddy PK, Leatherland JF. Does the time of feeding affect the diurnal rhythms of plasma hormone and glucose concentration adn hepatic glycogen content of rainbow trout. Fish Physiol Biochem. 1994;13(2):133–140. doi: 10.1007/BF00004338. [DOI] [PubMed] [Google Scholar]
- 40.Feng CL, Xu YP, Zhao GF, Zha JM, Wu FC, Wang ZJ. Relationship between BDE 209 metabolites and thyroid hormone levels in rainbow trout (Oncorhynchus mykiss) Aquat Toxicol. 2012;122:28–35. doi: 10.1016/j.aquatox.2012.05.008. [DOI] [PubMed] [Google Scholar]
- 41.Holloway AC, Sheridan MA, Van der Kraak G, Leatherland JF. Correlations of plasma growth hormone with somatostatin, gonadal steroid hormones and thyroid hormones in rainbow trout during sexual recrudescence. Comp Biochem Physiol B. 1999;123(3):251–260. doi: 10.1016/s0305-0491(99)00059-0. [DOI] [PubMed] [Google Scholar]
- 42.Noyes PD, Lema SC, Macaulay LJ, Douglas NK, Stapleton HM. Low level exposure to the flame retardant BDE-209 reduces thyroid hormone levels and disrupts thyroid signaling in fathead minnows. Environ Sci Technol. 2013;47:10012–10021. doi: 10.1021/es402650x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Muzzio AM, Noyes PD, Stapleton HM, Lema SC. Tissue distribution and thyroid hormone effects on mRNA abundance for membrane transporters Mct8, Mct10, and organic anion-transporting polypeptides (OATPs) in a teleost fish. Comp Biochem Phys A. 2013;167:77–89. doi: 10.1016/j.cbpa.2013.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lema SC, Dickey JT, Schultz IR, Swanson P. Dietary exposure to 2,2′,4,4′-tetrabromodiphenyl ether (PBDE-47) alters thyroid status and thyroid hormone-regulated gene transcription in the pituitary and brain. Environ Health Perspect. 2008;116(12):1694–1699. doi: 10.1289/ehp.11570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Crane HM, Pickford DB, Hutchinson TH, Brown JA. The effects of methimazole on development of the fathead minnow, Pimephales promelas, from embryo to adult. Toxicol Sci. 2006;93(2):278–285. doi: 10.1093/toxsci/kfl063. [DOI] [PubMed] [Google Scholar]
- 46.Brown CL, Stetson MH. Prolactin thyroid interaction in Fundulus heteroclitus. Gen Comp Endocrinol. 1983;50(2):167–171. doi: 10.1016/0016-6480(83)90217-4. [DOI] [PubMed] [Google Scholar]
- 47.Zhou T, John-Alder HB, Weis JS, Weis P. Endocrine disruption: thyroid dysfunction in mummichogs (Fundulus heteroclitus) from a polluted habitat. Mar Environ Res. 2000;50(1–5):393–397. doi: 10.1016/s0141-1136(00)00042-8. [DOI] [PubMed] [Google Scholar]
- 48.Munakata A, Amano M, Ikuta K, Kitamura S, Aida K. Involvement of sex steroids, luteinizing hormone and thyroid hormones in upstream and downstream swimming behavior of land-locked sockeye salmon Oncorhynchus nerka. Fish Sci. 2012;78(1):81–90. [Google Scholar]
- 49.Eales JG, Devlin R, Higgs DA, McLeese JM, Oakes JD, Plohman J. Thyroid function in growth-hormone-transgenic coho salmon (Oncorhynchus kisutch) Can J Zool. 2004;82(8):1225–1229. [Google Scholar]
- 50.Larsen DA, Beckman BR, Dickhoff WW. The effect of low temperature and fasting during the winter on metabolic stores and endocrine physiology (Insulin, insulin-like growth factor-I and thyroxine) of coho salmon, Oncorhynchus kisutch. Gen Comp Endocrinol. 2001;123(3):308–323. doi: 10.1006/gcen.2001.7677. [DOI] [PubMed] [Google Scholar]
- 51.Young G, Bjornsson BT, Prunet P, Lin RJ, Bern HA. Smoltification and seawater adaptation in coho salmon (Oncorhynchus kisutch) - Plasma prolactin, growth hormone, thyroin hormones, and cortisol. Gen Comp Endocrinol. 1989;74(3):335–345. doi: 10.1016/s0016-6480(89)80029-2. [DOI] [PubMed] [Google Scholar]
- 52.Babin PJ, Vernier JM. Plasma-lipoproteins in fish. J Lipid Res. 1989;30(4):467–489. [PubMed] [Google Scholar]
- 53.Santulli A, Cusenza L, Modica A, Curatolo A, Damelio V. Fish plasma-lipoproteins—comparative observations in Serranides and Sparides. Comp Biochem Physiol B. 1991;99(2):251–255. doi: 10.1016/0305-0491(91)90036-d. [DOI] [PubMed] [Google Scholar]
- 54.Zhou T, John-Alder HB, Weis P, Weis JS. Thyroidal status of mummichogs (Fundulus heteroclitus) from a polluted versus a reference habitat. Environ Toxicol Chem. 1999;18(12):2817–2823. [Google Scholar]
- 55.Despres N, Grant AM. Antibody interference in thyroid assays: a potential for clinical misinformation. Clin Chem. 1998;44(3):440–454. [PubMed] [Google Scholar]
- 56.Manzon RG, Neuls TM, Manzon LA. Molecular cloning, tissue distribution, and developmental expression of lamprey transthyretins. Gen Comp Endocrinol. 2007;151(1):55–65. doi: 10.1016/j.ygcen.2006.11.017. [DOI] [PubMed] [Google Scholar]
- 57.Bianco AC, Larsen PR. Intracellular pathways of iodothyronine metabolism. In: Braverman LE, Utiger RD, editors. The thyroid: a fundamental and clinical text. 9. Lippincott Williams & Wilkens; Philadelphia: 2005. p. 120. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.