Abstract
The goals of the study were to determine the extent to which the underlying structure of different types of well-being was multidimensional and whether well- and ill-being were influenced by similar or different genetic and environmental factors. Participants were 1226 male twins ages 51-60, from the Vietnam Era Twin Study of Aging. Measures included: psychological well-being, Multidimensional Personality Questionnaire Well-Being scale (MPQWB), life satisfaction, self-esteem, and depressive symptoms. A two-orthogonal-factor common pathway model fit the data well. Psychological well-being and self-esteem loaded most strongly on Factor 1, which was highly heritable (h2 = .79). Life satisfaction loaded most strongly on Factor 2, which was only moderately heritable (h2 = .32). Only MPQWB had measure-specific genetic influences. Depressive symptoms loaded on both factors, and only depressive symptoms had measure-specific common environmental influences. All measures had specific unique environmental influences. Results indicate that well-being is genetically and environmentally multidimensional and that ill-being has partial overlap with both latent factors.
Keywords: Well-being, Psychological well-being, Self esteem, Life satisfaction, CES-D, MPQ, Twins, Heritability, VETSA
Introduction
An important public health goal, and one shared by the World Health Organization, is to promote well-being and minimize ill-being throughout the life course (Federal Interagency Forum on Aging-Related Statistics 2004). Two schools of thought dominate well-being research: one focused more on hedonic/subjective well-being (i.e., evaluations of happiness, life satisfaction); the other on eudaimonic/psychological well-being (i.e. evaluations of self-realization, thriving, purposeful life engagement)(Keyes et al. 2002; Ryan and Deci 2001). (See “Method” section for more specific definitions). At an empirical level, however, well-being measures are often used and interpreted interchangeably because of relatively high statistical associations among measures. Adding to the complexity, several indicators of positive functioning—self-esteem and optimism—are used as indicators of well-being (Caprara et al. 2009; Diener et al.2003; Sprangers et al. 2010) and low levels of depressive symptoms are sometimes interpreted as the negative end of well-being even though happiness or satisfaction and ill-being likely do not represent the ends of a single bipolar dimension (Diener et al. 2006; Keyes 2002; Keyes et al. 2002; Ryff et al. 2006).
The current state of affairs raises concerns about the “jingle” and “jangle” fallacies; that is, when characteristics labeled similarly are actually different orwhenconstructs with different names turn out to be essentially the same (Block 1995; Kelley 1927). Twin studies and behavior genetics research can illuminate ways in which various types of well- and ill-being constructs can be “lumped” or “split” by examining the extent to which different environmental and genetic factors may explain the covariance among the measures, thereby addressing questions regarding their core independence and/or bipolarity. Twin studies have shown that—depending on the individual measure—20 to 73 % of the variance in well-being is influenced by genetic factors (Bartels and Boomsma 2009; Bartels et al. 2010; Bergeman et al. 1991; Caprara et al. 2009; Kendler et al. 2011; Kessler et al. 2004; Keyes et al. 2010; Mosing et al. 2009; Nes et al.2008; Nes et al. 2010; Roysamb et al. 2002; Roysamb et al.2003; Stubbe et al. 2005). To date, however, few twin or family studies have used multivariate behavior genetic approaches to examine the genetic and environmental multidimensionality of different types of well-being or to differentiate wellfrom ill-being.
Unidimensionality versus multidimensionality
The distinction between unidimensional and multidimensional is as obvious as it would seem. Finding two or more factors in a multivariate genetic analysis constitutes clear evidence of multidimensionality, but a single common latent factor can be ambiguous with respect to multidimensionality. For example, we would consider a single latent genetic factor with no significant measure-specific genetic influences to constitute evidence of unidimensionality. However, even with only a single latent genetic factor, if there were some significant measure-specific genetic influences that were independent of the common factor, we would argue that this scenario is a form of multidimensionality. The presence of independent genetic effects on a particular measure indicates that there must be more than one underlying set of genetic influences. Thus, we would consider this situation to indicate multidimensionality at the genetic level. Accordingly, although researchers who found only a single factor are technically correct in suggesting unidimensionality, we suggest below that some of those findings can actually be interpreted as indicating multidimensionality.
Multivariate twin studies of well-being
Bartels and Boomsma (2009) found that approximately half of the overlap among four measures of satisfaction and happiness was accounted for by genetic influences in common whereas the patterns of associations among unique environmental factors across measures were complex. We list measures used in each study in Table 1 and list items in Supplementary Material. Although there were common additive genetic influences, Bartels and Boomsma also found measure-specific non-additive/dominance effects on satisfaction and happiness. Similarly, Caprara et al. (2009) found that genetic influences were substantially shared among three indicators of well-being (optimism, life satisfaction, and self-esteem) whereas unique environmental influences were partly common but largely measure-specific. Keyes et al. (2010) conducted multivariate genetic analyses of psychological, emotional and social well-being (Keyes et al.2010; Snowden et al. 2010). They considered their emotional well-being indicator to be a measure of hedonic well-being (satisfaction, cheerfulness and happiness). The psychological well-being indicator assessed eudaimonic well-being (Kessler et al. 2004; Ryff 1989, 1995; Ryff and Keyes 1995). A single latent genetic factor (called mental well-being) emerged that accounted for 99 % of the genetic influences on psychological well-being and 61/65 % of the genetic influences on social and emotional well-being, respectively. There were also significant measure-specific additive genetic influences on both emotional and social well-being. These results, thus, suggest multidimensionality of the underlying genetic influences on types of well-being.
Table 1.
Measures used in multivariate twin studies of well- and ill-being (items in Supplementary Material)
| Comparisons of well-being measures | |
| Bartels and Boomsma 2009 |
|
| Caprara et al. 2009 |
|
| Keyes et al. 2010 | Mental Well-being (latent factor): |
|
|
| Comparisons of well- and ill-being items | |
| Kendler et al. 2011 |
|
| Mosing et al. 2010, 2009 |
|
| Nes et al. 2008 |
|
| Plomin et al. (1992) |
|
Multivariate twin studies of both well- and ill-being
Several twin studies have simultaneously examined the genetic and environmental architecture of well- and ill-being. Substantial genetic and environmental overlap between positive and negative orientations would provide support for their bipolarity whereas greater differentiation of genetic and environmental influences would provide support for their independence. Findings are inconclusive. Two studies provided some support for the view that, at a genetic level, well- and ill-being are bipolar; that is, genes associated with the propensity to feel positively about life also decrease the likelihood of feeling depressed (Mosing et al. 2010, 2009; Nes et al. 2008). In a study of young adult twins (ages 18-31), ill-being was assessed with the anxiety and depression scales from the abbreviated Symptom Checklist-25 (two items and three items, respectively); well-being was assessed with a single item which evaluated whether the twins were mostly satisfied or dissatisfied with life overall (Nes 2008). It is perhaps worth noting that with only five items on one measure and one on the other, the deck may have been somewhat stacked against finding multidimensionality. Associations between optimism, and a measure of mental health (primarily depression and anxiety) were examined in a combined sample of older Australian twins and Swedish twins (Mosing et al. 2010, 2009). High scores on the optimism scale reflected a positive orientation while low scores reflected pessimism. In both studies, well- and ill-being shared substantial common genetic influences but each measure was also influenced by unique environmental influences specific to the measure.
Associations between well- and ill-being were more complex in two other twin studies. Plomin et al. (1992) examined genetic overlap between life satisfaction, optimism, pessimism, and depressive symptoms in older adult twins. Life satisfaction was not heritable, but at the phenotypic level, optimism was significantly associated with satisfaction (r = .46). A common genetic factor significantly influenced both pessimism and depressive symptoms, but not optimism and depressive symptoms. Finally, Kendler et al. (2011) examined associations between mental well-being (i.e. emotional, psychological, and social well-being) and internalizing psychopathology (i.e., depression, anxiety, panic) in adult twins. When data from two time points were combined to represent stable risks associated with mental well-being and internalizing psychopathology, these two constructs shared substantial genetic influences (41-50 %) and modest, though significant, environmental influences. In addition, there were significant genetic influences specific to major depression, anxiety disorder, and emotional well-being (1995) and unique environmental influences specific to each measure. Based on our conceptualization, both of the latter two studies suggest multidimensionality of well- and ill-being.
Rationale for the present study
The extant literature suggests that more systematic behavior genetic work is needed to clarify associations among well- and ill-being constructs. The few twin studies that examine well- and ill-being constructs have tended to use different measures and samples with widely varying characteristics making crossstudy comparisons difficult. Few studies incorporated indicators of both hedonic and eudaimonic well-being or indicators of both well- and ill-being. The tendency in earlier studies has been toward lumping and splitting measures due to theoretical preconceptions (e.g., hedonic versus eudaimonic well-being) or face validity rather than empirical reasons. Because measures in some studies were combined prior to multivariate analysis, the possibility of observing multidimensionality may be reduced. Thus studies used a proliferation of measures, some of which were viewed as conceptually distinct, but most of which were moderately-to-highly inter-correlated. Despite these limitations, there was still evidence for multidimensionality in the genetic and environmental influences underlying well-and ill-being. Therefore, there is good reason to think that there is even greater complexity and multidimensionality to these constructs than previously believed.
In the present study we assessed well- and ill-being in middle-aged twin men ages 51-60 using four different measures of well-being (including measures thought to assess both hedonic, eudaimonic well-being) and a measure of depressive symptoms as an indicator of ill-being. The goal of the study was to determine the extent to which: (1) the underlying structure of different types of well-being were multidimensional; and (2) well- and ill-being were influenced by similar or different genetic and environmental factors (thus reflecting their bipolarity or independence). Based on our interpretation of the results of prior studies, we predicted that well- and ill-being would be genetically and environmentally multidimensional.
Method
Participants
Participants in the Vietnam Era Twin Study of Aging (VETSA) were recruited from 3322 twin pairs who participated in a previous large-scale study (Tsuang et al.2001); all participants were part of the Vietnam Era Twin Registry (Eisen et al. 1987). In order to be eligible, participants had to be 51-59 years old when recruited and both members of a twin pair had to agree to participate. Participants averaged 55 years old at the time of the VETSA assessment (mean age = 55.4; SD 2.47).
Most participants were married (79 %) and employed fulltime (78 %) with a median family income of 60-70 thousand dollars per year. Average education was 13.9 years. One quarter (25 %) of the men currently smoked and 59 % reported having at least one alcoholic drink in the past 2 weeks. With regard to health and lifestyle factors, 56 % had hypertension, 8 % diabetes, and about 16 % reported cardiovascular disease. Health and lifestyle characteristics were very comparable to those of the general population of men in the United States in this age group (Centers for Disease Control and Prevention 2003, 2007). Although all participants were military veterans, the majority (62 %) did not serve in combat or in Vietnam (Eisen et al. 1987).
Procedures and measures
Details of the data collection and sample are available in previous publications (Franz et al. 2011; Kremen et al. 2006). Although the VETSA focuses on genetic and environmental influences on cognitive aging, measures of well-being, satisfaction, and depressive symptoms were included in the assessment as important risk and preventive factors in aging (Kremen et al. 2006). Participants completed questionnaires at home and brought them to the test sites (University of California, San Diego or Boston University) on the testing day (99 % return rate). Of the 1237 VETSA participants, 1226 had questionnaire data (336 monozygotic [MZ] malemale pairs; 277 dizygotic [DZ] male-male pairs). Institutional Review Board approval was obtained at all sites; all participants provided written informed consent.
Well-being measures (see Supplementary Material for items on each measure)
Psychological well-being (PWB)
Psychological well- being was measured with the 18-item Psychological Well-Being Scale (Ryff and Keyes 1995). The PWB has been conceptualized as a measure of eudaimonic well-being and delineates six dimensions of well-being: autonomy; environmental mastery; personal growth; positive relations with others; purpose in life; and self-acceptance (Ryff 1989, 1995; Ryff and Keyes 1995). Because of low alphas for the individual dimensions (alphas ranged from .40 to .50), we used the total score for the scale as an indicator of general psychological well-being (α = .80). Items on the PWB were worded both positively and negatively (e.g., “In general I feel I am in charge of the situation in which I live,” “I like most aspects of my personality,” “In many ways I feel disappointed about my achievements in life”). Negatively worded items were reverse scored so that high scores reflect well-being. Questions dealt with how the participant felt about himself and his life; participants responded to each item on a scale from one (strongly disagree) to six (strongly agree).
Life satisfaction
General life satisfaction was assessed with a single item (“Using a scale from 0 to 10 where 0 means ‘the worst possible life overall’ and 10 means ‘the best possible life overall,’ how would you rate your life overall these days?”). This item is commonly used and viewed as valid in the epidemiological/survey literature (Brim et al. 1996; Nes et al. 2008; Schaie et al. 2004). Life satisfaction has been considered by some researchers to be an index of hedonic/subjective well-being (Diener 2005; Diener et al. 2003).
Rosenberg self-esteem scale
We measured self-esteem with the 10-item Rosenberg Self-Esteem Scale (Rosenberg 1965). Because of its emphasis on positive optimal functioning, the Rosenberg likely reflects eudaimonic well-being (Caprara et al. 2009; Schimmack et al. 2002). The scale assesses both positive (“I feel that I have a number of good qualities”) and negative (“All in all, I’m inclined to think I’m a failure”) general appraisals about one’s self (α = 0.90). Participants indicated on a scale from one (strongly disagree) to four (strongly agree) how the items reflected their general feelings. The Rosenberg has been used a measure of well-being in previous studies, is highly correlated with other measures of well-being, and has strong item overlap with PWB. In addition, conceptually it fits Diener’s definition of well-being as “an umbrella term for the different valuations people make regarding their lives, the events happening to them, their bodies and minds, and the circumstances in which they live” (Diener 2005).
Multidimensional Personality Questionnaire Well-Being (MPQWB)
MPQWB was assessed using the 11-item well-being scale (α = 0.80) from the Multidimensional Personality Questionnaire-form NZ. The NZ version is considered to be very similar to the Brief Form (Caspi 2000; Caspi et al. 1997; Krueger et al. 2000; Patrick et al.2002; Tellegen 1985; Tellegen et al. 1988). Psychometric properties and validity of the MPQ are well documented (Lykken and Tellegen 1996; Patrick et al. 2002; Tellegen 1985). A person high in MPQWB is someone who has a cheerful and happy disposition, feels good about himself and his life, and is optimistic about the future. MPQWB was intended as a trait-like measure of well-being. As suggested by the description of a person high in MPQWB, this measure includes elements of each of the other three well-being measures (see Supplementary Material for items). Thus, it does not fit neatly under the rubric or either eudaimonic or hedonic well-being. There are no negatively worded items on the scale. Participants responded whether each statement was true or false about them.
Ill-being
We assessed ill-being with the Center for Epidemiologic Studies Depression Scale (CESD; Radloff 1977). The CESD comprised 20 items involving an appraisal of the frequency of specific moods and behaviors during the past week; responses ranged from “rarely or none of the time” to “most or all of the time.” It has good reliability (α = 0.92) and is highly correlated with indicators of major depression (Rush et al. 2006). Most items on the CESD were worded negatively (“I felt sad”; “I was bothered by things that usually don’t bother me”) but four items were worded positively (“I was happy”, “I felt hopeful about the future”). Depressive symptom scores for the sample were comparable to other community-based non-patient samples (Franz et al. 2011; Radloff 1977).
Zygosity
Zygosity was determined using a combination of DNA testing (examination of 25 satellite markers), questionnaire, and blood group methods. There was 95 % agreement between the DNA and questionnaire methods (Eisen et al. 1989; Nichols and Bilbro 1966). Blood based zygosity was available for 92 % of the sample and was used as the gold standard when available.
Data analysis: multivariate statistical analysis methods
In order to elucidate the genetic and environmental relationships among well-being phenotypes, we fit a series of multivariate twin models. As extensions of the traditional univariate twin analysis, these models decomposed the variance of a phenotype, as well as the covariance between phenotypes into proportions attributable to additive genetic (A) influences, shared or common environmental (C) influences (i.e.,environmental factors that make members of a twin pair more similar to one another), and unique environmental (E) influences (i.e., environmental factors that make twins different from one another, including measurement error) (Eaves et al. 1978; Neale and Cardon 1992). Non-additive genetic influences (D) that are due to genetic dominance or epistasis can also be quantified in these models; however, due to issues of model identification D and C effects cannot be simultaneously represented in the classical twin design. As with all twin analyses, additive genetic influences are assumed to correlate 1.0 between monozygotic (MZ) twins because they share 100 % of their DNA.Dizygotic (DZ) twins share on average 50 % of their segregating DNA, and are therefore assumed to correlate .50 for additive genetic influences. The shared environment is assumed to correlate 1.0 between both members of a twin pair, regardless of their zygosity.
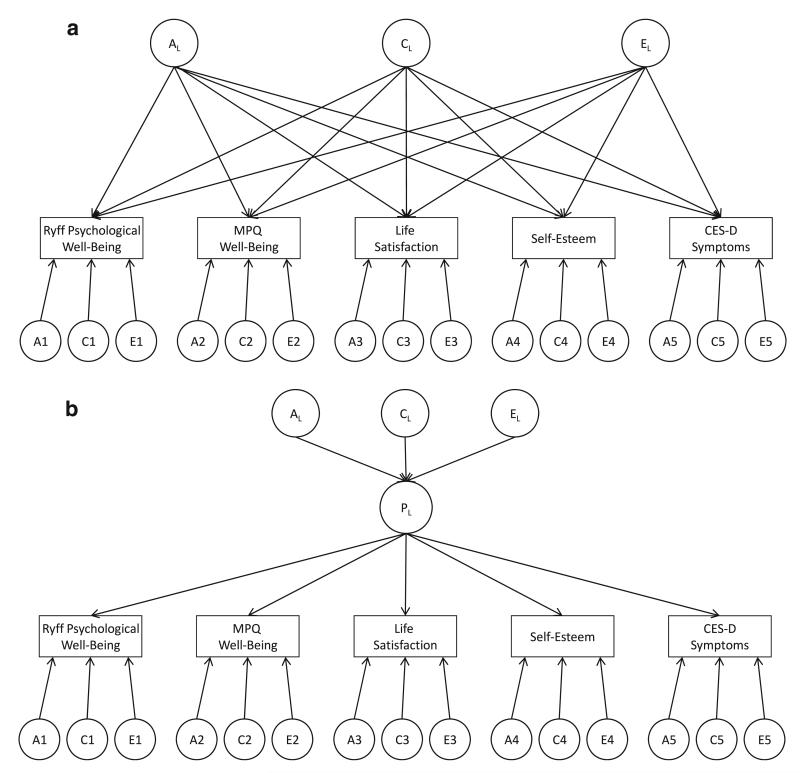
A multivariate Cholesky decomposition was first fit to the data in order to estimate the magnitude of genetic and environmental covariance between the variables of interest. These covariance estimates can then be used to calculate genetic and environmental correlations. Conceptually, genetic and environmental correlations represent the degree to which genetic and environmental influences of one phenotype are predictive of the influences for another phenotype (Carey 1988). In statistical terms, the genetic correlation (rG) between any two phenotypes is defined as their genetic covariance divided by the square root of the product of their individual genetic variances. Shared and unique environmental correlations are calculated in the same way utilizing the respective variance and covariance estimates. In addition to the multivariate Cholesky we fit independent pathway/biometric factors models (see Fig. 1a) (McArdle and Goldsmith 1990) and common pathway/psychometric factors models (see Fig. 1b) (Kendler et al. 1987) in order to determine the degree to which the genetic and environmental influences on our well-being phenotypes stem from the same or different factors. The independent pathway model imposes genetic and environmental factors on the respective covariance estimates, while also allowing for variable-specific genetic and environmental influences. These latent factors then act on each variable through separate, independent pathways and allow for the determination of whether observed genetic and environmental covariance is due to one or more common genetic or environmental factors. In the common pathway model, genetic and environmental influences act directly on a latent phenotype, and then on the variables of interest through a common pathway that is equivalent to a factor loading. The model is akin to a principal factors analysis in which genetic and environmental influences are identified for each factor, as well as for the residual variance of each variable. The independent pathway models were used to test for the presence of genetic and environmental factors without imposing formal latent phenotypes on the data. The common pathway model was then used to test for the presence of global well-being factors.
Fig. 1.
a Independent pathway model. b Common pathway model
Analyses were conducted using the raw data option of the maximum-likelihood based structural equation modeling software Mx (Neale et al. 2006). Prior to model fitting, all variables were transformed to approximate normality, and were then standardized to a mean of 0 and a variance of 1.0 in order to simplify the specification of start values and the appropriate ranges for model parameters. Overall model fit was determined using the likelihood-ratio Chi-square test (LRT). Calculated as the difference in the −2 log likelihood (−2LL) of two models, the LRT is distributed as a Chisquare with degrees of freedom equal to the difference in the number of parameters between the models. Non-significant LRT values (p >.05) indicate that a model does not result in a significant change in fit relative to the comparison model, and can therefore be considered as an accurate representation of, or good fit to, the data. In addition to the overall fit, models were compared using Akaike’s Information Criterion (AIC) and the Bayesian Information Criterion (BIC) (Akaike 1987; Schwartz 1978). Both statistics reflect the balances between goodness-of-fit and model parsimony, with the BIC also adjusting for sample size. Models with smaller/more negative AIC and BIC values are preferred. Based on Markon and Krueger (2004), when discrepancies between the statistics are observed, preference should be given to the BIC as it outperforms the AIC when dealing with more complex models and in larger samples.
Results
Table 2 presents the raw means and standard deviations, as well as the MZ and DZ cross-twin correlations for each phenotype examined. In addition, we present the standardized variance components (A, C, and E) for each phenotype as derived from the full Cholesky decomposition. Heritability estimates ranged from .19 for Life Satisfaction to .50 for PWB. Shared/common environmental influences contributed little to each phenotype, with the exception of the CESD total score (C = .13). In preliminary analyses univariate ACE models for each phenotype were all shown to provide good fits relative to saturated models, indicating that major assumptions of the twin design (i.e., equality of means and variances) were not in violation. Given that MZ correlations were more than twice the magnitude of the DZ correlations for four of the five phenotypes (which suggested the presence of non-additive genetic effects) we also fit an ADE Cholesky model to the data. Although some evidence for non-additive genetic effects was observed (estimates ranged from .13 to .32) the resulting A and D variance components were not significant based on 95 % confidence intervals for all of the phenotypes examined. Moreover, the fit of the ADE Cholesky relative to a multivariate saturated model was noticeably poorer than the fit of the ACE Cholesky based on LRT, AIC, and BIC values (results available upon request). We concluded that we were underpowered to adequately detect separate additive and non-additive effects, and proceeded with ACE models for the remaining analyses.
Table 2.
Descriptive statistics and standardized variance components derived from the ACE Cholesky decomposition
| Descriptive statistics | Cross-twin correlations |
Standardized variance components |
||||
|---|---|---|---|---|---|---|
| Mean (SD) | MZ | DZ | A | C | E | |
| Ryff psychological well-being | 4.73 (0.64) | .51 | .21 | .50 (.34; .58) | .01 (.00; .14) | .49 (.42; 57) |
| MPQ well-being | 7.72 (2.77) | .38 | .12 | .35 (.18; .45) | .02 (.00; .15) | .63 (.55; .72) |
| Life satisfaction | 7.61 (1.58) | .22 | .06 | .19 (.07; .28) | .02 (.00; .13) | .79 (.70; .88) |
| Self-esteem | 3.40 (0.48) | .40 | .18 | .35 (.19; .47) | .05 (.00; .19) | .60 (.52; .68) |
| CES-D depression | 8.30 (8.25) | .38 | .24 | .26 (.11; .41) | .13 (01; .26) | .61 (.53; .69) |
A additive genetic variance component, C common/shared environment variance component, E unique environment variance component
Multivariate twin analyses
Although the full ACE Cholesky was a good fit relative to the multivariate saturated model (LRT(80) = 98.33, p = .080, AIC = −61.67, BIC = −2495.31), we were able to improve substantially the fit of the model by constraining all common environmental influences to zero, with the exception of the influences specific to the CESD (Reduced Cholesky Model: LRT(94) = 104.29, p = .220, AIC = −83.71, BIC = −2515.15). All subsequent multivariate models were therefore tested assuming only additive genetic and non-shared environment covariance between the phenotypes, as well as common environmental influences limited to the CESD.
Table 3 presents the phenotypic, genetic and non-shared environmental correlations generated by the reduced Cholesky model for the five well- and ill-being measures. At the phenotypic level all five phenotypes were significantly correlated with one another. The absolute magnitude of these correlations (ignoring the sign) ranged from r = .70 between PWB and Self-Esteem, to r = .41 between Life Satisfaction and MPQWB. Genetic correlations among the measures were substantial, ranging in absolute magnitude from .59 to .95 and were all significant based on 95 % confidence intervals. The genetic correlations between PWB and self-esteem, PWB and the CESD, the CESD and self-esteem were particularly high (rG = .95, −.89, −.91 respectively) which suggests nearly complete genetic overlap among these three measures. Unique environmental correlations tended to be somewhat lower than genetic correlations,ranging in absolute magnitude from .27 to .50. The strongest unique environmental correlations were between PWB, self-esteem and the CESD. This suggests that the same type of life events would influence depressive symptoms, self-esteem and PWB.
Table 3.
Phenotypic, genetic, and environmental correlations from the reduced Cholesky model
| Ryff psychological well-being | MPQ well-being | Life satisfaction | Self-esteem | |
|---|---|---|---|---|
| Phenotypic correlations | ||||
| MPQ well-being | .51 (.46; .55) | |||
| Life satisfaction | .52 (.48; .56) | .41 (.36; .45) | ||
| Self-esteem | .70 (.67; .73) | .47 (.42; .51) | .45 (.41; .50) | |
| CES-D depression | −.58 (−.62; −.54) | −.41 (−.46; −.36) | −.49 (−.54; −.45) | −.53 (−.57; −.48) |
| Genetic correlations | ||||
| MPQ well-being | .72 (.61; .82) | |||
| Life satisfaction | .73 (.58; .90) | .79 (.59; .99) | ||
| Self-esteem | .95 (.89; 1.0) | .69 (.56; .81) | .72 (.53;. 91) | |
| CES-D depression | −.89 (−1.0; −.65) | −.74 (−.94; −.50) | −.80 (−.99; −.52) | −.91 (−1.0; −.66) |
| Unique environment correlations | ||||
| MPQ well-being | .35 (.26; .43) | |||
| Life satisfaction | .45 (.37; .53) | .27 (.18; .35) | ||
| Self-esteem | .50 (.42; .56) | .33 (.24; .41) | .36 (.28; .44) | |
| CES-D depression | −.48 (−.55; −.41) | −.30 (−.39; −.22) | −.45 (−.52; −.37) | −.40 (−.47; −.32) |
Table 4 presents the model fitting results for all independent pathway and common pathway models examined. Independent pathway models (models 1-8) were used to systematically test all possible variations in the genetic and environmental factor structures. These models indicated that the genetic covariance between the well-being phenotypes was best accounted for by two genetic factors, whereas only one factor was observed for the unique environmental influences (model 7). A model in which only one genetic and environmental factor were allowed (model 5) resulted in a significant reduction in fit relative to the Reduced Cholesky. Common pathway models (models 9-13) confirmed that a two-factor solution provided the best representation of the data. As with the independent pathway models, a one-factor common pathway model (model 9) resulted in a significant reduction in model fit relative to the reduced Cholesky. In contrast, a two-factor common pathway model (model 10) provided a good fit to the data. Reducing this model by fixing at zero parameters that accounted for less than 5 % of the variance in a phenotype (model 11) further improved the model fit, and resulted in the most negative AIC and BIC values of all models tested.
Table 4.
Multivariate model fitting results
| Model | −2LL | DF | LRT | ΔDF | p | AIC | BIC | |
|---|---|---|---|---|---|---|---|---|
| Independent pathway models | ||||||||
| 1. | 1 Genetic factor | 14911.04 | 6120 | 8.28 | 5 | .141 | −1.72 | −2519.16 |
| 2. | 2 Genetic factors | 14902.85 | 6116 | 0.09 | 1 | .765 | −1.91 | −2516.73 |
| 3. | 1 Unique environmental factor | 14909.25 | 6120 | 6.50 | 5 | .261 | −3.51 | −2520.05 |
| 4. | 2 Unique environmental factors | 14902.81 | 6116 | 0.05 | 1 | .818 | −1.95 | −2516.75 |
| 5. | 1 Genetic & 1 unique env. factor | 14924.83 | 6125 | 22.07 | 10 | .015 | 2.07 | −2520.41 |
| 6. | 2 Genetic and 2 unique env. factors | 14902.89 | 6117 | 0.12 | 2 | .942 | −3.88 | −2518.35 |
| 7. | 2 Genetic factors & 1 unique env. factor | 14909.62 | 6121 | 6.87 | 6 | .333 | −5.13 | −2521.49 |
| 8. | 1 Genetic factor & 2 unique env. factors | 14911.20 | 6121 | 8.45 | 6 | .207 | −3.55 | −2520.70 |
| Common pathway models | ||||||||
| 9. | 1 Factor | 14959.91 | 6129 | 57.15 | 14 | <.001 | 29.15 | −2509.39 |
| 10. | 2 Factors | 14907.57 | 6123 | 4.81 | 8 | .777 | −11.19 | −2525.78 |
| 11. | 2 Factors—reduced | 14911.03 | 6127 | 8.27 | 12 | .763 | − 15.73 | − 2530.57 |
| 12. | 2 Factors simple structure | 14927.67 | 6130 | 24.91 | 15 | .051 | −5.086 | −2527.14 |
| 13. | 2 Factors modified simple structure | 14919.95 | 6115 | 17.19 | 14 | .246 | −10.81 | −2529.37 |
Best-fitting model appears in bold. All models are tested against the fit of the reduced multivariate Cholesky. Models 12 and 13 are similar to the 2 Factors Reduced model in that residual genetic contributions to four of the five well-being phenotypes have been fixed at zero
−2LL negative 2 log-likelihood, DF degrees of freedom, LRT likelihood ratio test, p significance value of the LRT, AIC Akaike information criterion, BIC Bayesian information criterion, Env environmental
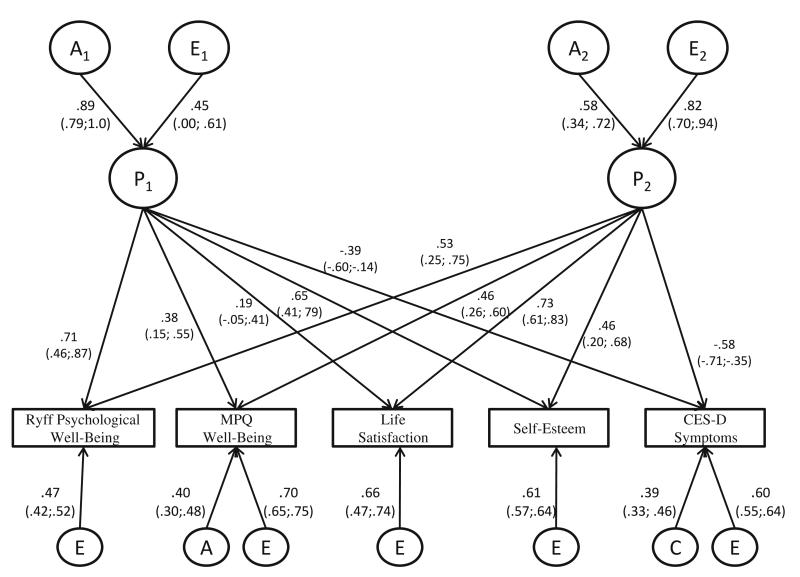
Standardized parameter estimates from model 11 are shown in Fig. 2. Additive genetic influences accounted for 79 % of the variance in the first latent factor (P1), with unique environmental influences accounted for the remaining variance (21 %). Although four of the five measures loaded significantly on this factor, it was most strongly associated with PWB and self-esteem. The second latent factor (P2) had a more modest heritability of .34; unique environmental influences accounted for a large amount of the variance (66 %). Life satisfaction and the CESD loaded most strongly on the second latent factor. Only MPQWB had significant specific residual genetic influences—accounting for 16 % of the phenotypic variance—which were not attributable to the two factors. In addition, all five measures had significant specific residual unique environmental influences not accounted for by the higher order factors; unique environmental influences include measurement error. These influences ranged from .49 (MPQWB) to .22 (PWB).
Fig. 2.
Standardized parameter estimates for the best-fitting multivariate model (Model 14). Circles with P1 and P2 represent the 2 factors; A1, E1 and A2, E2 represent the genetic (A) and unique environmental (E) influences (respectively) associated with each factor. Unnumbered circles with A, C and E represent genetic, common environmental and unique environmental influences specific to each measure. Standardized parameter estimates are beside each path with confidence intervals in parentheses. Squaring the parameter estimates yields the proportion of variance that is accounted for in the relative phenotype. Parameter estimates leading from the latent phenotypes to the measured variables are equivalent to factor loadings
In order to determine if a more simplified factor structure could be obtained, as well as if genetic and environmental correlations could be imposed between the latent phenotypes, we fit two additional variants of model 11. In model 12, we fit a simple factor structure by constraining at zero the smaller factor loading for each phenotype. Thus, PWB and self-esteem were allowed to load on first factor (P1), whereas MPQWB, life satisfaction, and CESD were allowed to load onto the second factor (P2). Genetic and environmental correlations between P1 and P2 were also freely estimated. These changes resulted in a substantial reduction in overall model fit, as well as poorer AIC and BIC values. In model 13, similar constraints and correlations were imposed; however, MPQWB was allowed to load onto both P1 and P2, given that these parameters were roughly similar to one another in the full two-factor model. This modified simple structure provided a good fit relative to the reduced Cholesky. Nevertheless, AIC and BIC values indicated that the model did not represent an improvement over model 11.
Discussion
Our results indicate that well-being is genetically and environmentally multidimensional, with two latent factors emerging from the multivariate analyses. These findings also provide evidence that ill- and well-being are not simply at opposite ends of a continuum, neither are they totally independent. The CESD loaded significantly on both latent factors, indicating that it is associated with each of these two dimensions of well-being in different ways. PWB and self-esteem loaded most strongly on the first factor, which also has the strongest genetic component (h2 = .79). This latent factor appears to involve predominantly self-appraisal (“I like most aspects of my personality;” “I take a positive attitude toward myself”). This first factor also seems to have a social comparative element to it that involves gauging oneself according to some yardstick for success or failure (e.g. “In many ways, I feel disappointed about my achievements in life”). The second latent factor had a modest but significant genetic component (h2 = .34) and was most strongly influenced by unique environmental effects. The life satisfaction measure loaded most highly on the second factor. We think of the second latent factor as representing global satisfaction, the feeling that the quality of one’s life on the whole is good. The strong unique environmental component of the second factor suggests that this component of well-being may vary more in response to life events. It may be that characteristics associated with the first factor, with its stronger genetic component account for the stability of well-being.
At first glance, the final model could be partly characterized in terms of the two dominant schools of thought about well-being: eudaimonia (first factor) and hedonia (second factor). However, with the exception of life satisfaction, all of the measures are influenced by genes associated with both latent factors. Thus, in keeping with findings at the phenotypic level (Ryan and Deci 2001; Ryff et al. 2006), the pattern of genetic and environmental influences indicates that these different types of well-being are connected but not entirely equivalent.
Evidence for multidimensionality was further supported by the additional measure-specific genetic influences on MPQWB and measure-specific common environmental influences on the CESD. The MPQWB also had significant loadings on both latent factors. This finding is consistent with the view that the measure includes elements of each of the other well-being measures. To our knowledge, some of the earliest evidence for the heritability of well-being came from a twin study of the MPQWB (Tellegen et al. 1988). The MPQ was developed within the empirical personality tradition as opposed to research focused specifically on well-being per se. It has not generally been included in multivariate studies of well-being, perhaps because its conceptualization of well-being is somewhat different from the constructs of hedonic or eudaimonic well-being. It does, however, represent a meaningful well-being construct that is likely to be useful in studies of well-being dimensions.
With regard to the jingle and jangle fallacies, given the degree of item overlap in the different measures, development of optimized well- and ill-being phenotypes would likely be improved by genetically-informed item-response theory analyses. A few examples of individual items strongly support the need for item-level analysis: (1) PWB: “I like most aspects of my personality”; Rosenberg Selfesteem: “I feel that I have a number of good qualities’; (2) PWB: “In many ways, I feel disappointed about my achievements in life”; CESD: “I thought my life had been a failure”; Rosenberg Self-esteem: “All in all, I am inclined to feel that I am a failure.” (3) PWB: “When I look at the story of my life, I am pleased with how things have turned out”; Rosenberg Self-esteem: “On the whole, I am satisfied with myself”; Life Satisfaction: “Worst/best possible life overall”.
Few genetic association studies have examined the genetics of well-being. However, the strong evidence for the heritability of well- and ill-being constructs does suggest that it should be possible to identify genes associated with well-being. A genome-wide linkage scan of 371 micro-satellite markers demonstrated a linkage signal for subjective happiness at two regions: marker D19S254 at 110 cM on chromosome 19 and marker D1S534 at 153 cM on chromosome 1q; these two linkage peaks did not overlap with regions found for depressive symptoms (Bartels et al. 2010). Our findings are consistent with the conclusion of Bartels et al. that the positive and negative spectrum of well-being may be influenced in part by independent genetic factors. Chakrabarti et al. found 4 SNPs in the cannabinoid receptor gene (CNR1) that modulate differential striatal responses to happy but not disgusted faces, suggesting that CNR1 may play a role in social reward responsivity, possibly influencing WB (Chakrabarti et al. 2006). The dopaminergic system has been linked with measures associated with both well- and ill-being (Sprangers et al. 2010; Sprangers and Schwartz 2008). Associations of oxytocin receptor genes with both positive and negative affect regulation have also been reported (Kim et al. 2010; Lucht et al. 2009; Saphire-Bernstein et al. 2011). Ill-being is often conceptualized as involving gene variants that are associated with sensitivity to stressful environmental conditions. However, the “differential sensitivity” framework of Belsky and colleagues (Belsky et al. 2009; Belsky and Pluess 2009) suggests that those same genes could be “well-being” genes in the presence of certain environments. In other words, greater sensitivity to the environment could promote well-being in supportive environments and ill-being under adverse conditions. Thus, it appears that there is substantial room for exploration of genes underlying well-being and this should be enhanced by the development of genetically optimized well-being phenotypes. Indeed, we suspect one impediment to success in gene finding could be the lack of clarity regarding the underlying genetic and environmental architecture of different well- and ill-being phenotypes.
Some limitations of the study should be considered. This sample included only middle-aged men from the United States so the results may not generalize to women, other age groups or other cultures (Oishi and Diener 2003). In addition, because there is substantial item overlap among well- and ill-being measures, further genetically-informative research is needed at an item level in order to distinguish whether correlations among measures reflect shared variances or tautologies based on item redundancy. Life Satisfaction was measured with one item only, and thus has unknown reliability which may affect estimates of its heritability.
In conclusion, an early study of the genetics of well-being (Lykken and Tellegen 1996) aroused concerns that efforts to improve well-being would be futile if well-being was “genetic.” Such nihilism is clearly unwarranted as there are also substantial environmental—and therefore potentially modifiable—environmental influences on these measures of well-being. Further study is needed to identify what genes and what kinds of environmental events most strongly affect different types of well- and ill-being. Here behavioral genetics studies (i.e. twin studies) can also make unique contributions to understanding the association between well- and ill-being because they allow us to differentiate environmental influences from genetic ones.
The study of the dimensionality of well- and ill-being has been limited in twin research on aging. The focus has been on depression, anxiety and distress, rather than more positive states. The paradox of well-being and the life course is that there is evidence for a “U” shaped curve for hedonic well-being across the life-course (Blanchflower and Oswald 2008; Stone et al. 2010); older adults tend to be higher in well-being than younger adults with the nadir occurring around age 50 (close to the age of this sample) for men in the United States (Yang 2008). One strength of the VETSA project is that it assessed multiple indicators of well-being in twin men starting right at this transitional period. Although no age effects were found in our narrow age range in these analyses, future VETSA longitudinal assessments may illuminate whether well-being is “U” shaped in this sample, what types of genetic factors and life events are associated with different well- and ill-being pathways, and the extent to which genetic and environmental factors influence changes in different aspects of well-being. Identification of the genetic and environmental multidimensionality of these related measures should permit the development of optimized measures of different components of well-being, help to identify common items or scales across studies allowing for improved analysis of predictors and outcomes of different types of well-being, and increase the likelihood of identifying genes and specific environmental influences associated with different components of well-being, potentially facilitating interventions. Our multivariate genetic analysis constitutes a step toward these goals.
Supplementary Material
Acknowledgments
The U.S. Department of Veterans Affairs has provided financial support for the development and maintenance of the Vietnam Era Twin (VET) Registry. Numerous organizations have provided invaluable assistance in the conduct of this study, including: Department of Defense; National Personnel Records Center, National Archives and Records Administration; Internal Revenue Service; National Opinion Research Center; National Research Council, National Academy of Sciences; the Institute for Survey Research, Temple University. Most importantly, the authors gratefully acknowledge the continued cooperation and participation of the members of the VET Registry and their families. This material is, in part, the result of work supported with resources of the VA San Diego Healthcare System. We also appreciate the time and energy of many staff and students without whom this study could not have been conducted. The project described was supported by awards AG018386, AG022381, AG022982 and AG018384 from the National Institute on Aging. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIA or the NIH.
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s10519-012-9538-x) contains supplementary material, which is available to authorized users.
Contributor Information
Carol E. Franz, Department of Psychiatry, University of California San Diego, MC0738, La Jolla, CA 92093, USA; Twin Research Laboratory, Center for Behavioral Genomics, University of California San Diego, La Jolla, CA, USA
Matthew S. Panizzon, Department of Psychiatry, University of California San Diego, MC0738, La Jolla, CA 92093, USA; Twin Research Laboratory, Center for Behavioral Genomics, University of California San Diego, La Jolla, CA, USA
Lindon J. Eaves, Virginia Institute for Psychiatric and Behavioral Genetics, Virginia Commonwealth University, Richmond, VA, USA
Wesley Thompson, Department of Psychiatry, University of California San Diego, MC0738, La Jolla, CA 92093, USA.
Michael J. Lyons, Department of Psychology, Boston University, Boston, MA, USA
Kristen C. Jacobson, Department of Psychiatry and Behavioral Neuroscience, University of Chicago, Chicago, IL, USA
Ming Tsuang, Department of Psychiatry, University of California San Diego, MC0738, La Jolla, CA 92093, USA; Center for Behavioral Genomics, University of California San Diego, La Jolla, CA, USA; Center of Excellence for Stress and Mental Health, VA San Diego Healthcare System, La Jolla, CA, USA; Institute of Genomic Medicine, University of California San Diego, La Jolla, CA, USA.
Stephen J. Glatt, Psychiatric Genetic Epidemiology & Neurobiology Laboratory (PsychGENe Lab), Departments of Psychiatry & Behavioral Sciences and Neuroscience & Physiology, Medical Genetics Research Center, SUNY Upstate Medical University, 750 East Adams Street, Syracuse, NY 13210, USA
William S. Kremen, Department of Psychiatry, University of California San Diego, MC0738, La Jolla, CA 92093, USA; Twin Research Laboratory, Center for Behavioral Genomics, University of California San Diego, La Jolla, CA, USA; Center of Excellence for Stress and Mental Health, VA San Diego Healthcare System, La Jolla, CA, USA
References
- Akaike H. Factor analysis and AIC. Psychometrika. 1987;52:317–332. [Google Scholar]
- Bartels M, Boomsma DI. Born to be happy? The etiology of subjective well-being. Behav Genet. 2009;39(6):605–615. doi: 10.1007/s10519-009-9294-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartels M, Saviouk V, de Moor MH, Willemsen G, van Beijsterveldt TC, Hottenga JJ, de Geus EJ, Boomsma DI. Heritability and genome-wide linkage scan of subjective happiness. Twin Res Hum Genet. 2010;13(2):135–142. doi: 10.1375/twin.13.2.135. [DOI] [PubMed] [Google Scholar]
- Belsky J, Pluess M. Beyond diathesis stress: differential susceptibility to environmental influences. Psychol Bull. 2009;135(6):885–908. doi: 10.1037/a0017376. [DOI] [PubMed] [Google Scholar]
- Belsky J, Jonassaint C, Pluess M, Stanton M, Brummett B, Williams R. Vulnerability genes or plasticity genes? Mol Psychiatry. 2009;14(8):746–754. doi: 10.1038/mp.2009.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergeman CS, Plomin R, Pedersen NL, McClearn GE. Genetic mediation of the relationship between social support and psychological well-being. Psychol Aging. 1991;6(4):640–646. doi: 10.1037//0882-7974.6.4.640. [DOI] [PubMed] [Google Scholar]
- Blanchflower DG, Oswald AJ. Is well-being U-shaped over the life cycle? Soc Sci Med. 2008;66(8):1733–1749. doi: 10.1016/j.socscimed.2008.01.030. [DOI] [PubMed] [Google Scholar]
- Block J. A contrarian view of the five-factor approach to personality description. Psychol Bull. 1995;117:187–215. doi: 10.1037/0033-2909.117.2.187. [DOI] [PubMed] [Google Scholar]
- Brim OG, Baltes PB, Bumpass LL, Cleary PD, Featherman DL, Hazzard WR, Kessler RC, Lachman ME, Markus HR, Marmot MG, Rossi AS, Ryff CD, Shweder RA. In: The midlife development inventory (MIDI) John D, Catherine T, editors. MacArthur Foundation Research Network on Successful Midlife Development (MIDMAC); Chicago: 1996. [Google Scholar]
- Caprara GV, Fagnani C, Alessandri G, Steca P, Gigantesco A, Cavalli Sforza LL, Stazi MA. Human optimal functioning: the genetics of positive orientation towards self, life, and the future. Behav Genet. 2009;39(3):277–284. doi: 10.1007/s10519-009-9267-y. [DOI] [PubMed] [Google Scholar]
- Carey G. Inference about genetic correlations. Behav Genet. 1988;18(3):329–338. doi: 10.1007/BF01260933. [DOI] [PubMed] [Google Scholar]
- Caspi A. The child is father of the man: personality continuities from childhood to adulthood. J Pers Soc Psychol. 2000;78(1):158–172. doi: 10.1037//0022-3514.78.1.158. [DOI] [PubMed] [Google Scholar]
- Caspi A, Begg D, Dickson N, Harrington H, Langley J, Moffitt TE, Silva PA. Personality differences predict health-risk behaviors in young adulthood: evidence from a longitudinal study. J Pers Soc Psychol. 1997;73(5):1052–1063. doi: 10.1037//0022-3514.73.5.1052. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention . MMWR CDC surveillance summaries. 2003. Public health and aging: trends in aging—United States and worldwide; pp. 101–106. [Google Scholar]
- Centers for Disease Control and Prevention . Health data for all ages. National Center for Health Statistics; 2007. [Google Scholar]
- Chakrabarti B, Kent L, Suckling J, Bullmore E, Baron-Cohen S. Variations in the human cannabinoid receptor (CNR1) gene modulate striatal responses to happy faces. Eur J Neurosci. 2006;23(7):1944–1948. doi: 10.1111/j.1460-9568.2006.04697.x. [DOI] [PubMed] [Google Scholar]
- Diener E. SINET. University of Illinois; 2005. Guidelines for national indicators of subjective well-being and ill-being; pp. 4–6. [Google Scholar]
- Diener E, Oishi S, Lucas RE. Personality, culture, and subjective well-being: emotional and cognitive evaluations of life. Annu Rev Psychol. 2003;54:403–425. doi: 10.1146/annurev.psych.54.101601.145056. [DOI] [PubMed] [Google Scholar]
- Diener E, Lucas RE, Scollon CN. Beyond the hedonic treadmill: revising the adaptation theory of well-being. Am Psychol. 2006;61(4):305–314. doi: 10.1037/0003-066X.61.4.305. [DOI] [PubMed] [Google Scholar]
- Eaves LJ, Last KA, Young PA, Martin NG. Model-fitting approaches to the analysis of human behavior. Heredity. 1978;41:249–320. doi: 10.1038/hdy.1978.101. [DOI] [PubMed] [Google Scholar]
- Eisen SA, True WR, Goldberg J, Henderson W, Robinette CD. The Vietnam Era Twin (VET) Registry: method of construction. Acta Geneticae Medicae et Gemellologiae. 1987;36:61–66. doi: 10.1017/s0001566000004591. [DOI] [PubMed] [Google Scholar]
- Eisen SA, Neuman R, Goldberg J, Rice J, True W. Determining zygosity in the Vietnam Era Twin Registry: an approach using questionnaires. Clin Genet. 1989;35:423–432. doi: 10.1111/j.1399-0004.1989.tb02967.x. [DOI] [PubMed] [Google Scholar]
- Federal Interagency Forum on Aging-Related Statistics . In: Older Americans 2004: key indicators of well-being. Statistics FIFoA-R, editor. U.S. Government Printing Office; Washington DC: 2004. [Google Scholar]
- Franz CE, York TP, Eaves LJ, Prom-Wormley E, Jacobson KC, Lyons MJ, Grant MD, Xian H, Panizzon MS, Jimenez E, Kremen WS. Adult romantic attachment, negative emotionality, and depressive symptoms in middle aged men: a multivariate genetic analysis. Behav Genet. 2011;41:488–498. doi: 10.1007/s10519-010-9428-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley EL. Interpretation of educational measurements. World; Yonkers, NY: 1927. [Google Scholar]
- Kendler KS, Heath AC, Martin NG, Eaves LJ. Symptoms of anxiety and depression in a volunteer twin population: the etiologic role of genetic and environmental factors. Arch Gen Psychiatry. 1987;43:213–221. doi: 10.1001/archpsyc.1986.01800030023002. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Myers JM, Maes HH, Keyes CL. The relationship between the genetic and environmental influences on common internalizing psychiatric disorders and mental well-being. Behav Genet. 2011;41(5):641–650. doi: 10.1007/s10519-011-9466-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Gilman SE, Thornton LM, Kendler KS. Health, well-being, and social responsibility in the MIDUS twin and sibling subsamples. In: Brim OG, Ryff CD, Kessler RC, editors. How healthy are we? A national study of well-being at mid-life. University of Chicago Press; Chicago: 2004. pp. 124–152. [Google Scholar]
- Keyes CL. The mental health continuum: from languishing to flourishing in life. J Health Soc Behav. 2002;43(2):207–222. [PubMed] [Google Scholar]
- Keyes CL, Shmotkin D, Ryff CD. Optimizing well-being: the empirical encounter of two traditions. J Pers Soc Psychol. 2002;82(6):1007–1022. [PubMed] [Google Scholar]
- Keyes CL, Myers JM, Kendler KS. The structure of the genetic and environmental influences on mental well-being. Am J Public Health. 2010;100(12):2379–2384. doi: 10.2105/AJPH.2010.193615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HS, Sherman DK, Sasaki JY, Xu J, Chu TQ, Ryu C, Suh EM, Graham K, Taylor SE. Culture, distress, and oxytocin receptor polymorphism (OXTR) interact to influence emotional support seeking. Proc Natl Acad Sci USA. 2010;107(36):15717–15721. doi: 10.1073/pnas.1010830107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremen WS, Thompson-Brenner H, Leung YJ, Grant MD, Franz CE, Eisen SA, Jacobson KC, Boake C, Lyons MJ. Genes, environment, and time: the Vietnam Era Twin Study of Aging (VETSA) Twin Research and Human Genetics. 2006;9:1009–1022. doi: 10.1375/183242706779462750. [DOI] [PubMed] [Google Scholar]
- Krueger RF, Caspi A, Moffitt TE. Epidemiological personology: the unifying role of personality in population-based research on problem behaviors. J Pers. 2000;68(6):967–998. doi: 10.1111/1467-6494.00123. [DOI] [PubMed] [Google Scholar]
- Lucht MJ, Barnow S, Sonnenfeld C, Rosenberger A, Grabe HJ, Schroeder W, Volzke H, Freyberger HJ, Herrmann FH, Kroemer H, Rosskopf D. Associations between the oxytocin receptor gene (OXTR) and affect, loneliness and intelligence in normal subjects. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33(5):860–866. doi: 10.1016/j.pnpbp.2009.04.004. [DOI] [PubMed] [Google Scholar]
- Lykken D, Tellegen A. Happiness is a stochastic phenomenon. Psychol Sci. 1996;7:186–189. [Google Scholar]
- Markon KE, Krueger RF. An empirical comparison of information-theoretic selection criteria for multivariate behavior genetic models. Behav Genet. 2004;3:593–610. doi: 10.1007/s10519-004-5587-0. [DOI] [PubMed] [Google Scholar]
- McArdle JJ, Goldsmith HH. Alternative common factor models for multivariate biometric analyses. Behav Genet. 1990;20:569–608. doi: 10.1007/BF01065873. [DOI] [PubMed] [Google Scholar]
- Mosing MA, Zietsch BP, Shekar SN, Wright MJ, Martin NG. Genetic and environmental influences on optimism and its relationship to mental and self-rated health: a study of aging twins. Behav Genet. 2009;39(6):597–604. doi: 10.1007/s10519-009-9287-7. [DOI] [PubMed] [Google Scholar]
- Mosing MA, Pedersen NL, Martin NG, Wright MJ. Sex differences in the genetic architecture of optimism and health and their interrelation: a study of Australian and Swedish twins. Twin Res Hum Genet. 2010;13(4):322–329. doi: 10.1375/twin.13.4.322. [DOI] [PubMed] [Google Scholar]
- Neale MC, Cardon LR. Methodology for genetic studies of twins and families. Kluwer Academic Publishers; Dordrecht: 1992. [Google Scholar]
- Neale MC, Boker SM, Xie G, Maes HH. Mx: statistical modeling. Department of Psychiatry. Medical College of Virginia; Richmond, VA: 2006. [Google Scholar]
- Nes RB, Czajkowski N, Roysamb E, Reichborn-Kjennerud T, Tambs K. Well-being and ill-being: shared environments, shared genes? The Journal of Positive Psychology. 2008;3:253–265. [Google Scholar]
- Nes RB, Czajkowski N, Tambs K. Family matters: happiness in nuclear families and twins. Behav Genet. 2010;40:577–590. doi: 10.1007/s10519-010-9365-x. [DOI] [PubMed] [Google Scholar]
- Nichols RC, Bilbro WCJ. The diagnosis of twin zygosity. Acta Genetica et Statistica Medica. 1966;16:265–275. doi: 10.1159/000151973. [DOI] [PubMed] [Google Scholar]
- Oishi S, Diener E. Culture and well-being: the cycle of action, evaluation, and decision. Pers Soc Psychol Bull. 2003;29(8):939–949. doi: 10.1177/0146167203252802. [DOI] [PubMed] [Google Scholar]
- Patrick CJ, Curtin JJ, Tellegen A. Development and validation of a brief form of the Multidimensional Personality Questionnaire. Psychol Assess. 2002;14:150–163. doi: 10.1037//1040-3590.14.2.150. [DOI] [PubMed] [Google Scholar]
- Plomin R, Scheier MF, Bergemen CS, Pedersen NL, Nesselroade JR, McClearn GE. Optimism, pessimism and mental health: a twin/adoption analysis. Personality Individ Differ. 1992;13:921–930. [Google Scholar]
- Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- Rosenberg M. The measurement of self-esteem. In: Rosenberg M (ed) Society and the adolescent self-image. Princeton University Press; Princeton: 1965. pp. 16–307. [Google Scholar]
- Roysamb E, Harris JR, Magnus P, Vitterso J, Tambs K. Subjective well-being: sex-specific effects of genetic and environmental factors. Pers Individ Differ. 2002;32:211–223. [Google Scholar]
- Roysamb E, Tambs K, Reichborn-Kjennerud T, Neale MC, Harris JR. Happiness and health: environmental and genetic contributions to the relationship between subjective well-being, perceived health, and somatic illness. J Pers Soc Psychol. 2003;85(6):1136–1146. doi: 10.1037/0022-3514.85.6.1136. [DOI] [PubMed] [Google Scholar]
- Rush BK, Barch DM, Braver TS. Accounting for cognitive aging: context processing, inhibition or processing speed? Aging Neuropsychol Cognit. 2006;13:588–610. doi: 10.1080/13825580600680703. [DOI] [PubMed] [Google Scholar]
- Ryan RM, Deci EL. On happiness and human potentials: a review of research on hedonic and eudaimonic well-being. Annu Rev Psychol. 2001;52:141–166. doi: 10.1146/annurev.psych.52.1.141. [DOI] [PubMed] [Google Scholar]
- Ryff CD. Happiness is everything, or is it? Explorations on the meaning of well-being. J Pers Soc Psychol. 1989;57:1069–1081. [Google Scholar]
- Ryff CD. Psychological well-being in adult life. Curr Dir Psychol Sci. 1995;4:99–104. [Google Scholar]
- Ryff CD, Keyes CL. The structure of psychological well-being revisited. J Pers Soc Psychol. 1995;69:719–727. doi: 10.1037//0022-3514.69.4.719. [DOI] [PubMed] [Google Scholar]
- Ryff CD, Dienberg Love G, Urry HL, Muller D, Rosenkranz MA, Friedman EM, Davidson RJ, Singer B. Psychological well-being and ill-being: do they have distinct or mirrored biological correlates? Psychother Psychosom. 2006;75(2):85–95. doi: 10.1159/000090892. [DOI] [PubMed] [Google Scholar]
- Saphire-Bernstein S, Way BM, Kim HS, Sherman DK, Taylor SE. Oxytocin receptor gene (OXTR) is related to psychological resources. Proc Natl Acad Sci USA. 2011;108(37):15118–15122. doi: 10.1073/pnas.1113137108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaie KW, Willis SL, Caskie GI. The Seattle Longitudinal Study: Relationship between personality and cognition. Aging Neuropsychol Cognit. 2004;11:304–324. doi: 10.1080/13825580490511134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schimmack U, Diener E, Oishi S. Life-satisfaction is a momentary judgment and a stable personality characteristic: the use of chronically accessible and stable sources. J Pers. 2002;70(3):345–384. doi: 10.1111/1467-6494.05008. [DOI] [PubMed] [Google Scholar]
- Schwartz G. Estimating the dimension of a model. Ann Stat. 1978;6:461–464. [Google Scholar]
- Snowden M, Dhingra SS, Keyes CL, Anderson LA. Changes in mental well-being in the transition to late life: findings from MIDUS I and II. Am J Public Health. 2010;100(12):2385–2388. doi: 10.2105/AJPH.2010.193391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprangers MA, Schwartz CE. Reflections on changeability versus stability of health-related quality of life: distinguishing between its environmental and genetic components. Health Qual Life Outcomes. 2008;6:89. doi: 10.1186/1477-7525-6-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprangers MA, Bartels M, Veenhoven R, Baas F, Martin NG, Mosing M, Movsas B, Ropka ME, Shinozaki G, Swaab D. Which patient will feel down, which will be happy? The need to study the genetic disposition of emotional states. Qual Life Res. 2010;19(10):1429–1437. doi: 10.1007/s11136-010-9652-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone AA, Schwartz JE, Broderick JE, Deaton A. A snapshot of the age distribution of psychological well-being in the United States. Proc Natl Acad Sci USA. 2010;107(22):9985–9990. doi: 10.1073/pnas.1003744107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stubbe JH, Posthuma D, Boomsma DI, De Geus EJ. Heritability of life satisfaction in adults: a twin-family study. Psychol Med. 2005;35(11):1581–1588. doi: 10.1017/S0033291705005374. [DOI] [PubMed] [Google Scholar]
- Tellegen A. Structure of mood and personality and their relevance to assessing anxiety, with an emphasis on self-report. In: Tuma AH, Maser JD, editors. Anxiety and the anxiety disorders. Erlbaum; Hillsdale: 1985. pp. 681–706. [Google Scholar]
- Tellegen A, Lykken DT, Bouchard TJ, Wilcox KJ, Segal N, Risch S. Personality similarity in twins reared apart and together. J Pers Soc Psychol. 1988;54:1031–1039. doi: 10.1037//0022-3514.54.6.1031. [DOI] [PubMed] [Google Scholar]
- Tsuang MT, Bar JL, Harley RM, Lyons MJ. The Harvard twin study of substance abuse: what we have learned. Harv Rev Psychiatry. 2001;9:267–279. [PubMed] [Google Scholar]
- Yang Y. Trends in U.S. adult chronic disease mortality, 1960-1999: age, period, and cohort variations. Demography. 2008;45(2):387–416. doi: 10.1353/dem.0.0000. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.