Abstract
Objective
Literature on the relationship of depressive symptoms and stress hormones after cancer diagnosis has been mixed, with some studies showing a relationship and other studies showing none. Time since diagnosis may explain these contradictory findings. This study examined the relationship of depressive symptoms to stress hormones in breast cancer patients using 12 month longitudinal data.
Methods
Patients with Stage II or III breast cancer (N=227) were assessed every 4 months from diagnosis/surgery to 12 months. They completed the Centers for Epidemiological Studies Depression scale (CES-D) Iowa Short Form and the Perceived Stress Scale (PSS) and blood samples were obtained to measure stress hormones (i.e., cortisol, adrenocorticotropin hormone (ACTH), norepinephrine, and epinephrine).
Results
Depressive symptoms were negatively related to cortisol levels (β=−0.023, p=0.002) but were positively related to rate of change in cortisol (β=0.003, p=0.003). Neither ACTH, epinephrine nor norepinephrine covaried with depressive symptoms (all ps>.05). When the CES-D and PSS were both used to predict cortisol, only the CES-D was significantly related (β= −.025, p=.017).
Conclusions
Depressive symptoms were negatively related to cortisol but this relationship changed from the time of diagnosis/surgery through 12 months. Cortisol may initially provide a buffering effect against depression during the stress of initial diagnosis and treatment but this relationship appears to change over time.
Keywords: Cancer, depression, stress, cortisol
Among women with breast cancer, 20–30% experience significant depressive symptoms (1). Depressive symptoms are associated with poorer survival (2). Alterations in the physiological stress response (such as differences in cortisol and the catecholamines) may be a pathway through which depressive symptoms affect disease course (3). Changes in the stress response have been documented in people with depression, such as increases in cortisol from the hypothalamic-pituitary-adrenal (HPA) axis and changes in the catecholamines, epinephrine, and norepinephrine. The majority of studies have shown no association between depressive symptoms and adrenocorticotropin hormone, however [ACTH, (4–6)].
Research from non-cancer populations may not apply to cancer patients due to stress response abnormalities associated with cancer diagnosis (7). Some cancer studies have shown positive relationships of morning cortisol and depressive symptoms (8, 9) but others have shown no relationship (10, 11). Depressive symptoms were more consistently related to evening cortisol (8, 11). Cortisol output has been found to be unrelated to depressive symptoms whereas cortisol slope was related to depressive symptoms (9–11). However, these data come from cross-sectional designs, and studying cancer patients across time could illuminate the source of the inconsistencies. The only longitudinal studies have been trials of psychological treatments in cancer patients, with some providing indirect evidence of the relationship of stress hormones and depression (12–14), although others showing no relationship (15–17).
This study investigates the longitudinal association of depressive symptoms with stress hormones in a sample of breast cancer patients. Previous research with this sample had shown that change over time in subjective stress was related to natural killer cell lysis (18). This suggests that the relationship of depressive symptoms and stress hormones may also change across time. In addition to investigating the relationship of depression and stress hormones, the potential role of subjective stress was examined. As depression and stress are related, these secondary analyses tested whether perceived stress was related to stress hormones and if perceived stress could account for the relationship of depression and stress hormones.
Methods
Participants and Procedures
The study was conducted following approval by the institutional review board. A convenience sample of patients (N=227) accrued between surgery and the start of adjuvant treatment and consented for participation in a randomized controlled trial (RCT) of a biobehavioral intervention; see previous report for a description of accrual procedures (19). Inclusion criteria included a recent diagnosis of stage II or III breast cancer. Exclusion criteria were refusal of cancer treatment, a diagnosis of mental retardation, untreated psychopathology (self-reported), or neurologic/immunological disorders. Participants were accrued between May 1994 and May 2000.
The mean of initial assessment was approximately 37 days (range: 14 to 101) following surgery. Blood was drawn between 8:00 and 11:00 am at each assessment to reduce diurnal variation. Patients were regularly instructed by the staff as well as study personnel to not eat after midnight though to have fluid intake. Participants sat for 10 minutes before blood collection. Catecholamines and ACTH were collected on 158 of the 227 participants as collection of catecholamines and ACTH began after the first 69 participants were accrued. Participants with catecholamine/ACTH data were more likely to be stage II than stage III (p=.026) and to have lumpectomy than mastectomy (p=.043). Blood was transported by courier and treated with heparin to prevent clotting. After transport, blood was centrifuged and serum was frozen (−80 degrees Celsius without preservatives and in 12×75 polypropylene tubes). Patients were then randomized to Intervention and Assessment or Assessment Only arms and the Intervention cohorts began treatment (20), which concluded at 12 months. Subsequent assessments occurred at 4, 8, and 12 months. As most women were undergoing active treatment and often visiting the medical centers, all assessments occurred during clinic visits and questionnaire and blood data were often collected during the same medical appointments (approximately 90% on the same day and another 4% within a week). Participants were recruited in 13 cohorts (waves) and endocrine analyses were run for each cohort within two weeks of the cohort completing the 12-month blood draw. All four samples for each cohort (baseline, 4-, 8- and 12-month) were run together. The intervention did not significantly affect depression in the entire sample but reduced depressive symptoms for those with high initial depressive symptoms (21).
Measures
Depressive symptoms
Depressive symptoms were measured using the Iowa short form (22) of the Center for Epidemiological Studies Depression Scale [CES-D, (23)]. The Iowa short form consists of 11 items from the full length (20 item) CES-D. Each item is rated on a 3-point scale from 0 = hardly ever or never to 2 = much or most of the time over the previous week. Total scores range from 0 to 22 and higher scores reflect greater depressive symptoms. Internal consistency was 0.74. Unlike other measures of depressive symptoms, the CES-D is relatively unaffected by physical symptoms and is, therefore, commonly used in research with medical patients (24). This short form of the CESD was also preferable to other measures as it includes only two physical symptoms, appetite and sleep changes.
Subjective stress
The Perceived Stress Scale (PSS) was used to measure subjective stress (25). The 10-item version was used and consists of 10 items measuring perceptions of one’s stress and ability to cope with stress (example item “How often have you felt nervous or stressed?”). Items are rated on a 0=never to 4= very often scale and, after reverse scoring for four items, are summed to create a total score in which total scores indicate greater perceptions of stress. The 10-item PSS has been shown to have adequate reliability and validity (26).
Cortisol
Cortisol levels in plasma were measured. All determinations were made using the Cortisol Coat-A-Count RIA (Diagnostic Products Corporation, Los Angeles, CA). Per the manufacturer, intra-assay variation is 4.3% and inter-assay variation is 5.2%. Sensitivity is 0.2 ug/dl. Normal levels for serum morning cortisol are between 5 and 23 ug/dl.
Adrenocorticotropin Hormone
ACTH was measured using the Immulite 1000 with reagents manufactured specifically for this instrument (Diagnostic Products Corporation, Los Angeles, CA). Per the manufacturer, intra-assay coefficient of variation is 5.6% and Inter-assay coefficient of variation is 7.8%. Sensitivity is 9 pg/ml. This assay was read and calculated with the System Luminometer 400 (Nichols Institute, San Clemente, CA).
Catecholamines
Norepinephrine and epinephrine determinations were made by HPLC with ElectroChemical Detection using Standards and Chemistry [Alumina extraction] purchased from ChromSystems, Munich, Germany (U.S. affiliate Thermo-Alko, Beverly, MA). C-18 Columns were purchased from the Waters Corporation (Waters Corporation, Milford, MA). Per manufacturer, intra-assay variation, inter-assay variation, and sensitivity for norepinephrine were 3%, 6%, and 15 pg/ml. For epinephrine, values were 6%, 13%, and 6 pg/ml.
Analytic Strategy
Hierarchical linear modeling was used to test the association of depressive symptoms with stress hormones and all analyses were conducted using SPSS. One model was constructed for each of the four, natural log transformed outcomes (cortisol, ACTH, norepinephrine, epinephrine). Outcome variables were log transformed consistent with recommendations that advise transformation due to skew in endocrine data (27). Depressive symptoms were time-varying and continuous in all models. The following variables were entered as controls: chemotherapy and radiation therapy (time-varying), estrogen and progesterone receptor status, time from surgery to baseline, age, income, and study arm. Unconditional growth models were constructed using linear and quadratic trajectories as the only predictors for each outcome. Quadratic effects were not included in subsequent models if the effects were not significant in the unconditional growth models. The quadratic effect was only significant for ACTH and was not included in the models for cortisol, epinephrine and norepinephrine. These models also included a random intercept coefficient and tested whether a random slope coefficient significantly improved model fit, however the random slope coefficient was not significant in any model. The random intercept was significant and was retained in all models. Then, a series of models were run using all control variables, depressive symptoms, and interaction terms with linear and, when indicated, quadratic trends. For significant effects, the antilog was used to transform coefficients back to regular unit values and the antilog was reported in text. We chose to use the continuous CESD score; a dichotomous variable created by a cut point would result in loss of power (28).
For significant main effects of depressive symptoms, secondary analyses examined the role of subjective stress. We first tested if perceived stress was related to stress hormones using the same hierarchical linear modeling outlined above; the same control variables were used. Second, both perceived stress and depressive symptoms were entered into a model to determine which variable significantly predicted the stress hormone outcome.
Results
The average participant was middle aged (mean age 50.58), white (90%), in a relationship (73%) and middle to high socioeconomic status (14.34 years of education, mean family income of $65,000/year). A slight majority had received radiation therapy (52%) and the majority received chemotherapy (87%). Table 1 lists the descriptive statistics for the endocrine measures as well as the psychological measures (CES-D and PSS). Table 2 lists the correlations of the CES-D with the log transformed stress hormones at each assessment. Non-parametric (Spearman’s rho) correlations of change in the CES-D from baseline to 12 months to change in the stress hormones from baseline to 12 months were as follows: cortisol −.026 (p=.74), ACTH .117 (p=.27), epinephrine .252 (p=.014), and norepinephrine −.085 (p=.41).
Table 1.
Means and standard deviations for measures of depressive symptoms, perceived stress and stress hormones across assessments. CES-D=Center for Epidemiological Studies Depression scale. PSS=10-item Perceived Stress Scale. Clinically significant distress cutoff for CESD was a score of 10.
| Baseline Mean (SD) |
4 months Mean (SD) |
8 months Mean (SD) |
12 months Mean (SD) |
|
|---|---|---|---|---|
| CES-D | 6.08 (3.69) | 4.90 (4.02) | 4.32 (3.77) | 4.12 (3.79) |
| Percent above clinical cutoff | 19.8% | 13.7% | 11.4% | 10.9% |
| PSS | 18.44 (6.99) | 15.69 (7.29) | 15.08 (6.80) | 14.92 (6.91) |
| Cortisol ug/dl | 11.02 (4.77) | 11.99 (5.44) | 13.18 (5.02) | 12.84 (5.18) |
| Median | 10.10 | 10.80 | 13.07 | 12.25 |
| ACTH pg/ml | 21.56 (11.72) | 18.71 (10.75) | 17.45 (9.27) | 18.62 (11.13) |
| Median | 19.75 | 14.95 | 14.30 | 15.95 |
| Epinephrine pg/ml | 29.31 (19.17) | 26.91 (15.70) | 26.32 (14.93) | 26.30 (15.37) |
| Median | 23.00 | 24.06 | 22.17 | 23.58 |
| Norepinephrine pg/ml | 330.79 (153.38) | 339.72 (169.44) | 372.44 (178.25) | 359.40 (177.33) |
| Median | 294.94 | 308.76 | 349.54 | 308.11 |
Table 2.
Non-parametric correlations of the natural log transformed stress hormones with the CES-D at each of the four assessments. CES-D=Center for Epidemiological Studies Depression Scale.
| Cortisol | ACTH | Epinephrine | Norepinephrine | |
|---|---|---|---|---|
| Baseline | ||||
| CES-D | −.082 | −.098 | −.042 | −.039 |
| 4-Month | ||||
| CES-D | −.109 | .030 | .001 | −.153 |
| 8-Month | ||||
| CES-D | −.060 | .068 | −.207* | −.055 |
| 12-Month | ||||
| CES-D | .002 | −.051 | −.248* | −.138 |
=p<.05
Primary Analyses
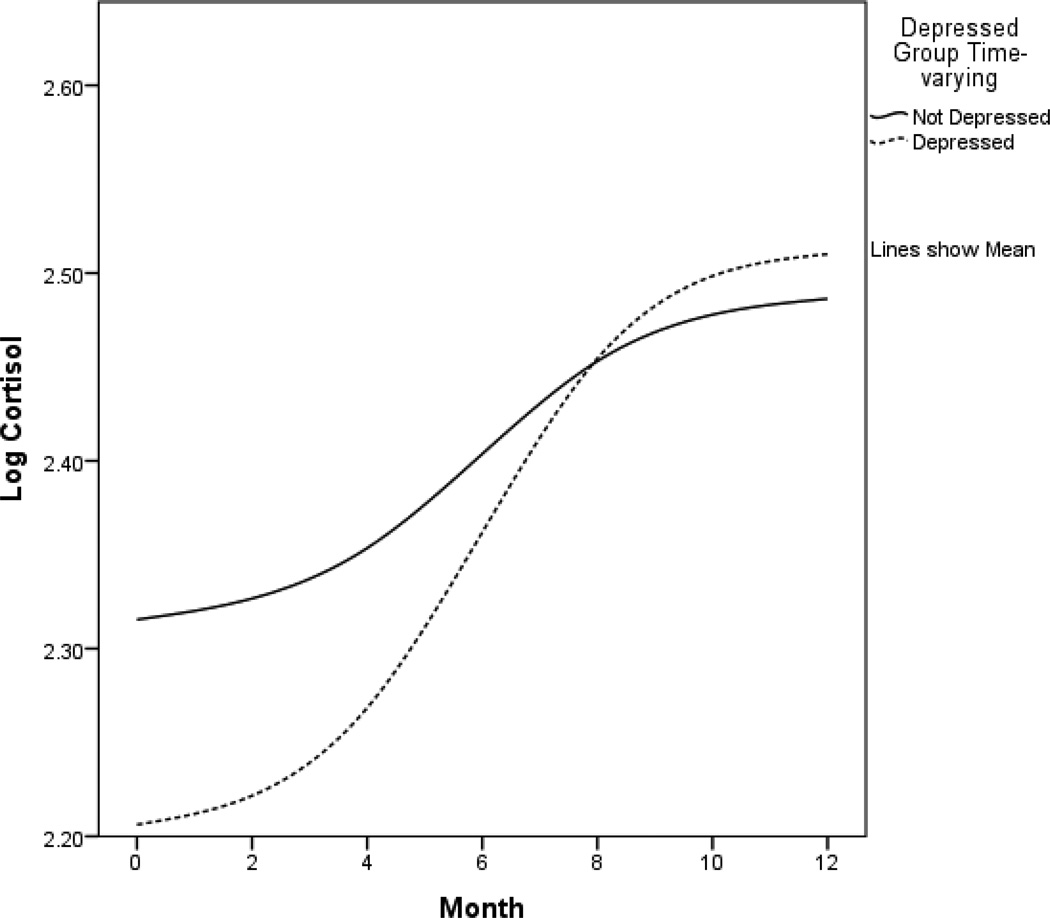
Table 3 summarizes the fixed effects from the final model for each outcome. For significant effects of depressive symptoms, the antilog was used to return the coefficients from Table 3 to the original units and are reported here in the text. Depressive symptoms and cortisol covaried, with higher depressive symptoms associated with lower cortisol levels (p=.002; antilog of coefficient from Table 3: −0.208, 95% CI: −0.252, −0.098). Also, depressive symptoms were positively associated with a linear rate of change in cortisol (p=.002; antilog of coefficient from Table 3: 0.004, 95% CI: 0.001, 0.005), indicating that higher depressive symptoms were associated with a more positive slope in cortisol over time. Figure 1 shows how the relationship of depressive symptoms and cortisol change over time. For graphical displays, a cutpoint of 10 and above was used to indicate significant depressive symptoms (29) although the continuous measure of depressive symptoms was used in all statistical analyses. Time-varying CES-D scores were used to create the groups in Figure 1. Although only linear change was included in the analyses, the lines in the graphs are not straight due to the changing effect of depressive symptoms over time and smoothing of the interpolation line. We also ran additional multiple regressions at each timepoint (baseline, 4, 8, 12), predicting both the natural log of cortisol (lnCort) and the level of cortisol predicted by the mixed effects model (pred) from CESD (with covariates listed above) to demonstrate that the CESD and cortisol were more strongly related at baseline than at later timepoints. Results indicated that the CESD and cortisol were most strongly related at baseline (lnCort: −.018, p=.065, CI: −.037, .001; pred: −.020, p<.001, CI: −.026, −.013) and 4 months (lnCort: −.020, p=.092, CI: −.043, .003; pred: −.015, p<.001, CI: −.022, −.008) than 8 months (lnCort: .001, p=.90, CI: −.015, .017; pred: −.001, p=.71, CI: −.008, .006) or 12 months (lnCort: .005, p=.50, CI: −.010, .021; pred: .006, p=.10, CI: −.001, .013).
Table 3.
Summary of fixed effects for final models of stress hormone trajectories.
| Effect | Estimate | P-value | 95% Confidence interval |
|---|---|---|---|
| Cortisol | |||
| Intercept | 2.211 | <0.001 | (1.911, 2.510) |
| Linear | 0.031 | 0.243 | (−0.021, 0.084) |
| CES-D | −0.023 | 0.002 | (−0.038, −0.008) |
| CES-D×Linear | 0.003 | 0.002 | (0.001, 0.005) |
| ACTH | |||
| Intercept | 3.010 | <0.001 | (2.465, 3.554) |
| Linear | −0.078 | 0.407 | (−0.262, 0.107) |
| Quadratic | 0.004 | 0.633 | (−0.013, 0.021) |
| CES-D | −0.011 | 0.361 | (−0.034, 0.012) |
| CES-D×Linear | 0.005 | 0.211 | (−0.003, 0.012) |
| CES-D×Quadratic | −0.0003 | 0.341 | (−0.001, 0.0003) |
| Epinephrine | |||
| Intercept | 3.389 | <0.001 | (2.984, 3.795) |
| Linear | −0.047 | 0.198 | (−0.118, 0.024) |
| CES-D | 0.003 | 0.778 | (−0.018, 0.024) |
| CES-D×Linear | −0.002 | 0.065 | (−0.005, 0.0001) |
| Norepinephrine | |||
| Intercept | 5.486 | <0.001 | (5.186, 5.787) |
| Linear | 0.071 | 0.007 | (0.019, 0.122) |
| CES-D | −0.004 | 0.569 | (−0.020, 0.011) |
| CES-D×Linear | −0.0002 | 0.797 | (−0.002, 0.002) |
The parameter estimates were calculated using hierarchical linear modeling and maximum likelihood estimation.
Outcomes are the natural log of the stress hormone and values reported in the table are for the original models of the transformed outcomes. Significant effects of depressive symptoms were back-transformed into the original units and are reported in the text. Controls are not shown for clarity. The linear term refers to monthly changes since the baseline assessment. CES-D=Center for Epidemiological Studies Depression Scale.
Figure 1.
Log-transformed cortisol trajectories. The y-axis refers to predicted cortisol level. Low and high depressive symptom groups were created using a cutpoint of 10 on the time-varying CES-D (CES-D at each assessment was used to create groups for that assessment) and are presented for graphical purposes only. The following variables were controls in all analyses: chemotherapy and radiation therapy, estrogen and progesterone receptor status, time from surgery to baseline, age, income, and study arm (intervention vs. assessment only). Sample sizes for the groups at each timepoint were as follows: baseline not depressed n=182, baseline depressed n=45; 4 months not depressed n=170, 4 months depressed n=27; 8 months not depressed n=171, 8 months depressed n=22; 12 months not depressed n=164, 12 months depressed n=20. CES-D=Center for Epidemiological Studies Depression Scale.
Depressive symptoms were not associated with ACTH levels (p=.31), linear rate of change in ACTH (p=.24) or quadratic rate of change in ACTH (p=.40), indicating that depressive symptoms were unrelated to the level of ACTH or slope of ACTH across time. Depressive symptoms were not associated with norepinephrine levels (p=.47) nor associated with differences in slope or linear rate of change in norepinephrine during this time period (p=.78).
Depressive symptoms were not associated with epinephrine levels (p=.56). Higher depressive symptoms showed a trend of being negatively associated with linear rate of change (slope) in epinephrine, indicating a more negative slope in epinephrine over time was associated with higher depressive symptoms (p=.065; antilog of coefficient from Table 3: −0.002, 95% CI: −0.005, 0.0001). However, this effect did not reach significance.
Secondary Analyses
As depressive symptoms were significantly associated with cortisol, secondary analyses first considered the relationship of subjective stress to cortisol. Subjective stress showed a trend towards significance when predicting cortisol (β= −.007, t=−1.760, p=.079) and change in cortisol over time (β=.001, t=1.870, p=.062). Secondly, when both depression and subjective stress were variables in the model, depressive symptoms were significantly related to overall level of cortisol (β=−.025, t=−2.390, p=.017) and were significantly related to linear change (slope) of cortisol (β=.003, t=2.233, p=.026). However, subjective stress was not related to overall level of cortisol (β=.003, t=.455, p=.65) nor linear change of cortisol over time (β=−.0003, t=−.346, p=.73).
Discussion
The present study investigated the covariation of stress hormones with depressive symptoms in a sample of breast cancer patients in the year following diagnosis and surgery. We confirmed our hypothesis that the relationship of depressive symptoms and stress hormones changed over time. Depressive symptoms were related to rate of change in cortisol but were unrelated to ACTH, epinephrine and norepinephrine. Secondary analyses suggested the relationship of depressive symptoms to cortisol was not accounted for by any relationship to subjective stress.
Results suggest depressive symptoms are initially related to lower cortisol and that the first year post-diagnosis is a period of change in the relationship of depressive symptoms and the levels of morning cortisol. Some research has suggested that heightened cortisol during stress can buffer against negative affect (30). As stress was highest near diagnosis in this sample (18), morning cortisol appeared to provide a buffering effect against depressive symptoms during this high stress time but the buffering effect faded over the first year as stress decreased. Depressive symptoms were also associated with greater increases in cortisol. As indicated by Figure 1 and the significant change in slope associated with depressive symptoms, the relationship of depressive symptoms to cortisol became weaker over time, consistent with cortisol providing a buffering effect only during initial stress. These results suggest that the relationship of depressive symptoms to stress hormones is in flux in the year following diagnosis.
Results showed ACTH, epinephrine and norepinephrine were unrelated to depressive symptoms, unlike cortisol. Past research on depressive symptoms and ACTH has shown disparate results (4). Even chronic stress (e.g., traumatic events, caregiving) does not have a consistent relationship to ACTH (31). For epinephrine and norepinephrine, some evidence suggests peripheral catecholamines may be elevated in people with depressive symptoms (6, 32) but the effect of an additional stressor (breast cancer treatment) may have hidden any association with norepinephrine or epinephrine.
The data are considered within the limitations of the study. The study was conducted within high volume, stressful oncology clinics. The majority of the sample was receiving chemotherapy so blood collection for count monitoring was routine. We were able to achieve appointments for the study patients in the morning hours, albeit not a specific time and time of awakening on the day of the blood draws was not available. We anticipate compliance with instructions to not eat before the blood draw, but do not know so for a fact. Our single assessment results for morning cortisol may differ when diurnal cortisol slope and afternoon and evening measures are used. We used serum collection for the catacholamines. A more general catecholamine measure could be achieved with 24-hour urine collection (35). While the latter is feasible in some settings, it was not in this clinical context. It is difficult to know how robust the effects reported are. The design employed a depressive symptom measure and the large and homogeneous sample increased power. Other designs, such as contrasting smaller groups comprised of patients with or without a diagnosis of major depression (4) or more heterogeneous groups, would be a useful compliment to these findings.
In summary, the results suggest that changes in the stress response may be one mechanism through which depressive symptoms affect health and outcomes in breast cancer survivors (33). The physiological stress response can influence cancer outcome through immune function (3) as well as more directly (34). The study showed a negative relationship between stress hormones and depressive symptoms that differ from previous research (8–11) in cancer samples. However, previous cancer studies were conducted at a variety of points after diagnosis whereas this study spanned the first year. Essentially, the changes in cortisol related to depressive symptoms were decreased amounts of cortisol during a stressful time period and not elevation of morning cortisol. Overall, the relationships between psychological variables such as depressive symptoms and stress hormones are neither obvious nor simple, though they are important for understanding stress and coping in both the healthy and those with disease.
Acknowledgments
We thank the patients of the Stress and Immunity Breast Cancer Project for their participation and commitment and the research staff, especially Dale Kiss, and graduate and undergraduate assistants for their expertise and many contributions. The OSU Clinical Research Center also provided important assistance.
Sources of support: National Institute of Mental Health (R01MH51487), National Cancer Institute (R01CA92704, K05 CA098133, KA24 CA93670, P01 CA95426), with additional support from the American Cancer Society (PBR-89 and PF-07-169-01-CPPB), the Longaberger Company-American Cancer Society (PBR-89A), the U.S. Army Medical Research Acquisition Activity (DAMD17-94-J-4165, DAMD17-96-1-6294, and DAMD17-97-1-7062), the OSU Comprehensive Cancer (P30 CA16058), and the Walther Cancer Institute.
Abbreviations
- ACTH
adrenocorticotropin hormone
- CES-D
Centers for Epidemiological Studies Depression scale
Footnotes
Conflict of Interest Statement: The authors have no declared conflicts of interest.
References
- 1.Zabora J, BrintzenhofeSzoc K, Curbow B, Hooker C, Piantadosi S. The prevalence of psychological distress by cancer site. Psychooncol. 2001;10:19–28. doi: 10.1002/1099-1611(200101/02)10:1<19::aid-pon501>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 2.Pinquart M, Duberstein PR. Depression and cancer mortality: a meta-analysis. Psychol Med. 2010:1–14. doi: 10.1017/S0033291709992285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reiche EM, Morimoto HK, Nunes SM. Stress and depression-induced immune dysfunction: implications for the development and progression of cancer. Int Rev Psychiatry. 2005;17:515–527. doi: 10.1080/02646830500382102. [DOI] [PubMed] [Google Scholar]
- 4.Holsboer F. The corticosteroid receptor hypothesis of depression. Neuropsychopharmacol. 2000;23:477–501. doi: 10.1016/S0893-133X(00)00159-7. [DOI] [PubMed] [Google Scholar]
- 5.Koslow SH, Maas JW, Bowden CL, Davis JM, Hanin I, Javaid J. CSF and urinary biogenic amines and metabolites in depression and mania. A controlled, univariate analysis. Arch Gen Psychiat. 1983;40:999–1010. doi: 10.1001/archpsyc.1983.01790080081011. [DOI] [PubMed] [Google Scholar]
- 6.Wong ML, Kling MA, Munson PJ, Listwak S, Licinio J, Prolo P, Karp B, McCutcheon IE, Geracioti TD, Jr, DeBellis MD, Rice KC, Goldstein DS, Veldhuis JD, Chrousos GP, Oldfield EH, McCann SM, Gold PW. Pronounced and sustained central hypernoradrenergic function in major depression with melancholic features: relation to hypercortisolism and corticotropin-releasing hormone. Proc Natl Acad Sci U S A. 2000;97:325–330. doi: 10.1073/pnas.97.1.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abercrombie HC, Giese-Davis J, Sephton S, Epel ES, Turner-Cobb JM, Spiegel D. Flattened cortisol rhythms in metastatic breast cancer patients. Psychoneuroendocrino. 2004;29:1082–1092. doi: 10.1016/j.psyneuen.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 8.Jehn CF, Kuehnhardt D, Bartholomae A, Pfeiffer S, Krebs M, Regierer AC, Schmid P, Possinger K, Flath BC. Biomarkers of depression in cancer patients. Cancer. 2006;107:2723–2729. doi: 10.1002/cncr.22294. [DOI] [PubMed] [Google Scholar]
- 9.Sephton SE, Dhabhar FS, Keuroghlian AS, Giese-Davis J, McEwen BS, Ionan AC, Spiegel D. Depression, cortisol, and suppressed cell-mediated immunity in metastatic breast cancer. Brain Behav Immun. 2009;23:1148–1155. doi: 10.1016/j.bbi.2009.07.007. [DOI] [PubMed] [Google Scholar]
- 10.Carlson LE, Campbell TS, Garland SN, Grossman P. Associations among salivary cortisol, melatonin, catecholamines, sleep quality and stress in women with breast cancer and healthy controls. J Behav Med. 2007;30:45–58. doi: 10.1007/s10865-006-9082-3. [DOI] [PubMed] [Google Scholar]
- 11.Lutgendorf SK, Weinrib AZ, Penedo F, Russell D, DeGeest K, Costanzo ES, Henderson PJ, Sephton SE, Rohleder N, Lucci JA, 3rd, Cole S, Sood AK, Lubaroff DM. Interleukin-6, cortisol, and depressive symptoms in ovarian cancer patients. J Clin Oncol. 2008;26:4820–4827. doi: 10.1200/JCO.2007.14.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Antoni MH, Lehman JM, Kilbourn KM, Boyers AE, Culver JL, Alferi SM, Yount SE, McGregor BA, Arena PL, Harris SD, Price AA, Carver CS. Cognitive-behavioral stress management intervention decreases the prevalence of depression and enhances benefit finding among women under treatment for early-stage breast cancer. Health Psychol. 2001;20:20–32. doi: 10.1037//0278-6133.20.1.20. [DOI] [PubMed] [Google Scholar]
- 13.Hernandez-Reif M, Ironson G, Field T, Hurley J, Katz G, Diego M, Weiss S, Fletcher MA, Schanberg S, Kuhn C, Burman I. Breast cancer patients have improved immune and neuroendocrine functions following massage therapy. J Psychosom Res. 2004;57:45–52. doi: 10.1016/S0022-3999(03)00500-2. [DOI] [PubMed] [Google Scholar]
- 14.Phillips KM, Antoni MH, Lechner SC, Blomberg BB, Llabre MM, Avisar E, Gluck S, DerHagopian R, Carver CS. Stress Management Intervention Reduces Serum Cortisol and Increases Relaxation During Treatment for Nonmetastatic Breast Cancer. Psychosom Med. 2008;70:1044–1049. doi: 10.1097/PSY.0b013e318186fb27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carlson LE, Speca M, Patel KD, Goodey E. Mindfulness-based stress reduction in relation to quality of life, mood, symptoms of stress and levels of cortisol, dehydroepiandrosterone sulfate (DHEAS) and melatonin in breast and prostate cancer outpatients. Psychoneuroendocrino. 2004;29:448–474. doi: 10.1016/s0306-4530(03)00054-4. [DOI] [PubMed] [Google Scholar]
- 16.Nunes DFT, Rodriguez AL, Hoffmann FD, Luz C, Braga APF, Muller MC, Bauer ME. Relaxation and guided imagery program in patients with breast cancer undergoing radiotherapy is not associated with neuroimmunomodulatory effects. J Psychosom Res. 2007;63:647–655. doi: 10.1016/j.jpsychores.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 17.vanderPompe G, Duivenvoorden HJ, Antoni MH, Visser A, Heijnen CJ. Effectiveness of a short-term group psychotherapy program on endocrine and immune function in breast cancer patients: An exploratory study. J Psychosom Res. 1997;42:453–466. doi: 10.1016/s0022-3999(96)00393-5. [DOI] [PubMed] [Google Scholar]
- 18.Thornton LM, Andersen BL, Crespin TR, Carson WE. Individual trajectories in stress covary with immunity during recovery from cancer diagnosis and treatments. Brain Behav Immun. 2007;21:185–194. doi: 10.1016/j.bbi.2006.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Andersen BL, Farrar WB, Golden-Kreutz DM, Glaser R, Emery CF, Crespin TR, Shapiro CL, Carson WE., 3rd Psychological, behavioral, and immune changes after a psychological intervention: A clinical trial. J Clin Oncol. 2004;22:3570–3580. doi: 10.1200/JCO.2004.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Andersen BL, Golden-Kreutz DM, Emery CF, Thiel DL. Biobehavioral intervention for cancer stress: Conceptualization, components, and intervention strategies. Cogn Behav Pract. 2009;16:253–265. [Google Scholar]
- 21.Thornton LM, Andersen BL, Schuler TA, Carson WE. A psychological intervention reduces inflammatory markers by alleviating depressive symptoms: secondary analysis of a randomized controlled trial. Psychosom Med. 2009;71:715–724. doi: 10.1097/PSY.0b013e3181b0545c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kohout FJ, Berkman LF, Evans DA, Cornoni-Huntley J. Two shorter forms of the CES-D Depression Symptoms Index. Journal of Aging and Health. 1993;5:179–193. doi: 10.1177/089826439300500202. [DOI] [PubMed] [Google Scholar]
- 23.Radloff LS. The CES-D Scale: A self-report depression scale for research in the general population. Appl Psych Meas. 1977;1:385–401. [Google Scholar]
- 24.Devins GM, Orme CM, Costello CG, Binik YM, et al. Measuring depressive symptoms in illness populations: Psychometric properties of the Center for Epidemiologic Studies Depression (CES-D) scale. Psychology & Health. 1988;2:139–156. [Google Scholar]
- 25.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24:385–396. [PubMed] [Google Scholar]
- 26.Cohen S, Williamson G. Perceived stress in a probability sample of the United States. In: Oskamp SSS, editor. The social psychology of health. Newbury Park, CA: Sage; 1988. pp. 31–67. [Google Scholar]
- 27.Nicolson N. Handbook of Physiological Research Methods in Health Psychology. Thousand Oaks, CA: Sage Publications; 2007. Measurement of cortisol; pp. 37–62. [Google Scholar]
- 28.Cohen J. The cost of dichotomization. Applied Psychological Measurement. 1983;7:249–253. [Google Scholar]
- 29.Andresen EM, Carter WB, Malmgren JA, Patrick DL. Screening for depression in well older adults: Evaluation of a short form of the CES-D. Am J Prev Med. 1994;10:77–84. [PubMed] [Google Scholar]
- 30.Het S, Schoofs D, Rohleder N, Wolf OT. Stress-induced cortisol level elevations are associated with reduced negative affect after stress: indications for a mood-buffering cortisol effect. Psychosom Med. 2012;74:23–32. doi: 10.1097/PSY.0b013e31823a4a25. [DOI] [PubMed] [Google Scholar]
- 31.Miller GE, Chen E, Zhou ES. If It Goes Up, Must It Come Down? Chronic Stress and the Hypothalamic-Pituitary-Adrenocortical Axis in Humans. Psychol Bull. 2007;133:25–45. doi: 10.1037/0033-2909.133.1.25. [DOI] [PubMed] [Google Scholar]
- 32.Koslow SH, Maas JW, Bowden CL, Davis JM, Hanin I, Javaid J. CSF and urinary biogenic amines and metabolites in depression and mania. A controlled, univariate analysis. Archives of General Psychiatry. 1983;40:999–1010. doi: 10.1001/archpsyc.1983.01790080081011. [DOI] [PubMed] [Google Scholar]
- 33.Gidron Y, Ronson A. Psychosocial factors, biological mediators, and cancer prognosis: a new look at an old story. Current Opin Oncol. 2008;20:386–392. doi: 10.1097/CCO.0b013e3282fbcd0d. [DOI] [PubMed] [Google Scholar]
- 34.Moran TJ, Gray S, Mikosz CA, Conzen SD. The glucocorticoid receptor mediates a survival signal in human mammary epithelial cells. Cancer Res. 2000;60:867–872. [PubMed] [Google Scholar]
- 35.Dimsdale JE, Ziegler MG. What do plasma and urinary measures of catecholamines tell us about human response to stressors? Circulation. 1991;83:II36–II42. [PubMed] [Google Scholar]