Abstract
Background
The findings form studies on the relationship between vitamin D and type 2 diabetes were inconsistent.
Objectives
To elucidate the association between vitamin D consumption and type 2 diabetes risk by conducting a meta-analysis.
Methods
We conducted a systematic literature search to identify prospective cohort studies of vitamin D intake and type 2 diabetes risk prior to November 2012. Eligible studies were retrieved via both computer searches and manual review of references. The summary risk estimates were calculated based on the highest versus the lowest categories.
Results
Meta-analysis of 4 prospective cohort studies involving 187, 592 participants and 9, 456 incident cases showed an absence of significant association between total vitamin D intake and type 2 diabetes risk. The combined RR was 0.93 (95% CI: 0.85–1.01). The associations were similar for subgroup analyses, a combined RR respectively was 0.94 (95% CI: 0.77–1.08), 0.91 (95% CI: 0.77–1.08), 0.93 (95% CI: 0.84–1.02), and 0.92 (95% CI: 0.84–1.01) for the intake of dietary vitamin D, supplemental vitamin D, total vitamin D in USA and total vitamin D for women only.
Conclusions
Our results support that there was no association between vitamin D intake and type 2 diabetes.
Keywords: Vitamin D, Diet, Type 2 Diabetes, Meta-analysis
Introduction
Diabetes mellitus is a group of metabolic diseases in which a person is characterized by high blood sugar producing the classical symptoms of frequent urination, increased thirst and increased hunger1. Type 2 diabetes is caused by insulin resistance, a disease in which cells do not respond to the insulin properly, sometimes coupled with relatively insulin deficiency2. Type 2 diabetes mellitus is one of the 10 most prevalent diagnosed diseases in a representative US population of men older than 50 years of age, with a further increase of average 7 million people affected by diabetes each year3. There will be approximately 438 million developing diabetes by 2030, accounting for 4.4% of all age groups worldwide4. Smoking, ageing, obesity and physical inactivity are the wellknown risk factors for diabetes 5. Currently, feasible preventive measures of this disease remain limited. Vitamin D is a group of fat-soluble prohormones enhancing the absorption and metabolism of calcium and phosphorous. A large number of evidence indicated that vitamin D might have important roles in cardiovascular disease, autoimmune disorders, cancers and type 2 diabetes6, 7. In the past few years, the roles of vitamin D in the etiology of type 2 diabetes have received considerable attentions in research fields. There is mounting evidence from cross-sectional studies8, 9, or even stronger study designs such as randomized controlled trial studies (RCTs)6. As we know, a cohort study is undertaken to support the existence of association between suspected cause and disease achieving a more and long-term result, while a RCT study often needs to be end at the scheduled termination time, and the follow-up time is often shorter. However, findings from prospective cohort studies on the association between vitamin D and Type 2 diabetes are inconsistent10–13. To the best of our knowledge, there is no a comprehensive assessment of the relation between vitamin D intakes and type 2 diabetes risks by summarizing the prospective cohort studies.
In response, we conducted this first meta-analysis by pooling together the results from all published prospective cohort studies. Our purpose was to examine the potential association between vitamin D intake and risk of type 2 diabetes.
Methods
Literature Search
We attempted to conduct this study in line with the Meta-analysis of Observational Studies in Epidemiology guidelines for meta-analyses of observational studies14. Our literature search was conducted with a systematic literature search at November 2012, which included MEDLINE, PubMed, Science Direct, Springer link, EMBASE and Chinese National Knowledge Infrastructure (CNKI). The keywords of our search were as following:
#1. (vitamin D) OR (vit D) OR VD OR ergocalciferol*
#2. (Type 2 diabetes) OR diabetes mellitus OR T2DM OR (impaired glucose tolerance) OR (impaired fasting glucose) OR (abnormal glucose metabolism)
#3. #1 and #2
The search was limited on human studies, without any other restriction. References listed in the searched papers were used for additional screening of relevant data. This process was performed by two investigators independently. Any disagreement among the investigators was resolved by consensus. When necessary, we contacted the authors of original studies for additional data. Furthermore, we have also searched the Cochrane Online, the Clinical Trials Online and contacted the organization of Chinese medical doctorate dissertation database to acquire related unpublished reports.
Inclusion and exclusion criteria
The study can be included as a candidate if it met the following criteria: 1) It is an original prospective cohort study on human subject which have mentioned the association between vitamin D and type 2 diabetes; 2) The primary outcome clearly was defined as type 2 diabetes; 3) Only the most recent study would be included if multiple publications were on the same population study or the same results were published in different journals; 4) The studies provided relative risk (RR) and its 95% confidence interval (95% CI), or studies had raw data available in the paper for calculating these parameters; 5) The RR and the corresponding 95% CI extracted from the literature were compared to obtain the highest and the lowest amount of vitamin D intake; 6) In the included studies, the participants should generally be healthy people in the researcher's region; 7) As we know, the association between type 2 diabetes and vitamin D intake was a slow-progressed effect, a short term follow-up may not expose such an effect. Based on the original articles, we therefore have defined that the follow-up period of cohort participants should not be short than five years. The exclusion criteria were: duplicates data, no usable data reported and Communication letters, reviews, editorials, abstracts and conference proceedings published in non-peer-reviewed journals15.
Data extraction
Data extracted from the selected papers included the following information: the name of the first author, publication year, gender, location of the study, duration of follow-up, sample size, the number of type 2 diabetes cases, the consumption level of vitamin D, adjustments and risk estimates with 95% CI. When more than one data set presented in one study, the one adjusted by more potential confounders was used to meet with the optimal control of confounding factors. All data was extracted by two reviewers independently according to the pre-specified selection criteria. Disagreement was resolved by discussion with co-authors by reviewing the full text.
The quality of each study was completed by the other two investigators independently. All the evaluated work was processed by reporting following crucial components of eligible studies: a clearly stated aim, clear examination of exposure and outcome, clear definition of participant characteristics, study duration, sufficient duration of follow-up, person-years of follow-up, no selective loss during follow-up and control for potential confounding factors. If a study did not clearly provide one of these key points information, we did not consider that it had been performed, but it's probably underestimated the reported characteristics.
Statistical analysis
The combined risk estimates were based on comparisons of the highest intake category with the lowest intake category (including persons who have no consumption of vitamin D). Between-studies heterogeneity was measured using the Q statistic. P<0.10 was regarded as significantly heterogeneous and random effects models were used for analyses estimating the pooled RR, 95% confidence intervals, and corresponding p-values for heterogeneity, otherwise, fixed effects models were used16. I2 statistic was also applied to measure the percentage of the total variation across studies caused by heterogeneity17, 18. Furthermore, forest plots were also examined to assess the relationship between vitamin D intake and type 2-diabetes. Subgroup analyses were conducted on the basis of geographical region (USA), gender (women), dietary vitamin D intake and two on supplemental vitamin D intake.
To investigate whether a single study has an effect on the overall risk estimate, we conducted a sensitivity analysis by excluding one study in each turn. In addition, we applied the Begg adjusted rank correlation test and the Egger's regression asymmetry test to assess the possible bias captured by the funnel plot19, 20. It should be noted both tests have low power to detect the potential biases; especially the numbers of studies are very limited. Therefore, we set p = 0.1 as our statistical penalty in these two tests. P<0.1 indicated possible publication bias16.
All statistical analyses were using the commands in the Stata statistical software package (version, 11.0, Stata Corporation, College Station, TX, USA). All statistical tests were two sided.
Results
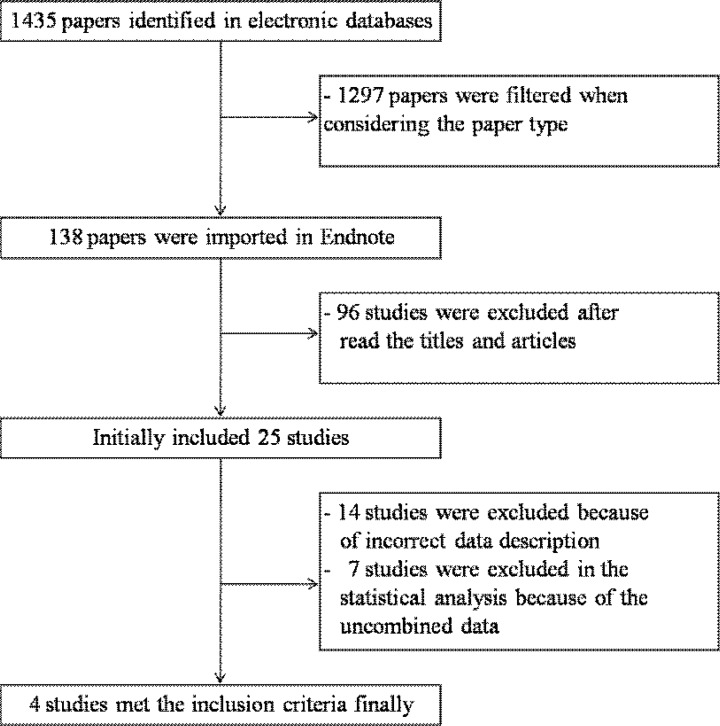
We have retrieved 1435 articles initially, 1297 papers were excluded after firstly screening the paper type or subjects. 96 relative papers were exclusion after reading the titles and abstracts. While, the other 17 studies were excluded, because the doses of vitamin D could not be summarized in the highest versus lowest category due to the limited data in the original studies. Therefore, 25 articles were remained. Of the remaining 25 studies, 14 papers reported results without enough information (e.g.: raw data and p value) to estimate the effect sizes and their CIs. The other 7 studies were excluded based on the following reasons: The results were only presented with median and ranges. Moreover, there had not any adjusted or unadjusted odds ratio or risk ratio that could be summarized for meta-analysis. In addition, as is known to all, a fixed cycle is very important in a follow-up study. If we cannot find any fixed follow-up cycle definitions in some original studies, we considered that these data may be unbelievable, and these studies should not be included. Finally, we identified a total of four publications with prospective cohort studies on vitamin D intake and the risk of type 2 diabetes according to our above mentioned criteria. The articles selection flow chat was presented in figure 1.
Figure 1.
The flow chart of study selection
They were involving 187, 592 participants and 9, 456 incident cases. The number of cases diagnosed in the original studies ranged from 1, 114 to 4, 843, and the number of participants ranged from 10, 066 to 83,779. The identification of diabetes was mainly based on self-reports of physician diagnosis, but the majority of cases were confirmed in validation studies. Participants in the study reported by Kirii et al10. were divided to men and women individually for observation and analysis. Thus, it was considered two studies when the observed items were pooled. In other words, there were four papers with five data sets in our meta-analysis. The characteristics of the included studies are presented in table 1. The four prospective cohort studies were published between 2000 and 2012.
Table 1.
Characteristics of studies that analyzed vitamin D intake and type 2 diabetes.
| First authorand year | Study location | Cases/Subjects | Yearsa | Items | Analytical comparisonb |
RR (95% CI)/ Trend p |
Adjustments |
| K. Kirii 2009 [10] | Japan | 1114/59796 | 5.0 | Vitamin D(Men) | Highest vs. Lowest |
0.96 (0.74–1.23)0.35 | Age, BMI , smoking, family history of diabetes, history of hypertension, alcohol intake, area, exercise frequency, consumption of coffee, energy-adjusted magnesium and total energy |
| Vitamin D(Women) | Highest vs. Lowest |
0.88 (0.67–1.16)0.67 | |||||
| De Boer IH 2008 [11] | USA | 2291/33951 | 7.0 | Total vitamin D | >400IU/day vs.<200IU/day |
0.95 (0.83–1.08)0.47 | Age, BMI smoking, family history of diabetes, race, ethnicity, education, calcium intake, alcohol intake, physical activity, waist circumference. |
| Pittas AG 2006 [12] | USA | 4843/83739 | 20 | Total vitamin D | >800 IU/day vs. <200 IU/day |
0.87(0.69–1.09)0.67 | Age, BMI, smoking, family history of diabetes, hypertension, physical activity, caffeine, alcohol, and state of residence, type of fat, cereal fiber, glycemie load, magnesium and retinol. |
| Dietary vitamin D | >400 IU/day vs. <100 IU/day |
1.00(0.78–1.29)0.25 | |||||
| Supplemental vitamin D |
>400 IU/day vs <100 IU/day |
0.87(0.75–1.00)0.04 | |||||
| Liu S 2005 [13] | USA | 1208/10066 | 8.8 | Total vitamin D | Quintiles of intake Q5 VS. Q1 |
0.92 (0.72–0.99)0.02 | Age, smoking, exercise, total calories, alcohol, multivitamin, parental history of myocardial infarction, dietary intakes of total fat, cholesterol, protein, and glycemic load and calcium. |
| Dietary vitamin D | Q5 VS. Q1 | 0.92 (0.79–1.09)0.29 | |||||
| Supplemental vitamin D |
Q5 VS. Q1 | 1.00 (0.83–1.20)0.16 | |||||
the period of following-up
analytical comparison based on comparisons of the highest intake category with the lowest intake category
Of the included studies, three studies were conducted in the United States11–13 and the other one in Japan10. The length of follow-up period ranged from 5 to 20 years. Only one study involved men and women10, the remaining three studies consisted of women only11–13. Of the studies, four reported results on total vitamin D intake, two on dietary vitamin D intake12, 13 and two on supplemental vitamin D intake12, 13. Despite of the different number of food items in the food frequency questionnaires (FFQs) across studies, most studies used validated FFQs to define dietary information pertaining to vitamin D products consumption. All the included studies considered the lowest or no consumption of vitamin D as the reference. Although the confounding factors varied across studies, the major adjusted confounders in our included studies included the most essential confounders such as age, BMI, smoking and family history of diabetes.
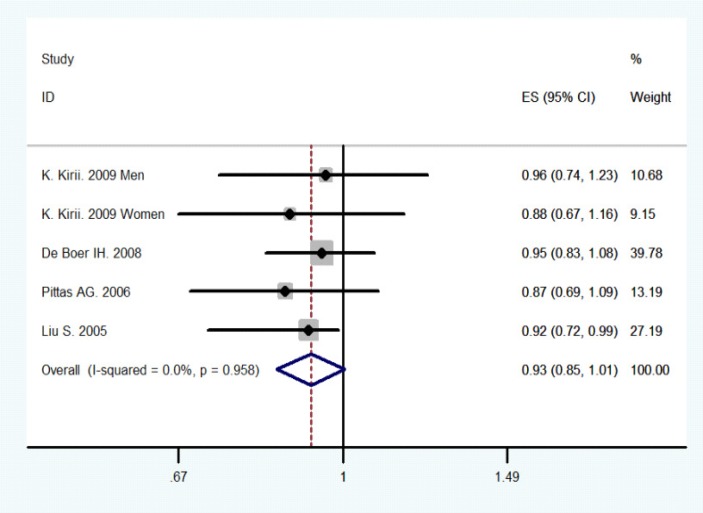
Figure 2 revealed that the combined results of all studies for the highest versus the lowest category of total vitamin D intake. Of these selected studies, only one study found a statistically significant inverse association between total vitamin D intake and type 2 diabetes risks. The other studies reported no significant association between the two. The combined RR of Type 2 diabetes was 0.93 (95% CI 0.85–1.01) (10–13), comparing the highest with the lowest category of total vitamin D intake. No heterogeneity was detected across studies (P-value for heterogeneity =0.958, I2=0 %; figure 1). We did not conduct the dose-response analysis of studies on vitamin D intake because the eligible studies did not provide sufficient information on the category data of vitamin D intake
Figure 2.
Meta-analysis of vitamin D intake and type 2 diabetes (the highest versus the lowest category).
The size of the square is proportional to the percent weight of each study in the meta-analysis; the horizontal lines represent 95% CI. Studies are ordered by the year of publication. CI: confidence interval; ES: effect size.
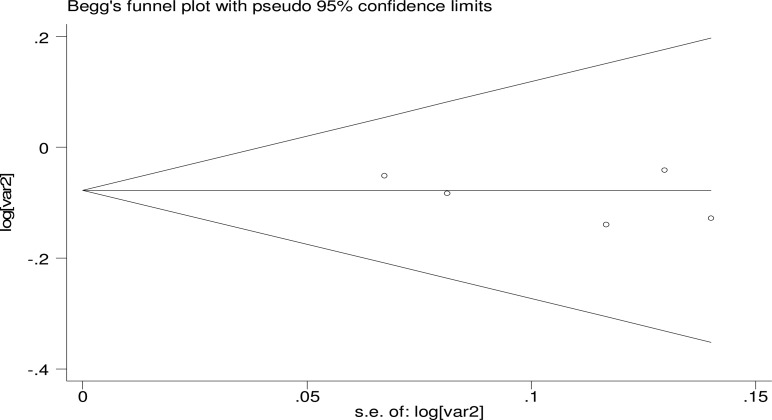
To investigate the robustness of our findings, we conducted the sensitivity analyses by omitting one study at each turn and calculating the combined RR for the remaining studies yielded consistent results, with a narrow range from 0.90 (95% CI, 0.81–1.61) to 0.92 (95% CI, 0.79–1.64). Thus, we did not find that any single study substantially influence the combined risk estimate. In addition, there was no evidence of publication bias with regard to consumption of vitamin D in relation to risk of Type 2 diabetes, as indicated by a P-value of 0.462 by Begg rank correlation test (figure 2) and a P-value of 0.320 by Egger linear regression test.
When the studies were stratiûed by geographical region, no significant association was observed in USA (RR = 0.93, 95% CI: 0.84–1.02, p-value for heterogeneity = 0.804, I2 = 0 %) (11–13). In the subgroup analysis for women, the summary RR was 0.92 (95% CI 0.84–1.01) (10–13). To be noted, there was no variability across the studies included women only (p-value for heterogeneity = 0.902, I2 = 0.0 %). Summary associations were similar between studies on dietary vitamin D intake (RR = 0.94, 95% CI: 0.82–1.08; p-value for heterogeneity = 0.584, I2 =11.2%) (12–13) and studies on supplemental vitamin D intake (RR = 0.91, 95% CI: 0.77–1.08; p- value for heterogeneity = 0.142, I2 =53.6 %) (12–13).
Discussion
It is well-known that the major function of vitamin D is to maintain calcium and phosphorus homeostasis and promote bone mineralization6. Moreover, Vitamin D may have a beneficial effect on insulin action directly via stimulating the expression of insulin receptor resulting in enhancing insulin responsiveness for glucose transport, or indirectly through its role in regulating extracellular calcium and ensuring normal calcium influx6, 21. In experimental studies, vitamin D has been demonstrated to improve pancreatic beta cell function and peripheral insulin sensitivity22, 23. However, in humans, findings on the association between vitamin D intake and the risk of type 2 diabetes from prospective studies are limited and inconsistent10–13.
To the best of our knowledge, this is the first meta-analysis of cohort studies on the potential relationship between vitamin D intake and type 2 diabetes risk. Our present study is a meta-analysis based on the epidemiological studies which included large-scale and long-term follow-up results. As we know, a cohort study is undertaken to support the existence of association between suspected cause and disease. Prospective cohort study can not be interfered by investigators, and it is more suitable for long-term follow-up and owns high strength evidence. Whereas, RCT often needs to be end at the scheduled termination time, and the follow-up time is often shorter. On the other hand, a well-designed cohort study always can minimize the interference or potential confounding factors of the participants through the corrected multivariable analyses. While these function cannot be conducted in a RCT study. In addition, there were obvious interventions on whether participants accepted vitamin D in RCT studies, while the researchers did not interfere with participants in cohort studies, this is well conformed with the actual situation than RCT.
Here, we pooled four prospective cohort studies involving 187, 592 participants and 9, 456 incident cases to get a more stable and creditable result. The results suggested no significant overall association between consumption of vitamin D and type 2 diabetes incidence. Furthermore, among the subgroup analyses based on women, USA continent, dietary vitamin D intake and supplemental vitamin D intake, no significant associations were observed. To be noted, no substantial heterogeneity was detected in those studies included in our present analysis. On the basis of Egger's and Begg's tests, we have shown an absence of publication bias in these meta-analyses. In addition, sensitivity analyses showed none of the studies considerably affected the summary associations between vitamin D intake and the risk of type 2 diabetes.
Our results compare favorably with most of studies included in our analysis, in which they reported that subjects consuming vitamin D were not associated with type 2 diabetes risk. We did not perform the dose-response analysis of vitamin D intake for two reasons. On one hand, our findings did not show any relationship with risk of type 2 diabetes in our analysis. On the other hand, the four eligible prospective cohort studies did not provide sufficient information for a dose-response analysis on the category data of vitamin D intake, number of cases, person-years, and logarithm of RR and its corresponding standard error.
The publication bias is a major concern in a meta-analysis. Although we do not have enough statistical power to formally test for publication bias, there was no evidence for the smaller cohorts to overestimate effect estimates compared with the larger studies. In our analysis, no evidence of publication bias was observed by setting the penalty as p value less than 0.1, though the tests of bias have low statistical power. More importantly, the associations between consumption of vitamin D and risk of type 2 diabetes appeared to be consistent across most studies. Thereof, the likelihood that these findings are largely a result of selective publication seems to be minimal. However, a potential bias could not be excluded completely.
We have several important strengths in the present analysis. This is the first systematic epidemiologic assessment from prospective cohort studies to investigate the relationship between vitamin D intake and type 2 diabetes. Because individual study has low statistical power, our meta-analysis of four large prospective cohort studies involving enlarged sample size enhancing the statistical power to detect more stable association and provide more reliable estimation. The studies included in our meta-analysis were prospective cohort studies with a large sample size and long-term follow-up periods, which increased the statistical power to quantitatively assess the overall associations of vitamin D intake and incidence of type 2 diabetes. Furthermore, the prospective study designs could minimize selection bias and recall bias24. Moreover, the results were further analyzed based on the subgroups and yielded a similar conclusion, and no evidence of heterogeneity in our analyses probably indicated the robustness of our findings. Additionally, the included original studies reported the dietary intake levels of vitamin D and supplemented vitamin D, whereas, due to the unpooled data, our review mentioned the dietary intake levels of calcium and vitamin D.
Several potential limitations need to be taken into account in our study when considering its contributions. First, residual confounders always raise a major concern in the epidemiology studies. Although a wide range of potential confounding factors, including demographic and lifestyle factors were performed adjustment in original studies, whereas dietary factors were not sufficiently considered 25. We could not exclude the possibility that the unmeasured or unknown dietary factor may contribute to the results not entirely accurate. Second, the primary studies reported consumption of vitamin D by different category which might cause random misclassifications. However, we reported the summary relative risk estimates based on the highest vitamin D intake compared with the lowest intake categories which possibly reduce the bias. Third, the strength of the association may have been weakened by possible misclassification bias. Misclassification of vitamin D intake by using the method of self-administered questionnaires is inevitable. Moreover, because the confirmations of type 2 diabetes in the original studies were based on self-reports, misclassification of type 2 diabetes cases is also likely to occur. Additionally, our results were based on only four studies included in this meta-analysis. The pooled risk estimate may be affected by individual studies. However, our sensitivity test showed the findings were consistent, which indicated a somewhat high degree of robustness of our findings. At last, due to the unmagable data or other reasons above mentioned, we have excluded several potential related studies and this effect may induce potential selection bias on our analysis. Therefore, more large-scale and high quality studies are warranted to enhance our results.
Conclusion
Our meta-analysis of all relevant prospective cohort studies showed an absence of significant association between vitamin D intake and type 2 diabetes risk. Further studies are required to better confirm the findings.
Figure 3.
Begger plot for the assessment of potential publication bias for vitamin D intake and type 2 diabetes. Var 2: relative risk
References
- 1.Yang W, Lu J, Weng J. China National Diabetes and Metabolic Disorders Study Group. Prevalence of diabetes among men and women in China. N Engl J Med. 2010;362:1090–1101. doi: 10.1056/NEJMoa0908292. [DOI] [PubMed] [Google Scholar]
- 2.Hsieh MC, Lee TC, Cheng SM, Tu ST, Yen MH, Tseng CH. The influence of type 2 diabetes and glucose-lowering therapies on cancer risk in the Taiwanese. Exp Diabetes Res. 2012;2012:413782. doi: 10.1155/2012/413782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27:1047–1053. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- 4.Mokdad AH, Ford ES, Bowman BA, et al. Prevalence of obesity, diabetes, and obesity-related health risk factors, 2001. JAMA. 2003;289:76–79. doi: 10.1001/jama.289.1.76. [DOI] [PubMed] [Google Scholar]
- 5.Hu FB, Manson JE, Stampfer MJ, et al. Diet, lifestyle, and the risk of type 2 diabetes mellitus in women. N Engl J Med. 2001;345:790–797. doi: 10.1056/NEJMoa010492. [DOI] [PubMed] [Google Scholar]
- 6.Pittas AG, Lau J, Hu FB, Dawson-Hughes B. The role of vitamin D and calcium in type 2 diabetes. A systematic review and meta-analysis. J Clin Endocrinol Metab. 2007;92:2017–2029. doi: 10.1210/jc.2007-0298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bischoff-Ferrari HA, Giovannucci E, Willett WC, Dietrich T, Dawson-Hughes B. Estimation of optimal serum concentrations of 25-hydroxyvitamin D for multiple health outcomes. Am J Clin Nutr. 2006;84:18–28. doi: 10.1093/ajcn/84.1.18. [DOI] [PubMed] [Google Scholar]
- 8.Chiu KC, Chu A, Go VL, Saad MF. Hypovitaminosis D is associated with insulin resistance and beta cell dysfunction. Am J Clin Nutr. 2004;79:820–825. doi: 10.1093/ajcn/79.5.820. [DOI] [PubMed] [Google Scholar]
- 9.Scragg R, Sowers M, Bell C. Serum 25-hydroxyvitamin D, diabetes, and ethnicity in the Third National Health and Nutrition Examination Survey. Diabetes Care. 2004;27:2813–2818. doi: 10.2337/diacare.27.12.2813. [DOI] [PubMed] [Google Scholar]
- 10.Kirii K, Mizoue T, Iso H, et al. Calcium, vitamin D and dairy intake in relation to type 2 diabetes risk in a Japanese cohort. Diabetologia. 2009;52:2542–2550. doi: 10.1007/s00125-009-1554-x. [DOI] [PubMed] [Google Scholar]
- 11.de Boer IH, Tinker LF, Connelly S, et al. Calcium plus vitamin D supplementation and the risk of incident diabetes in the Women's Health Initiative. Diabetes Care. 2008;31:701–707. doi: 10.2337/dc07-1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pittas AG, Dawson-Hughes B, Li T, et al. Vitamin D and calcium intake in relation to type 2 diabetes in women. Diabetes Care. 2006;29:650–656. doi: 10.2337/diacare.29.03.06.dc05-1961. [DOI] [PubMed] [Google Scholar]
- 13.Liu S, Song Y, Ford ES, Manson JE, Buring JE, Ridker PM. Dietary calcium, vitamin D, and the prevalence of metabolic syndrome in middle-aged and older U.S. women. Diabetes Care. 2005;28:2926–2932. doi: 10.2337/diacare.28.12.2926. [DOI] [PubMed] [Google Scholar]
- 14.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 15.Tong X, Dong JY, Wu ZW, Li W, Qin LQ. Dairy consumption and risk of type 2 diabetes mellitus: a meta-analysis of cohort studies. Eur J Clin Nutr. 2011;65:1027–1031. doi: 10.1038/ejcn.2011.62. [DOI] [PubMed] [Google Scholar]
- 16.Li F, An SL, Zhou Y, et al. Milk and dairy consumption and risk of bladder cancer: a meta-analysis. Urology. 2011;78:1298–1305. doi: 10.1016/j.urology.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 17.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 18.Orsini N, Bellocco R, Greenland S. Generalized least squares for trend estimation of summarized dose-response data. Stata Journal. 2006;6:40–57. [Google Scholar]
- 19.Bgger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101. [PubMed] [Google Scholar]
- 21.Maestro B, Campion J, Davila N, Calle C. Stimulation by 1,25-dihydroxyvitamin D3 of insulin receptor expression and insulin responsiveness for glucose transport in U-937 human promonocytic cells. Endocr J. 2000;47:383–391. doi: 10.1507/endocrj.47.383. [DOI] [PubMed] [Google Scholar]
- 22.Palomer X, Gonzalez-Clemente JM, Blanco-Vaca F, Mauricio D. Role of vitamin D in the pathogenesis of type 2 diabetes mellitus. Diabetes Obes Metab. 2008;10:185–197. doi: 10.1111/j.1463-1326.2007.00710.x. [DOI] [PubMed] [Google Scholar]
- 23.Tai K, Need AG, Horowitz M, Chapman IM. Vitamin D, glucose, insulin, and insulin sensitivity. Nutrition. 2008;24:279–285. doi: 10.1016/j.nut.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 24.He K, Song Y, Daviglus ML, et al. Fish consumption and incidence of stroke: a meta-analysis of cohort studies. Stroke. 2004;35:1538–1542. doi: 10.1161/01.STR.0000130856.31468.47. [DOI] [PubMed] [Google Scholar]
- 25.Dong JY, Xun P, He K, Qin LQ. Magnesium intake and risk of type 2 diabetes: meta-analysis of prospective cohort studies. Diabetes Care. 2011;34:2116–2122. doi: 10.2337/dc11-0518. [DOI] [PMC free article] [PubMed] [Google Scholar]