Abstract
Background
All research involving human participants should be reviewed by a competent and independent institutional research and ethics committee. Research conducted at Makerere University College of Health Sciences should be subjected to a rigorous review process by the ethics committee in order to protect human participants' interests, rights and welfare.
Objective
To evaluate researchers' knowledge about the functions and ethical review process of the College of Health Sciences research and ethics committee.
Methods
A cross sectional study. 135 researchers consented to participate in the study, but 70 questionnaires were answered giving a 52% response.
Results
Age ranged between 30 to 61 years, majority of participants 30–39 years. Most of the respondents do agree that the
REC functions include Protocol review 86%, protection of research participants 84.3%, and monitoring of ongoing research. During ethical review, the RECpays special attention to scientific design [79.7%] and ethical issues [75.3%], but less to the budget and literature review. More than 97% of the respondents believe that the REC is either average or very good, while 2.8% rank it below average.
Conclusion
Respondents knew the major functions of the committee including protection of the rights and welfare of research participants, protocol review and monitoring of on going research, and the elements of protocol review that are given more attention include ;scientific design and ethical issues. Overall performance of the REC was ranked as average by respondents. The committee should limit delays in approval and effectively handle all functions of the committee.
Introduction
Makerere University is one of the leading research institutions in Uganda and the region with world class research particularly in the fields of medicine and social sciences. Thus, as a national and international standard, all research involving human participants conducted in the institution should be reviewed by a competent and independent institutional research and ethics committee. 1–5 to ensure protection of human participants interests, welfare and rights against harm and exploitation by some researchers6–10.
However, like any service provider, a number of criticisms are lodged on the research and ethics committee , and administrators' for making shallow, rushed and biased reviews of protocols, favorings selected colleagues, concealing conflict of interest, making unreasonable requests for changes, imposing excessive bureaucratic requirements, delaying the review process and incompetence. Other accusations include failure to ensure that the research design includes adequate monitoring of data and any additional safeguards necessary to protect welfare of vulnerable persons, failing to conduct continuing review of research at intervals appropriate to the degree of risk, failing to monitor ongoing research and to make research related decisions with appropriate questions11–13.
For adequate protection of welfare and rights of research participants, all the interacting parties in research should share a common goal, such that the application of these principles is not in a hierarchical way but in a circular fashion of interaction with the research and ethics committee members, investigators, sponsors, research participants, community, institutions and governments forming a circle of trust4. It links all parties equally, and each demonstrates dedication, both individually and collectively, through education and cooperation to uphold human dignity during research.
It should be noted that many researchers regard ethical review as a road block to doing research, and slowing the progress of science, imposed by Institutions and institutional review committees14. They also have minimal knowledge about institutional review committees, the ethical reviewprocess, elements of ethical review, research regulatory guidelines and laws. The faculty of medicine research and ethics committee has ensured protection of research participants as well as upholding all the prescribed functions of ethics committees, However, the faculty review committee had never been evaluated and was not aware of the researchers' knowledge about its functions, membership, proceedings and overall performance regarding protection of human research participants.
This study was therefore aimed assessing their knowledge and attitudes about their research and ethics committee so that it can make rational changes and adjustments to improve the dispensation of their services.
Methods
This was a cross sectional study at Makerere University Faculty of Medicine and Mulago Teaching Hospital. The survey population included all faculty, Mulago hospital staff involved in research and the graduate student.
The survey sample was drawn from the Faculty of Medicine staff, graduate students and Mulago Hospital clinicians database by simple random sampling. All departments that do research were included. Only researchers who had never served as members of the research and ethics committee and who consented were included. The data collection instrument was first pre-tested and relevant corrections made before use for data collection.
After consenting, the researchers were handed the structured questionnaire for filling at their own convenience. A special box was left at an accessible but safe point in each department where filled questionnaires had to be dropped for the investigator to collect, and this was done to maintain protection of privacy and confidentiality of the respondents.
Ethical review and approval was sought from the Faculty of Medicine Research and Ethics Committee. Informed consent was obtained from all the respondents before data collection and appropriate measures were taken to minimize risks and maintain confidentiality.
The data was first analyzed descriptively by SPSS Statistical package; averages for questions that required quantitative answers and frequency tables for questions that required a choice among several given alternatives were calculated, bar graphs and pie chats were constructed. ANOVA was used to test for differences between means of variables on researcher's knowledge about the REC and ethical review11.
Results
A total of 135 questionnaires were administered to researchers who consented to participate in the survey, an effort was put to get back the fully filled ones, only seventy researchers (70) returned the questionnaires which were fully answered giving a 52% response rate, the remaining 65 questionnaires were never returned. The male to female ratio of respondents was 1.3:1. Most of the respondents knew about the membership of the committee, how the members are appointed, ethical review process and the frequency of the meetings.
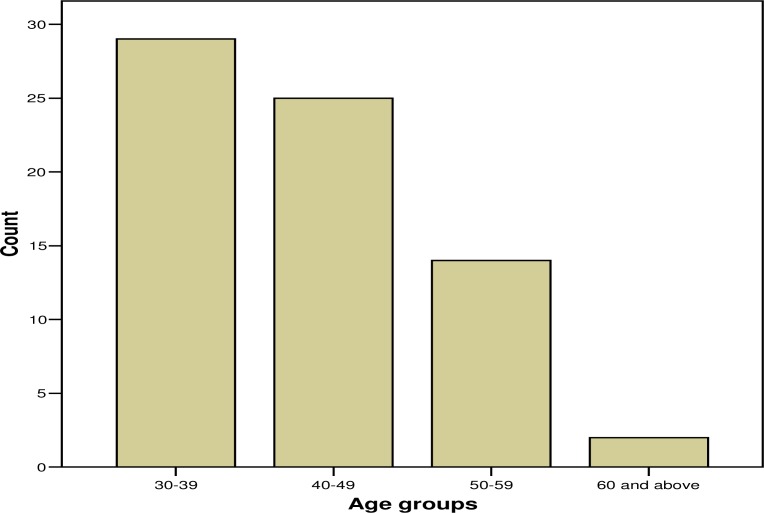
The age ranged from 30 to 61 years,with the majority of participants in the age range 30–39 as shown in figure 1.
Figure 1.
Age groups of respondents in the survey
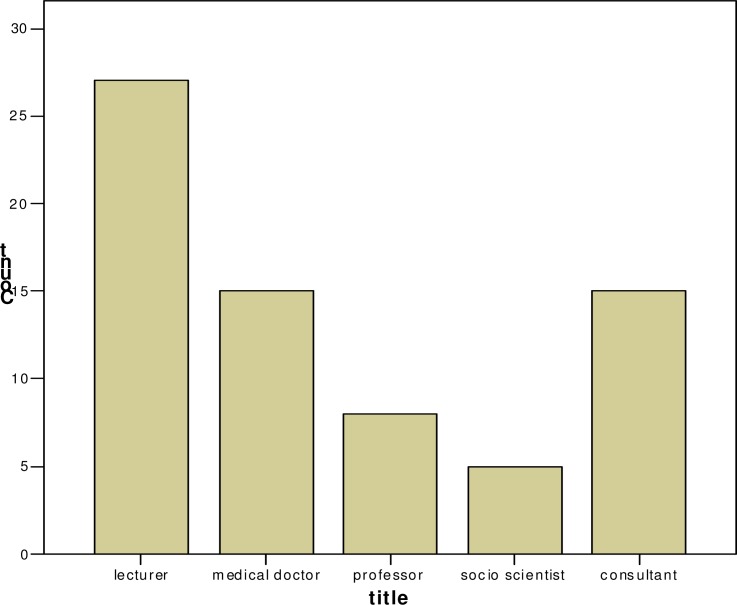
Most of the participants were academic staff of the faculty of medicine, postgraduates and researchers from Mulago hospital as shown in figure 2.
Figure 2.
Title of respondent
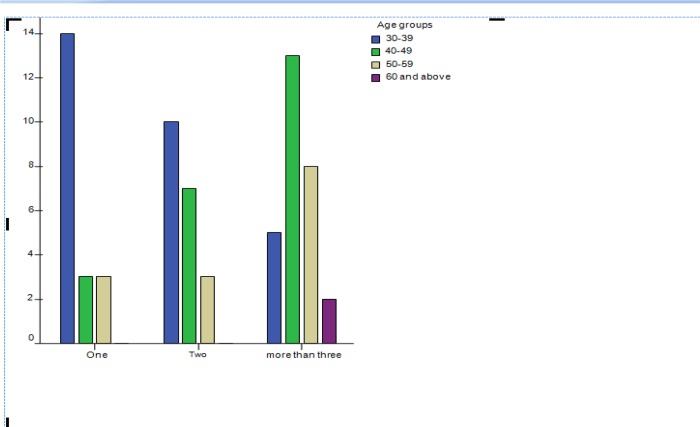
As shown in figure 3, it is those researchers in the age range 40–49 that have had more than three protocols going through the faculty review committee;the majority of participants have had two or more protocols reviewed.
Figure 3.
Age group and number of research protocols reviewed by the review committee
Discussion
The response rate among researchers in this study was 52%, which is fairly reasonable as compared to 49% by a similar study in Australian and 38%, in what scientists want from their institutional review committees11, 12. There is also a 98%, 59% and 24% response rate in what institutional review committees look like of California State University USA12. All the respondents had previous experience with the Faculty of Medicine review committee and had at least presented a research protocol for review.
The age range was 30 to 61 years and the majority of respondents were in the age group 30–39, this also compares with the ages of the majority staff of Makerere faculty of Medicine and Mulago Hospital. The male to female ratio was 1.3:1; this is almost equal because of the policy of gender balance and women emancipation held by the University. Most researchers were academic staff, but since the committee serves the faculty of Medicine and Mulago teaching Hospital with all the, doctors, consultant, postgraduates and social scientists, who conduct research.
The study revealed that most researchers consider protocol review 63[86.6%], protection of human subjects 59[84%], monitoring of ongoing research 40[57.1%] and discipline of researchers 44[62.9%] to be the major functions of the Ethics committee. Other functions included policy advice to the institution 34[48.6%], staff (researchers) and community education on ethical issues15–17. It has never been a function of the committee to harass researchers although such a belief is also reflected elsewhere11–12.
The researchers were also aware of the discipline function of the committee which is in agreement with other studies where the committee has powers to, suspend, or discontinue researcher's work, in addition to making them face suspension, or loss of privilege to conduct research12. A small percentage of researchers (12.9%) knew all the above as functions of the ethics committee. Hence need for the faculty committee to step up publicity and education of researchers about its functions and the ethical review process, outlining the guidelines.
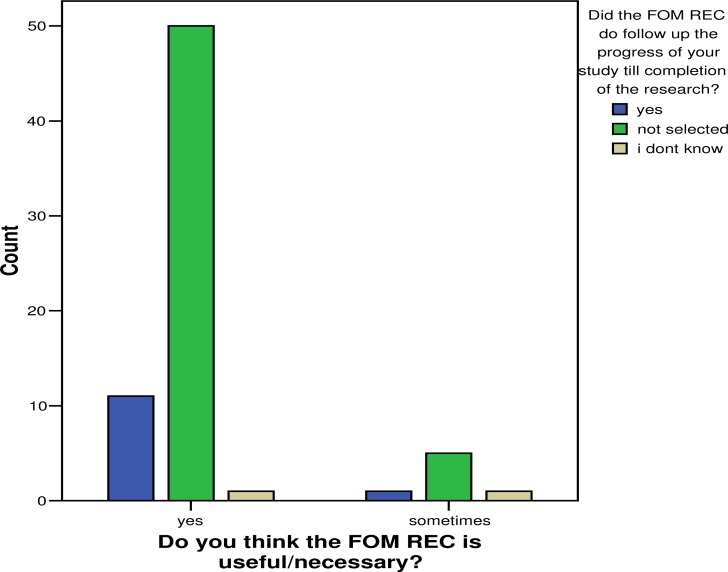
Whereas researchers are aware of major function of the committee, they went ahead to indicate that some aspects are not well accomplished including monitoring of ongoing research giving an opportunity for researchers to deviate and or violate the approved protocols, which is aggravated by planning research long before it is conducted and unpredictable circumstances leading to a need to change in the procedures once it is instituted11. This is worsened by the perception that the committee causes unnecessary delays in approving proposals or any protocol amendments.
Although, any research proposal that deviates from that approved by the committee should be viewed as being unethical because it is in breach of local and international guidelines. All amendments of the protocol must be approved by the review committee before being implemented. Making it necessary for continuous monitoring of ongoing research projects till completion as a safeguard against deviations and violations from the required research practices11. Thus monitoring is the only sure mechanism to limit unethical practices by some researchers following approval of their protocols.
Despite the fact that the committee did not follow up or monitor the progress of their research, 50 of the participants (71%) still thought that the review committee was necessary, bearing in mind the fact that it fulfilled other roles adequately.It's a common practice that the review committee pays little attention to the proposed budget and literature review during protocol review (14.3%,2.43%) respectively.Howeverspecial attention is given to, scientific design [79.7%], ethical issues[75.3%] the care and protection of research subjects [56.5%] which is also part of the ethical issues. It is necessary that the committee pays attention to all elements of the review process, since each has a particular influence on the protection of the research participants.
Conclusion
The survey cuts across the range of researchers in the Faculty of Medicine and Mulago Hospital who are fairly knowledgeable about the functions of the review committee, but ignorant about a few others. It's important to note that the majority know the major functions like protection of the rights and welfare of human research participants.
These respondents also confirmed the fact that monitoring of approved protocols and ongoing research by the review committee is not very strictly adhered to as a major function of the committee, which is a weakness in the performance of the committee and a loophole for possible abuse of research ethics by researchers.
The faculty of medicine research and ethics committee sticks to elements of ethical protocol review, especially, the scientific design, and ethical issues. This ensures protection of Human subjects as well as complying with the national and international ethical codes1–5.
The committee also still has other weaknesses like delay in approval of protocols and timely communication to the researchers. Hence the overallperformance which was ranked as average from the majority of our respondents.
Recommendations
The faculty review committee should improve its publicity among researchers, by carrying out its mandate of educating researchers, staff and the community about the ethical guidelines for review, and should make greater efforts to educate researchers as a way of limiting the unethical research practices this should be supported by good communication.
The committee should as well strengthen its ability to monitor ongoing research to counter any deviations and violations from the required ethical practices. The committee should limit delays in review and approval of protocols. The researchers should be continuously educated about all functions of the ethics committees, and the review process, so as to limit and curb any ethical violations and deviations.
Figure 4.
Follow up of on going research by review committee and its usefulness
Table 1.
Gender of the participants
| Valid | Frequency | Percent | Valid Percent |
| Male | 39 | 55.7 | 55.8 |
| Female | 31 | 44.2 | 44.2 |
| Total | 70 | 99.9 | 100.0 |
Table 2.
Knowledge of respondents about the functions of the faculty review committee
| Function | Selected | % | Not selected | % | Total | % |
| Protocol Review | 63 | 90 | 7 | 10 | 70 | 100 |
| Policy advice on research | 34 | 48.6 | 36 | 51.4 | 70 | 100 |
| Staff advice on research | 36 | 51.4 | 34 | 48.6 | 70 | 100 |
| Protection of research participants |
59 | 84.3 | 11 | 15.7 | 70 | 100 |
| Harass Researchers | 6 | 8.6 | 64 | 91.4 | 70 | 100 |
| Discipline researchers | 44 | 62.9 | 26 | 37.1 | 70 | 100 |
| Monitor on going research | 40 | 57.1 | 30 | 42.9 | 70 | 100 |
| All the Above | 9 | 12.9 | 61 | 87.1 | 70 | 100 |
Table 3.
Knowledge of researchers about Elements of the research protocol review, the review committee pays special attention to during protocol review process [n=70].
| Scientific design and conduct of the study |
Recruitment of research subjects |
Care and protection of research participants |
Ethical issues |
Community Budget considerations |
Literature review |
||
| Selected | 79.7% | 51.5% | 56.5% | 75.3% | 44.9% | 14.3% | 24.3% |
| Not Selected | 20.3% | 48.5% | 43.5% | 24.6% | 55.1% | 85.7% | 75.7% |
| Total | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 |
Acknowledgements
We wish to express our deepest appreciation to Professor Solomon Benator, Director of IRENSA and the facilitators University of Cape Town, Forgaty Foundation (NIH) for the sponsorship. Special thanks to Dr. Ian Munabi (Makerere College of Health Sciences), for the assistance and expertise he rendered in data analysis. Thanks to all researchers of Makerere College of Health Sciences and Mulago Hospital for accepting to participate in the survey. Finally thanks to Gertrude Nansimbe for all her secretarial work on this study.
References
- 1.Uganda National Council for Science and Technology, author. National guidelines for research involving humans as research participants. 2007. Mar, www.uncst.go.ug.
- 2.Trials of War Criminals before the Nuremberg Military Tribunals under Control Council Law No. 10. Washington D.C.: U.S. G.P.O; The Nuremberg Code 1947; pp. 1949–1953. Nuremberg 1946 – April 1949. [Google Scholar]
- 3.World medical association, author. Ethical principles for medical research involving human subjects. Declaration of Helsinki. 2000. http://www.wma.net/en/30publications/10policies/b3/17c.pdf.
- 4.The National commission for the protection of human subjects of Biomedical and behavioral research. 1979. Apr 18, Belmont report. [Google Scholar]
- 5.The Council for International Organization of medical sciences (CIOMS), author Internaational ethical guidelines for biomedical research involving human subjects. 2002 [PubMed] [Google Scholar]
- 6.Dyer O. GP suspended for enrolling patients in drug trials without consent. BMJ. 2003;326:304. [PMC free article] [PubMed] [Google Scholar]
- 7.Mudur G. John Hopkins admits scientist used Indian patients as guinea pigs. BMJ. 2001;323:7323. [PMC free article] [PubMed] [Google Scholar]
- 8.Lenzer J. Secret report surfaces showing that Pfizer was at fault in Nigerian drug tests. BMJ. 2006;332:1233. doi: 10.1136/bmj.332.7552.1233-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kovac C. Nigerians to sue US drug company over meningitis treatment. BMJ. 2001;323:592. (15 September) [Google Scholar]
- 10.Wise J. Pfizer accused of testing new drug without ethical approval. BMJ. 2001;322:194. [PMC free article] [PubMed] [Google Scholar]
- 11.Mcneill Paul M, Bergland Catherine A, et al. Do Australian Researchers Accept Committee Review and Conduct of Ethical Research? Soc Sci Med. 1992;35(3):317–322. doi: 10.1016/0277-9536(92)90028-o. [DOI] [PubMed] [Google Scholar]
- 12.Keith - Spiegel Patricia, Koocher Gerald P. What scientists want from their Research Ethics Committee? Journal of Empirical Research on Human Research Ethics. 2006;1(1):67–68. doi: 10.1525/jer.2006.1.1.67. [DOI] [PubMed] [Google Scholar]
- 13.Operational guidelines for ethics committees that review biomedical research. TDR/ETHICS/2000.1.
- 14.Devries Raymond, Anderson Mellissa S, Marfinson Brian C. Normal Misbehavior: Scientists Talk about the Ethics of Research. Journal of Imperial Research on Human Research Ethics. :43–56. doi: 10.1525/jer.2006.1.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thabane Lehana, Childs Aaron, Lefontaine Amanda. Determining the level of statistician Participation on Canadian -Based Research Ethics Board. IRB: Ethics and Human Research. 2005;27(2):11–14. [PubMed] [Google Scholar]
- 16.Jansen LA. Local IRBs, Multicentre Trials and the Ethics of Internal Amendments IRB. Ethics and Human Research. 2055;27(2):7–11. [PubMed] [Google Scholar]
- 17.Schuklenk Udo. Protecting the Vulnerable Testing Times for Clinical Research Ethics. Social Sciences and Medicine. 2005;51:969–977. doi: 10.1016/s0277-9536(00)00075-7. [DOI] [PubMed] [Google Scholar]