Abstract
Aim/background
We sought to determine the tolerance level and complication rates of the vaginal vault to combined high-dose-rate intra-cavitary brachytherapy with concomitant chemo-radiotherapy.
Patients and methods
A retrospective review of medical records of all the patients who received definitive chemo-radiotherapy for cervical cancer between 1998 and 2002 was undertaken. The records were reviewed for doses and for radiation-associated early and late sequelae of the vagina, rectum and bladder. Cumulative biological effective dose was calculated for two reference vaginal surface points.
Results
Fifty patients were included. Average age at diagnosis was 54 years. Median follow-up was 59 months. There were no recorded instances of acute grade IV toxicity. Maximal high-dose-rate vaginal surface dose (upper central point) was 103 Gy, and maximal brachytherapy lateral surface dose was 70 Gy. Maximal cumulative biological effective dose for the lateral surface reference point was 465.5 Gy3, and the maximal cumulative biological effective dose for the superior reference point was 878.6 Gy3. There were no cases of vaginal necrosis or fistulas, and no cases of grade IV late vaginal, rectal or bladder toxicity. No correlation was found between the maximal vaginal surface dose and vaginal, rectal or bladder toxicity.
Conclusions
The maximal surface HDR brachytherapy dose of 103 Gy and the maximal cBED of 878.6 Gy3 were not associated with fistula or necrosis or other grade 3–4 vaginal complications. Concomitant chemo-radiotherapy, including pelvic radiotherapy and high-dose-rate intracavitary brachytherapy, is relatively safe for cervical cancer patients.
Keywords: Tolerance, Vagina, Irradiation, High-dose-rate, Brachytherapy
1. Background
The treatment of locally advanced uterine-cervix cancer includes concomitant chemo-radiotherapy and intracavitary brachytherapy as definitive treatment. Radiotherapy doses should be optimized to achieve maximal tumor control but great caution needs to be taken to avoid life-threatening or disabling early and late complications. Brachytherapy allows for creating dose escalation which is possible since the tolerance dose of the proximal vagina (vault), uterine-cervix and uterus is high.1,2
Numerous factors need to be taken into account when planning the treatment, apart from the patient's habitus and tumor configuration. Procedural factors associated with source placement are often important factors affecting outcome. Vaginal shortening can occur during treatment; therefore, doses to more distal areas of the vagina, which are considered to be less tolerant than the proximal vagina, may increase during treatment.3 These factors may prevent the use of optimal brachytherapy, smaller applicators and mini ovoids, which might cause the vaginal vault mucosa tolerance dose to become a dose-limiting factor.1 Moreover, the shift from intracavitary low-dose rate (LDR) brachytherapy to intracavitary high-dose-rate (HDR) brachytherapy has led to some uncertainty. As opposed to LDR brachytherapy, there is no standardized way to deliver HDR brachytherapy, and treatments vary considerably between institutions. Dwell time patterns change from patient to patient, to allow optimization of dose distributions according to tumor geometry.4 Fractionation of HDR with four to six fractions can be applied to achieve nominal complication rates similar to LDR. However, HDR complication rates are likely to be susceptible to minor changes of dose and biological parameters, due to the amplified biological effects.1
The development of complications is multifactorial and includes medical comorbidities, total dose of radiation, dose per fraction, number of fractions, dose rate, radiation field, radiation technique, and whether surgery was performed prior to radiotherapy.1 There are only a few reports of vaginal necrosis secondary to radiotherapy, most reported in patients treated with adjuvant concomitant chemoradiotherapy.5–7 Publications often lack important components of treatment planning, doses, vaginal surface dose, complications, treatment failure, and other vital clinical data.4 Since the advent of 3D planning, the rectum and bladder have been given new restrictions according to volume, while the vaginal vault tolerance dose is still in the dark. Moreover, definitive treatment for cervical cancer includes concomitant chemotherapy and external beam radiotherapy (EBRT) together with HDR brachytherapy, and all should be taken into account when calculating the exposed doses of the mucosa (vagina, rectum, and bladder). The addition of chemotherapy is expected to narrow the therapeutic window when combined treatment is applied. More studies are needed to evaluate the outcome of definitive treatment for uterine-cervix cancer, encompassing both detailed dosimetric and clinical aspects of treatment, to serve as a guide for non-compromising treatment of this potentially curable disease.1
Severe complications after radiotherapy for cervical cancer may occur decades after treatment completion.6 There is a paucity of information regarding late toxicity after chemo-radiation for locally advanced cervical cancer, while total vaginal necrosis after treatment is rarely reported.8 The purpose of the current study was to determine the tolerance levels and late complication rates of the vaginal vault to combined concomitant chemotherapy with external pelvic irradiation and intracavitary HDR brachytherapy, mostly late occurring vaginal necrosis. Complications of adjacent organs were also recorded, as well as survival, recurrences and other clinical data.
2. Patients and methods
A single center, retrospective study of the medical records of all consecutive patients with carcinoma of the uterine cervix treated between May 1998 and May 2002 with concomitant external beam radiotherapy, weekly chemotherapy, and intracavitary HDR brachytherapy was conducted. All the patients were treated with curative intent.
The medical records of 50 consecutive locally advanced patients were reviewed for radiotherapy doses and radiation-associated late sequelae of the proximal vagina (vault) with an emphasis on late-occurring vaginal necrosis. Complications of the rectum and bladder were also recorded. Vaginal patency was graded according to vaginal examination recorded in the medical files. The recorded examinations were reviewed and scored by an onco-gynecologist into four levels (level 0 – no toxicity; 1 – mild; 2 – moderately obliterated and shortened vagina; level 3 – complete vaginal obliteration). Vaginal, rectal and bladder toxicity were graded according to the Common Terminology Criteria for Adverse Events (CTCAE) Version 4.0 (September 2009). Data collected included demographics, follow-up, time and site of recurrence, mortality, sexual activity, and the presence and site of other malignancies.
The HDR planning process was based on 2D planning. In order to evaluate the doses received by the vaginal mucosa, two sets of points were defined for each ovoid: 5 points on the uppermost and 5 points on the lateral surface of the ovoid opposite the five active dwell positions at a distance equal to the radius of the ovoid. For each patient, the total vaginal dose for the whole HDR treatment was calculated on the surface of the ovoids at the lateral and the upper central point according to the method in our previous publication by Nevelsky et al.9
The cumulative biological effective dose (cBED) was calculated for two reference vaginal surface points. The equation used for calculation for cBED is presented in Fig. 1. The α/β ratio used was 3 for late responding tissues. The total physical dose was calculated as the sum of the physical external dose and the physical maximal brachytherapy dose (as calculated according to the above cited method).
Fig. 1.
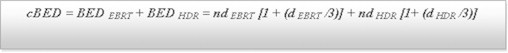
Equation used for calculation of cumulative biological effective dose (cBED). HDR: high-dose-rate brachytherapy; EBRT: external beam radiotherapy. The α/β ratio used was 3 for late responding tissue.
3. Statistical analysis
The statistical software package used was SPSS-18. Descriptive statistics are presented in terms of mean and median. As the maximal total dose and the maximal brachytherapy dose were not normally distributed by the Kolmogorov–Smirnov Z test, the Mann–Whitney U test and Kruskal–Wallis test were used for differences between groups. A value of P < 0.05 was considered as significant.
4. Results
Patients. Average age at diagnosis of the 50 consecutive patients who comprised the study population was 54.3 ± 12.9 years (range, 30–87 years). Median follow-up was 59 months (range, 4–132 months). Patient characteristics are summarized in Table 1. In two patients, the initial planned treatment was surgery but, due to the extent of the disease found during the procedure, it was decided to abort the operation and the patients were referred for definitive chemo-radiotherapy. One patient underwent surgery due to a pelvic abscess, and the diagnosis of cervical cancer was established during this procedure; this patient underwent unilateral salpingo-oophorectomy due to the proximity to the abscess and then was referred for definitive chemo-radiotherapy. Seven patients were lost to follow-up for evaluation of long-term toxicity; however, data of overall survival were available for all the patients and was based on the results of the population census as recorded in the population registry of the Ministry of the Interior.
Table 1.
Patients’ characteristics (n = 50).
| n of patients | |
|---|---|
| Histology | |
| Squamous cell carcinoma | 39 |
| Adenocarcinoma | 2 |
| Other | 5 |
| FIGO stage | |
| 1B1 | 1 |
| 1B2 bulky | 13 |
| 2B | 29 |
| 3B | 6 |
| Unknown | 1 |
| Grade | |
| 1 | 3 |
| 2 | 16 |
| 3 | 19 |
| Unknown | 12 |
Treatment. Forty-eight patients received concomitant chemo-radiotherapy (pelvic EBRT) followed by HDR brachytherapy. One patient received EBRT and HDR brachytherapy without chemotherapy because of Crohn's disease. One patient received concomitant chemo-radiotherapy (pelvic EBRT) at a different institution; the chemotherapy regimen given was not documented in the medical record. For all patients, EBRT was performed prior to HDR brachytherapy. Cisplatin at a dose of 40 mg/m2 was used concomitantly with pelvic irradiation in all except two patients in whom carboplatin was used (area under the curve of 2) due to renal insufficiency. Not all the patients received the planned chemotherapy cycles: acute gastrointestinal toxicity (diarrhea) was the main indication to discontinue chemotherapy.
EBRT. Patients were planned to be treated with external radiotherapy to the pelvis at a dose of 45–50.4 Gy (25–28 fractions, 1.8 Gy per fraction, 5 fractions per week) using 6 MV or 18 MV beams from a Varian Clinac 1800 linear accelerator (Varian Medical Systems, Inc., Palo Alto CA), according to our department's protocol. The actual doses were 39.6–50.4 Gy (average, 45–47 Gy). Four patients received 46 Gy, 2 Gy per fraction. Depending on the patient's body habitus, either two opposing (anterior–posterior) fields (true for most patients) or the 4-field box technique (for larger body habitus) were used. Twenty-one (42%) patients had gross parametrium involvement and an EBRT boost was added. Median boost dose was 10 Gy given as 2 Gy per fraction, 5 fractions per week (range, 5.4–10 Gy). The boost was given with a central block in the vagina and uterine-cervix area.
HDR brachytherapy. HDR brachytherapy was performed using the MicroSelectron system (Nucletron, an Elekta company (Elekta AB, Stockholm, Sweden) using Ir192 source. Prior to the first HDR brachytherapy fraction, patients underwent intrauterine stent insertion. Brachytherapy was performed with gauze packing. Intrauterine applications were performed in the lithotomy position. Nucletron (Elekta) “Fletcher-Suit like” tandem and ovoid applicators were used, with tandem curvatures of 15°, 30°, or 45°, and ovoid cap diameters of 16, 20, or 25 mm. The HDR fractionation scheme was planned for five fractions of 5–5.5 Gy each (depending on clinical evaluation of the tumor size). The Nucletron (Elekta) PLATO BPS v.13.7 treatment planning system was used. Treatment doses were prescribed at point “A”. Doses according to the International Commission on Radiation Units and Measurements (ICRU) rectal and bladder reference points, and point “B” doses (ICRU Report 38) were recorded for each treatment.10 As a standard, five active dwell positions separated by 5 mm steps were used in each ovoid to reproduce a traditional 2 cm LDR Cs137 source. Manual optimization of dwell times was performed in both the “ovoids” and the “tandem” to minimize rectal and bladder doses while retaining the traditional “pearshape” dose distribution. Brachytherapy sessions were conducted once a week. Two patients did not complete five HDR brachytherapy sessions (3 and 4 sessions out of 5 planned sessions). In the patient who received three HDR sessions, the intrauterine stent was displaced after the third session; she was given another single LDR fraction of 18 Gy to point “A”. One patient received four sessions of 6.25 Gy and another patient received five sessions of 5.9 Gy.
Fig. 2 summarizes the brachytherapy doses for the lateral ovoid point and the maximal brachytherapy point (upper central point) for each patient. The maximal HDR vaginal surface dose (upper central point) was 103 Gy. The maximal brachytherapy lateral surface dose was 70 Gy.
Fig. 2.
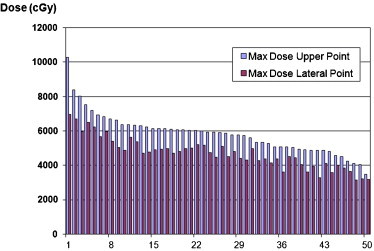
Brachytherapy doses for the lateral ovoid point and the upper central point (cGy).
Cumulative biological effective dose. The cBED for all patients is presented in Fig. 3. The cBED calculated using the lateral surface reference point was a median of 269 Gy3 (range, 168.5–465.5 Gy3). The cBED calculated using the upper central reference point was 368.5 Gy3 (range, 226–878.6 Gy3).
Fig. 3.
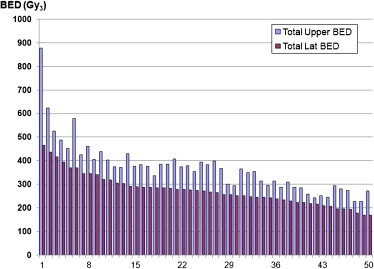
Cumulative biological effective dose (cBED) for all patients (Gy3).
Vaginal, rectal and bladder toxicity. Seven patients were lost to follow-up for evaluation of long-term toxicity. There were no fistulas (recto-vaginal or cysto-vaginal) or CTCAE grade IV late vaginal, rectal or bladder toxicity. Rectal and bladder toxicity are summarized in Table 2. There were no cases of necrosis of the distal vagina or vaginal vault. No relationship was found between the total physical dose of EBRT plus HDR/or maximal HDR brachytherapy dose and the severity of vaginal, rectal or bladder complications (P = NS).
Table 2.
Late rectal and bladder complications.
| CTCAE gradea | Gastrointestinal | n of patients |
|---|---|---|
| 1–2 | Diarrhea | 9 |
| 2 One patient had grade 3 proctitis |
Proctitisb | 4 |
| 3 | Incomplete bowel obstruction | 1 |
Notes: No correlation was found to total physical dose of EBRT and HDR or maximal HDR brachytherapy dose (P = NS).
Common Terminology Criteria for Adverse Events (CTCAE) Version 4.0.
Proven by endoscopy.
Detailed vaginal examinations recorded in the medical files, which enabled the assessment of late vaginal patency (≥12 months), were available for 25 patients: 7 (28%) patients had no obliteration, 7 (28%) patients had mild shortened vagina, 7 (28%) patients had moderately obliterated and shortened vagina, and 4 (16%) patients suffered from complete vaginal obliteration.
Data of intercourse were available for 12 patients during long-term follow-up (≥12 months), and four of these patients indicated being sexually active. Eight patients indicated that they were not sexually active, four due to partner issues (partner's medical condition or no partner). One patient indicated that she suffered from dyspareunia.
Recurrence, second malignancy and overall survival. Thirteen patients had recurrent disease, nine of whom had distant recurrences. Two patients were staged at diagnosis as FIGO 1B2 bulky disease, two were FIGO 2B, and five patients were FIGO 3B. Nine of 43 (20%) patients had a second primary malignancy. In two patients, the secondary malignancy was within the irradiation field: one patient had a bladder carcinoma in situ and one patient was diagnosed with ovarian cancer one year after completion of treatment. Overall survival rate was 25% for 50 patients.
5. Discussion
In the current study, the tolerance dose of the vaginal vault and late complications were assessed based on clinical data of 50 patients. Median follow-up was 59 months, as a tertiary institution some patients after a period of time (usually 5 years) are referred back to their community physician. The maximal surface HDR brachytherapy dose of 103 Gy and the maximal cBED of 878.6 Gy3 were not associated with fistula or necrosis or other grade 3–4 vaginal complications. These findings are similar to those reported by Toita et al.11 In their study, there were no cases of vaginal ulceration, fistula or necrosis at a median follow-up of 48 months with a vaginal cBED ranging from 185.5 to 618 Gy3 (median, 324 Gy3). The cBED in that study was calculated according to the dose prescribed to point “A”, which is probably lower than the calculated vaginal surface dose which was used to calculate cBED in the current study.9 A retrospective analysis of 222 consecutive patients receiving radical treatment for invasive cervical cancer reported a higher rate of recto-vaginal cases of fistula, the investigators found a strong association between the risk of developing a fistula and the BED at rectal reference point.12
The sum of physical doses of EBRT (without the boost) and maximal HDR dose in the current study was 148 Gy. This sum of physical doses was conducted for a “historical control” and should not be used in practice.1,2 However, the value is similar to that reported by Au and Grigsby,1 238 Gy for LDR brachytherapy.
No correlation was found between the values of the maximal HDR surface doses calculated and vaginal, rectal or urinary complications, indicating that these complications might follow a stochastic model.
Radiobiologically-wise, HDR-brachytherapy is expected to result in more normal tissue toxicity than LDR-brachytherapy.13 Yet our study had similar complication rates to those reported by Au and Grigsby1 for LDR brachytherapy. In that study as well, there were no grade 4 complications. In the current study, all patients were treated by using tandem and ovoid applicators. More studies are needed to evaluate the doses when a ring applicator is used, as it may result in a slightly narrower distribution, leading to a higher vaginal dose and potentially higher toxicity.14
The primary endpoint of our study was the evaluation of late vaginal toxicity (primary – vaginal necrosis) according to the HDR intracavitary brachytherapy doses that were given. The cBED was calculated based on both external beam dose and HDR-brachytherapy dose. When using the lateral surface reference point, the cBED was a median of 269 Gy3 (range, 168.5–465.5 Gy3) and the cBED calculated using the upper central reference point was 368.5 Gy3 (range, 226–878.6 Gy3) without vaginal cuff necrosis and/or fistula, at a median follow-up of 59 months. Our study indicates that the vaginal vault tolerance to HDR intracavitary brachytherapy is high, similar to the reports of Au and Grigsby1 for LDR brachytherapy. It seems that the tolerance of the vaginal cuff is greater than cBED of 368.5 Gy3. However, the study has a small patient population and conclusions should be drawn with caution.
We also reported the rates of vaginal obliteration; however, we do not consider this as an endpoint for estimating vaginal vault tolerance. The development of vaginal obliteration also depends on other cofactors, such as vaginal involvement, length of the treated vagina, vaginal washings after treatment, regular dilation, sexual activity, etc. Owing to the retrospective nature of this study, this information was only partially available. In our practice, all patients are routinely instructed to use vaginal dilators. Vaginal obliteration in this young patient population is a disabling side effect, and efforts should be made to avoid this complication.
Combinations of dose-specification methods, insertion techniques and normal tissue dose relationships from different clinical systems can be dangerous and should be avoided. There are many differences between HDR treatment plans and it is very challenging (or impossible) to evaluate a true biological–clinical effect. Thus, there is a need for a spectrum of maximal surface doses that are safe without causing vaginal-vault necrosis and acceptable long-term complication rates. Hopefully, data of long-term follow-up of 3D image-guided brachytherapy will be available in the near future to aid in the determination of appropriate tolerance levels of the vaginal mucosa.
Although our study is a retrospective report of 2D technique, it should be emphasized that 2D is still employed in some countries in which this disease is prevalent. Since our data provide spatial information of maximal dose, it is of utmost importance, as this information is not provided by the DVH. According to our study, the maximal surface HDR brachytherapy dose of 103 Gy and the maximal cBED of 878.6 Gy3 were not associated with fistula or necrosis or other grade 3–4 vaginal complications. Concomitant chemo-radiotherapy, including pelvic radiotherapy and high-dose-rate intracavitary brachytherapy, is relatively safe for cervical cancer patients.
Conflict of interest
None declared.
Financial disclosure
None declared.
Acknowledgements
The authors thank Mrs. Ronit Leiba MA, Biostatistician, Quality Improvement Unit, Rambam Health Care Campus, Haifa, Israel, for her statistical help.
Footnotes
This study was presented as an Oral Presentation at the Annual Meeting of the Israeli Society of Clinical and Radiation Oncology (ISCORT) in Eilat, Israel, in January 2012. It was also presented as a Poster Presentation at ESTRO 31 in Barcelona, Spain, May 9–13, 2012.
References
- 1.Au S.P., Grigsby P.W. The irradiation tolerance dose of the proximal vagina. Radiother Oncol. 2003;67:77–85. doi: 10.1016/s0167-8140(02)00384-5. [DOI] [PubMed] [Google Scholar]
- 2.Hintz B.L., Kagan A.R., Chan P. Radiation tolerance of the vaginal mucosa. Int J Radiat Oncol Biol Phys. 1980;6:711–716. doi: 10.1016/0360-3016(80)90227-8. [DOI] [PubMed] [Google Scholar]
- 3.Katz A., Njuguna E., Rakowsky E., Sulkes A., Sulkes J., Fenig E. Early development of vaginal shortening during radiation therapy for endometrial or cervical cancer. Int J Gynecol Cancer. 2001;11:234–235. doi: 10.1046/j.1525-1438.2001.01019.x. [DOI] [PubMed] [Google Scholar]
- 4.Mai J., Erickson B., Rownd J., Gillin M. Comparison of four different dose specification methods for high-dose-rate intracavitary radiation for treatment of cervical cancer. Int J Radiat Oncol Biol Phys. 2001;51:1131–1141. doi: 10.1016/s0360-3016(01)01771-0. [DOI] [PubMed] [Google Scholar]
- 5.Micha J.P., Goldstein B.H., Rettenmaier M.A., Caillouette J.T., Fee M.J., Brown J.V., 3rd Pelvic radiation necrosis and osteomyelitis following chemoradiation for advanced stage vulvar and cervical carcinoma. Gynecol Oncol. 2006;101:349–352. doi: 10.1016/j.ygyno.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 6.Gellrich J., Hakenberg O.W., Oehlschläger S., Wirth M.P. Manifestation, latency and management of late urological complications after curative radiotherapy for cervical carcinoma. Onkologie. 2003;26:334–340. doi: 10.1159/000072091. [DOI] [PubMed] [Google Scholar]
- 7.Matthews K.S., Rocconi R.P., Straughn J.M., Jr. Complete uterine necrosis following chemoradiation for advanced cervical cancer: a case report. Gynecol Oncol. 2007;106:265–267. doi: 10.1016/j.ygyno.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 8.Güth U., Ella W.A., Olaitan A., Hadwin R.J., Arora R., McCormack M. Total vaginal necrosis: a representative example of underreporting severe late toxic reaction after concomitant chemoradiation for cervical cancer. Int J Gynecol Cancer. 2010;20:54–60. doi: 10.1111/IGC.0b013e3181c4a63f. [DOI] [PubMed] [Google Scholar]
- 9.Nevelsky A., Bar-Deroma R., Cokal G.Y., Rosenblatt E., Kuten A. Definition of vaginal doses in intrauterine high-dose-rate brachytherapy. Brachytherapy. 2004;3:101–105. doi: 10.1016/j.brachy.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 10.International Commission on Radiation Units and Measurement (ICRU) ICRU Publications; Bethesda, MD: 1985. ICRU Report 38: dose and volume specification for reporting intracavitary therapy in gynecology. [Google Scholar]
- 11.Toita T., Kakinohana Y., Ogawa K. Combination external beam radiotherapy and high-dose-rate intracavitary brachytherapy for uterine cervical cancer: analysis of dose and fractionation schedule. Int J Radiat Oncol Biol Phys. 2003;56:1344–1353. doi: 10.1016/s0360-3016(03)00288-8. [DOI] [PubMed] [Google Scholar]
- 12.Lebioda A. Rectovaginal fistula risk doses in patients with cervical cancer. Rep Pract Oncol Radiother. 2004;9:37–43. [Google Scholar]
- 13.Kubo H.D., Glasgow G.P., Pethel T.D., Thomadsen B.R., Williamson J.F. High dose-rate brachytherapy treatment delivery: report of the AAPM Radiation Therapy Committee Task Group No. 59. Med Phys. 1998;25:375–403. doi: 10.1118/1.598232. [DOI] [PubMed] [Google Scholar]
- 14.Viswanathan A.N., Beriwal S., De Los Santos J.F. American Brachytherapy Society consensus guidelines for locally advanced carcinoma of the cervix. Part II: High-dose-rate brachytherapy. Brachytherapy. 2012;11:47–52. doi: 10.1016/j.brachy.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]


