Abstract
Despite many studies investigating exercise-induced hypoalgesia, there is limited understanding of the optimal intensity of aerobic exercise in producing hypoalgesic effects across different types of pain stimuli. Given that not all individuals are willing or capable of engaging in high intensity aerobic exercise, whether moderate intensity aerobic exercise is associated with a hypoalgesic response and whether this response generalizes to multiple pain induction techniques needs to be substantiated.
Purpose
This study’s purpose is to test for differences in the magnitude of pressure and heat pain modulation induced by moderate (MAE) and vigorous (VAE) intensity aerobic exercise.
Methods
Twelve healthy young males and 15 females completed one training session and three testing sessions consisting of 25 minutes of either 1) stationary cycling at 70% heart rate reserve (HRR), 2) stationary cycling at 50% HRR, or 3) quiet rest (control). Pain testing was conducted on both forearms prior to and immediately following each condition and included the following tests: pressure pain thresholds (PPT), suprathreshold pressure pain test, static continuous heat test, and repetitive pulse heat pain test. Repeated measures ANOVAs were conducted on each pain measure.
Results
VAE and MAE reduced pain ratings during static continuous heat stimuli and repetitive heat pulse stimuli, with VAE producing larger effects. VAE also increased PPTs, while neither exercise influenced suprathreshold pressure pain ratings.
Conclusion
These results suggest that MAE is capable of producing a hypoalgesic effect using continuous and repetitive pulse heat stimuli. However, a dose-response effect was evident as VAE produced larger effects than MAE.
Keywords: aerobic exercise, exercise-induced analgesia, pressure pain, thermal pain, temporal summation of pain
Numerous studies have demonstrated that an acute bout of aerobic exercise temporarily reduces experimentally induced pain (See Naugle et al. for a review, 25); a phenomenon termed exercise-induced analgesia or exercise-induced hypoalgesia (EIH: 15,16). Human pain perception is a dynamic and multifaceted construct that cannot be adequately assessed with a single stimulus modality (i.e., pressure, thermal, electrical). Furthermore, different temporal patterns of stimulation within a single modality can be used to assess different aspects of the pain experience (e.g. A-delta fiber mediated sharp, well localized sensations called “first pain” v. C-fiber mediated late burning sensations termed “second pain”) (29). Along these lines, past studies indicate that experimental pain measures correlate only moderately across stimulus modalities and tests (10,27), demonstrating the importance of multimodal pain assessment. However, this practice has rarely been utilized in past EIH research and caution should be made in generalizing findings based on responses to one type of experimental pain test.
A recent meta-analytic review of the hypoalgesic effects of aerobic exercise reported a broad range of effect sizes (i.e., .04 to 1.47), which was likely a function of both the intensity of exercise and the form or modality of pain induction technique (25). For example, using temporal summation (TS) of heat pain, Vierck et al. showed that vigorous intensity aerobic exercise reduces the magnitude of late pain responses to stimulation of C-fiber nociceptors (38). In the TS pain induction model, brief noxious heat pulses held at a constant intensity are delivered to the skin at intervals of 3 seconds or less. This temporal pattern of stimulation is characterized by C-fiber mediated late burning sensations that intensify with time (34). However, using a continuous thermal pain model, Ruble et al. found no effect of treadmill exercise at 75% of VO2max on pain perception in response to continuous static hot and cold stimuli (31). Thus, given that the generality of EIH among different experimental pain procedures remains unclear, the present study was designed to evaluate the hypoalgesic effect of exercise among a range of pain induction techniques, including TS of heat pain and continuous static heat pain, in the same sample of participants. This information could potentially provide insights into the pain pathways involved with EIH.
Furthermore, the potential application of aerobic exercise as a form of pain management requires a comprehensive understanding of the intensity thresholds required to elicit EIH. Indeed, not all individuals are willing or capable of engaging in high-intensity aerobic exercise. Only one study has investigated potential thresholds for both intensity and duration of the aerobic exercise required to elicit exercise-induced hypoalgesia (12). Hoffman and colleagues discovered that pain intensity ratings for pressure applied to the index finger were reduced following 30 minutes of treadmill running at 75% VO2max (12). However, a hypoalgesic effect of treadmill running was not found at 75% VO2max for 10 minutes or 50% VO2max for 30 minutes. Importantly, the data for 50% VO2max exercise revealed a reduction in pain ratings following exercise that approached significance and had a moderate effect size with only 12 participants. Thus, the failure to detect a hypoalgesic effect of moderately intense exercise may have been due to a lack of power from an insufficient number of subjects. Additionally, intensity thresholds for aerobic EIH have not been tested with other pain induction methods. As such, additional studies are needed to clarify the intensity of aerobic exercise required to elicit EIH.
The purpose of this study was twofold. The first purpose was to investigate the effect of submaximal aerobic exercise on experimental pain produced by different pain induction procedures that are commonly used in human pain research. The second purpose was to examine whether the effect of aerobic exercise on pain sensitivity and perception is dependent on the intensity of the exercise. To address these aims a series of pain induction protocols was administered pre and post moderate aerobic exercise, vigorous aerobic exercise, and quiet rest. Pain measures included pressure pain thresholds, ratings of suprathreshold pressure pain, pain ratings during static continuous heat pain stimuli, and magnitude of late heat pain responses during a series of noxious heat pulses. Based on the results of a recent meta-analysis (25), we hypothesized that 1) aerobic exercise would produce a hypoalgesic effect among all pain modalities and 2) the largest effect would be associated with the exercise of higher intensity.
METHODS
Participants
Participants were twenty-seven (15 female) healthy young adults. Participant descriptors are presented in Table 1. Participants were recruited through posted advertisements in the local community. Exclusion criteria included: 1)inability to reliably rate pain intensity, 2) current use of narcotics or tobacco products, 3) uncontrolled hypertension, 4) neurological disease with significant changes in somatosensory and pain perception at intended stimulation sites, 5) the known presence of or any signs or symptoms of cardiovascular disease, pulmonary disease, or metabolic disease, 6) serious psychiatric conditions (e.g., schizophrenia and bipolar disorder), and 7) not physically ready to exercise without a medical exam as indicated by the Physical Activity Readiness Questionnaire (PAR-Q: 32). Session exclusion criteria included active infectious disease or febrile condition (e.g., sinusitis, influenza) and severe uncontrolled hypertension. Additionally, participants were instructed to refrain from use of coffee or any pain medications prior to the experimental sessions.
Table 1.
Effect sizes (Cohen’s d) demonstrating the magnitude of the hypoalgesic effect of each condition for each pain induction technique
| Pain Test | Condition
|
||
|---|---|---|---|
| Control | MAE | VAE | |
| Pressure pain threshold | −0.01 | 0.24 | 1.07 |
| Suprathreshold pressure pain | −0.26 | 0.30 | 0.10 |
| Continuous heat pain at 30 s | 0.25 | 0.59 | 0.83 |
| Pulse heat pain – mag. of late sensations* | 0.11 | 0.35 | 0.47 |
Note. A positive effect size represents a reduction in pain sensitivity following exercise or rest.
Effect size for ratings of late pain sensations after pulse 10; mag=magnitude; MAE=moderate aerobic exercise; VAE=vigorous aerobic exercise.
Procedures
The University’s Institutional Review Board (IRB) approved all procedures and participants signed an IRB-approved informed consent form. Participants completed a screening/training session followed by three randomized experimental sessions. All sessions were separated by a minimum of 48 hours and conducted at approximately the same time of day (± 2 hours). All sessions began after two stable blood pressure readings separated by 5 minutes.
Screening and training session
To determine eligibility, participants completed the PAR-Q, a health history questionnaire, supplemented by clarification by interview, height and weight measurement, and a resting heart rate (HR) and blood pressure measurement. Once eligibility was determined, participants completed a training session designed to 1) teach them the continuous pain rating system, and 2) determine the individualized temperatures of the thermal stimuli for the heat pain testing protocols such that participants would experience moderate pain (i.e., 50/100 on a 0–100 visual analogue scale). Additionally, the experimental pain testing procedures were conducted once during the training session to ensure familiarity with the testing protocols.
Experimental sessions
Participants completed three experimental sessions in randomized order consisting of one of the following conditions: vigorous intensity aerobic exercise, moderate intensity aerobic exercise, and quiet rest. At the beginning of each session, participants were fitted with a Polar Heart Rate monitor (FT7) (Polar Electro, Lake Success, NY), which monitored and collected heart rate (HR) at rest (sitting) and during exercise. Heart rate was similarly measured during all experimental sessions. During each session, four different pain tests were administered on each forearm followed by 25 minutes of exercise or quiet rest. Blood pressure was immediately taken upon completion of exercise or quiet rest followed by the administration of the same 4 pain tests in the same order as the pre-exercise pain tests. The administration of the 4 pain tests pre and post exercise took under 10 minutes to complete. Figure 1 shows a timeline of an experimental session.
Figure 1.

Timeline of procedures during the experimental sessions. The bidirectional arrows between the pressure pain tests and the heat pain tests indicate that these tests were conducted in counterbalanced order. The site of pain testing alternated between left and right arms, so that one arm was never tested consecutively. Participants maintained the same pain testing order for every session pre and post exercise and quiet rest. PPT=pressure pain threshold; PPS= pressure pain suprathreshold test; R=right forearm; L=left forearm.
Experimental Session 1: Acute bout of vigorous aerobic exercise
This session tested for changes in pain sensitivity and perception (as described under experimental pain measures) following 25 minutes of vigorous stationary cycling. Participants cycled the first 5 minutes at an intensity of up to 50% heart rate reserve (warm-up period), followed by 20 minutes at 70% heart rate reserve (HRR) (28). The speed and/or resistance of the cycle ergometer were adjusted to meet the prescribed intensity (target HR zone) throughout the exercise bout. The following data were recorded every five minutes during exercise: 1) the wattage of the cycle ergometer, 2) ratings of perceived exertion (RPE) using Borg’s 6–20 scale (1), and 3) heart rate with a heart rate monitor. A target HR was determined for each participant using the Karvonen formula (14). The Karvonen formula is related to the percent of age-predicted maximal heart rate but allows for differences in resting HR with the following formula: . Age predicted maximal heart rate = 220-age.
Experimental session 2: Acute bout of moderate intensity aerobic exercise
This session tested for changes in pain sensitivity following 25 minutes of moderate intensity stationary cycling. This session was identical to the vigorous exercise session, however, following the 5 minute warm-up period participants cycled for 20 minutes at an intensity of 50–55% HRR (27).
Experimental session 3: Quiet Rest – control condition
This session tested for changes in pain sensitivity and perception following 25 minutes of quiet rest. Participants remained in a seated position for the entire 25 minutes and were allowed to read. Heart rate was recorded every five minutes.
Psychophysical Pain Testing
Participants were administered 4 different pain tests to each forearm pre and post exercise or quiet rest, including: 1) pressure pain thresholds, 2) suprathreshold pressure pain trial, 2) continuous static heat pain trial, and 4) pulse heat pain trial. The order of the pain tests are shown in Figure 1 and were conducted as follows: 1) a pressure pain test administered to both forearms, 2) a continuous or pulse heat pain test administered to one forearm, 3) a continuous or pulse heat pain test administered to the other forearm, 4) a pressure pain test administered to both forearms 5) a continuous or pulse heat pain test administered to one forearm, 6) a continuous or pulse heat pain test administered to the other forearm. The site of pain testing alternated between left and right arms, so that one arm was never tested consecutively. Additionally, the order of the pressure and heat pain tests, as well as the bodily site (right v. left arm) was counterbalanced among participants. Participants maintained the same pain testing order for every session pre and post exercise and quiet rest.
Pressure Pain Thresholds (PPT)
Pressure pain threshold was assessed with a handheld algometer (Jtech, Heber City, Utah) on the right and left ventral forearm, approximately 8 cm distal to the elbow. The tip of the algometer consisted of a rubber flat 1.0 cm2 probe. Pressure stimuli were delivered to the forearm at an approximate rate of 0.5 kg/s. Participants were instructed to respond verbally when the pressure sensation first became painful, at which the algometer was removed. The amount of pressure applied to the forearm did not exceed 5 kg. Pressure pain threshold was defined as the amount of pressure in kilograms at which the participant first reported experiencing pain.
Suprathreshold Pressure Pain Test
Ratings of suprathreshold pressure stimuli were also assessed on the right and left ventral forearm with the same handheld algometer used for PPTs. The site of the pressure pain threshold and suprathreshold assessments were always separated by a minimum of 1 cm on the forearm and the same sites were used for pre and post assessments. Pressure stimuli were delivered to the forearm at an approximate rate of 0.5 kg/s, until 5 kg was applied. This level of pressure was chosen to assess stimulus intensity without producing excessive discomfort. Immediately after each trial, participants rated the intensity of the stimulus on a 0 to 100 visual analogue scale (VAS), with “0” indicating “no pain” and “100” indicating “intolerable pain”.
Continuous Heat Pain Test
Contact heat stimuli were delivered by a computer controlled Peltier-based thermode (32 mm × 32 mm; TSA-2001, Ramat Yishai, Israel) to the right and left ventral forearms. For each 30-second continuous heat pain trial, the thermode was first brought to a neutral temperature (32°C) and then ramped (2.0°C/s) to the individualized temperature (44–49°C) determined during the training session and maintained at that temperature for 30 s. The thermode position was altered slightly between each heat trial (i.e., continuous and pulse heat pain trials). The intensity of the pain produced by the contact thermode was rated every 5 s on a 0 to 100 VAS.
Repetitive Pulse Heat Pain Test
Brief repetitive suprathreshold heat pulses were delivered to the right and left ventral forearms. Each trial consisted of a series of 10 heat pulses, with each pulse delivered at a rate of 10°C/s. The peak to peak inter-pulse interval was 2.5 seconds. The baseline temperature was 38°C and the target temperature was the individualized temperature that produced moderate pain as determined during the training session (45°C – 51.5°C). Participants were instructed to rate the intensity of the late pain sensations experienced after each pulse (i.e., pain felt between the pulses not during each pulse, termed “second pain”) with a 0–100 scale. Prior work has shown that during a series of nociceptive pulses administered at a frequency greater than .3 Hz, C-fiber mediated responses of nociceptive dorsal horn neurons and the sensation of second pain progressively increase (29, 37). Thus, the pulse heat pain test permitted the assessment of the effect of exercise on the magnitude and temporal summation (TS) of C-fiber mediated heat pain sensations (i.e., late burning heat pain sensations).
Data Analysis
Descriptive statistics were calculated for HR, percentage of HRR, and RPE for each exercise session. One participant’s average HRR% for the vigorous aerobic exercise was only 55%; therefore, this participant’s data were not included in the statistical analyses. During the suprathreshold pressure pain test, one participant did not report experiencing any pressure pain after the target pressure of 5 kg was reached; thus this participant’s data was removed from the suprathreshold pressure pain analyses. During the PPT test, two participants did not report experiencing pain before the upper limit of pressure for the test was reached; therefore their data were not included in the PPT analyses.
Preliminary testing using separate mixed model ANOVAs for each condition (moderate aerobic exercise, vigorous aerobic exercise, Control) indicated that the effect of exercise on experimental pain did not differ between forearms or sex for any of the psychophysical pain tests. Thus, the data for each pain test was averaged between the two forearms and sex was not included as a factor for further analyses. The data for PPTs and ratings of suprathreshold pressure pain were analyzed with 3 (condition: moderate aerobic exercise, vigorous aerobic exercise, Control) × 2 (time: pre, post exercise) repeated measures ANOVAs. Data for the continuous heat pain test were analyzed with a 3 (session: moderate aerobic exercise, vigorous aerobic exercise, Control) × 2 (trials: pre, post exercise) × 6 (time: 5s, 10s, 15s, 20s, 25s, 30s) repeated measures ANOVA. Data for the magnitude of the late pain sensations during the pulse heat trial were analyzed with a 3 (session: moderate aerobic exercise, vigorous aerobic exercise) × 2 (trials: pre, post exercise) × 10 (pulse: 1,2,3,4,5,6,7,8,9,10) repeated measures ANOVA with repeated measures on the last three factors. If the sphericity assumption was violated, then Greenhouse-Geisser degrees of freedom corrections were applied to obtain the critical p-value. Post-hoc comparisons were made with Tukey’s HSD procedure. A level of p ≤ .05 was used for all statistical analyses. The effect size (ES) for each pain test was calculated using Cohen’s d, defined as the mean for the pretest minus the mean for the posttest, divided by the pooled within group standard deviation (d=[Xpretest − Xposttest]/pooled standard deviation). Effect sizes were calculated so that reductions in pain resulted in positive effect sizes. Due to the within subjects designs of the studies, the effect sizes were adjusted as recommended by Morris and DeShon (24). Finally, bivariate correlations were conducted to determine if exercise-induced hypoalgesic responses were consistent across the experimental pain tests for each condition (moderate exercise, vigorous exercise, quiet rest). For each condition, the exercise-induced hypoalgesic response for each pain test was calculated by subtracting the pretest score from the posttest score. For the continuous heat pain test and pulse heat pain test, pain ratings at 30s and after pulse 10, respectively, were used to calculate the EIH response.
RESULTS
Descriptive Characteristics
The average age of participants was 21.78 (SD=4.14). The RPE and heart data indicated that on average participants exercised at a moderate pace during the moderate aerobic exercise session (average HRR%=52.62, SD=2.42; average HR=123.68, SD=6.63; average RPE=11.95, SD=1.82) and at a vigorous pace during the vigorous aerobic exercise session (average HRR%=69.61, SD=4.31; average HR=142.07, SD=5.35; average RPE=14.49, SD=1.46).
PPTs
Figure 2a shows the means and standard errors of the pre- and posttests for each condition. The repeated measures ANOVA revealed a significant main effect of Trial (p=.03), which was superseded by a significant session × trial interaction, F(2,46)=4.11, p=.023. The follow-up tests indicated that PPTs increased significantly following the vigorous aerobic exercise, but not after moderate aerobic exercise and quiet rest. As displayed in Table 2, VAE produced a large effect size, d=1.07. Furthermore, PPTs at pretest during the vigorous aerobic exercise session were significantly lower than the posttest PPTs during the moderate aerobic exercise session. Given this finding, it was possible that the significant effect of vigorous exercise was due to the lower pretest PPT value. Thus, we examined the correlations between the pretest PPT values and the exercise-induced increases in PPT (posttest – pretest). The correlational analysis was not significant (p=0.44, r=0.108), suggesting that while the lower PPT values before VAE were unexpected, they likely did not influence the exercise induced changes. Additionally, further inspection of the data indicated that while the overall test-retest reliability was good (> .80) four participants exhibited high variability in pre-exercise PPT values between days of testing (±1kg). Therefore, these four participants were removed and the data was reanalyzed. The session x trial interaction was still significant at p=.047. Follow-up tests indicated only a significant difference between the pre and posttest PPTs during the vigorous exercise session (Control: pre=2.78±1.08, post=2.80±.09; moderate AE: pre=2.85±1.03, post=3.022±1.12; vigorous AE: pre=2.73±.98, post=3.05±1.17). The effect sizes only changed a little from the original analysis: Control ES=.09 v. −.01, moderate AE ES=.37 v. 0.24, vigorous AE ES=.94 v. 1.07. Thus, we are confident that the significant effect of vigorous exercise on PPT’s was not attributable to the lower pretest value.
Figure 2.
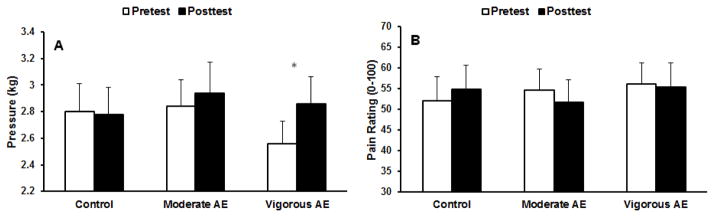
(A) Means and standard errors (SE) for pressure pain thresholds for pre- and post-tests for each exercise condition. (B) Means and SE for ratings of suprathreshold pressure pain for pre- and post-tests for each exercise condition. *p<.05.
Table 2.
Bivariate correlations among EIH responses to experimental pain tests for each condition
| PPT | SPP | CHP | PHP | |
|---|---|---|---|---|
| Control condition | ||||
| Pressure pain threshold | 1 | −.086 | −.099 | −.185 |
| Suprathershold presssure pain | 1 | −.049 | .321 | |
| Continuous heat pain | 1 | .221 | ||
| Pulse heat pain | 1 | |||
| Moderate Aerobic Exercise condition | ||||
| Pressure pain threshold | 1 | −.522* | −.227 | −.212 |
| Suprathershold presssure pain | 1 | −.060 | .236 | |
| Continuous heat pain | 1 | .221 | ||
| Pulse heat pain | 1 | |||
| Vigorous Aerobic Exercise condition | ||||
| Pressure pain threshold | 1 | −.154 | .072 | −.281 |
| Suprathershold presssure pain | 1 | .303 | .153 | |
| Continuous heat pain | 1 | .092 | ||
| Pulse heat pain | 1 | |||
Note. PPT= Pressure pain threshold; SPP= Suprathershold presssure pain; CHP= Continuous heat pain; PHP= Pulse heat pain;
significant at p<.05..
Suprathreshold pressure pain test
Means and standard errors of suprathreshold pressure pain ratings for pre and post tests for each condition are shown in Figure 2b. No effects were significant (session × trial, p=.07), indicating that acute exercise did not affect suprathreshold pressure pain ratings. The effect sizes for each exercise condition, while in the expected direction (indicating pain reduction), were small.
Continuous heat pain test
The repeated measures ANOVA revealed significant main effects of trial (p=.001) and time (p=.048), which were superseded by a significant condition × trial × time interaction, F(3.38,84.45)=5.73, p=.001. The post-hoc tests revealed that pain intensity ratings reported at 25 s and 30 s during the 30-second constant heat trials were reduced following moderate aerobic exercise. Similarly, pain ratings reported at 20–30 s during the 30-second heat trial were reduced following vigorous aerobic exercise. Figure 3 shows the average pain intensity ratings across the 30-second trial for the pre- and posttests for each condition. In Table 2, effect sizes are displayed for the pain ratings at 30 s, given that the hypoalgesic effect of exercise was most evident at this time point. This data revealed a moderate effect size for moderate aerobic exercise and a large effect size for vigorous aerobic exercise.
Figure 3.
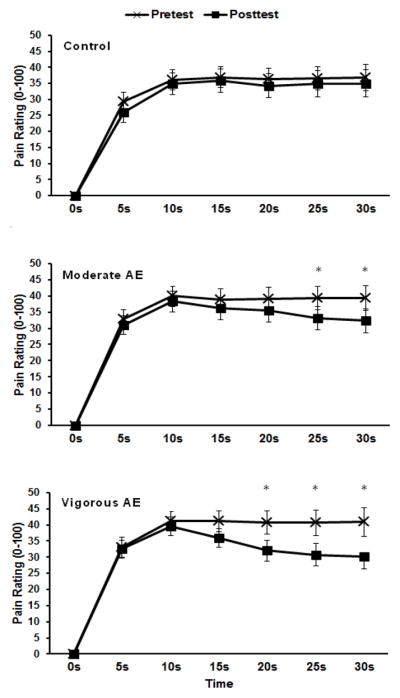
Means and SE for pain intensity ratings across the 30 s continuous heat pain trials for pre- and post-tests during the control, moderate aerobic exercise (AE), and vigorous aerobic exercise conditions (AE). *p<.05.
Pulse heat pain test
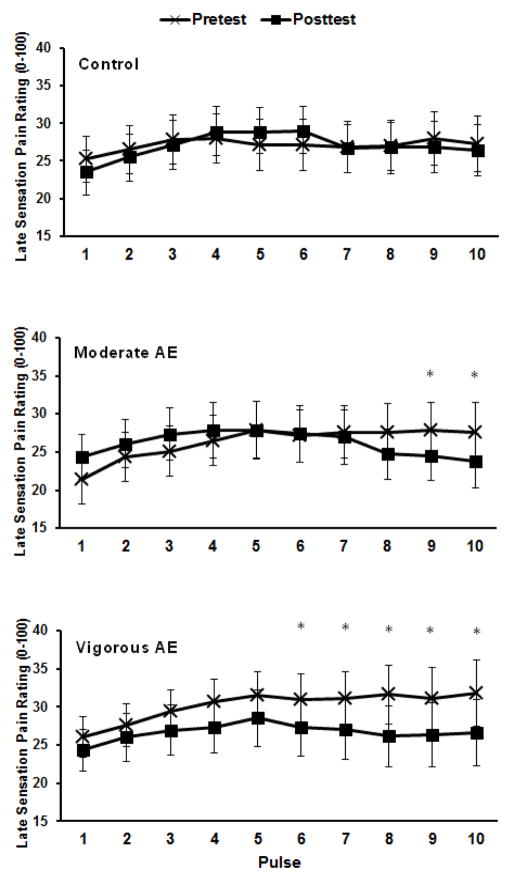
The ANOVA for the magnitude of the late pain sensations during the pulse heat trials showed a significant condition × trial × pulse interaction, F(5.65,135)=2.53, p=.026. Post-hoc tests indicated that late pain sensations were reduced during the pulse heat pain trial following: 1) moderate aerobic exercise after pulses 9 and 10, and 2) vigorous aerobic exercise after pulses 6 – 10 (See Figure 4). In Table 1, effect sizes are displayed for the late pain sensations at pulse 10, given that the hypoalgesic effect of exercise was most evident at this time point.
Figure 4.
Means and SE for the late sensation pain intensity ratings following each pulse for the pulse heat pain trials for pre- and post-tests during the control, moderate aerobic exercise (AE), and vigorous aerobic exercise conditions (AE). *p<.05.
Correlations among EIH responses to experimental pain tests for each condition
The bivariate correlations among experimental pain tests for each condition are presented in Table 2. The EIH responses did not significantly correlate between the different experimental pain tests for any condition, with only a single exception. During the moderate aerobic exercise session, individuals who demonstrated greater EIH during the PPT test also exhibited greater EIH during the pressure pain suprathreshold test.
DISCUSSION
The purpose of this study was to investigate experimentally-induced pain evoked by four different pain induction procedures before and after aerobic exercise that varied in intensity. The principle findings were as follows: 1) vigorous intensity aerobic exercise reduced pain during pressure pain thresholds, static continuous heat stimuli, and repetitive heat pulse stimuli, but did not influence suprathreshold pressure pain ratings, 2) moderate intensity aerobic exercise reduced pain only during static continuous heat stimuli and repetitive heat pulse stimuli, and 3) a dose-response effect was evident as vigorous intensity exercise produced larger effects than moderate intensity exercise. Furthermore, the correlational data generally indicated that EIH responses were not consistent across experimental pain tests for moderate or vigorous aerobic exercise, demonstrating the importance of multi-modal/stimulation pain assessment when studying EIH.
Aerobic exercise and pressure pain
The results of the present study support prior work showing that vigorous intensity aerobic exercise increases pressure pain thresholds (17,22) and that moderate intensity aerobic exercise fails to alter pressure pain sensitivity (12). However, in contrast to Hoffman et al. (12) and Koltyn et al. (17), vigorous aerobic exercise did not reduce the perception of suprathreshold pressure pain. Several methodological differences between the studies could account for these discrepant findings. First, the duration of vigorous exercise was slightly different between the studies (Hoffman & Koltyn studies: 30 minutes v. current study: 20 minutes). Importantly, Hoffman et al. revealed potential thresholds for the duration of aerobic exercise required to elicit hypoalgesia, in which pressure pain ratings were altered following 30 but not 10 minutes of vigorous exercise. Secondly, both Hoffman et al. and Koltyn et al. applied a constant pressure pain stimulus to the index finger, whereas we applied a dynamic pressure stimulus which progressively increased in intensity on the forearm. Third, we used a muscle pain stimulus whereas the other studies used a cutaneous pain stimulus.
Interestingly, we showed that vigorous aerobic exercise alters pressure pain threshold but not suprathreshold pressure pain ratings. Pressure pain thresholds signify the lowest boundary of painful sensations in musculoskeletal structures (3). As the intensity of extrinsic pressure and the subsequent pain increases, so does the tangible risk of injury. Therefore, significant reductions in pain sensitivity at increasing suprathreshold, compared to threshold, levels of pain could place individuals at a greater risk of injury. In the current study, the suprathreshold pressure pain test was the only test that progressively increased in stimulation intensity and consistently evoked high levels of pain sensations (i.e. more than 50% of participants rated pain >60/100 and approximately 60% rated pain > 50/100). Perhaps, the warning capacity of the pain system is preserved following exercise for higher intensity and potentially injurious stimuli, thereby protecting against tissue damage. Alternatively, given that suprathreshold heat pain was reduced by exercise, a methodological explanation may also be possible. Specifically, the suprathreshold heat pain tests used an individualized protocol (i.e., temperature was tailored for each individual to produce a moderate level of pain) whereas the suprathreshold pressure pain test utilized a fixed protocol (i.e., 5 kg of pressure was applied to all participants). Individualized compared to fixed pain test protocols are better suited for within-individual comparisons (19) because they produce a similar level of pre-intervention pain among participants and can minimize floor and ceiling effects. Indeed, inspection of the baseline suprathreshold pressure pain rating data revealed large variability between participants, with average ratings ranging between 17 and 90. Future EIH research should consider individualized suprathreshold pressure pain protocols when making within subject comparisons.
Aerobic exercise and continuous static heat pain
We showed that vigorous and moderate intensity aerobic exercise reduces perception of pain during continuous static heat stimuli. Our results are in contrast to Ruble et al. who found that treadmill running at 75% VO2max produced no significant changes in pain intensity ratings of continuous, static hot and cold stimuli delivered to the palm for 2 minutes (31). While we cannot explain the discrepant results between the two studies, some methodological differences between the two studies did exist including bodily site of pain stimulation (palm vs. forearm) and method of rating pain (i.e., in Ruble’s study participants could see previous pain ratings).
Previous studies have evaluated the temporal fluctuations in specific heat pain qualities during 30 s of static heat pain stimulation (9). Sharp stinging pain sensations peak in the first 10 s and then abruptly attenuate to a very low level of pain (>10/100) for the remaining 20 s of the trial. In contrast, burning pain sensations do not adapt with time but rather continue through the entire 30 s of heat stimulation. Specific heat pain sensations have been equivocally linked with distinct nociceptive mechanisms (2,20). Sharp stinging sensations (“first pain”) are associated with A-delta type-2 nociceptor activation. Attenuation of sharp sensations during prolonged heat stimulation is likely caused by adaptation of activity in these nociceptors (35,36). On the other hand, burning pain sensations (“2nd pain”) involve activation of polymodal C-fiber nociceptors which do not adapt over time to static heat stimulation (9). Thus, the last 15 s of the 30-s continuous heat pain trial likely represented pain sensations mediated by C-fiber activation. As such, our data suggest that C-fiber mediated pain sensations may be more susceptible to the hypoalgesic effects of exercise, as pain was reduced following exercise only during the last 10 s of the 30-s heat pain trial. While this hypothesis is based on speculation, it is in also accordance with Vierck et al. who suggested that the source of nociceptor input may be an important factor in exercise-induced hypoalgesia (36). Indeed, prior research indicates that exercise-induced hypoalgesia may function through the activation of the endogenous opioid system and A-fiber compared to C-fiber mediated pain sensations are less susceptible to opioid inhibition (5,30).
Aerobic exercise and repetitive pulse heat pain
Supporting prior work (38), we also showed that aerobic exercise reduces the magnitude of late pain sensations during the static pulse heat trials. Similar to the continuous heat pain trials, pain reduction was observed only in the latter end of the trials (i.e., pulses 6–10). A single brief noxious heat pulse evokes A-delta mediated sharp-stinging pain sensations followed by a C-fiber mediated burning pain sensations. We specifically instructed participants to rate the late burning pain sensations experienced following each pulse, not the pain felt during each pulse. Price and colleagues revealed that during a train of brief repetitive heat stimuli, first pain intensity decreases with each successive heat pulse as a consequence of heat induced suppression of A-delta type-2 nociceptors (29). On the other hand, C-fiber mediated second pain sensations progressively increase with each successive pulse when inter-pulse intervals are less than 3 s (temporal summation of pain: 29,34). Thus, pulses during the latter half of the repetitive pulse heat trials likely evoked more distinct C-fiber mediated late pain sensations compared to the earlier pulses. Consequently, data from the heat pulse trials also support the notion that pain sensations elicited by C-fiber input are preferentially attenuated by acute aerobic exercise.
Effect of moderate v. vigorous aerobic exercise on experimentally-induced pain
As hypothesized, aerobic exercise produced a dose response effect on pain perception during the continuous and pulse static heat stimuli, with the more intense exercise producing larger effects. Furthermore, vigorous exercise produced large hypoalgesic effects during the PPTs, while moderate aerobic exercise produced only a small, but non-significant hypoalgesic effect. However, we cannot completely exclude the possibility that the significant pain reduction in response to vigorous exercise was due to the lower pre-exercise value. Nonetheless, we showed for the first time that moderately intense aerobic exercise (i.e., ~50% of HRR) is capable of producing an analgesic effect in healthy adults, although this effect was dependent on pain induction technique. This knowledge could have important implications for the use of exercise in the management of pain, particularly in deconditioned individuals (e.g., older adults, fibromyalgia patients). Importantly, the present study only included young to middle aged healthy individuals; therefore, caution is warranted in the generalization of these results to other age groups and individuals with chronic pain. In fact, several studies suggest that vigorous aerobic exercise fails to produce a hypoalgesic effect in individuals with central pain inhibitory deficiencies, such as fibromyalgia patients and chronic fatigue syndrome (4,22,38). However, a recent study by Newcomb and colleagues revealed that both preferred and prescribed moderate intensity aerobic exercise is capable of producing hypoalgesia in fibromyalgia patients (26). Future research needs to systematically investigate the intensity thresholds for aerobic exercise as a pain reduction treatment in older and chronic pain populations.
The most widely ascribed mechanism for EIH involves the activation of the endogenous opioid system during exercise. Several animal studies have shown that exercise of sufficient intensity and duration results in the release of central and peripheral beta endorphins, which have been associated with reductions in pain sensitivity (8,11,33). Data from our heat pain tests support this mechanism. Specifically, C-fiber v. A-fiber input is more susceptible to opioid inhibition (5,30) and the heat pain sensations that were likely C-fiber mediated were preferentially inhibited following exercise. Another potential mechanism involves the activation of group III and IV muscle afferents in skeletal muscle during exercise (23). Animal studies show that activation of these afferents increases pain thresholds (13,35). In relation to our data, perhaps a threshold of muscle afferent input is required to increase pressure pain thresholds and stationary biking at 70% HRR reserve reached this threshold whereas biking at 50% HRR did not. Other potential mechanisms of EIH that have been discussed include the activation of nonopioid pain inhibitory systems by exercise (e.g, endocannabinoids, serotonin; 6,13,21,39) and the interaction between pain modulatory and cardiovascular systems (See Kolytn & Umeda for a review, 18). Unfortunately, each of these proposed mechanisms for EIH have received mixed support, illustrating the complexity of this phenomenon. Furthermore, the animal studies collectively indicate that EIH is likely produced by multiple analgesic systems, each of which may preferentially alter different types of nociceptive input. Indeed, prior research suggests that different descending pain pathways exert differential influence on nociceptive input from cutaneous and deep tissues (40). Along these lines, moderate intensity exercise may activate an analgesic system that preferentially modulates cutaneous v. muscle input or heat v. pressure pain. In conclusion, this study confirms that moderate intensity aerobic exercise is likely insufficient to elicit EIH using pressure stimuli on healthy adults. However, we provided evidence that vigorous and moderate aerobic exercise reduce pain perception during static continuous and pulse heat stimulation, with vigorous exercise producing larger effects. Furthermore, during the heat pain trials, aerobic exercise appeared to preferentially alter late pain sensations, which are likely C-fiber mediated and strongly implicated in chronic pain states. Finally, given the lack of correlation between EIH responses among the different experimental pain tests, future research with larger sample sizes is needed to understand factors influencing exercise-induced hypoalgesic response patterns and their clinical significance.
Acknowledgments
Funding:
This research was supported by NIH Grant T32 T32NS045551-06.
Footnotes
Conflict of Interest
There are not actual or potential conflicts of interest for any of the authors.
The results of the present study do not constitute endorsement by ACSM.
References
- 1.Borg G. Borg’s Perceived Exertion and Pain Scales. Champaign, IL: Human Kinetics; 1998. [Google Scholar]
- 2.Campbell JN, LaMotte RH. Latency to detection of first pain. Brain Research. 1983;266:203–8. doi: 10.1016/0006-8993(83)90650-9. [DOI] [PubMed] [Google Scholar]
- 3.Chapman CR, Casey KL, Dubner R, Foley KM, Gracely RH, Reading AE. Pain measurement: an overview. Pain. 1985;22:1–31. doi: 10.1016/0304-3959(85)90145-9. [DOI] [PubMed] [Google Scholar]
- 4.Cook DB, Stegner AJ, Ellingson LD. Exercise alters pain sensitivity in Gulf War veterans with chronic musculoskeletal pain. J Pain. 2010;11:764–772. doi: 10.1016/j.jpain.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 5.Cooper BY, Vierck CJ, Jr, Yeomans DC. Selective reduction of second pain sensations by systemic morphine in humans. Pain. 1986;24:93–116. doi: 10.1016/0304-3959(86)90030-8. [DOI] [PubMed] [Google Scholar]
- 6.Dietrich A, McDaniel WF. Endocannabinoids and exercise. Br J Sports Med. 2004;38:536–541. doi: 10.1136/bjsm.2004.011718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goldfarb AH, Hatfield BD, Armstrong D, Potts J. Plasma beta-endorphin concentration: response to intensity and duration of exercise. Med Sci Sports Exerc. 1990;22(2):241–244. [PubMed] [Google Scholar]
- 8.Goldfarb AH, Jamurtas AZ. Beta-endorphin response to exercise. An update. Sports Med. 1997;24:8–16. doi: 10.2165/00007256-199724010-00002. [DOI] [PubMed] [Google Scholar]
- 9.Hashmi JA, Davis KD. Women experience greater heat pain adaptation and habituation than men. Pain. 2009;145:350–357. doi: 10.1016/j.pain.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 10.Hastie BA, Riley JL, III, Robinson ME, Glover T, Campbell CM, Staud R, Fillingim RB. Cluster analysis of multiple experimental pain modalities. Pain. 2005;116:227–237. doi: 10.1016/j.pain.2005.04.016. [DOI] [PubMed] [Google Scholar]
- 11.Hoeger Bement M, Sluka KA. Low-intensity exercise reverses chronic muscle pain in the rat in a naloxone-dependent manner. Arch Phys Med Rehabil. 2005;86:1736–1740. doi: 10.1016/j.apmr.2005.03.029. [DOI] [PubMed] [Google Scholar]
- 12.Hoffman MD, Shepanski M, Ruble S, Valic Z, Buckwalter J, Clifford P. Intensity and duration threshold for aerobic exercise-induced analgesia to pressure pain. Arch Phys Med Rehabil. 2004;85:1183–1187. doi: 10.1016/j.apmr.2003.09.010. [DOI] [PubMed] [Google Scholar]
- 13.Hoffman P, Skarphedinsson JO, Delle M, Thoren P. Electrical stimulation of the gastrocnemius muscle in the spontaneously hypertensive rat increases the pain threshold: Role of different serotonergic receptions. Acta Physiol Scand. 1990;138:125–131. doi: 10.1111/j.1748-1716.1990.tb08824.x. [DOI] [PubMed] [Google Scholar]
- 14.Karvonen MJ, Kentala E, Mustala O. The effects of training on heart rate; a longitudinal study. Ann Med Exp Biol Fenn. 1957;35:307–315. [PubMed] [Google Scholar]
- 15.Koltyn K. Analgesia following exercise. Sports Med. 2000;29:85–98. doi: 10.2165/00007256-200029020-00002. [DOI] [PubMed] [Google Scholar]
- 16.Koltyn K. Exercise-induced hypoalgesia and intensity of exercise. Sports Med. 2002;32:477–487. doi: 10.2165/00007256-200232080-00001. [DOI] [PubMed] [Google Scholar]
- 17.Koltyn K, Garvin A, Gardiner R, Nelson T. Perception of pain following aerobic exercise. Med Sci Sport Exerc. 1996;28:1418–1421. doi: 10.1097/00005768-199611000-00011. [DOI] [PubMed] [Google Scholar]
- 18.Koltyn K, Umeda M. Exercise, hypoalgesia, and blood pressure. Sports Med. 2006;36:207–214. doi: 10.2165/00007256-200636030-00003. [DOI] [PubMed] [Google Scholar]
- 19.Kong JT, Johnson KA, Balise RR, Mackey S. Test-retest reliability of thermal temporal summation using an individualized protocol. J Pain. 2013;14:79–88. doi: 10.1016/j.jpain.2012.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lamotte RH, Cambell JN. Comparison of responses of warm and nociceptive C-fiber afferents in monkey with human judgments of thermal pain. J Neurophysiol. 1978;41:509–528. doi: 10.1152/jn.1978.41.2.509. [DOI] [PubMed] [Google Scholar]
- 21.McMurray RG, Forsythe WA, Mar MH, Hardy CJ. Exercise intensity-related responses of beta-endorphin and catecholamines. Med Sci Sports Exerc. 1987;19(6):570–574. [PubMed] [Google Scholar]
- 22.Meeus M, Roussel N, Truijen S, Nijs J. Reduced pressure pain thresholds in response to exercise in Chronic Fatigue Syndrome but not in Chronic Low Back Pain: An experimental study. J Rehab Med. 2010;42:884–890. doi: 10.2340/16501977-0595. [DOI] [PubMed] [Google Scholar]
- 23.Mense S, Simons DG. Muscle Pain: Understanding its nature, diagnosis, and treatment. Philadelphia, PA: Lippincott Williams & Wilkins; 2001. pp. 1–385. [Google Scholar]
- 24.Morris SB, DeShon RP. Combining effect size estimates in meta-analysis with repeated measures and independent-groups designs. Psychological Methods. 2002;7:105–125. doi: 10.1037/1082-989x.7.1.105. [DOI] [PubMed] [Google Scholar]
- 25.Naugle KM, Fillingim RB, Riley JL., 3rd A meta-analytic review of the hypoalgesic effects of exercise. J Pain. 2012;13(12):1139–1150. doi: 10.1016/j.jpain.2012.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Newcomb LW, Koltyn K, Morgan WP, Cook DB. Influence of preferred versus prescribed exercise on pain in fibromyalgia. Med Sci Sports Exerc. 2011;43:1106–1113. doi: 10.1249/MSS.0b013e3182061b49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Neziri AY, Curatolo M, Nuesch E, Scaramozzino P, Andersen OK, Arendt-Nielsen L, Juni P. Factor analysis of responses to thermal, electrical, and mechanical painful stimuli supports the importance of multi-modal pain assessment. Pain. 2011;152:1146–55. doi: 10.1016/j.pain.2011.01.047. [DOI] [PubMed] [Google Scholar]
- 28.Pollock ML, Gaesser GA, Butcher J, Despres JP, Dishman RK, Franklin BA, et al. ACSM position stand: The recommended quantity and quality of exercise for developing and maintaining cardiorespiratory and muscular fitness, and flexibility in healthy adults. Med Sci Sport Exerc. 1998;30:975–991. doi: 10.1097/00005768-199806000-00032. [DOI] [PubMed] [Google Scholar]
- 29.Price DD, Hu JW, Dubner R, Gracely RH. Peripheral suppression of first pain and central summation of second pain evoked by noxious heat pulses. Pain. 1977;3:57–68. doi: 10.1016/0304-3959(77)90035-5. [DOI] [PubMed] [Google Scholar]
- 30.Price D, Von der Gruen A, Miller J, Rafii A, Price C. A psychophysical analysis of morphine analgesia. Pain. 1985;22:261–269. doi: 10.1016/0304-3959(85)90026-0. [DOI] [PubMed] [Google Scholar]
- 31.Ruble S, Hoffman M, Shepanski M, Valic Z, Buckwalter J, Clifford P. Thermal pain perception after aerobic exercise. Arch Phys Med Rehabil. 2005;86:1019–10123. doi: 10.1016/j.apmr.2004.09.024. [DOI] [PubMed] [Google Scholar]
- 32.Shephard R. PAR-Q, Canadian Home Fitness Test and exercise screening alternatives. Sports Med. 1988;5:185–195. doi: 10.2165/00007256-198805030-00005. [DOI] [PubMed] [Google Scholar]
- 33.Stagg NJ, Mata HP, Ibrahim MM, Henriksen EJ, Porreca F, Vanderah TW, Malan PT. Regular exercise reverses sensory hypersensitivity in a rat neuropathic pain model: role of endogenous opioids. Anesthesiology. 2011;114:940–948. doi: 10.1097/ALN.0b013e318210f880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Staud R, Robinson ME, Price DD. Temporal summation of second pain and its maintenance are useful for characterizing widespread central sensitization of fibromyalgia patients. J Pain. 2007;8(11):893–901. doi: 10.1016/j.jpain.2007.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Treede RD, Meyer RA, Campbell JN. Myelinated mechanically insensitive afferents from monkey hairy skin: heat-response properties. J Neurophysiol. 1998;80:1082–1093. doi: 10.1152/jn.1998.80.3.1082. [DOI] [PubMed] [Google Scholar]
- 36.Treede RD, Meyer RA, Raja SN, Campbell JN. Evidence for two different heat transduction mechanisms in nociceptive primary afferents innervating monkey skin. J Physiol. 1995;483:747–758. doi: 10.1113/jphysiol.1995.sp020619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vierck C, Cannon RL, Fry G, Maixner W, Whitsel BL. Characteristics of temporal summation of second pain sensations elicited by brief contact of glabrous skin by a preheated thermode. J Neurophysiol. 1997;87:992–1002. doi: 10.1152/jn.1997.78.2.992. [DOI] [PubMed] [Google Scholar]
- 38.Vierck C, Staud R, Price D, Cannon R, Mauderli A, Martin A. The effect of maximal exercise on temporal summation of second pain (windup) in patients with Fibromyalgia Syndrome. J Pain. 2001;2:334–244. doi: 10.1054/jpai.2001.25533. [DOI] [PubMed] [Google Scholar]
- 39.Yao T, Andersson S, Thoren P. Long-lasting cardiovascular depressor response following sciatic stimulation in spontaneously hypertensive rats: evidence for the involvement of central endorphin and serotonin systems. Brain Res. 1982;244:295–303. doi: 10.1016/0006-8993(82)90088-9. [DOI] [PubMed] [Google Scholar]
- 40.Yu XM, Hua M, Mense S. The effects of intracerebroventricular injection of naloxone, phentolamine and methysergide on the transmission of nociceptive signals in rat dorsal horn neurons with convergent cutaneous-deep input. Neuroscience. 1991;44:715–723. doi: 10.1016/0306-4522(91)90090-b. [DOI] [PubMed] [Google Scholar]