Abstract
Objective
Little is known regarding whether exercise-induced hypoalgesia (EIH) produced by isometric exercise is influenced by psychological factors or systematically varies across multiple experimental psychophysical pain tests. Thus, this study sought to determine the influence of experimental pain test, psychological factors, and sex on the hypoalgesic response of submaximal isometric exercise.
Methods
Healthy young males (n=12) and females (n=15) completed one training and two testing sessions consisting of quiet rest (control condition) or a 3-minute isometric handgrip performed at 25% of maximum voluntary contraction. Pain testing was conducted on both forearms prior to and following exercise and quiet rest. The pain tests included: pressure pain thresholds (PPT), suprathreshold pressure pain test, static prolonged heat test, and temporal summation of heat pain. Participants completed the Pain Catastrophizing Scale during the training session and the State-Trait Anxiety Inventory –State version prior to each session. The data were analyzed with mixed model ANOVAs, partial pearson correlations, and hierarchical regression analyses.
Results
Isometric exercise increased PPTs for men and women, reduced pain perception during static prolonged heat stimuli for women, and reduced temporal summation of pain for men and women. Greater pain catastrophizing was associated with smaller reductions in temporal summation following isometric exercise.
Conclusions
These findings demonstrate that the hypoalgesic response to submaximal isometric exercise is partially a function of sex and experimental pain test. Furthermore, the relationship between EIH and pain catastrophizing was psychophysical pain test specific, with greater pain catastrophizing predicting diminished EIH only during the temporal summation of pain trials.
Keywords: exercise-induce hypoalgesia, exercise analgesia, pressure pain, thermal pain, temporal summation
Introduction
Short duration submaximal and maximal isometric exercises produce a pain-inhibitory response in healthy adults [1–4]. Substantial evidence has shown the hypoalgesic effect of isometric exercise using pressure pain stimuli [See 5 for a review], while less is known about the effect of isometric exercise on responses to heat pain stimuli. Most recently, Koltyn et al. showed that submaximal isometric exercise reduces temporal summation of heat pain in men and women [6]. Additionally, Staud and colleagues demonstrated that submaximal isometric exercise decreases pain intensity ratings of brief heat stimuli only in healthy women [7]. The effect of isometric exercise on sustained heat stimuli remains unknown. Importantly, the use of psychophysical pain tests with sustained stimulation has been gaining interest, as such stimulation patterns are thought to more closely emulate natural/clinical pain [8,9]. Furthermore, several studies have indicated that experimental pain measures correlate only moderately across stimulus modalities and tests [10,11], demonstrating the importance of multimodal pain assessment; a practice that has been rare in isometric exercise-induced hypoalgesia (EIH) research.
Even though accumulating evidence indicates that psychological factors, such as pain catastrophizing and anxiety, play a significant role in shaping pain experiences [12,13], little is known about their influence on EIH. Pain catastrophizing is characterized by a set of maladaptive emotional/cognitive processes that involve perceptions of helpless, rumination, and magnification of painful sensations. In particular, several studies in the last decade have shown that pain catastrophizing is disruptive of endogenous pain inhibitory systems using conditioned pain modulation (CPM) [14] and counter irritation paradigms [15]. Moreover, some data suggests that catastrophizing may specifically interfere with opioid-related pain inhibitory circuits [13]. Interestingly, no research has investigated the impact of pain catastrophizing on EIH; a pain inhibitory model hypothesized to be regulated by activation of endogenous opioid systems [16].
The present study explored the influence of several variables on the hypoalgesic response to isometric exercise, including pain induction technique, psychological factors (i.e., anxiety, pain catastrophizing), and sex. Modality and duration of painful stimuli are important factors that often influence the outcomes of experimental pain testing. Indeed, responses to pressure pain, heat pain, and temporal summation of pain likely represent distinct dimensions of pain perception that may be under the influence of different mechanisms [10]. Therefore, the first purpose of the study was to gain a better understanding of the hypoalgesic effect of isometric exercise on multiple experimental pain tests, including threshold and suprathreshold pressure pain, sustained heat pain, and temporal summation of heat pain. The second purpose was to examine the relationship between psychological variables and EIH with each pain induction technique. Finally, because prior work has shown that sex differences exist in EIH [17], we examined the effect of isometric exercise on pain sensitivity in both men and women. We hypothesized that submaximal isometric exercise would reduce pain sensitivity, regardless of pain induction technique. Furthermore, we hypothesized that higher pain catastrophizing and state anxiety would be associated with reduced EIH.
METHODS
Participants
Participants included twenty-seven (12 men; average BMI=23±2.8) healthy young adults. Participants were recruited through posted advertisements in the local community. Exclusion criteria included: 1) inability to reliably rate pain intensity, 2) current use of narcotics or tobacco products, 3) uncontrolled hypertension, 4) neurological disease with significant changes in somatosensory and pain perception at intended stimulation sites, 5) the known presence of or any signs or symptoms of cardiovascular disease, pulmonary disease, or metabolic disease, 6) serious psychiatric conditions (e.g., schizophrenia and bipolar disorder), and 7) not physically ready to exercise without a medical exam as indicated by the Physical Activity Readiness Questionnaire (PAR-Q) [18]. Session exclusion criteria included active infectious disease or febrile condition (e.g., sinusitis, influenza) and severe uncontrolled hypertension. Additionally, participants were instructed to refrain from use of caffeinated beverages or any pain medications on days of experimental sessions.
Procedures
The University’s Institutional Review Board (IRB) approved all procedures and participants signed an IRB-approved informed consent form. Participants completed a screening/training session followed by two randomized experimental sessions. All sessions were conducted at approximately the same time of day (± 2 hours) and separated by a minimum of 48 hours (i.e., average number of days between sessions was 4.76 days ± 1.69). All sessions began with two stable blood-pressure readings separated by 5 minutes.
Screening and training session
To determine eligibility, participants completed the PAR-Q, a health history questionnaire, supplemented by clarification by interview, height and weight measurement, and blood pressure measurement. Eligible participants completed a training session designed to familiarize them with the pain tests to be conducted during the experimental sessions and to determine the individualized temperatures of the thermal stimuli for the heat pain testing protocols such that participants would experience moderate pain during the experimental sessions. For this purpose, trains of increasing heat stimuli were applied to the forearm until participants experienced a moderate level of pain (40–60 on a 0–100 visual analogue scale). Participants also completed a packet of questionnaires which included the Pain Catastrophizing Scale (PCS) [19].
During the training session, maximal voluntary contraction (MVC) of hand flexor muscles was also determined. Participants placed their dominant arm on a table surface with the elbow at a 90° angle. Participants were asked to squeeze the dynamometer as hard as possible for 5 seconds. This procedure was repeated three times with a one-minute rest between trials. The maximum of the three MVC’s was used to calculate the percent of MVC used for the isometric hand grip exercise.
Experimental sessions
Participants completed two randomized and counterbalanced experimental sessions consisting of either submaximal isometric exercise or quiet rest. At the beginning of each session, participants completed the state version of the State-Trait Anxiety Inventory [20]. Then participants were fitted with a Polar Heart Rate monitor (FT7) (Polar Electro, Lake Success, NY), which monitored and collected heart rate (HR) at rest (sitting) and during exercise. During each session, four different pain tests were administered on each forearm followed by 22 minutes of quiet rest and then either isometric exercise or an additional 3 minutes of rest. Blood pressure was immediately taken upon completion of exercise or rest followed by the administration of the same 4 pain tests in the same order as the pre-exercise pain tests. A timeline of the experimental sessions is shown in Figure 1.
Figure 1.
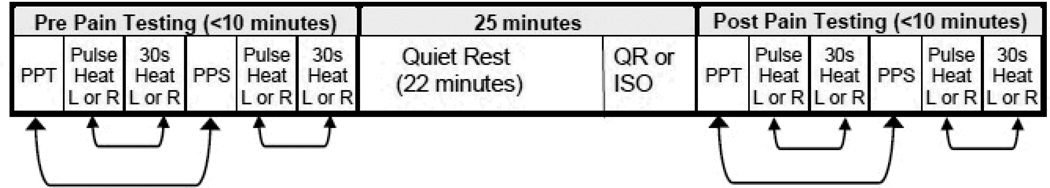
Timeline of procedures during the experimental sessions. The bidirectional arrows between the pressure and heat pain tests indicate that these tests were conducted in counterbalanced order. The site of pain testing alternated between left and right forearms, so that one arm was never tested consecutively. Participants maintained the same pain testing order for each session pre and post exercise and quiet rest. PPT=pressure pain threshold; PPS= pressure pain suprathreshold test; R=right forearm; L=left forearm; QR=quiet rest; ISO=isometric exercise.
Submaximal isometric exercise session (isometric)
This session tested for changes in pain sensitivity following a 3-minute trial of submaximal isometric handgrip exercise at 25% of MVC. We chose a duration of 3 minutes for the handgrip task because prior research has shown handgrips of this duration to produce the largest EIH effects (as opposed to 1 and 5 minute handgrips) [25]. Twenty-two minutes separated the pre-exercise pain assessments and the initiation of the isometric contraction, during which subjects sat quietly. This period of rest was included to prevent within-session adaptation, as prior work has shown complete recovery of primary afferent responsiveness after 10 minutes of no stimulation [21]. The isometric hand grip exercise was performed with the dominant arm resting on the table surface with the elbow at a 90° angle. Participants were able to see the dynamometer read-out and adjust their effort as necessary to maintain a level of force production at 25% of their MVC. Ratings of perceived exertion (RPE) using Borg’s 6–20 RPE scale [22] and HR were assessed every 20 s during the isometric exercise trial.
Quiet Rest session (control condition)
This session tested for changes in pain sensitivity following 25 minutes of quiet rest. Heart rate was recorded every five minutes.
Psychophysical Pain Testing
The pre- and post-pain testing included the administration of 4 different pain tests to each forearm including: 1) pressure pain thresholds, 2) ratings of suprathreshold pressure pain stimuli, 3) prolonged heat pain stimuli, and 4) temporal summation (TS) of heat pain. The order of the pain tests was conducted as follows: 1) a pressure pain test administered to both forearms, 2) a prolonged or TS heat pain test administered to one forearm, 3) a prolonged or TS heat pain test administered to the other forearm, 4) a pressure pain test administered to both forearms 5) a prolonged or TS heat pain test administered to one forearm, 6) a prolonged or TS heat pain test administered to the other forearm. The site of pain testing alternated between the exercised and inactive forearms, so that one arm was never tested consecutively. Additionally, the order of the pressure pain tests (threshold v. suprathreshold test), heat pain tests (prolonged heat v. TS), and bodily site (exercised v. inactive arm) was counterbalanced among participants. Participants maintained the same pain testing order for the pre- and post-tests for every session.
Pressure Pain Thresholds (PPT)
Pressure pain threshold was assessed with a handheld algometer that had a rubber, flat 1.0 cm2 probe (Jtech, Heber City, Utah). Pressure stimuli were delivered to the right and left ventral forearm approximately 8 cm distal to the elbow at an approximate rate of 0.5 kg/s. Participants responded verbally when the pressure sensation first became painful, at which point the algometer was removed. The amount of pressure applied to the forearm did not exceed 5 kg. Pressure pain threshold was defined as the amount of pressure in kilograms at which the participant first reported experiencing pain.
Ratings of Suprathreshold Pressure Stimuli
Suprathreshold pressure stimuli were also delivered to the right and left ventral forearm with the same handheld algometer used for PPTs (Jtech, Heber City, Utah). Pressure pain threshold and suprathreshold assessments were always separated by a minimum of 1 cm on the forearm and the same sites were used for pre and post assessments. Pressure stimuli were delivered at an approximate rate of 0.5 kg/s, until 5 kg was applied. This level of pressure was chosen to assess pain perception without producing excessive discomfort. Immediately after each trial, participants rated the intensity of the stimulus on a 0 to 100 visual analogue scale (VAS), with “0” indicating “no pain” and “100” indicating “intolerable pain”.
Prolonged Heat Pain
Contact heat stimuli were delivered by a computer controlled Peltier-based thermode (30 mm × 30 mm; TSA-2001, Ramat Yishai, Israel) to the right and left ventral forearms. For each 30-second prolonged heat pain trial, the thermode was first brought to a neutral temperature (33°C) and then ramped (1.5°C/s) to the individualized temperature (44–49°C) determined during the training session and maintained at that temperature for 30 s. The thermode position was altered slightly between each heat trial (i.e., prolonged and TS heat pain trials). The intensity of the pain produced by the contact thermode was rated every 5 s on a 0 to 100 VAS.
Temporal Summation of Heat Pain
Brief repetitive suprathreshold heat pulses were delivered to the right and left ventral forearms. Each trial consisted of a series of 10 heat pulses, with each pulse delivered at a rate of 10°C/s. The peak to peak inter-pulse interval was approximately 2.5 seconds. The baseline temperature was 38°C and the target temperature was the individualized temperature determined during the training session (45°C – 51.5°C). Participants were instructed to rate the intensity of the late pain sensations experienced after each pulse (i.e., pain felt between the pulses not during each pulse, termed second pain) with a 0–100 scale. This pain test permitted the assessment of the effect of exercise on the temporal summation (TS) of C-fiber mediated heat pain (i.e., late heat pain sensations, often termed “second pain”). Temporal summation was calculated by subtracting the pain rating following the first pulse from the highest inter-pulse pain rating. This score captures the maximum amount of temporal summation across the 10 pulses.
Psychological Questionnaires
Pain Catastrophizing
The Pain Catastrophizing Scale [PCS: 19] consists of 13 items rated on a 5-point likert scale and assesses the trait-like psychological construct of an individual’s tendency to engage in pain-related catastrophizing. Specifically, this questionnaire asks the respondents to reflect upon past painful experiences and to rate the degree to which they experienced negative thoughts or feelings about pain. The PCS measures three dimensions of catastrophizing including rumination, helplessness, and magnification. The total score is calculated by summing item responses and represents an individual’s propensity to engage in pain-related catastrophizing. Higher scores indicate greater pain catastrophizing.
State-Trait Anxiety Inventory (STAI) – State Version
State anxiety levels at the beginning of each session were measured with the STAI-S. The STAI has extensive normative data and is a frequently used measure of anxiety in pain studies [20]. The STAI consists of 20 items which evaluate how respondents feel “right now” at this moment. Higher scores indicate greater state anxiety.
Data Analysis
Descriptive statistics were calculated for PCS, STAI, thermode test temperatures for the TS and prolonged heat pain tests, target force production (kg) during the isometric handgrip, and HR and RPE for each session. Independent t-tests determined whether differences existed between men and women on the PCS, thermode test temperatures, and target force production. Heart rate and RPE during the isometric session were analyzed with 2-way ANOVAs with sex as the between subjects factor and time (every 20s of the 180s handgrip) as the within subject factor. STAI was analyzed with a 2(sex) × 2(session) ANOVA.
During the threshold pressure pain test, two participants did not report experiencing pain after the upper limit of pressure for the test was reached; therefore their data were not included in the PPT analyses. The data for PPT and ratings of suprathreshold pressure pain were analyzed with 2(sex) × 2(forearm: exercised, inactive) × 2(session: Isometric, Control) × 2(trial: pre, post exercise) mixed model ANOVAs with repeated measures on the last three factors. Temporal summation was analyzed with a 2(sex) × 2(forearm) × 2(session) × 2(trial) mixed model ANCOVA with repeated measures on the last three factors and thermode temperature as a covariate. Data for the prolonged heat pain test were analyzed with a 2(sex) × 2(forearm: exercised, inactive) × 2(session: Isometric, Control) × 2(trial: pre, post exercise) × 6(time: 5s, 10s, 15s, 20s, 25s, 30s) mixed model ANCOVA with repeated measures on the last three factors and thermode temperature as a covariate. If the sphericity assumption was violated, then Greenhouse-Geisser degrees of freedom corrections were applied to obtain the critical p-value. Post-hoc comparisons were made with Tukey’s HSD procedure. The effect size (ES) for each pain test was calculated using Cohen’s d, defined as the mean for the pretest minus the mean for the posttest, divided by the pooled within group standard deviation (d=[Xpretest – Xposttest]/pooled standard deviation). Effect sizes were calculated so that reductions in pain sensitivity resulted in positive effect sizes. The effect sizes were adjusted for the within subjects design as recommended by Morris and DeShon [23]. Following the recommendations of Cohen [24], d=0.20 is considered a small effect, d=0.50 is considered a medium effect, and d=0.80 is considered a large effect.
Partial Pearson correlation tests were performed to examine the relationships between the pain test change scores, PCS, and state anxiety, while controlling for sex. Change scores were created for each pain test, whereby pretest values were subtracted from the posttest values. For the prolonged heat pain test, change scores were calculated from the average pain rating for each trial. Furthermore, change scores were averaged across forearms. Hierarchical linear regression analysis was conducted on each change score to determine the contribution of sex, PCS, and state anxiety as predictors of exercise-induced hypoalgesic effects. Sex was added in the first step of the regression and the psychological variables were added in the second step. A level of p ≤ .05 was used for all statistical analyses. Statistical analyses were performed with SPSS version 20 (SPSS Inc., Chicago, IL, USA).
RESULTS
Results are reported as mean ±standard deviation. Target force for the isometric handgrip was significantly greater for men (10.8 kg ±1.67) compared to women (6.19 kg ±1.20; p=.001). Sex differences on the PCS approached significance (p=.058), with women (9.33±4.12) trending towards greater PCS scores compared to men (5.17±4.10). Furthermore, no significant differences existed between men and women on thermode test temperature for the TS heat pain test (p=.343, Men=48.92 C°±2.33, Women=48.13 C° ±1.88) and the prolonged heat pain test (p=.168, Men=47.33 C° ±1.93, Women =46.37 C° ±1.61), even though men trended toward having higher temperatures. Average state anxiety levels were generally low and did not significantly differ between sessions (p=.592: Control=25.93±7.61, Isometric=26.85±8.25) or sex (p= .077: Men=23.83±5.23, Women=28.43±9.00). The 2-way ANOVA conducted on HR during the isometric exercise revealed a main effect of time (p<.001), with HR progressively increasing the first minute of the exercise and then remaining stable for the last 2 minutes of the trial. Initial HR was 73.93 ±11.52 and increased to 80.75 ±11.21 at 180s. The main effect of sex and the sex by time interaction were not significant (p’s>.05). Similarly, a main effect of time was revealed for RPE during the isometric handgrip, (p<.001), in which RPE significantly increased every 20 s. The initial RPE at 20 s was 9.86±1.73 and increased to 16.27±1.78 at 180 s. The main effect of sex and the sex by time interaction were not significant (p’s>.05).
PPTs
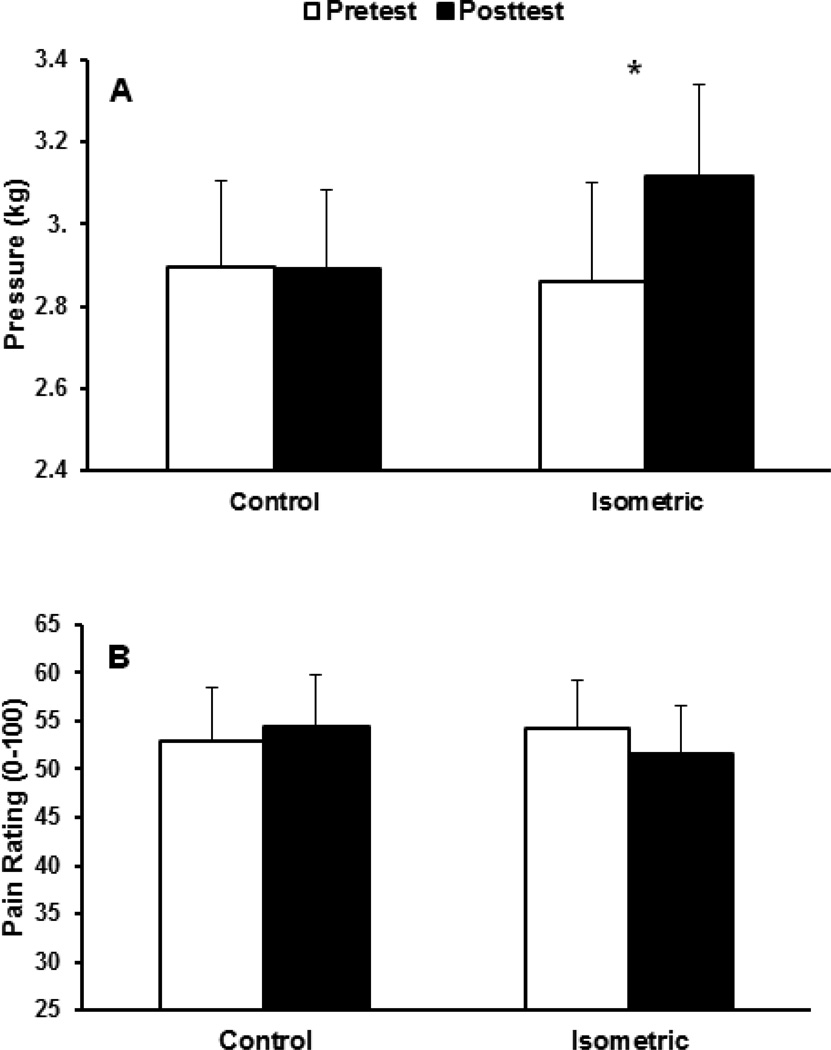
The mixed model ANOVA revealed a significant session by trial interaction, p=.014. Post-hoc tests indicated that PPTs increased from pre to posttest during the isometric exercise session, while no significant changes were evident during the control session. No other effects were significant (p’s >.05), indicating no EIH differences as a function of sex or exercised vs. inactive arms. Figure 2a shows the means and standard errors of the pre- and posttests for each session, averaged across forearms. As displayed in Table 1, isometric exercise produced moderate effect sizes for men and women.
Figure 2.
(A) Means and standard errors (SE) for pressure pain thresholds for pre- and post-tests for each session, averaged across forearms. (B) Means and SE for ratings of suprathreshold pressure pain for pre- and post-tests for each session, averaged across forearms. *p<.05.
Table 1.
Effect sizes (Cohen’s d) demonstrating the magnitude of the hypoalgesic effect (i.e. pre-post changes) for each condition and each pain induction technique for men and women
| Control | Isometric | |||
|---|---|---|---|---|
| Pain Test | Men | Women | Men | Women |
| Pressure pain threshold | −0.31 | 0.24 | 0.47 | 0.57 |
| Suprathreshold pressure pain | −0.04 | −0.10 | 0.07 | 0.22 |
| Prolonged heat pain | 0.29 | 0.13 | 0.12 | 0.83 |
| Temporal summation | 0.09 | −0.38 | 0.99 | 0.43 |
Note. A positive effect size represents a reduction in pain sensitivity following exercise or rest.
Suprathreshold pressure pain
Means and standard errors of suprathreshold pressure pain ratings for pre and post tests for each session are shown in Figure 2b. The ANOVA revealed no significant effects (all p’s >.05), indicating that the isometric exercise did not affect suprathreshold pressure pain ratings. The effect size for isometric exercise, while in the expected direction (indicating pain reduction), was small.
Prolonged heat pain
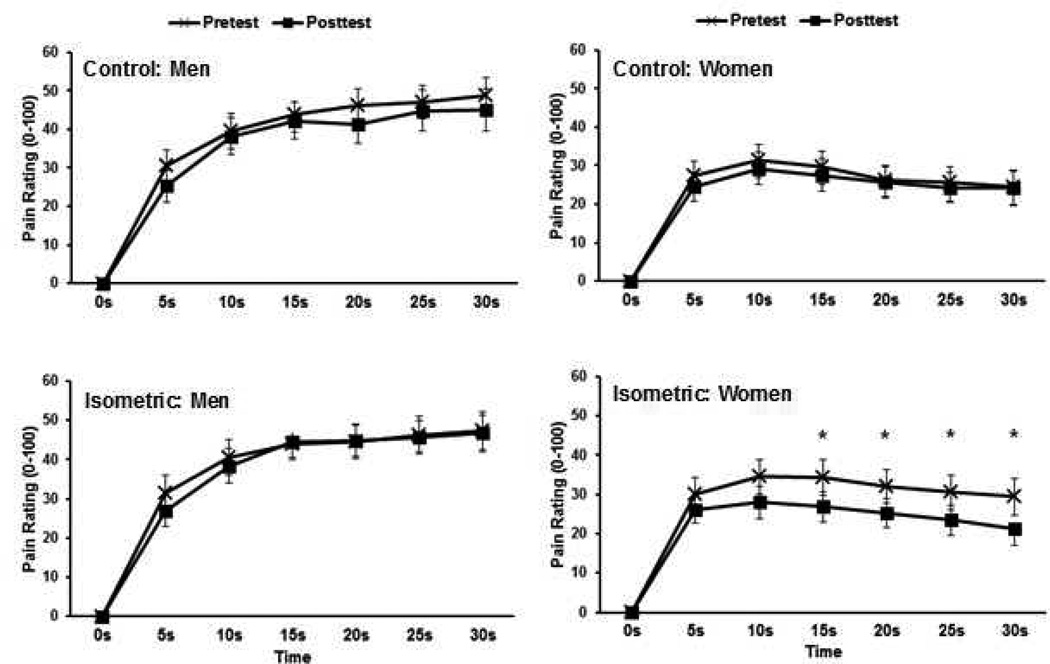
The mixed model ANCOVA revealed a significant main effect of sex (p=.018) and sex × seconds interaction (p=.004), which was superseded by a significant sex × session × trial × time interaction, p=.046. The post-hoc tests revealed that women’s pain intensity ratings decreased during the 30-second prolonged heat pain trial at seconds 15–30 following isometric exercise, but not during the control session. No pre-post differences were evident for males or during the exercise or control sessions. Figure 3 shows men’s and women’s average pain intensity ratings across the 30-s trial (averaged across forearms) for the pre- and posttests for each session. Additionally, no significant differences were found between the exercised and inactive forearms. In Table 1, effect sizes are displayed for the average pain rating across the entire 30-s trial for each session and sex. This data shows that the isometric exercise produced a large hypoalgesic effect for women and a small effect for men.
Figure 3.
Means and SE for men’s and women’s pain intensity ratings across the 30-second prolonged heat pain trials for pre- and post-tests during the control and isometric sessions. Data is averaged across forearms. *p<.05.
Temporal summation of heat pain
The ANOVA for TS revealed a significant main effect of trial (p=.024), which was superseded by a significant session × trial interaction, p=.008. During the isometric exercise session, TS significantly decreased from pretest to posttest (pre=14.6, SE=2.8, post=11.15, SE=2.23), while no changes were evident for the control session (pre=10.5, SE=1.76, post=10.6, SE=1.75). No other effects were significant (p’s >.05), indicating no EIH differences as a function of sex or exercised vs. inactive forearms. Isometric exercise produced a large hypoalgesic effect for men and a moderate effect for women (See Table 1).
Correlations between psychological measures and exercise-induced hypoalgesic effects
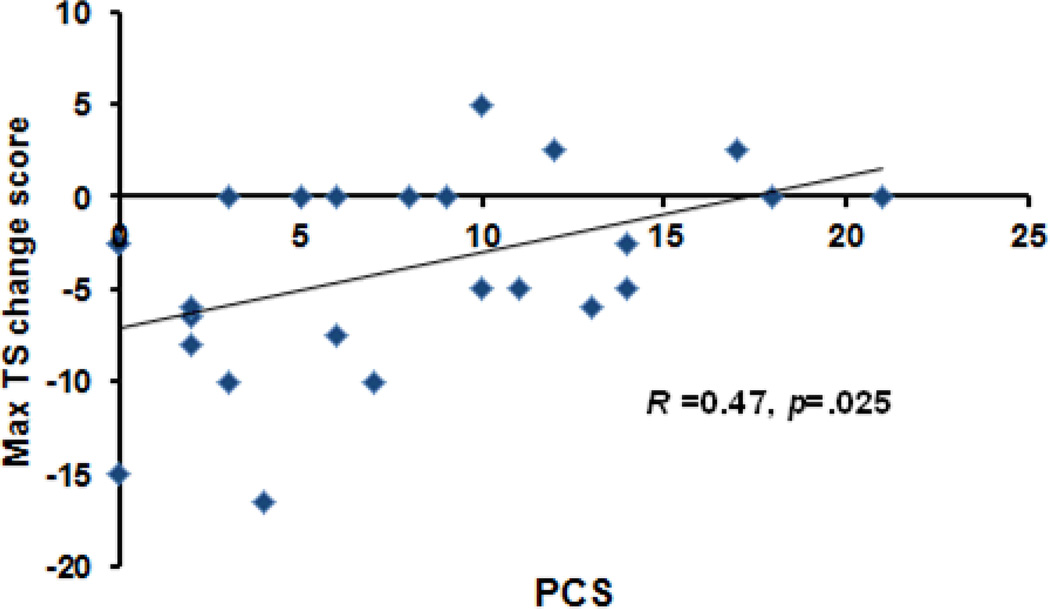
Table 2 shows the partial correlation coefficients for PCS, STAI-S, and exercise-induced hypoalgesic effects for each pain test. A significant correlation emerged between PCS and the TS change score during the isometric session (p=.025, r=.467: See Figure 4). No other significant correlations were found (p’s > .05). A multiple regression test demonstrated that after controlling for sex, PCS predicted changes in TS following isometric exercise (p=.032; Beta=.462), accounting for 18.0% of the variance. Individuals who reported greater pain catastrophizing exhibited smaller reductions in temporal summation following isometric exercise. PCS was not a significant predictor of pain sensitivity changes from pre to posttest for any other pain test. STAI was not associated with pain sensitivity changes from any pain test in either condition.
Table 2.
Partial Pearson correlation coefficients for PCS, state anxiety, and pain test change scores, while controlling for sex
| Control | Isometric | |||
|---|---|---|---|---|
| Pain test | STAI | PCS | STAI | PCS |
| Pressure pain threshold | 0.16 | −0.23 | 0.32 | −0.24 |
| Suprathreshold pressure pain | 0.09 | 0.16 | −0.14 | 0.05 |
| Prolonged heat pain | 0.05 | 0.29 | 0.16 | −0.21 |
| Temporal summation | 0.26 | 0.14 | −0.30 | 0.47a |
Note.
=significant at p< 0.05.
Figure 4.
Scatterplot showing the relationship between pain catastrophizing (PCS) and changes in the magnitude of temporal summation following isometric exercise.
Discussion
This study sought to determine the influence of pain induction technique, psychological factors, and sex on the hypoalgesic response of submaximal isometric exercise. Three key findings emerged from this data: 1) the hypoalgesic response to isometric exercise varied across pain induction methods, 2) pain catastrophizing predicted the exercise-induced hypoalgesic response to TS of heat pain, and 3) sex differences in EIH emerged only during the prolonged heat pain test.
Effect of Isometric Exercise on Reponses to Different Experimental Pain Tests
Previous EIH research has directed little attention toward the effect of experimental pain test on EIH induction and magnitude within the same experimental set-up. This study was the first to test the efficacy of isometric exercise in producing hypoalgesia across multiple experimental pain tests, including pressure pain threshold and suprathresholds, prolonged heat pain, and TS of heat pain. Supporting prior work testing single pain modalities, a submaximal isometric handgrip increased PPTs [2,25], reduced temporal summation of heat pain in men and women [6], and reduced heat pain sensitivity in women [7]. The magnitude of pain reduction varied by sex and pain test. Women showed the largest effect size during the prolonged heat pain test, while men showed the largest effect during the TS test. Also in line with previous studies [1,2,7], pain reduction for these three measures was present in both the contracting and contralateral muscles, suggesting a non-local, centrally-mediated inhibitory mechanism.
Contrary to our hypothesis, isometric exercise failed to alter suprathreshold pain ratings during a dynamic pressure pain test. These results are in contrast to prior studies showing reduced suprathreshold pressure pain intensity ratings following a 25% of MVC handgrip held for multiple durations [25] and a 40–50% of MVC handgrip held for 2 minutes [2]. The reasons for these differing results remain unclear, but may be due to methodological variations between the studies, including different pain testing sites (i.e., skin of the forefinger vs muscles of the forearm) and different pain induction methods (i.e., 2-minute constant pressure stimulus vs 10-sec dynamic pressure stimulus). In the current study, the suprathreshold pressure pain test was the only test that progressively increased in stimulation intensity and consistently evoked high levels of pain. From a biological perspective, pain serves as an early warning signal to protect against tissue injury [26]. Perhaps the protective function of pain is preserved following exercise for pain stimuli perceived to pose a tangible risk of injury, thereby protecting against damage to musculoskeletal structures. However, a linear relationship between pain intensity and tissue damage is often lacking and this hypothesis requires additional research. Alternatively, the lack of significant pain reduction may have been caused by the large between-subject variability in pre-exercise pain ratings during the suprathreshold pressure pain test (i.e., ranging from 10 to 90). Noteably, the suprathreshold heat pain tests used individualized protocols (i.e., customized temperature for each subject) and produced similar pre-exercise pain levels (30 to 50), which likely minimized ceiling and floor effects. Future EIH research should consider using individualized suprathreshold pressure pain protocols when making within subject comparisons. Nonetheless, our data suggest that not all pain models are equally modulated by the hypoalgesic effects of isometric exercise.
Isometric EIH and Psychological Factors
To our knowledge, the current study is the first to examine the relationship between pain catastrophizing and EIH. The results only partially supported our hypothesis, as pain catastrophizing was associated with the degree of EIH only for the TS pain test. Specifically, individuals reporting higher levels of pain catastrophizing exhibited smaller reductions in TS following isometric exercise. These findings are in line with previous studies showing that pain catastrophizing is disruptive of opioid-dependent pain inhibitory systems [14,15,27]. Importantly, human and animal studies have documented the involvement of endogenous opioids in pain reduction following exercise [16,28]. Furthermore, C-fiber compared to A-fiber mediated pain sensations are more susceptible to opioid inhibition [29,30] and TS is known to be dependent on C-fiber afferents [31]. In contrast, both A-delta and C-fibers likely mediated pain induced by the other pain tests [32,33]. Consequently, reduction of TS following isometric exercise may rely more heavily on endogenous opioid mechanisms, causing exercise-induced inhibition of this pain response to be more susceptible to interference from pain catastrophizing. Also in line with prior investigations [34,35], women in the current study reported higher levels of pain catastrophizing compared to men. These differences in pain catastrophizing could explain why the magnitude of reduction in TS following exercise was large for men (d=0.99) and only moderate for women (d=0.43).
In contrast to pain catastrophizing, state anxiety was not related to the degree of EIH among any of the pain tests. Previous studies have found an association between anxiety and endogenous analgesia assessed with CPM [12], with greater anxiety related to reduced CPM. However, our results corroborate previous EIH studies [4,17] and suggest no relationship exists between state anxiety levels and EIH. Notably, this finding should be interpreted with caution as participants in the current study reported relatively low levels of state anxiety prior to both experimental sessions. Thus, our results may not generalize to high levels of anxiety, which would be more likely to interfere with efficient EIH.
Isometric EIH and Sex Differences
Finally, we assessed sex differences in EIH among the 4 pain testing paradigms. The few studies investigating sex differences in EIH have produced mixed results [4,17,25,36]. For example, Koltyn et al. found that men and women exhibited reduced pressure pain sensitivity on the forefinger following a maximal isometric handgrip, whereas only women showed reduced pressure pain sensitivity following a submaximal handgrip at 40–50% of MVC [17]. In contrast, Hoeger-Bement observed no sex differences in pressure pain sensitivity following submaximal isometric handgrips performed at 25% of MVC [4]. Our results suggest that sex-related variations in EIH may be partially dependent on the experimental pain test. Indeed, we observed sex differences in EIH only during the prolonged heat pain test, with significant pain reduction found for women and no changes observed for men. These results are in agreement with Staud et al. who showed that a submaximal handgrip decreased pain intensity ratings of 5-second suprathreshold heat pain stimuli on the contracting and contralateral muscles of women (men were not tested) [7]. The reason for the sex differences in EIH during the prolonged heat pain test remains unclear. However, it has been suggested that effective central inhibitory mechanisms to attenuate sustained pain would be more biologically advantageous for women to cope with natural pain including menstrual and childbirth pain [37]. Several studies show that fluctuations in sex hormones influence the effectiveness of endogenous pain inhibitory systems [38,39,40], with high estradiol and low progesterone levels (i.e., related to ovulatory phase of menstrual cycle) associated with greater pain inhibition and greater endogenous opioid neurotransmission in supraspinal regions involved in pain inhibition. However, Hoeger-Bement et al. found that the menstrual cycle phase does not influence the magnitude of EIH for pressure pain threshold and suprathreshold ratings [41]. In the current study, menstrual phase and sex hormone levels were not assessed. Overall, the collective evidence indicates EIH is more consistently observed in women, which is in contrast to other tests of pain inhibitory function such as conditioned pain modulation) [43,44]. Thus, EIH and conditioned pain modulation may be regulated by different mechanisms. Furthermore, the existence of sex differences in EIH likely depends on complex interactions among several factors including contraction intensity, and pain testing site or modality.
Limitations of Present Study
Several limitations of this study should be acknowledged. First, the participants in the current study were healthy young adults. Therefore, generalization of these results to individuals with chronic pain and older adults is limited. Prior research has shown that individuals with fibromyalgia syndrome often do not exhibit hypoalgesia following an acute bout of exercise [3,7], a dysfunction that partially explains the symptom flares that commonly occur following exercise in FMS patients [42]. Furthermore, high levels of pain catastrophizing are present in FMS patients [13] and often lead to physical inactivity. Based on the results of the current study, future research is warranted to investigate the role that pain catastrophizing plays in the dysfunctional EIH found in FMS patients. Secondly, the cross-sectional nature of this study renders it possible that the association between EIH and pain catastrophizing may be bidirectional, in which ineffective inhibitory mechanisms lead to greater pain catastrophizing. Third, we tested an isometric contraction of a specific intensity and duration. The relationships among the psychological variables, pain modality, and sex may differ for isometric exercise performed under different intensity and duration parameters. Fourth, EIH following isometric exercise tends to be short-lasting with a progressive decline in magnitude following exercise cessation [5]. Thus, given that the duration of the pre- and post-pain testing lasted almost 10 minutes, the effect sizes for each pain test may have been smaller in magnitude than if just one pain test had been administered pre and post exercise. Fifth, we did not control for the menstrual cycle phase of female subjects. Finally, based on evaluation of our effect size data for sex differences in EIH, the current study may not have been sufficiently powered to detect small effects in sex differences in EIH.
Summary and Future Directions
In summary, the present data argue against a generalized reduction in pain sensitivity following submaximal isometric exercise. Rather, EIH was partially a function of sex and experimental pain test. In contrast to other pain modulatory tests (e.g., CPM) [43,44], women consistently exhibit more effective EIH compared to men, particularly during tests of prolonged pain. Furthermore, the relationship between EIH and pain catastrophizing was pain test specific, with greater pain catastrophizing predicting diminished EIH only during the temporal summation of heat pain trials. Our results suggest that a battery of pain tests assessing different modalities and temporal profiles of stimulation are needed to comprehensively assess an individual’s response to isometric exercise and inferences regarding EIH using only one pain modality should be made with caution. Future research should study the mechanistic and clinical implications of individual differences in EIH and determine which pain tests used to assess EIH have the most clinical relevance. For example, do individual differences in EIH predict health-related quality of life outcomes, such as everyday clinical pain, physical functioning, and pain experiences specifically related to regular physical activity? This knowledge could have important implications regarding the identification of high-risk individuals for persistent pain, declining physical activity levels, and functional disability.
Acknowledgments
Funding: This research was supported by NIH Grant T32 T32NS045551-06.
Footnotes
Conflict of Interest: There are not actual or potential conflicts of interest for any of the authors.
References
- 1.Kosek E, Lundberg L. Segmental and plurisegmental modulation of pressure pain thresholds during static muscle contractions in healthy individuals. Eur J Pain. 2007;7:251–258. doi: 10.1016/S1090-3801(02)00124-6. [DOI] [PubMed] [Google Scholar]
- 2.Koltyn K, Umeda M. Contralateral attenuation of pain after short-duration submaximal isometric exercise. J Pain. 2007;8:887–892. doi: 10.1016/j.jpain.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 3.Lannersten L, Kosek E. Dysfunction of endogenous pain inhibition during exercise with painful muscles in patients with shoulder myalgia and fibromyalgia. Pain. 2010;151:77–86. doi: 10.1016/j.pain.2010.06.021. [DOI] [PubMed] [Google Scholar]
- 4.Hoeger Bement M, Dicapo J, Rasiarmos R, Hunter S. Dose response of isometric contractions on pain perception in healthy adults. Med Sci Sports Exerc. 2008;40:1880–1889. doi: 10.1249/MSS.0b013e31817eeecc. [DOI] [PubMed] [Google Scholar]
- 5.Naugle KM, Fillingim RB, Riley JL., III A meta-analytic review of the hypoalgesic effects of exercise. J Pain. 2012;13:1139–1150. doi: 10.1016/j.jpain.2012.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koltyn KF, Knauf MT, Brellenthin AG. Temporal summation of heat pain modulated by isometric exercise. Eur J Pain. 2012;17:1005–1011. doi: 10.1002/j.1532-2149.2012.00264.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Staud R, Robinson M, Price D. Isometric exercise has opposite effects on central pain mechanisms in fibromyalgia patients compared to normal controls. Pain. 2005;118:176–184. doi: 10.1016/j.pain.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 8.Strulov L, Zimmer EZ, Granot M, Tamir A, Jakobi P, Lowenstein L. Pain catastrophizing, response to experimental heat stimuli, and post-cesarean section pain. J Pain. 2007;8:273–279. doi: 10.1016/j.jpain.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 9.Tousignant-Laflamme Y, Page S, Goffaux P, Marchand S. An experimental model to measure excitatory and inhibitory pain mechanisms in humans. Brain Research. 2008;1230:73–79. doi: 10.1016/j.brainres.2008.06.120. [DOI] [PubMed] [Google Scholar]
- 10.Hastie BA, Riley JL, III, Robinson ME, Glover T, Campbell CM, Staud R, Fillingim RB. Cluster analysis of multiple experimental pain modalities. Pain. 2005;116:227–237. doi: 10.1016/j.pain.2005.04.016. [DOI] [PubMed] [Google Scholar]
- 11.Neziri AY, Curatolo M, Nuesch E, Scaramozzino P, Andersen OK, Arendt-Nielsen L, Juni P. Factor analysis of responses to thermal, electrical, and mechanical painful stimuli supports the importance of multi-modal pain assessment. Pain. 2011;152:1146–1155. doi: 10.1016/j.pain.2011.01.047. [DOI] [PubMed] [Google Scholar]
- 12.Granot M, Weissman-Fogel I, Crispel Y, Pud D, Granovsky Y, Sprecher E, Yarnitsky D. Determinants of endogenous analgesia magnitude in diffuse noxious inhibitory control (DNIC) paradigm: Do conditioning stimulus painfulness, gender and personality variables matter? Pain. 2008;136:143–149. doi: 10.1016/j.pain.2007.06.029. [DOI] [PubMed] [Google Scholar]
- 13.Campbell CM, Edwards RR. Mind-body interactions in pain: the neurophysiology of anxious and catastrophic pain-related thoughts. Translational Research. 2009;153:97–101. doi: 10.1016/j.trsl.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goodin BR, McGuire L, Allshouse M, Stapleton L, Haythornthwaite JA, Burns N, Mayes LA, Edwards RR. Associations between catastrophizing and endogenous pain-inhibitory processes: Sex differences. J Pain. 2009;10:180–190. doi: 10.1016/j.jpain.2008.08.012. [DOI] [PubMed] [Google Scholar]
- 15.Weissman-Fogel I, Sprecher E, Pud D. Effects of catastrophizing on pain perception and pain modulation. Exp Brain Res. 2008;186:79–85. doi: 10.1007/s00221-007-1206-7. [DOI] [PubMed] [Google Scholar]
- 16.Stagg NJ, Mata HP, Ibrahim MM, Henriksen EJ, Porreca F, Vanderah TW, Malan PT. Regular exercise reverses sensory hypersensitivity in a rat neuropathic pain model: role of endogenous opioids. Anesthesiology. 2011;114:940–948. doi: 10.1097/ALN.0b013e318210f880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koltyn K, Trine M, Stegner A, Tobar D. Effect of isometric exercise on pain perception and blood pressure in men and women. Med Sci Sport Exerc. 2001;33:282–290. doi: 10.1097/00005768-200102000-00018. [DOI] [PubMed] [Google Scholar]
- 18.Shephard R. PAR-Q, Canadian Home Fitness Test and exercise screening alternatives. Sports Med. 1988;5:185–195. doi: 10.2165/00007256-198805030-00005. [DOI] [PubMed] [Google Scholar]
- 19.Sullivan MJ, Bishop SR, Pivik J. The Pain Catastrophizing Scale: Development and validation. Psychol Assess. 1995;7:524–532. [Google Scholar]
- 20.Spielberger CD. Manual for the State-Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists press; 1983. [Google Scholar]
- 21.La Motte RH, Campbell JN. Comparison of the responses of warmth and nociceptive C fiber afferents in monkey with human judgments of thermal pain. J Neurophys. 1978;41:509–528. doi: 10.1152/jn.1978.41.2.509. [DOI] [PubMed] [Google Scholar]
- 22.Borg G. Borg’s Perceived Exertion and Pain Scales. Champaign, IL: Human Kinetics; 1998. [Google Scholar]
- 23.Morris SB, DeShon RP. Combining effect size estimates in meta-analysis with repeated measures and independent-groups designs. Psychological Methods. 2002;7:105–125. doi: 10.1037/1082-989x.7.1.105. [DOI] [PubMed] [Google Scholar]
- 24.Cohen J. Statistical power analysis for the behavioral sciences. 2nd ed. Hillsdale NJ: Lawrence Erlbaum; 1988. [Google Scholar]
- 25.Umeda M, Newcomb L, Ellingson L, Koltyn K. Examination of the dose-response relationship between pain perception and blood pressure elevations induced by isometric exercise. Biol Psychol. 2010;85:90–96. doi: 10.1016/j.biopsycho.2010.05.008. [DOI] [PubMed] [Google Scholar]
- 26.Gibson SJ, Farrell M. A review of age differences in the neurophysiology of nociception and the perceptual experience of pain. Clin J Pain. 2004;20:227–239. doi: 10.1097/00002508-200407000-00004. [DOI] [PubMed] [Google Scholar]
- 27.King CD, Goodin B, Kindler LL, Caudle RM, Edwards RR, Gravenstein N, Riley JL, III, Fillingim RB. Reduction of conditioned pain modulation in humans by naltrexone: an exploratory study of the effects of pain catastrophizing. J Behav Med. 2012;36:315–327. doi: 10.1007/s10865-012-9424-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Droste C, Greenlee M, Schreck M. Experimental pain thresholds and plasma beta-endorphin levels during exercise. Med Sci Sports Exerc. 1991;23:334–342. [PubMed] [Google Scholar]
- 29.Price D, Von der Gruen A, Miller J, Rafii A, Price C. A psychophysical analysis of morphine analgesia. Pain. 1985;22:261–269. doi: 10.1016/0304-3959(85)90026-0. [DOI] [PubMed] [Google Scholar]
- 30.Cooper BY, Vierck CJ, Jr, Yeomans DC. Selective reduction of second pain sensations by systemic morphine in humans. Pain. 1986;24:93–116. doi: 10.1016/0304-3959(86)90030-8. [DOI] [PubMed] [Google Scholar]
- 31.Staud R, Robinson ME, Price DD. Temporal summation of second pain and its maintenance are useful for characterizing widespread central sensitization of fibromyalgia patients. J Pain. 2007;8:893–901. doi: 10.1016/j.jpain.2007.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Staahl C, Drewes AM. Experimental human pain models: A review of standardized methods for preclinical testing of analgesics. Basic & Clinical Pharmacology & Toxicology. 2004;95:97–111. doi: 10.1111/j.1742-7843.2004.950301.x. [DOI] [PubMed] [Google Scholar]
- 33.Hashmi JA, Davis KD. Effect of static and dynamic heat pain stimulus profiles on the temporal dynamics and interdependence of pain qualities, intensity, and affect. J Neurophysiol. 2008;100:1706–1715. doi: 10.1152/jn.90500.2008. [DOI] [PubMed] [Google Scholar]
- 34.Jensen I, Nygren A, Gamberale F, Goldie I, Westerholm P. Coping with long-term musculoskeletal pain and its consequences: Is gender a factor? Pain. 1994;57:167–172. doi: 10.1016/0304-3959(94)90220-8. [DOI] [PubMed] [Google Scholar]
- 35.Keefe FJ, Lefebvre JC, Egert JR, Affleck G, Sullivan MJ, Caldwell DS. The relationship of gender to pain, pain behavior, and disability in osteoarthritis patients: The role of catastrophizing. Pain. 2000;87:325–334. doi: 10.1016/S0304-3959(00)00296-7. [DOI] [PubMed] [Google Scholar]
- 36.Sternber W, Bokat C, Kass L, Alboyadjian A, Gracely R. Sex-dependent components of the analgesia produced by athletic competition. J Pain. 2001;2:65–74. doi: 10.1054/jpai.2001.18236. [DOI] [PubMed] [Google Scholar]
- 37.Hashmi JA, Davis KD. Women experience greater heat pain adaptation and habituation than men. Pain. 2009;145:350–357. doi: 10.1016/j.pain.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 38.Rezaii T, Hirschberg AL, Carlstrom K, Ernberg M. The influence of menstrual phases on pain modulation in healthy women. J Pain. 2012;13:646–655. doi: 10.1016/j.jpain.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 39.Tousignant-Laflamme Y, Marchand S. Excitatory and inhibitory pain mechanisms during the menstrual cycle in healthy women. Pain. 2009;146:47–65. doi: 10.1016/j.pain.2009.06.018. [DOI] [PubMed] [Google Scholar]
- 40.Smith YR, Stohler CS, Nichols TE, Bueller JA, Koeppe RA, Zubieta JK. Pronociceptive and antinociceptive effects of estradiol through endogenous opioid neurotransmission in women. J Neurscience. 2006;26:5777–5785. doi: 10.1523/JNEUROSCI.5223-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hoeger Bement M, Rasiarmos R, Dicapo J, Lewis A, Keller ML, Harkins AL, Hunter S. The role of the menstrual cycle phase in pain perception before and after an isometric fatiguing contraction. Eur J Appl Physiol. 2009;106:105–112. doi: 10.1007/s00421-009-0995-8. [DOI] [PubMed] [Google Scholar]
- 42.Van Oosterwijck J, Nijs J, Meeus M, Lefever I, Huybrechts L, Lambrecht L, Paul L. Pain inhibition and post-exertional malaise in myalgic encephalolyelitis/chronic fatigue syndrome: An experimental study. J Intern Med. 2010;268:265–278. doi: 10.1111/j.1365-2796.2010.02228.x. [DOI] [PubMed] [Google Scholar]
- 43.Fillingim RB, King CD, Ribeiro-Dasilva MC, Rahim-Williams B, Riley JL., III Sex, gender, and pain: A review of recent clinical and experimental findings. J Pain. 2009;10:447–485. doi: 10.1016/j.jpain.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Popescu A, LeResche L, Truelove EL, Drangsholt MT. Gender differences in pain modulation by diffuse noxious inhibitory controls: A systematic review. Pain. 2010;150:309–318. doi: 10.1016/j.pain.2010.05.013. [DOI] [PubMed] [Google Scholar]