Abstract
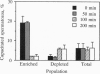
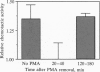
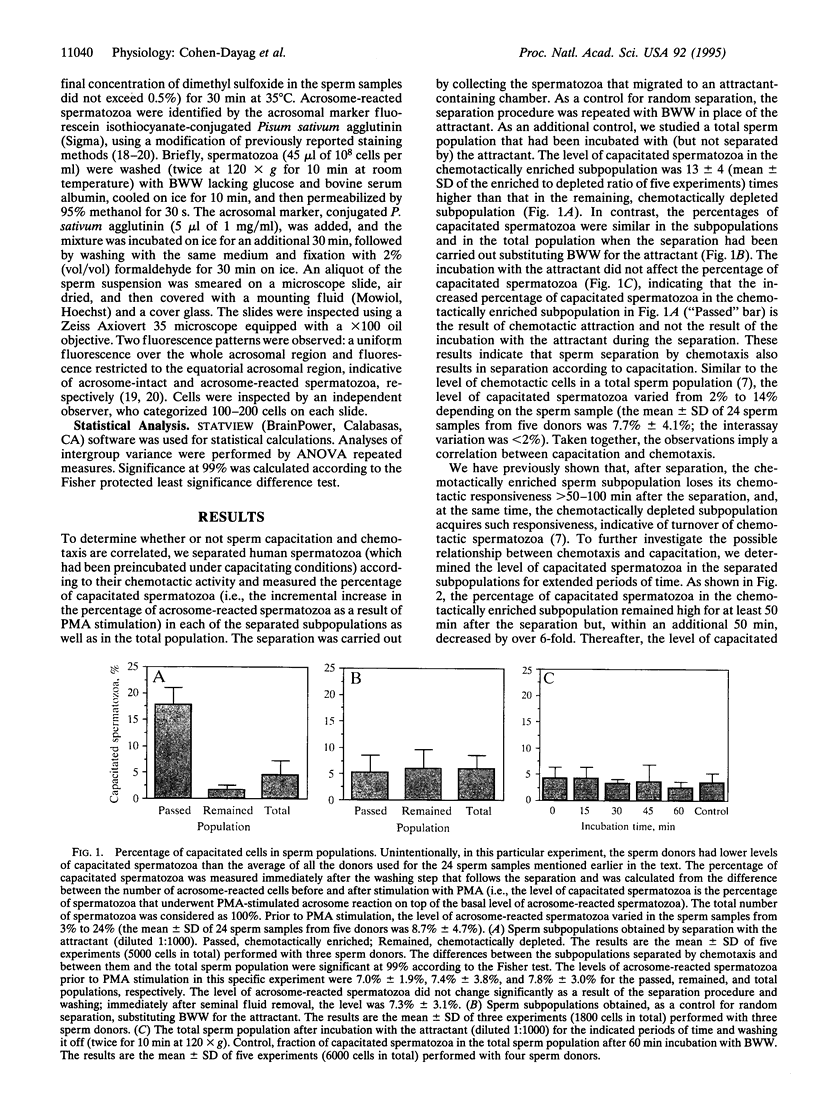
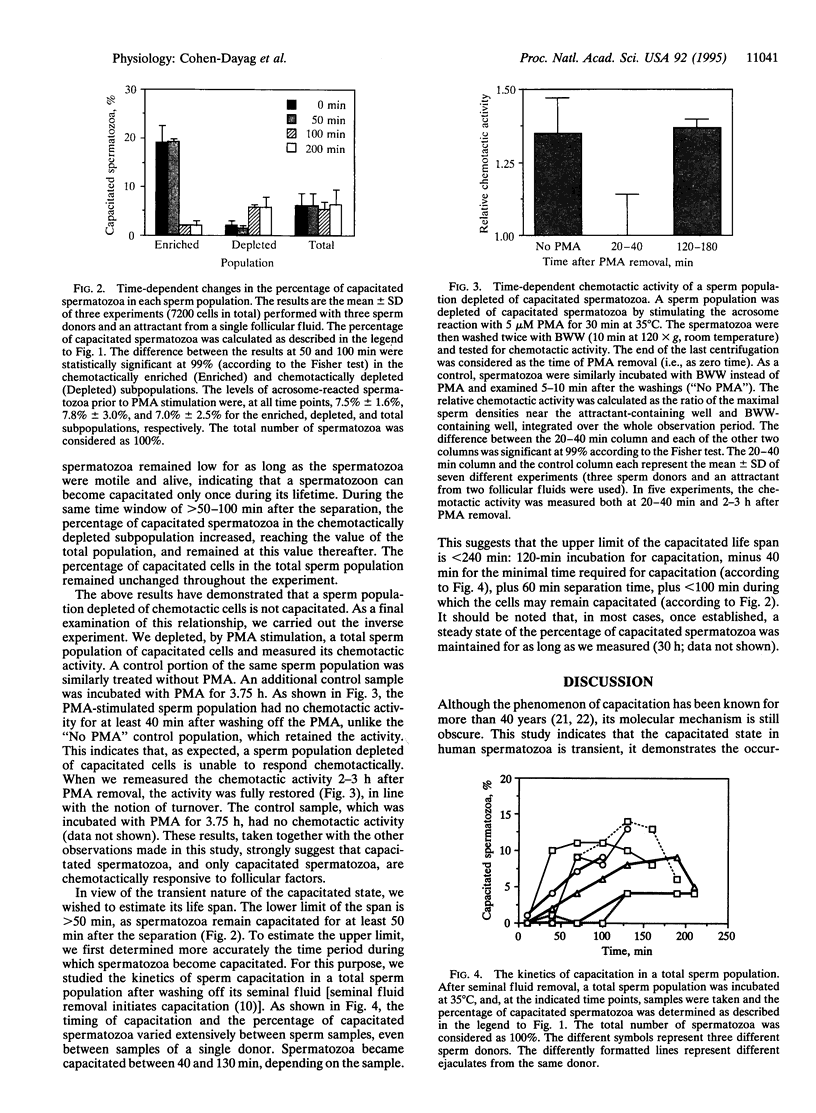
In humans, only a small fraction (2-12%) of a sperm population can respond by chemoattraction to follicular factors. This recent finding led to the hypothesis that chemotaxis provides a mechanism for selective recruitment of functionally mature spermatozoa (i.e., of capacitated spermatozoa, which possess the potential to undergo the acrosome reaction and fertilize the egg). This study aimed to examine this possibility. Capacitated spermatozoa were identified by their ability to undergo the acrosome reaction upon stimulation with phorbol 12-myristate 13-acetate. Under capacitating conditions, only a small portion (2-14%) of the spermatozoa were found to be capacitated. The spermatozoa were then separated according to their chemotactic activity, which resulted in a subpopulation enriched with chemotactically responsive spermatozoa and a subpopulation depleted of such spermatozoa. The level of capacitated spermatozoa in the former was approximately 13-fold higher than that in the latter. The capacitated state was temporary (50 min < life span < 240 min), and it was synchronous with the chemotactic activity. A continuous process of replacement of capacitated/chemotactic spermatozoa within a sperm population was observed. Spermatozoa that had stopped being capacitated did not become capacitated again, which indicates that the capacitated state is acquired only once in a sperm's lifetime. A total sperm population depleted of capacitated spermatozoa stopped being chemotactic. When capacitated spermatozoa reappeared, chemotactic activity was restored. These observations suggest that spermatozoa acquire their chemotactic responsiveness as part of the capacitation process and lose this responsiveness when the capacitated state is terminated. We suggest that the role of sperm chemotaxis in sperm-egg interaction in vivo may indeed be selective recruitment of capacitated spermatozoa for fertilizing the egg.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AUSTIN C. R. Observations on the penetration of the sperm in the mammalian egg. Aust J Sci Res B. 1951 Nov;4(4):581–596. doi: 10.1071/bi9510581. [DOI] [PubMed] [Google Scholar]
- Bedford J. M. Significance of the need for sperm capacitation before fertilization in eutherian mammals. Biol Reprod. 1983 Feb;28(1):108–120. doi: 10.1095/biolreprod28.1.108. [DOI] [PubMed] [Google Scholar]
- Bedford J. M. The contraceptive potential of fertilization: a physiological perspective. Hum Reprod. 1994 May;9(5):842–858. doi: 10.1093/oxfordjournals.humrep.a138604. [DOI] [PubMed] [Google Scholar]
- Benoff S., Cooper G. W., Hurley I., Mandel F. S., Rosenfeld D. L. Antisperm antibody binding to human sperm inhibits capacitation induced changes in the levels of plasma membrane sterols. Am J Reprod Immunol. 1993 Sep-Oct;30(2-3):113–130. doi: 10.1111/j.1600-0897.1993.tb00612.x. [DOI] [PubMed] [Google Scholar]
- Benoff S., Cooper G. W., Hurley I., Napolitano B., Rosenfeld D. L., Scholl G. M., Hershlag A. Human sperm fertilizing potential in vitro is correlated with differential expression of a head-specific mannose-ligand receptor. Fertil Steril. 1993 Apr;59(4):854–862. [PubMed] [Google Scholar]
- Benoff S. Preliminaries to fertilization. The role of cholesterol during capacitation of human spermatozoa. Hum Reprod. 1993 Dec;8(12):2001–2006. doi: 10.1093/oxfordjournals.humrep.a137971. [DOI] [PubMed] [Google Scholar]
- Bielfeld P., Anderson R. A., Mack S. R., De Jonge C. J., Zaneveld L. J. Are capacitation or calcium ion influx required for the human sperm acrosome reaction? Fertil Steril. 1994 Dec;62(6):1255–1261. doi: 10.1016/s0015-0282(16)57195-7. [DOI] [PubMed] [Google Scholar]
- Bielfeld P., Faridi A., Zaneveld L. J., De Jonge C. J. The zona pellucida-induced acrosome reaction of human spermatozoa is mediated by protein kinases. Fertil Steril. 1994 Mar;61(3):536–541. [PubMed] [Google Scholar]
- Breitbart H., Lax J., Rotem R., Naor Z. Role of protein kinase C in the acrosome reaction of mammalian spermatozoa. Biochem J. 1992 Jan 15;281(Pt 2):473–476. doi: 10.1042/bj2810473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkman L. J. Characterization of hyperactivated motility by human spermatozoa during capacitation: comparison of fertile and oligozoospermic sperm populations. Arch Androl. 1984;13(2-3):153–165. doi: 10.3109/01485018408987514. [DOI] [PubMed] [Google Scholar]
- Cohen-Dayag A., Eisenbach M. Potential assays for sperm capacitation in mammals. Am J Physiol. 1994 Nov;267(5 Pt 1):C1167–C1176. doi: 10.1152/ajpcell.1994.267.5.C1167. [DOI] [PubMed] [Google Scholar]
- Cohen-Dayag A., Ralt D., Tur-Kaspa I., Manor M., Makler A., Dor J., Mashiach S., Eisenbach M. Sequential acquisition of chemotactic responsiveness by human spermatozoa. Biol Reprod. 1994 Apr;50(4):786–790. doi: 10.1095/biolreprod50.4.786. [DOI] [PubMed] [Google Scholar]
- Cross N. L., Morales P., Overstreet J. W., Hanson F. W. Induction of acrosome reactions by the human zona pellucida. Biol Reprod. 1988 Feb;38(1):235–244. doi: 10.1095/biolreprod38.1.235. [DOI] [PubMed] [Google Scholar]
- De Jonge C. J., Han H. L., Lawrie H., Mack S. R., Zaneveld L. J. Modulation of the human sperm acrosome reaction by effectors of the adenylate cyclase/cyclic AMP second-messenger pathway. J Exp Zool. 1991 Apr;258(1):113–125. doi: 10.1002/jez.1402580113. [DOI] [PubMed] [Google Scholar]
- De Jonge C. J., Han H. L., Mack S. R., Zaneveld L. J. Effect of phorbol diesters, synthetic diacylglycerols, and a protein kinase C inhibitor on the human sperm acrosome reaction. J Androl. 1991 Jan-Feb;12(1):62–70. [PubMed] [Google Scholar]
- Eisenbach M., Ralt D. Precontact mammalian sperm-egg communication and role in fertilization. Am J Physiol. 1992 May;262(5 Pt 1):C1095–C1101. doi: 10.1152/ajpcell.1992.262.5.C1095. [DOI] [PubMed] [Google Scholar]
- Eisenbach M., Tur-Kaspa I. Human sperm chemotaxis is not enigmatic anymore. Fertil Steril. 1994 Aug;62(2):233–235. doi: 10.1016/s0015-0282(16)56869-1. [DOI] [PubMed] [Google Scholar]
- Furuya S., Endo Y., Osumi K., Oba M., Suzuki S. Effects of modulators of protein kinase C on human sperm capacitation. Fertil Steril. 1993 Jun;59(6):1285–1290. [PubMed] [Google Scholar]
- Grunert J. H., De Geyter C., Nieschlag E. Objective identification of hyperactivated human spermatozoa by computerized sperm motion analysis with the Hamilton-Thorn sperm motility analyser. Hum Reprod. 1990 Jul;5(5):593–599. doi: 10.1093/oxfordjournals.humrep.a137151. [DOI] [PubMed] [Google Scholar]
- Hoshi K., Yanagida K., Aita T., Yoshimatsu N., Sato A. Changes in the motility pattern of human spermatozoa during in vitro incubation. Tohoku J Exp Med. 1988 Jan;154(1):47–56. doi: 10.1620/tjem.154.47. [DOI] [PubMed] [Google Scholar]
- Liu D. Y., Baker H. W. Tests of human sperm function and fertilization in vitro. Fertil Steril. 1992 Sep;58(3):465–483. doi: 10.1016/s0015-0282(16)55247-9. [DOI] [PubMed] [Google Scholar]
- Makler A., Reichler A., Stoller J., Feigin P. D. A new model for investigating in real-time the existence of chemotaxis in human spermatozoa. Fertil Steril. 1992 May;57(5):1066–1074. doi: 10.1016/s0015-0282(16)55026-2. [DOI] [PubMed] [Google Scholar]
- Mendoza C., Carreras A., Moos J., Tesarik J. Distinction between true acrosome reaction and degenerative acrosome loss by a one-step staining method using Pisum sativum agglutinin. J Reprod Fertil. 1992 Aug;95(3):755–763. doi: 10.1530/jrf.0.0950755. [DOI] [PubMed] [Google Scholar]
- Morales P., Overstreet J. W., Katz D. F. Changes in human sperm motion during capacitation in vitro. J Reprod Fertil. 1988 May;83(1):119–128. doi: 10.1530/jrf.0.0830119. [DOI] [PubMed] [Google Scholar]
- ROBINSON K. L., COEY W. E. A brown discoloration of pig fat and vitamin E deficiency. Nature. 1951 Dec 8;168(4284):997–998. doi: 10.1038/168997a0. [DOI] [PubMed] [Google Scholar]
- Ralt D., Goldenberg M., Fetterolf P., Thompson D., Dor J., Mashiach S., Garbers D. L., Eisenbach M. Sperm attraction to a follicular factor(s) correlates with human egg fertilizability. Proc Natl Acad Sci U S A. 1991 Apr 1;88(7):2840–2844. doi: 10.1073/pnas.88.7.2840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralt D., Manor M., Cohen-Dayag A., Tur-Kaspa I., Ben-Shlomo I., Makler A., Yuli I., Dor J., Blumberg S., Mashiach S. Chemotaxis and chemokinesis of human spermatozoa to follicular factors. Biol Reprod. 1994 Apr;50(4):774–785. doi: 10.1095/biolreprod50.4.774. [DOI] [PubMed] [Google Scholar]
- Robertson L., Wolf D. P., Tash J. S. Temporal changes in motility parameters related to acrosomal status: identification and characterization of populations of hyperactivated human sperm. Biol Reprod. 1988 Nov;39(4):797–805. doi: 10.1095/biolreprod39.4.797. [DOI] [PubMed] [Google Scholar]
- Rotem R., Paz G. F., Homonnai Z. T., Kalina M., Lax J., Breitbart H., Naor Z. Ca(2+)-independent induction of acrosome reaction by protein kinase C in human sperm. Endocrinology. 1992 Nov;131(5):2235–2243. doi: 10.1210/endo.131.5.1425422. [DOI] [PubMed] [Google Scholar]
- Rotem R., Paz G. F., Homonnai Z. T., Kalina M., Naor Z. Protein kinase C is present in human sperm: possible role in flagellar motility. Proc Natl Acad Sci U S A. 1990 Sep;87(18):7305–7308. doi: 10.1073/pnas.87.18.7305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez S. S., Katz D. F., Owen D. H., Andrew J. B., Powell R. L. Evidence for the function of hyperactivated motility in sperm. Biol Reprod. 1991 Feb;44(2):375–381. doi: 10.1095/biolreprod44.2.375. [DOI] [PubMed] [Google Scholar]
- Tesarik J., Mendoza C., Carreras A. Fast acrosome reaction measure: a highly sensitive method for evaluating stimulus-induced acrosome reaction. Fertil Steril. 1993 Feb;59(2):424–430. doi: 10.1016/s0015-0282(16)55707-0. [DOI] [PubMed] [Google Scholar]
- Yanagimachi R. The movement of golden hamster spermatozoa before and after capacitation. J Reprod Fertil. 1970 Oct;23(1):193–196. doi: 10.1530/jrf.0.0230193. [DOI] [PubMed] [Google Scholar]