Abstract
HDL and apolipoprotein A1 (apoA1) concentrations inversely correlate with risk of death from ischemic heart disease; however, the role of apoA1 in the myocardial response to ischemia has not been well defined. To test whether apoA1, the primary HDL apolipoprotein, has an acute anti-inflammatory role in ischemic heart disease, we induced myocardial infarction via direct left anterior descending coronary artery ligation in apoA1 null (apoA1−/−) and apoA1 heterozygous (apoA1+/−) mice. We observed that apoA1+/− and apoA1−/− mice had a 52% and 125% increase in infarct size as a percentage of area at risk, respectively, compared with wild-type (WT) C57BL/6 mice. Mitochondrial oxidation contributes to tissue damage in ischemia–reperfusion injury. A substantial defect was present at baseline in the electron transport chain of cardiac myocytes from apoA1−/− mice localized to the coenzyme Q (CoQ) pool with impaired electron transfer (67% decrease) from complex II to complex III. Administration of coenzyme Q10 (CoQ10) to apoA1 null mice normalized the cardiac mitochondrial CoQ pool and reduced infarct size to that observed in WT mice. CoQ10 administration did not significantly alter infarct size in WT mice. These data identify CoQ pool content leading to impaired mitochondrial function as major contributors to infarct size in the setting of low HDL/apoA1. These data suggest a previously unappreciated mechanism for myocardial stunning, cardiac dysfunction, and muscle pain associated with low HDL and low apoA1 concentrations that can be corrected by CoQ10 supplementation and suggest populations of patients that may benefit particularly from CoQ10 supplementation.
Introduction
Plasma concentrations of HDL and its major protein, apoA1, are both inversely associated with cardiovascular disease morbidity and mortality (1, 2). apoA1 is a single polypeptide of ∼28,000 kDa primarily synthesized in the liver and small intestine (3, 4). Human individuals with apoA1 deficiency (5) and apoA1-deficient mice (6) fail to form normal HDL particles, cannot effectively transport cholesterol from tissues back to liver and, as a result, are predisposed to premature coronary artery disease and death (7, 8). More recently, novel therapeutic approaches to treat coronary artery disease, such as the administration of apoA1 or its analogs to alter the development of atherosclerosis, were investigated in animal models and in humans (9, 10).
Recent studies (11–14) show that apoA1 possesses anti-inflammatory and antioxidant properties, in addition to its role in reverse cholesterol transport. Given the role of inflammation (15–17), redox state, and mitochondrial electron transport (18–20) in determining cardiac function during postmyocardial infarction, we sought to determine whether apoA1 has acute anti-inflammatory properties that modulate myocardial infarct size. In fact, we found that apoA1 deficiency sharply increased infarct size. The pathology was traced to a functional defect in mitochondrial electron transport that arose from a deficiency in the coenzyme Q (CoQ)10 pool of apoA1 null (apoA1−/−) mice. This defect was reversed by coenzyme Q10 (CoQ10) i.p. injection, which normalized infarct size in apoA1−/− mice. Our study adds to the emerging role of HDL in modulating cardiac mitochondrial function (21) and in defining cardiac damage after reperfusion of ischemic myocardium.
Materials and Methods
Left anterior descending coronary artery ligation/reperfusion and quantification of area at risk and infarct size.
Animal protocols were approved by the Animal Research Committee using mice housed in the Association for Assessment and Accreditation of Laboratory Animal Care International–approved facilities of the Cleveland Clinic and Northeast Ohio Medical University. Mice were maintained on LabDiet 5008 (27% protein, 17% fat, and 56% carbohydrate by calories; 3.5 kcal/g energy value) (LabDiet). Mice were all littermates generated from apoA1heterozygous (apoA1+/−) mice (The Jackson Laboratory). In indicated experiments, mice were administered CoQ10 i.p. (0.1 mg/g body weight per day in 10% Tween 20) or a control solution of 10% Tween for 3 d before initiating ischemia because this dose of CoQ10 generates a peak myocardial CoQ10 content within 4 d (22). Anterior wall myocardial infarction was performed as described previously (15). Briefly, anterior wall myocardial infarction was induced in 20–25 g male littermate wild-type (WT) C57BL/6J, apoA1+/−, or apoA1−/− mice by ligation of the left anterior descending coronary artery (LAD) (with 7-0 Prolene). Blanching and dysfunction of the anterior wall verified LAD ligation. After 30 min of LAD ligation, the knot was cut at the level of the myocardium, and subsequently mice underwent reperfusion for 3 h. Successful reperfusion was verified by return of red color to the tissue that was initially blanched at the time of LAD ligation and gross evidence of some recovery of anterior wall motion. Mice were continuously ventilated. The area at risk and infarct size was analyzed using Evan’s blue dye and 1% 2,3,5-triphenyltetrazolium chloride (TTC) at 37°C, respectively. At the time of death, the LAD was ligated again, and Evan’s blue dye (1 g/L) was infused to define the area of myocardium not at risk (area of Evan’s blue dye exclusion). The heart was then harvested and sectioned into 3 pieces defined as base, mid, and apex. The sections were incubated in TTC solution for 15 min, rinsed, and then placed in formalin overnight. The infarct size as a percentage of area at risk was calculated as the area of myocardium that was TTC-stain positive divided by the area of myocardium that was not stained by Evan’s blue dye.
Determination of reactive oxygen species production in vivo.
Reactive oxygen species (ROS) production was assessed in vivo using hydroethidine dye (23, 24) as described previously (25) with detection at 600 nm after excitation at 520 nm (26). Hydroethidine (10 mg/kg) was injected into the jugular vein of the anesthetized and previously infarcted mouse and allowed to circulate for 3 h. Serial sections of embedded heart (n = 5) were cut and collected at 600 μm intervals, and 5 randomly selected areas within the infarct zone were viewed by confocal microscopy in a blinded manner. The sum of the fluorescence intensity for each region was divided by the total number of pixels analyzed and expressed as relative fluorescence units.
Terminal deoxynucleotidyl transferase–mediated biotinylated dUTP nick end labeling assay.
Heart sections were stained with the In Situ Cell Death Detection kit (Roche Applied Science) per the instructions of the manufacturer and costained with 4′,6-diamidino-2-phenylindole. Terminal deoxynucleotidyl transferase–mediated biotinylated dUTP nick end labeling–positive cells were counted at 40× magnification in 5 randomly selected areas within the infarct zone and expressed as positive cells per square millimeter and then compared between WT and apoA1−/− mice. At least 10 sections were analyzed throughout the entire longitudinal axis of the hearts (n = 5 hearts per group).
HDL assay.
HDL was quantified in duplicate in serum using the Abnova colorimetric HDL assay kit (KA1656).
Mitochondrial techniques.
Three mouse hearts were pooled, finely minced, and placed in Chappell-Perry (CP1) buffer [100 mmol/L potassium chloride (KCl), 50 mmol/L MOPS, 5 mmol/L magnesium sulfate, 1 mmol/L EGTA, and 1 mmol/L ATP], and trypsin was added (1 mg/g wet weight) and then homogenized with a polytron tissue processor (Brinkmann Instruments) for 2.5 s at a rheostat setting of 3.5. The polytron homogenate was incubated for 10 min at 4°C with stirring. CP2 buffer (CP1 with 2% FA–free BSA) was added to arrest trypsin digestion. Additional mixing and homogenization used 2 strokes with a loose-fitting pestle and 2 strokes with a tight-fitting pestle. The homogenate was cleared (500 × g, 10 min), and recovered mitochondria (3000 × g, 10 min) were washed twice before resuspension in buffer containing 100 mmol/L KCL, 50 mmol/L MOPS, and 0.5 mmol/L EGTA. Mitochondrial protein concentration was measured by the Lowry method, using BSA as a standard.
Oxygen consumption was measured using a Clark-type oxygen electrode at 30°C. Mitochondria were incubated in a solution containing 80 mmol/L KCl, 50 mmol/L MOPS, 1 mmol/L EGTA, 5 mmol/L potassium phosphate, and 1 mg/mL defatted, dialyzed BSA, pH 7.4. Glutamate (complex I substrate, 20 mmol/L) plus malate (2 mmol/L), succinate (complex II substrate, 20 mmol/L) plus rotenone (7.5 μmol/L), and N,N,N′,N′-tetramethyl p-phenylenediamine (TMPD)-ascorbate (complex IV substrate, 1 mmol/L-10 mmol/L) plus rotenone (7.5 μmol/L) were used. State 3 (ADP-stimulated) respiration, state 4 (ADP-limited) respiration, respiratory control ratios, the ADP-to-oxygen ratio, and dinitrophenol-uncoupled respiration were determined. Endogenous substrates were depleted by addition of 0.1 mmol/L ADP before glutamate stimulated respiration.
Enzyme activities were measured in detergent-solubilized mitochondria as described previously (27, 28): 1) nicotinamide adenine dinucleotide cytochrome c reductase, rotenone-sensitive; 2) succinate cytochrome c reductase, antimycin A–sensitive; 3) complex II, thenoyltrifluroacetone-sensitive; and 4) complex III, antimycin A–sensitive and 5) citrate synthase.
Net hydrogen peroxide production from mitochondria was measured using oxidation of the fluorogenic indicator Amplex red (Invitrogen) in the presence of HRP. Glutamate and succinate were used as substrates at the same concentration used to measure oxidative phosphorylation (29).
CoQ10 assay.
CoQ10 concentrations were determined by reversed-phase HPLC on a C18 column (30) with slight modification. Heart mitochondria were isolated by differential centrifugation as described above, and a defined amount of CoQ9 was added before CoQ10 was extracted at 4°C with methanol n-hexane (2:3, v:v) and evaporated to dryness under nitrogen gas before resuspension in mobile phase (methanol, n-hexane, 72:28 v:v) for resolution at 1 mL/min at 24°. CoQ10 was detected at 275 nm and quantified using the CoQ9 internal standard.
Statistical analyses.
All data are expressed as means ± SDs with statistical analysis by SPSS software (Windows version 10.0; SPSS). The effect of apoA1 on infarct size was tested by 2-factor ANOVA with comparisons between groups performed using Bonferroni’s correction. Pearson’s correlation coefficient was calculated based on the number of apoA1 alleles present. Comparisons between 2 groups were statistically evaluated by Student’s t test. A value of P ≤ 0.05 was considered significant.
Results
HDL concentrations and infarct size in mice vary by apoA1 gene dose.
We quantified HDL concentrations as a function of apoA1 genotype. As anticipated, the serum concentration of HDL corresponded to the gene dose of apoA1. HDL contents were 10.5 ± 0.71 mg/dL (n = 4), 33.0 ± 2.0 mg/dL (n = 4), and 60.3 ± 3.0 mg/dL (n = 5) in apoA1−/−, apoA1+/−, and WT mice, respectively, and are consistent with previous reports (6, 31).
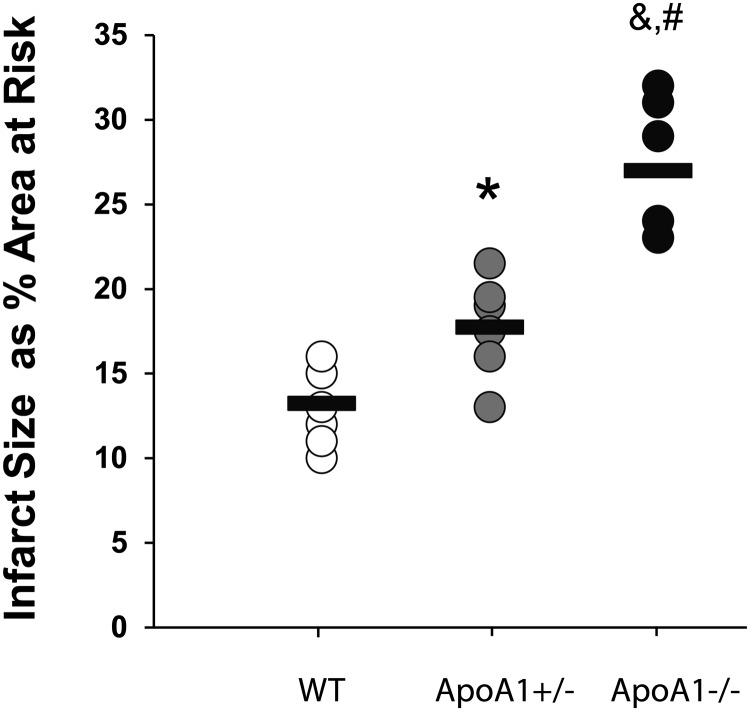
Loss of apoA1 and HDL affected outcomes after chronic ligation of the proximal LAD in which ∼80–90% of apoA1−/−, but not WT, mice died within the first 24 h (data not shown). Using an ischemia (30 min)–reperfusion (3 h) model, we observed no differences in the area at risk after LAD ligation between WT, apoA1+/−, and apoA1−/− mice after LAD ligation (50 ± 11%, 50 ± 3%, and 52 ± 4%, respectively). Infarct size as a percentage of the area at risk was significantly (P < 0.05) affected by the concentration of apoA1, with those in the apoA1−/− mice larger than those of apoA1+/− and WT mice, and those in the apoA1+/− mice larger than in WT mice (Fig. 1). The largest infarcts were present in the apoA1−/− mice compared with apoA1+/− and WT mice (Fig. 1), and these differences were significant compared with WT [WT vs. any apoA1 deletion (combined apoA1+/− and apoA1−/−), P = 0.001; and apoA1+/− vs. apoA1−/−, P < 0.01].
FIGURE 1.
Infarct size as a percentage of the area at risk resulting from left anterior descending coronary artery ligation for 30 min, followed by reperfusion for 3 h in apoA1−/−, apoA1+/−, and WT mice. Values are individual data points, with bars representing means, n = 6. *Different from WT, P < 0.01; &different from WT, P < 0.001; #different from apoA1+/−, P < 0.001. apoA1−/−, apoA1 null; apoA1+/−, apoA1 heterozygous; WT, wild-type.
apoA1 concentrations do not alter myocardial ROS content.
We determined whether the increase in infarct size in the apoA1−/− mice was due to increased production of ROS, because ROS play a major pathogenic role in ischemic injury. Three hours after reperfusion, there was no significant increase in mean ROS in apoA1−/− mice compared with WT mice (mean ± SD: 62 ± 42 and 40 ± 28, respectively, n = 8 mice per group).
apoA1 content does not alter myocardial apoptosis.
To determine whether increased apoptosis was responsible for the observed injury in apoA1−/− mice, terminal deoxynucleotidyl transferase–mediated biotinylated dUTP nick end labeling staining was used. The number of apoptotic cardiac myocytes (positive cells per square millimeter) was not different between apoA1−/− and WT mice (mean ± SD: 20 ± 14 and 12 ± 11, respectively, n = 5 per group). The lack of difference in apoptotic cardiac myocyte cell death suggests that the majority of the increased death in the absence of apoA1 is secondary to increased necrosis.
Mitochondrial oxidative phosphorylation is compromised by loss of apoA.
Mitochondrial oxidative metabolism was measured using glutamate, succinate, duroquinol, and TMPD-ascorbate as substrates for complexes I through IV, respectively. Oxygen consumption under ADP-stimulated (state 3) and ADP-limited (state 4) conditions are shown in Supplemental Table 1. State 3 respiration, state 4 respiration, respiratory control ratio, and the ADP-to-oxygen ratio were similar in WT and apoA1−/− mice when glutamate was used as the substrate (Supplemental Table 1). However, there was a decrease in both state 3 and uncoupled respiration in the apoA1−/− mice using a complex II substrate (succinate plus rotenone). The decreased coupling of respiration observed in apoA1−/− mice indicated by the decrease in the respiratory control ratio was mainly due to a decrease in state 3 respiration rather than an increase in state 4 respiration. Dinitrophenol-uncoupled respiration was decreased in apoA1−/− mice, localizing the respiratory defect to the electron transport chain.
Substrates that donate electrons to specific electron transport complexes were used under conditions of maximal ADP-stimulated respiration to further localize the sites of defects within the electron transport chain. Maximal ADP-stimulated respiration, measured using succinate as substrate for complex II, was decreased in apoA1−/−mice (Supplemental Table 1). These data uncover a defect in the pathway from complex II → CoQ pool → complex III → cytochrome c → cytochrome oxidase → oxygen. To assess the function of the electron transport distal to complex II, duroquinol (electron donor to complex III) and TMPD-ascorbate (electron donor to cytochrome oxidase via cytochrome c) were used. Maximal ADP-stimulated respiration was not affected in the apoA1−/− mice when duroquinol or TMPD-ascorbate were substrates (Supplemental Table 1). Maximal ADP-stimulated respiration was also similar in WT and apoA1−/− mice with a complex I substrate (which, like complex II, donates electrons to complex III), confirming the integrity of the electron transport chain other than at complex II.
Electron transport is compromised by inadequate CoQ.
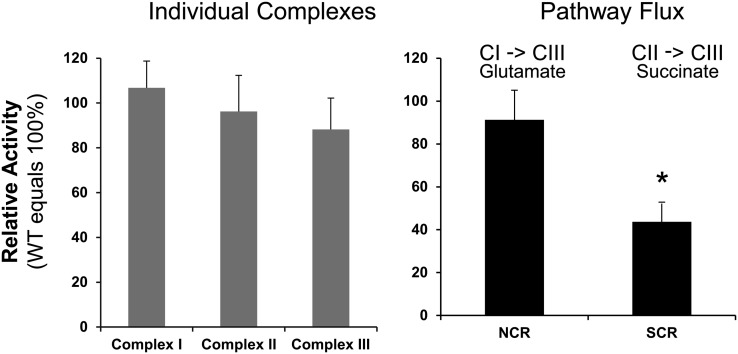
Nicotinamide adenine dinucleotide cytochrome c reductase, a measure of the activity of the electron transport segment I → CoQ → III, was similar in apoA1−/− and WT mice (Fig. 2). The activity of succinate cytochrome c reductase (antimycin A sensitive) was markedly decreased in apoA1−/− mice, localizing a defect to either complex II, CoQ, or complex III of the electron transport chain (Fig. 2; Supplemental Table 2). However, the activity of complex III was not changed compared with WT mice, and the activity of complex II itself was surprisingly normal (Fig. 2A). Thus, the defect in electron transport in mitochondria of apoA1−/− mice must be localized to the CoQ pool that transfers electrons from complex II to complex III. The activity of citrate synthase, a mitochondrial matrix enzyme, was similar in apoA1−/− and WT mice (Fig. 2B), indicating that mitochondrial integrity was not compromised in apoA1−/− mice.
FIGURE 2.
Maximal electron flux in individual electron transport complexes in mitochondria (left) and from complex I to complex III using glutamate as a substrate and complex II to complex III using succinate as a substrate (right) in apoA1−/− mice relative to WT mice. Values are means ± SDs; n = 4 (apoA1−/−) or 5 (WT). *Different from WT, P < 0.05. apoA1−/−, apoA1 null; CI, complex 1; CII, complex II; CIII, complex III; NCR, NADH-cytochrome C reductase; SCR, succinate-cytochrome C reductase; WT, wild-type.
Cardiac CoQ10 concentrations are reduced in apoA1−/− mice.
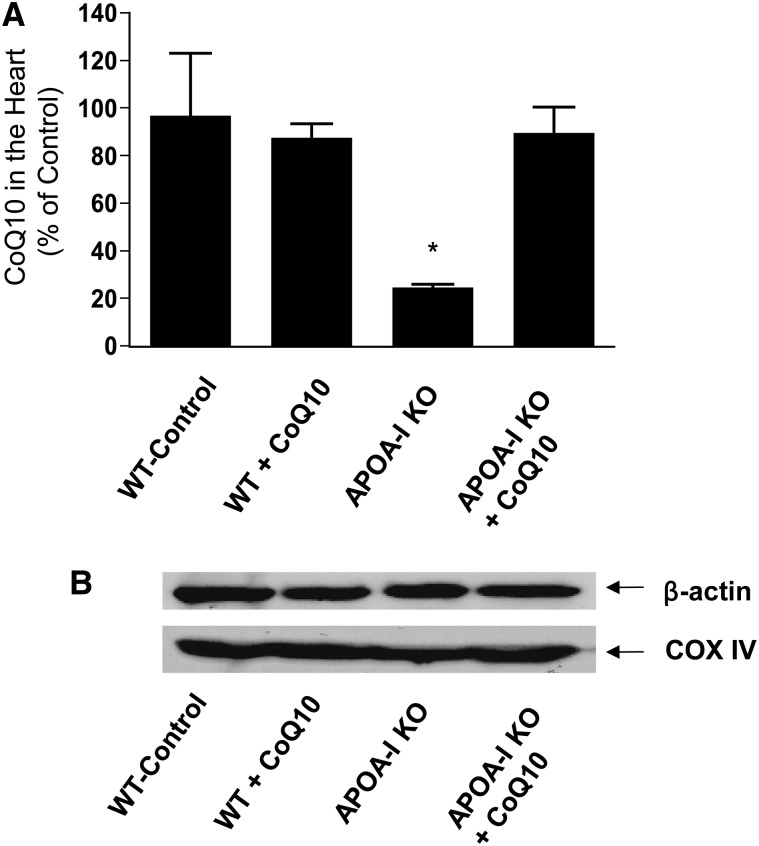
The foregoing analyses suggest that a defect exists in the mitochondria of apoA1−/− mice consistent with an inability of the CoQ pool to adequately support complex II function. We determined whether the cardiac pool of CoQ10 was indeed sensitive to circulating HDL and apoA1 concentrations and found that the lack of apoA1 reduced the cardiac CoQ10 pool by almost 75% (Fig. 3A). This was a selective reduction because Western blotting for cytochrome oxidase showed that mitochondrial mass was not diminished in the apoA1−/− mice (Fig. 3B). We sought to determine whether the cardiac pool of CoQ10 could be manipulated by administration of exogenous CoQ10 (22). CoQ10 concentrations did not increase in hearts of WT mice, but this supplementation did raise cardiac CoQ10 concentrations in the apoA1−/− mice to WT levels (Fig. 3A).
FIGURE 3.
CoQ is deficient in cardiac mitochondria of apoA1 null mice and is normalized by supplementation (A). Mitochondria were isolated by differential centrifugation from the hearts of WT or apoA1 null mice supplemented with CoQ10 or not by i.p. injection daily for 3 d before harvest (mean ± SD; n = 3 per group; *P < 0.05 vs. other groups). CoQ10 concentrations were quantitated after extraction by HPLC and UV absorption in relation to a CoQ9 internal standard. Representative Western blot (performed 3 times) for cytochrome oxidase in different groups shows that mitochondrial mass is not altered among groups or by CoQ10 supplementation (B). APOA-I KO, apoA1 null mice; CoQ, coenzyme Q; CoQ10, coenzyme Q10; COX IV, cytochrome c oxidase subunit IV; WT, wild-type mice.
CoQ10 supplementation reduced infarct size in apoA1−/−mice.
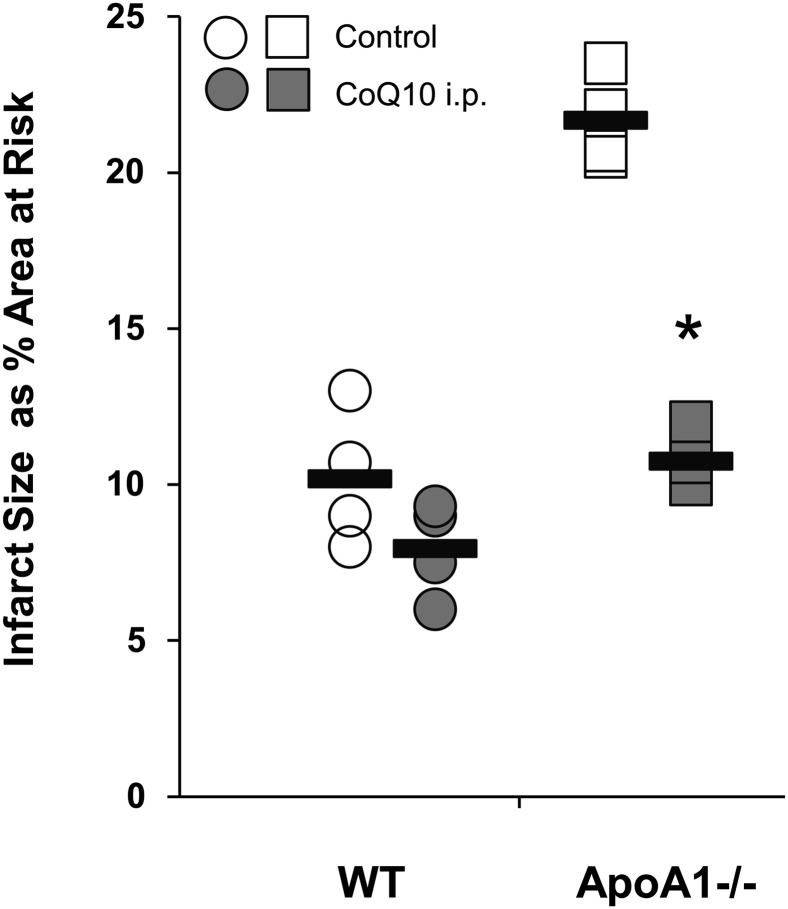
The ready manipulation of the cardiac CoQ10 pool is a potential therapy to reverse the adverse effects of apoA1/HDL deficiency on infarct size. Indeed, administration of CoQ10 to apoA1−/− mice completely corrected the defect in apoA1−/− mice because CoQ10 supplementation fully reduced infarct size as a percentage area at risk to that of WT mice (Fig. 4). We found that CoQ10 injected into WT mice were no different than WT mice that received vehicle (P = 0.15), suggesting that the CoQ10 content of WT mitochondria is sufficient.
FIGURE 4.
Infarct size in apoA1−/−mice was reduced to that of WT mice by CoQ10 supplementation. WT (circles) and apoA1−/−(squares) mice were administered vehicle or CoQ10 (0.1 mg · g−1 · d−1) i.p. for 3 d before left anterior descending coronary artery ligation. Infarct size as a function of area at risk was determined as in Figure 1 (mean ± SD; n = 4 per group; *P < 0.05 vs. corresponding untreated mice). Values are individual data points, with bars representing means. ApoA1−/−, apoA1 null; CoQ10, coenzyme Q10; WT, wild-type.
Discussion
For >3 decades, population studies demonstrated an inverse correlation between plasma apoA1 and HDL concentration and coronary artery disease (32, 33). Many of these same clinical populations’ low HDL concentrations are associated with increased mortality, which to date is presumed to be secondary to an increased risk of myocardial infarction in which decreased HDL concentrations place a greater burden of atherosclerotic coronary artery disease at presentation (34, 35). Here, we showed that in fact reducing HDL concentrations, even just by half, acutely alters infarct size. Processes occurring during the ischemia or reperfusion are affected by the previous history of circulating HDL concentrations, and we trace this to a sharply reduced cardiac CoQ10 pool that is insufficient to support electron transport from complex II to complex III. This deficit can more than double infarct size.
Our initial studies indicated that anterior wall myocardial infarction led to substantial cardiac dysfunction to such a degree that mice could not successfully be weaned from the ventilator in the apoA1−/− mice. These initial findings suggested that there was a significantly greater tissue death and perhaps increased strain on surviving myocardium in the absence of apoA1. This increased death led us to assess the effects of apoA1 in an acute ischemia–reperfusion model with 30 min ischemia and 3 h reperfusion. Our data indicate that necrotic, but not apoptotic, cell death was enhanced in apoA1-deficient mice in this model.
Current literature suggests that the increased death in apoA1−/− mice from reperfusion injury should be due to the loss of free-radical scavenging by apoA1 itself (14). However, our data demonstrate that a defect that persists after mitochondrial isolation is present in the hearts of apoA1−/− mice. This defect is the relevant change responsive to circulating apoA1 concentrations because correction of this defect by CoQ10 supplementation normalized infarct size. CoQ10 is a facile antioxidant, but neither reduction of circulating apoA1 nor cardiac mitochondrial CoQ10 content enhanced cardiac ROS production detectable by hydroethidine staining.
Given that there was CoQ10 in the diet of the mice, the fact that i.p. supplementation restored CoQ10 concentrations in the myocardium in the absence of an increase in apoA1 or HDL suggests that apoA1 could have an as-yet undefined role in CoQ10 absorption or trafficking from the gastrointestinal tract. Abnormal gastrointestinal absorption was postulated in patients with heart failure due to intestinal edema, and the increasing doses of CoQ10 supplementation were shown to increase plasma concentrations of CoQ10 and be associated with increases in cardiac function (36). Furthermore, emulsification of CoQ10 was shown to enhance the oral bioavailability of CoQ10 (37). The potential role for apoA1 in the bioavailability of oral CoQ10 remains to be determined.
Cardiac mitochondria of apoA1−/− mice exhibit a defect in succinate-driven respiration that, notably, exists before the onset of ischemic injury and may predispose mice with low HDL concentrations to heightened responses to other insults. This defect in oxidative phosphorylation was limited to the pathway from complex II → CoQ → complex III even while the electron transport chain from complex I → CoQ → complex III remained intact. Differential interaction of complex I and complex II with the CoQ pool was first described (38) in CDC-like kinase 1 (clk-1) mutants of Caenorhabditis elegans defective in the last step of CoQ synthesis (39). These mutants display defective electron transport (38), although in this organism, the decrease in respiration was with glutamate, a complex I donor, and not succinate (40). clk-1 Mutants have an increased lifespan (41), suggesting that defective coupling of the CoQ pool to complex I is protective. This outcome is in line with the protective effect of blocking electron flow from complex I into complex III during ischemia (19). In contrast, the current observation suggests that a blockade of electron flow from complex II into complex III exacerbates ischemic damage. Ischemia by itself allows cytochrome c to escape from mitochondria, with a concomitant reduction in electron transport from complex III to cytochrome oxidase (42). Therefore, reduced apoA1 imposes an additional stress on mitochondrial electron transport in cardiac tissue subjected to reperfusion after ischemia.
How apoA1 affects tissue CoQ10 is undefined because our understanding of the flux of CoQ10 from circulation to tissues is incomplete. CoQ10 circulates primarily in VLDL and LDL (43, 44). HDL normally does not contain CoQ10 but does in animals supplemented with CoQ10 (43). In fact, compared with LDL, CoQ10 flux through VLDL—its major carrier—and HDL are highly dynamic in these supplementation experiments (43). The VLDL compartment was modeled to be the major source of circulating CoQ10 (44), and lack of apoA1 either directly slows movement of the coenzyme to tissues or indirectly disrupts VLDL formation and function.
The implications of our observations are many. The finding of low HDL or apoA1 concentrations associated with decreased cardiac myocyte mitochondrial function offers a novel mechanism for myocardial stunning and potentially shock in patients who present with myocardial infarction. Only recently have studies begun to investigate the association of HDL on outcomes in patients who present with myocardial infarction (34, 45). Our findings suggest novel mechanisms for adverse outcomes in patients with low HDL and demonstrate that repletion of the CoQ10 pool in these patients may substantially improve outcomes.
3-Hydroxy-3-methylglutary-CoA reductase inhibitors (statins) significantly decrease myocardial infarction, stroke, and death in multiple clinical trials (46, 47). Unfortunately, many patients are intolerant of their effects secondary to muscle pain and fatigue. CoQ, like cholesterol, is a 3-hydroxy-3-methylglutary-CoA product, and statin therapy decreases circulating CoQ. Despite this, CoQ supplementation has not consistently inhibited these side effects (48, 49). Low CoQ10 concentrations in patients on statins who have subsequent ischemic events could result in a larger ischemic insult. Our findings indicate that low HDL concentrations and the associated deficient CoQ pool could be used to identify patients who will be intolerant to statins or at risk of larger ischemic damage. Thus, our findings suggest a population that may respond to CoQ supplementation to minimize or treat the consequences of statins and ischemia.
In summary, our findings demonstrate an important link between circulating apoA1 concentrations and cardiac mitochondrial function. Low apoA1/HDL decreases the CoQ10 pool, which in turn decreases electron transfer from complex II to complex III. This deficiency clearly has relevant implications in the setting of acute myocardial infarction and defines novel mechanisms for the recent findings, such as improved functional status associated with higher HDL concentrations (50) or a worse outcome in patients with non-ischemic heart failure associated with low HDL concentrations (51).
Supplementary Material
Acknowledgments
E.J.L. and M.S.P. designed the research; A.R.D., T.M.M., E.J.L., and M.S.P. conducted the research; T.M.M., G.Y., and C.L. provided essential reagents and materials; A.R.D., G.Y., C.L., T.M.M., E.J.L., and M.S.P. analyzed the data; T.M.M., E.J.L., and M.S.P. wrote the paper; M.S.P. and E.J.L. had primary responsibility for the final content. All authors read and approved the final manuscript.
Footnotes
Abbreviations used: apoA1+/−, apoA1 heterozygous; apoA1−/−, apoA1 null; clk-1, CDC-like kinase 1; CoQ, coenzyme Q; CoQ10, coenzyme Q10; KCl, potassium chloride; LAD, left anterior descending coronary artery; MOPS, 3-(N-morpholino)-propanesulfonic acid; ROS, reactive oxygen species; TMPD, N,N,N′,N′-tetramethyl p-phenylenediamine; TTC, 2,3,5-triphenyltetrazolium chloride; WT, wild-type.
References
- 1.Boden WE. High-density lipoprotein cholesterol as an independent risk factor in cardiovascular disease: assessing the data from Framingham to the Veterans Affairs High–Density Lipoprotein Intervention Trial. Am J Cardiol. 2000;86:19L–22L. [DOI] [PubMed] [Google Scholar]
- 2.Gordon T, Castelli WP, Hjortland MC, Kannel WB, Dawber TR. High density lipoprotein as a protective factor against coronary heart disease. The Framingham Study. Am J Med. 1977;62:707–14. [DOI] [PubMed] [Google Scholar]
- 3.Baker HN, Delahunty T, Gotto AM, Jr, Jackson RL. The primary structure of high density apolipoprotein-glutamine-I. Proc Natl Acad Sci USA. 1974;71:3631–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Delahunty T, Baker HN, Gotto AM, Jr, Jackson RL. The primary structure of human plasma high density apolipoprotein glutamine I (ApoA-I). I. The amino acid sequence of cyanogen bromide fragment II. J Biol Chem. 1975;250:2718–24. [PubMed] [Google Scholar]
- 5.Matsunaga T, Hiasa Y, Yanagi H, Maeda T, Hattori N, Yamakawa K, Yamanouchi Y, Tanaka I, Obara T, Hamaguchi H. Apolipoprotein A-I deficiency due to a codon 84 nonsense mutation of the apolipoprotein A-I gene. Proc Natl Acad Sci USA. 1991;88:2793–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Williamson R, Lee D, Hagaman J, Maeda N. Marked reduction of high density lipoprotein cholesterol in mice genetically modified to lack apolipoprotein A-I. Proc Natl Acad Sci USA. 1992;89:7134–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Karathanasis SK, Zannis VI, Breslow JL. A DNA insertion in the apolipoprotein A-I gene of patients with premature atherosclerosis. Nature. 1983;305:823–5. [DOI] [PubMed] [Google Scholar]
- 8.Ordovas JM, Schaefer EJ, Salem D, Ward RH, Glueck CJ, Vergani C, Wilson PW, Karathanasis SK. Apolipoprotein A-I gene polymorphism associated with premature coronary artery disease and familial hypoalphalipoproteinemia. N Engl J Med. 1986;314:671–7. [DOI] [PubMed] [Google Scholar]
- 9.Choi BG, Vilahur G, Yadegar D, Viles-Gonzalez JF, Badimon JJ. The role of high-density lipoprotein cholesterol in the prevention and possible treatment of cardiovascular diseases. Curr Mol Med. 2006;6:571–87. [DOI] [PubMed] [Google Scholar]
- 10.Navab M, Anantharamaiah GM, Reddy ST, Hama S, Hough G, Grijalva VR, Yu N, Ansell BJ, Datta G, Garber DW, et al. Apolipoprotein A-I mimetic peptides. Arterioscler Thromb Vasc Biol. 2005;25:1325–31. [DOI] [PubMed] [Google Scholar]
- 11.Hyka N, Dayer JM, Modoux C, Kohno T, Edwards CK, III, Roux-Lombard P, Burger D. Apolipoprotein A-I inhibits the production of interleukin-1beta and tumor necrosis factor-alpha by blocking contact-mediated activation of monocytes by T lymphocytes. Blood. 2001;97:2381–9. [DOI] [PubMed] [Google Scholar]
- 12.Jiao YL, Wu MP. Apolipoprotein A-I diminishes acute lung injury and sepsis in mice induced by lipoteichoic acid. Cytokine. 2008;43:83–7. [DOI] [PubMed] [Google Scholar]
- 13.Marchesi M, Booth EA, Davis T, Bisgaier CL, Lucchesi BR. Apolipoprotein A-IMilano and 1-palmitoyl-2-oleoyl phosphatidylcholine complex (ETC-216) protects the in vivo rabbit heart from regional ischemia-reperfusion injury. J Pharmacol Exp Ther. 2004;311:1023–31. [DOI] [PubMed] [Google Scholar]
- 14.Marchesi M, Booth EA, Rossoni G, Garcia RA, Hill KR, Sirtori CR, Bisgaier CL, Lucchesi BR. Apolipoprotein A-IMilano/POPC complex attenuates post-ischemic ventricular dysfunction in the isolated rabbit heart. Atherosclerosis. 2008;197:572–8. [DOI] [PubMed] [Google Scholar]
- 15.Askari AT, Brennan ML, Zhou X, Drinko J, Morehead A, Thomas JT, Topol EJ, Hazen SL, Penn MS. Myeloperoxidase and plasminogen activator inhibitor-1 play a central role in ventricular remodeling after myocardial infarction. J Exp Med. 2003;197:615–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jolly SR, Kane WJ, Hook BG, Abrams GD, Kunkel SL, Lucchesi BR. Reduction of myocardial infarct size by neutrophil depletion: effect of duration of occlusion. Am Heart J. 1986;112:682–90. [DOI] [PubMed] [Google Scholar]
- 17.Vasilyev N, Williams T, Brennan ML, Unzek S, Zhou X, Heinecke JW, Spitz DR, Topol EJ, Hazen SL, Penn MS. Myeloperoxidase-generated oxidants modulate left ventricular remodeling but not infarct size after myocardial infarction. Circulation. 2005;112:2812–20. [DOI] [PubMed] [Google Scholar]
- 18.Carrea FP, Lesnefsky EJ, Repine JE, Shikes RH, Horwitz LD. Reduction of canine myocardial infarct size by a diffusible reactive oxygen metabolite scavenger. Efficacy of dimethylthiourea given at the onset of reperfusion. Circ Res. 1991;68:1652–9. [DOI] [PubMed] [Google Scholar]
- 19.Chen Q, Camara AK, Stowe DF, Hoppel CL, Lesnefsky EJ. Modulation of electron transport protects cardiac mitochondria and decreases myocardial injury during ischemia and reperfusion. Am J Physiol Cell Physiol. 2007;292:C137–47. [DOI] [PubMed] [Google Scholar]
- 20.Zuo L, Chen YR, Reyes LA, Lee HL, Chen CL, Villamena FA, Zweier JL. The radical trap 5,5-dimethyl-1-pyrroline N-oxide exerts dose-dependent protection against myocardial ischemia-reperfusion injury through preservation of mitochondrial electron transport. J Pharmacol Exp Ther. 2009;329:515–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Somers SJ, Frias M, Lacerda L, Opie LH, Lecour S. Interplay between SAFE and RISK pathways in sphingosine-1-phosphate-induced cardioprotection. Cardiovasc Drugs Ther. 2012;26:227–37. [DOI] [PubMed] [Google Scholar]
- 22.Lichtman JH, Bigger JT, Jr, Blumenthal JA, Frasure-Smith N, Kaufmann PG, Lesperance F, Mark DB, Sheps DS, Taylor CB, Froelicher ES. Depression and coronary heart disease: recommendations for screening, referral, and treatment: a science advisory from the American Heart Association Prevention Committee of the Council on Cardiovascular Nursing, Council on Clinical Cardiology, Council on Epidemiology and Prevention, and Interdisciplinary Council on Quality of Care and Outcomes Research: endorsed by the American Psychiatric Association. Circulation. 2008;118:1768–75. [DOI] [PubMed] [Google Scholar]
- 23.Kondo T, Reaume AG, Huang TT, Carlson E, Murakami K, Chen SF, Hoffman EK, Scott RW, Epstein CJ, Chan PH. Reduction of CuZn-superoxide dismutase activity exacerbates neuronal cell injury and edema formation after transient focal cerebral ischemia. J Neurosci. 1997;17:4180–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Murakami K, Kondo T, Kawase M, Li Y, Sato S, Chen SF, Chan PH. Mitochondrial susceptibility to oxidative stress exacerbates cerebral infarction that follows permanent focal cerebral ischemia in mutant mice with manganese superoxide dismutase deficiency. J Neurosci. 1998;18:205–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Manabe Y, Anrather J, Kawano T, Niwa K, Zhou P, Ross ME, Iadecola C. Prostanoids, not reactive oxygen species, mediate COX-2-dependent neurotoxicity. Ann Neurol. 2004;55:668–75. [DOI] [PubMed] [Google Scholar]
- 26.Rothe G, Valet G. Flow cytometric assays of oxidative burst activity in phagocytes. Methods Enzymol. 1994;233:539–48. [DOI] [PubMed] [Google Scholar]
- 27.Hoppel CL, Kerr DS, Dahms B, Roessmann U. Deficiency of the reduced nicotinamide adenine dinucleotide dehydrogenase component of complex I of mitochondrial electron transport. Fatal infantile lactic acidosis and hypermetabolism with skeletal-cardiac myopathy and encephalopathy. J Clin Invest. 1987;80:71–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lesnefsky EJ, Tandler B, Ye J, Slabe TJ, Turkaly J, Hoppel CL. Myocardial ischemia decreases oxidative phosphorylation through cytochrome oxidase in subsarcolemmal mitochondria. Am J Physiol. 1997;273:H1544–54. [DOI] [PubMed] [Google Scholar]
- 29.Chen Q, Vazquez EJ, Moghaddas S, Hoppel CL, Lesnefsky EJ. Production of reactive oxygen species by mitochondria: central role of complex III. J Biol Chem. 2003;278:36027–31. [DOI] [PubMed] [Google Scholar]
- 30.Boitier E, Degoul F, Desguerre I, Charpentier C, Francois D, Ponsot G, Diry M, Rustin P, Marsac C. A case of mitochondrial encephalomyopathy associated with a muscle coenzyme Q10 deficiency. J Neurol Sci. 1998;156:41–6. [DOI] [PubMed] [Google Scholar]
- 31.Plump AS, Azrolan N, Odaka H, Wu L, Jiang X, Tall A, Eisenberg S, Breslow JL. ApoA-I knockout mice: characterization of HDL metabolism in homozygotes and identification of a post-RNA mechanism of apoA-I up-regulation in heterozygotes. J Lipid Res. 1997;38:1033–47. [PubMed] [Google Scholar]
- 32.Castelli WP, Doyle JT, Gordon T, Hames CG, Hjortland MC, Hulley SB, Kagan A, Zukel WJ. HDL cholesterol and other lipids in coronary heart disease. The cooperative lipoprotein phenotyping study. Circulation. 1977;55:767–72. [DOI] [PubMed] [Google Scholar]
- 33.Wilson PW, Garrison RJ, Castelli WP, Feinleib M, McNamara PM, Kannel WB. Prevalence of coronary heart disease in the Framingham Offspring Study: role of lipoprotein cholesterols. Am J Cardiol. 1980;46:649–54. [DOI] [PubMed] [Google Scholar]
- 34.Roe MT, Ou FS, Alexander KP, Newby LK, Foody JM, Gibler WB, Boden WE, Ohman EM, Smith SC, Jr, Peterson ED. Patterns and prognostic implications of low high-density lipoprotein levels in patients with non-ST-segment elevation acute coronary syndromes. Eur Heart J. 2008;29:2480–8. [DOI] [PubMed] [Google Scholar]
- 35.Salonen JT, Salonen R, Seppanen K, Rauramaa R, Tuomilehto J. HDL, HDL2, and HDL3 subfractions, and the risk of acute myocardial infarction. A prospective population study in eastern Finnish men. Circulation. 1991;84:129–39. [DOI] [PubMed] [Google Scholar]
- 36.Langsjoen PH, Langsjoen AM. Supplemental ubiquinol in patients with advanced congestive heart failure. Biofactors. 2008;32:119–28. [DOI] [PubMed] [Google Scholar]
- 37.Xia S, Xu S, Zhang X, Zhong F, Wang Z. Nanoliposomes mediate coenzyme Q10 transport and accumulation across human intestinal Caco-2 cell monolayer. J Agric Food Chem. 2009;57:7989–96. [DOI] [PubMed] [Google Scholar]
- 38.Kayser EB, Sedensky MM, Morgan PG, Hoppel CL. Mitochondrial oxidative phosphorylation is defective in the long-lived mutant clk-1. J Biol Chem. 2004;279:54479–86. [DOI] [PubMed] [Google Scholar]
- 39.Jonassen T, Larsen PL, Clarke CF. A dietary source of coenzyme Q is essential for growth of long-lived Caenorhabditis elegans clk-1 mutants. Proc Natl Acad Sci USA. 2001;98:421–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kayser EB, Morgan PG, Sedensky MM. Mitochondrial complex I function affects halothane sensitivity in Caenorhabditis elegans. Anesthesiology. 2004;101:365–72. [DOI] [PubMed] [Google Scholar]
- 41.Jonassen T, Marbois BN, Faull KF, Clarke CF, Larsen PL. Development and fertility in Caenorhabditis elegans clk-1 mutants depend upon transport of dietary coenzyme Q8 to mitochondria. J Biol Chem. 2002;277:45020–7. [DOI] [PubMed] [Google Scholar]
- 42.Chen Q, Yin G, Stewart S, Hu Y, Lesnefsky EJ. Isolating the segment of the mitochondrial electron transport chain responsible for mitochondrial damage during cardiac ischemia. Biochem Biophys Res Commun. 2010;397:656–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mohr D, Bowry VW, Stocker R. Dietary supplementation with coenzyme Q10 results in increased levels of ubiquinol-10 within circulating lipoproteins and increased resistance of human low-density lipoprotein to the initiation of lipid peroxidation. Biochim Biophys Acta. 1992;1126:247–54. [DOI] [PubMed] [Google Scholar]
- 44.Tomono Y, Hasegawa J, Seki T, Motegi K, Morishita N. Pharmacokinetic study of deuterium-labelled coenzyme Q10 in man. Int J Clin Pharmacol Ther Toxicol. 1986;24:536–41. [PubMed] [Google Scholar]
- 45.Correia LC, Rocha MS, Esteves JP. HDL-cholesterol level provides additional prognosis in acute coronary syndromes. Int J Cardiol. 2009;136:307–14. [DOI] [PubMed] [Google Scholar]
- 46.Reiner Z. Statins in the primary prevention of cardiovascular disease. Nat Rev Cardiol. 2013;10:453–64. [DOI] [PubMed] [Google Scholar]
- 47.Taylor F, Huffman MD, Macedo AF, Moore TH, Burke M, Davey Smith G, Ward K, Ebrahim S. Statins for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2013;1:CD004816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mabuchi H, Nohara A, Kobayashi J, Kawashiri MA, Katsuda S, Inazu A, Koizumi J. Effects of CoQ10 supplementation on plasma lipoprotein lipid, CoQ10 and liver and muscle enzyme levels in hypercholesterolemic patients treated with atorvastatin: a randomized double-blind study. Atherosclerosis. 2007;195:e182–9. [DOI] [PubMed] [Google Scholar]
- 49.Päivä H, Thelen KM, Van Coster R, Smet J, De Paepe B, Mattila KM, Laakso J, Lehtimäki T, von Bergmann K, et al. High-dose statins and skeletal muscle metabolism in humans: a randomized, controlled trial. Clin Pharmacol Ther. 2005;78:60–8. [DOI] [PubMed] [Google Scholar]
- 50.Landi F, Russo A, Cesari M, Pahor M, Bernabei R, Onder G. HDL-cholesterol and physical performance: results from the ageing and longevity study in the sirente geographic area (ilSIRENTE Study). Age Ageing. 2007;36:514–20. [DOI] [PubMed] [Google Scholar]
- 51.Iwaoka M, Obata JE, Abe M, Nakamura T, Kitta Y, Kodama Y, Kawabata K, Takano H, Fujioka D, Saito Y, et al. Association of low serum levels of apolipoprotein A-I with adverse outcomes in patients with nonischemic heart failure. J Card Fail. 2007;13:247–53. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.