Abstract
Emerging literature suggests that men’s diets may affect spermatogenesis as reflected in semen quality indicators, but literature on the relation between meat intake and semen quality is limited. Our objective was to prospectively examine the relation between meat intake and indicators of semen quality. Men in subfertile couples presenting for evaluation at the Massachusetts General Hospital Fertility Center were invited to participate in an ongoing study of environmental factors and fertility. A total of 155 men completed a validated food-frequency questionnaire and subsequently provided 338 semen samples over an 18-mo period from 2007–2012. We used linear mixed regression models to examine the relation between meat intake and semen quality indicators (total sperm count, sperm concentration, progressive motility, morphology, and semen volume) while adjusting for potential confounders and accounting for within-person variability across repeat semen samples. Among the 155 men (median age: 36.1 y; 83% white, non-Hispanic), processed meat intake was inversely related to sperm morphology. Men in the highest quartile of processed meat intake had, on average, 1.7 percentage units (95% CI: −3.3, −0.04) fewer morphologically normal sperm than men in the lowest quartile of intake (P-trend = 0.02). Fish intake was related to higher sperm count and percentage of morphologically normal sperm. The adjusted mean total sperm count increased from 102 million (95% CI: 80, 131) in the lowest quartile to 168 million (95% CI: 136, 207) sperm in the highest quartile of fish intake (P-trend = 0.005). Similarly, the adjusted mean percentages of morphologically normal sperm for men in increasing quartiles of fish intake were 5.9 (95% CI: 5.0, 6.8), 5.3 (95% CI: 4.4, 6.3), 6.3 (95% CI: 5.2, 7.4), and 7.5 (95% CI: 6.5, 8.5) (P-trend = 0.01). Consuming fish may have a positive impact on sperm counts and morphology, particularly when consumed instead of processed red meats.
Introduction
One in 6 couples trying to conceive experience infertility (1, 2), and abnormalities in semen quality are identified in ∼50% of couples evaluated for infertility (3). Although body weight (4, 5) and smoking (6) are well-characterized risk factors for low semen quality and male factor infertility, few other modifiable risk factors are currently known. Emerging literature suggests that men’s diets may affect spermatogenesis as reflected in altered semen quality (7–9). Red meats, for example, are an important source of saturated fat, which was previously identified as related to low sperm concentration (10) and total sperm count (11). On the other hand, fish intake is an important source of long-chain omega-3 (ω-3; n−3) FAs, which appear to play an important role in spermatogenesis (12) and were previously associated with higher sperm morphology in a cross-sectional study (10).
The existing literature on the relation between meat intake and semen quality indicators is scarce and limited to cross-sectional and case-control studies (13–17). We have previously reported that processed meat intake is associated with lower total sperm count among physically active healthy young men (17). Others have reported higher processed meat intake among oligoasthenoteratospermic and asthenospermic men (16). To further evaluate this issue, we prospectively examined the association between intake of meat and fish in relation to semen quality indicators among men attending a fertility clinic in Boston, Massachusetts.
Materials and Methods
Study population.
Men in subfertile couples presenting for evaluation at the Massachusetts General Hospital Fertility Center were invited to participate in the Environment and Reproductive Health (EARTH) Study, an ongoing study of environmental factors and fertility (18). Men from couples using their own gametes for intrauterine insemination or assisted reproductive technologies, aged 18–55 y and without a history of vasectomy, were eligible. A FFQ was introduced in April 2007 and was completed by 188 of the 246 men (76%) recruited through March 2012. Of these, 161 men produced ≥1 semen samples after the completion of the FFQ. We excluded men with incomplete semen analysis data (n = 5) and azoospermic men (n = 1). We further excluded all semen samples (47 samples from 8 men) that were collected >18 mo after the FFQ completion to minimize any influence that misclassification of meat intake because of changes in intake over time might have on the associations. After exclusions, 155 men with a total of 338 semen samples were included in the analysis; 57 men provided 1 sample, 51 men provided 2 samples, and 47 men provided ≥3 samples (the maximum number of samples per man was 6).
At enrollment, trained research nurses administered a general health questionnaire (asking about demographics, lifestyle, and reproductive history) and completed an anthropometric assessment at the clinic. The study was approved by the human subject committees of the Harvard School of Public Health and Massachusetts General Hospital, and informed consent was obtained from all participants.
Semen analysis.
We considered the following outcomes: sperm concentration, progressive sperm motility, sperm morphology, ejaculate volume, total sperm count, and total normal sperm count. Semen samples were obtained on site by masturbation and collected into a sterile plastic container. Men were instructed to abstain from ejaculation for at least 48 h before the sample was produced and to report the specific time of abstinence; 18 men (19 semen samples) did not report their last ejaculation date and were assigned to the most common abstinence time category (2–3 d). Semen samples were liquefied at 37°C for 20 min before analysis. Sperm morphology was determined by using Kruger strict criteria and results were expressed as percentage of normal spermatozoa (19). Assessment of sperm morphology is monitored by weekly evaluation of AQC sperm morphology smears (Fertility Solutions). Deviations from acceptable ranges of variation trigger re-evaluation and, if needed, retraining of personnel. In addition to these periodic procedures, the lab performs a quarterly competency evaluation of all the technicians and proficiency testing by an outside evaluator every 6 mo. Sperm concentration and motility were assessed with a computer-aided semen analysis system (Ceros, version 14; Hamilton-Thorne Biosciences). Ejaculate volume was measured with a graduated serological pipet. Results for sperm motility were expressed as percentage of progressive motile spermatozoa (20). Total sperm count (million) was calculated as concentration × volume, and total normal count (million) was defined as concentration × volume × % morphologically normal.
Dietary assessment.
Participants completed a previously validated 131-item FFQ (21). They were asked to report how often, on average, they consumed specific foods during the previous year. The FFQ had 9 categories for intake frequency that ranged from never to ≥6 times/d. The nutrient content of each food and the specific portion size was calculated with a nutrient database based on data from the USDA (22) with additional information from manufacturers. The options for dose of ω-3 FA supplements in the FFQ changed halfway through the study to document use of lower-dose supplements. This change, however, precluded combining data on ω-3 FA supplement dose across questionnaires. Assessment of meat intake using this questionnaire was validated against prospectively collected diet records representing 1 y of diet (23). The deattenuated correlation coefficient between meat intake assessed with the FFQ and the prospectively collected diet records ranged from 0.56 for poultry to 0.83 for processed red meat (23). Total meat intake was defined as the sum of processed red meat, unprocessed red meat, organ meat, poultry, and fish intake. Processed red meat was defined as hamburger, hot dog, bacon, or other processed red meats (such as salami and bologna). A serving of processed meat was 1 hamburger patty, 2 slices of bacon, or 2 oz (57 g) of sausage. Unprocessed red meat was defined as beef, pork, or ham consumed as sandwiches, mixed dishes, or main dishes. An example of a serving of unprocessed red meat was a 4–6 oz (113–170 g) of steak or lamb roast. Organ meat was defined as liver from beef, calf, pork, chicken, or turkey. A serving size for beef, calf, or pork liver was 4 oz (113 g) and 1 oz (28 g) for chicken or turkey liver. Poultry was defined as chicken or turkey cooked with or without skin, as a main dish, sandwich, or frozen dinner. Fish intake was defined as dark meat fish (e.g., canned tuna, salmon), white meat fish (e.g., cod, haddock), or shellfish (e.g., shrimp, scallops). A serving of canned tuna was 3–4 oz (85–113 g), whereas a serving of dark or white meat fish was 3–5 oz (85–142 g). Two data-derived dietary patterns previously related to semen quality measures (15), the “Prudent Pattern” and the “Western Pattern,” were also calculated as summary measures of overall food choices.
Statistical analysis.
We first summarized participant characteristics and compared them across quartiles of meat intake by using a Kruskal-Wallis test for continuous measures and an extended Fisher’s exact test for categorical variables. Linear mixed models were used to examine the relation between meat intake and semen quality indicators while adjusting for potential confounders and accounting for within-person correlations in semen quality indicators across repeated samples. Specifically, in these regression models we compared semen quality indicators (total sperm count, sperm concentration, progressive motility, morphology, and semen volume) for men in increasing quartiles of meat intake in relation to men in the lowest quartile of intake (reference). All exposure variables were first considered as quartiles and when the distribution was too narrow and a large percentage of men unexposed, we categorized them as tertiles or, in more extreme cases such as organ meat intake, as exposed and unexposed. Total sperm count and sperm concentration were log-transformed to more closely approximate a normal distribution. Results for these indicators were back-transformed to allow presentation of results in the original scale. Robust estimators of the variance (24) were used in the computation of 95% CIs. Population marginal means (25) were used to present marginal population averages adjusted for the covariates in the model. Tests for linear trend were performed by using the median values of meat intake in each quartile as a continuous variable.
Baseline characteristics previously reported as risk factors for low semen quality (age, smoking, BMI) and those associated with both meat intake and semen quality indicators in this population (BMI, diet patterns, total energy intake) were considered as potential confounders. In addition, we forced terms for abstinence time and a history of previous infertility exam into the model. Abstinence time was strongly associated with semen quality indicators but not with meat or fish intake. Following convention in semen quality studies, however, we included abstinence time in our multivariate adjusted models to improve the precision of effect estimates (26). Because the majority of the men in this study had been evaluated for infertility prior to joining the study (at Massachusetts General Hospital or a different center), thereby unblinding to participants their outcome status, we decided to include this term to take account for the possibility that knowledge of semen quality indicators could influence subsequent diet. Based on these criteria, all models were adjusted for age (continuous), BMI (continuous), abstinence time (<2, 2–3, 3–4, or ≥4 d), history of previous infertility exam (yes vs. no), race (white vs. other), smoking status (never smoked vs. other), dietary patterns (continuous), and caloric intake (continuous). We examined the possibility that fish and processed red meat intake were confounding each other’s relations with semen quality indicators by further adjusting for fish intake in the processed meat model and vice versa. To examine whether intake of nutrients for which specific meats were one of the top 10 contributors of intake in this population were mediating observed associations, we included terms for intake of these nutrients to the final multivariate models. We interpreted attenuation of the associations as evidence of mediation. We estimated the effects of substituting fish for other types of meat as the difference between their regression coefficients in the same model and calculated the corresponding 95% CIs by using the estimated covariance matrix for the regression coefficients (27). Finally, we assessed effect modification of dietary associations with semen quality indicators by BMI (<25 kg/m2 and ≥25 kg/m2) and smoking status (never and ever smoked) by using cross-product terms. We analyzed the data by using SAS (version 9.2; SAS Institute), and 2-sided P values ≤ 0.05 were considered statistically significant.
Results
Men included in the analysis were primarily Caucasian (83%), with a mean age of 36.1 y (95% CI: 33.0, 39.2) and mean BMI of 27.0 kg/m2 (95% CI: 24.2, 29.1). Most men had never smoked (63%) and 76% had previously been evaluated for infertility, with 36% receiving a diagnosis of male factor infertility. The median sperm concentration was 56.4 × 106/mL (26.1–109 × 106/mL), percentage of progressively motile sperm was 25.0% (14.0–37.0%), and percentage of morphologically normal sperm was 6.0% (4.0–8.0%) for each man’s first sample. The number of semen samples produced by each man was not related to meat intake, infertility diagnosis, or semen quality indicators. The mean time between the FFQ return and collection of the semen sample was 158 d (82–258 d) for each man’s first sample and 266 d (160–408 d) for each man’s last sample. Intake of processed meat was 0.46 ± 0.38 servings/d and 0.26 ± 0.20 servings/d of fish. Meat intake was positively related to BMI, intake of fat and protein from animal sources, and total caloric intake (Table 1). Intake of poultry (31%) and processed meats (31%) accounted for more than half of total meat intake. A total of 4 men reported not consuming any meat.
TABLE 1.
Characteristics of 155 men from the Environment and Reproductive Health Study by quartile of meat intake1
| Total meat intake |
|||||
| Quartile 1 (lowest) | Quartile 2 | Quartile 3 | Quartile 4 (highest) | P2 | |
| Participants, n | 38 | 39 | 39 | 39 | |
| Range, servings/d | 0–0.96 | 0.97–1.39 | 1.40–1.80 | 1.81–4.97 | |
| Demographics | |||||
| Age, y | 36.9 (33.5, 39.2) | 35.2 (32.8, 38.3) | 36.7 (32.9, 40.9) | 36.7 (32.9, 40.9) | 0.51 |
| Race/ethnicity | 0.78 | ||||
| White, not Hispanic | 32 (84.2) | 32 (84.1) | 34 (87.2) | 31 (79.5) | |
| Black | 1 (2.6) | 0 (0.00) | 2 (5.1) | 1 (2.6) | |
| Asian | 3 (7.9) | 3 (7.7) | 2 (5.1) | 2 (5.1) | |
| Hispanic or Latino | 2 (5.3) | 4 (10.3) | 1 (2.6) | 5 (12.8) | |
| BMI, kg/m2 | 25.6 (22.7, 27.9) | 26.9 (24.0, 27.9) | 26.9 (24.2, 30.9) | 28.7 (27.1, 29.5) | 0.004 |
| Smoker | 0.42 | ||||
| Never smoked | 21 (55.3) | 25 (64.1) | 29 (74.4) | 23 (59.0) | |
| Past smoker | 14 (36.8) | 13 (33.3) | 10 (25.6) | 13 (33.3) | |
| Current smoker | 3 (7.9) | 1 (2.6) | 0 (0.00) | 3 (7.7) | |
| Abstinence interval <2 d | 13 (34.2) | 10 (25.6) | 13 (33.3) | 11 (28.2) | 0.85 |
| Diet | |||||
| Total energy intake, kcal/d | 1510 (1240, 1830) | 1960 (1630, 2230) | 2090 (1790, 2450) | 2530 (2100, 2940) | <0.0001 |
| Caffeine intake, mg/d | 141 (38, 310) | 201 (103, 281) | 125 (44, 240) | 186 (77, 252) | 0.44 |
| Alcohol intake, g/d | 13.3 (2.1, 20.0) | 12.0 (5.0, 21.2) | 8.2 (2.0, 18.1) | 10.0 (5.2, 17.2) | 0.67 |
| Saturated fat, % energy | 9.3 (7.8, 10.7) | 10.7 (8.8, 12.0) | 11.4 (9.2, 12.4) | 10.5 (9.0, 12.4) | 0.006 |
| Monounsaturated fat, % energy | 11.0 (8.6, 12.8) | 12.3 (10.6, 14.7) | 12.3 (10.8, 14.2) | 13.2 (11.6, 14.9) | 0.006 |
| Polyunsaturated fat, % energy | 5.6 (4.6, 6.5) | 5.6 (4.7, 6.7) | 5.7 (4.6, 7.1) | 6.2 (5.3, 6.6) | 0.56 |
| trans fat, % energy | 0.9 (0.7, 1.1) | 1.0 (0.8, 1.2) | 1.0 (0.8, 1.2) | 1.1 (0.9, 1.3) | 0.10 |
| ω-3 FA intake from foods, g/d | 1.1 (0.9, 1.3) | 1.1 (1.0, 1.3) | 1.1 (1.0, 1.4) | 1.3 (1.1, 1.6) | 0.005 |
| Animal fat intake, % energy | 11.0 (8.6, 12.8) | 12.3 (10.6, 14.7) | 12.3 (10.8, 14.2) | 13.2 (11.6, 14.9) | <0.0001 |
| Protein intake, % energy | 14.7 (13.4, 16.2) | 15.8 (14.1, 16.6) | 16.4 (15.2, 17.6) | 18.1 (16.1, 20.9) | <0.0001 |
| Animal protein, % energy | 8.1 (6.0, 9.9) | 9.2 (8.0, 11.3) | 10.5 (9.0, 12.7) | 12.6 (10.6, 14.8) | <0.0001 |
| Prudent pattern score | −0.7 (−1.1, −0.2) | −0.2 (−0.6, 0.3) | 0.1 (−0.6, 0.6) | 0.3 (−0.3, 1.2) | <0.0001 |
| Western pattern score | −0.8 (−1.1, −0.5) | −0.3 (−0.8, 0.1) | −0.1 (−0.4, 0.3) | 0.7 (0.3, 1.6) | <0.0001 |
| Reproductive history | |||||
| Male factor infertility diagnosis | 13 (34.2) | 13 (33.3) | 20 (51.3) | 10 (25.6) | 0.13 |
| Previous infertility exam | 28 (73.7) | 27 (69.2) | 34 (87.2) | 29 (74.4) | 0.26 |
| Undescended testes | 0 (0.0) | 3 (7.7) | 2 (5.1) | 1 (2.6) | 0.52 |
| Varicocele | 5 (13.2) | 4 (10.3) | 3 (7.7) | 3 (7.7) | 0.81 |
| Any reproductive surgery3 | 2 (5.3) | 4 (10.3) | 4 (10.3) | 6 (15.4) | 0.57 |
Values are medians (IQRs) or n (%) unless indicated otherwise.
From the Kruskal-Wallis test for continuous variables, Fisher’s exact test for categorical variables.
Report of any of the following: orchidopexy, varicocelectomy, hydrocelectomy, hernia repair, urethral repair, hypospadias repair, prostatectomy, sympathectomy, bladder neck surgery, vasectomy, or other reproductive surgery.
Total meat intake was unrelated to semen quality indicators (Table 2). Processed red meat intake, on the other hand, was inversely related to sperm morphology (Table 2). Compared with men in the lowest quartile of processed red meat intake, men in the highest quartile had 23.2% fewer morphologically normal sperm. Adjustment for animal protein, animal fat, cholesterol, saturated fat, trans fat, monounsaturated fat, iron, zinc, or vitamin B-12 intake did not explain these associations (data not shown). Intake of poultry and unprocessed red meats was unrelated to semen quality indicators (Table 2).
TABLE 2.
Adjusted semen quality indicators in 155 men (338 semen samples) according to intake of different meat types from the Environment and Reproductive Health Study1
| Meat intake (servings/d) | Participants | Total sperm count | Sperm concentration | Progressive motility | Sperm morphology | Ejaculate volume |
| n | million | million/mL | % motile | % normal | mL | |
| Total meat | ||||||
| Quartile 1 [0.00–0.96] | 38 | 120 (92, 157) | 55.6 (42.6, 72.6) | 27.9 (22.4, 33.3) | 6.9 (5.6, 8.2) | 2.5 (2.1, 2.9) |
| Quartile 2 [0.97–1.39] | 39 | 124 (99, 155) | 48.9 (38.5, 62.3) | 25.6 (20.6, 30.6) | 6.3 (5.3, 7.3) | 2.8 (2.5, 3.2) |
| Quartile 3 [1.40–1.80] | 39 | 106 (81, 138) | 42.5 (32.2, 56.2) | 23.0 (18.4, 27.6) | 5.7 (4.7, 6.6) | 2.7 (2.4, 3.1) |
| Quartile 4 [1.81–4.97] | 39 | 135 (99, 185) | 52.1 (36.9, 73.5) | 29.1 (24.7, 33.6) | 6.1 (5.1, 7.2) | 2.9 (2.4, 3.4) |
| P-trend | 0.65 | 0.84 | 0.62 | 0.46 | 0.37 | |
| Processed red meat2 | ||||||
| Quartile 1 [0.00–0.23] | 39 | 116 (90, 151) | 50.6 (37.8, 67.7) | 27.2 (22.5, 32.0) | 7.2 (6.1, 8.3) | 2.6 (2.2, 3.1) |
| Quartile 2 [0.24–0.37] | 37 | 154 (121, 196) | 63.6 (50.0, 80.8) | 31.0 (24.3, 37.6) | 6.7 (5.5, 7.9) | 2.7 (2.4, 3.1) |
| Quartile 3 [0.38–0.55] | 39 | 132 (106, 164) | 50.9 (39.4, 65.7) | 24.3 (20.7, 27.9) | 5.5 (4.7, 6.4)* | 2.8 (2.5, 3.2) |
| Quartile 4 [0.56–2.79] | 40 | 92 (68, 123) | 37.3 (27.3, 50.8) | 23.3 (18.6, 28.1) | 5.5 (4.6, 6.5)* | 2.8 (2.3, 3.2) |
| P-trend | 0.12 | 0.07 | 0.09 | 0.02 | 0.72 | |
| Unprocessed red meat3 | ||||||
| Quartile 1 [0.00–0.15] | 44 | 109 (87, 137) | 45.9 (35.7, 59.2) | 23.6 (19.8, 27.5) | 6.0 (4.9, 7.0) | 2.7 (2.3, 3.1) |
| Quartile 2 [0.16–0.23] | 27 | 134 (101, 179) | 56.3 (41.5, 76.5) | 30.0 (22.1, 38.0) | 6.9 (5.6, 8.2) | 2.8 (2.3, 3.3) |
| Quartile 3 [0.24–0.35] | 42 | 132 (106, 164) | 55.7 (43.8, 70.9) | 28.2 (24.3, 32.0) | 6.4 (5.6, 7.2) | 2.6 (2.3, 2.9) |
| Quartile 4 [0.36–1.29] | 42 | 116 (86, 156) | 43.4 (31.9, 59.2) | 25.3 (20.6, 30.0) | 5.9 (4.9, 6.9) | 2.9 (2.5, 3.3) |
| P-trend | 0.97 | 0.59 | 0.93 | 0.74 | 0.38 | |
| Organ meat4 | ||||||
| None | 125 | 117 (103, 134) | 49.4 (42.8, 57.1) | 25.9 (23.4, 28.4) | 5.9 (5.4, 6.5) | 2.7 (2.5, 2.9) |
| Any [0.02–0.94] | 30 | 140 (104, 189) | 50.1 (37.4, 67.3) | 28.7 (23.5, 33.9) | 7.5 (6.3, 8.7) | 3.0 (2.6, 3.5) |
| P (comparing 2 groups) | 0.28 | 0.93 | 0.34 | 0.02 | 0.15 | |
| Poultry5 | ||||||
| Quartile 1 [0.00–0.21] | 42 | 103 (79, 135) | 45.7 (35.0, 59.9) | 27.4 (22.3, 32.5) | 7.0 (5.9, 8.2) | 2.6 (2.2, 3.0) |
| Quartile 2 [0.22–0.41] | 37 | 145 (113, 186) | 52.4 (39.6, 69.2) | 25.7 (21.6, 29.7) | 6.1 (5.1, 7.0) | 3.0 (2.7, 3.3) |
| Quartile 3 [0.42–0.64] | 36 | 107 (81, 142) | 46.5 (34.2, 63.2) | 25.6 (20.1, 31.1) | 5.7 (4.7, 6.7) | 2.6 (2.2, 2.9) |
| Quartile 4 [0.65–2.82] | 40 | 136 (107, 172) | 54.3 (42.4, 69.5) | 26.9 (22.6, 31.1) | 6.0 (5.0, 7.0) | 2.8 (2.4, 3.2) |
| P-trend | 0.45 | 0.51 | 0.96 | 0.35 | 0.88 |
Values are means (95% CIs). *Different from men in the lowest category, P < 0.05. Semen quality indicators were adjusted for age, total energy intake, BMI, race, smoking status, abstinence interval, previous infertility diagnosis, and dietary patterns.
Includes hamburgers, hot dogs, bacon, and other processed meats (e.g., salami, bologna, etc.).
Includes beef, pork, and ham consumed as a mixed or main dish.
Includes liver and chicken liver.
Includes chicken or turkey cooked with or without skin, as a main dish, sandwich, or frozen dinner.
In contrast to processed red meat, organ meat intake was positively related to sperm morphology (Table 2). Compared with nonconsumers (n = 125), men who reported consuming organ meats had 24.5% higher normal sperm morphology in the multivariable-adjusted model. Further adjustment for intakes of cholesterol, manganese, zinc, iron, retinol, or vitamin B-12 did not change this association. However, intake of copper attenuated the association, with an adjusted mean difference in percentage of morphologically normal sperm of 1.0 (95% CI: −0.4, 2.4).
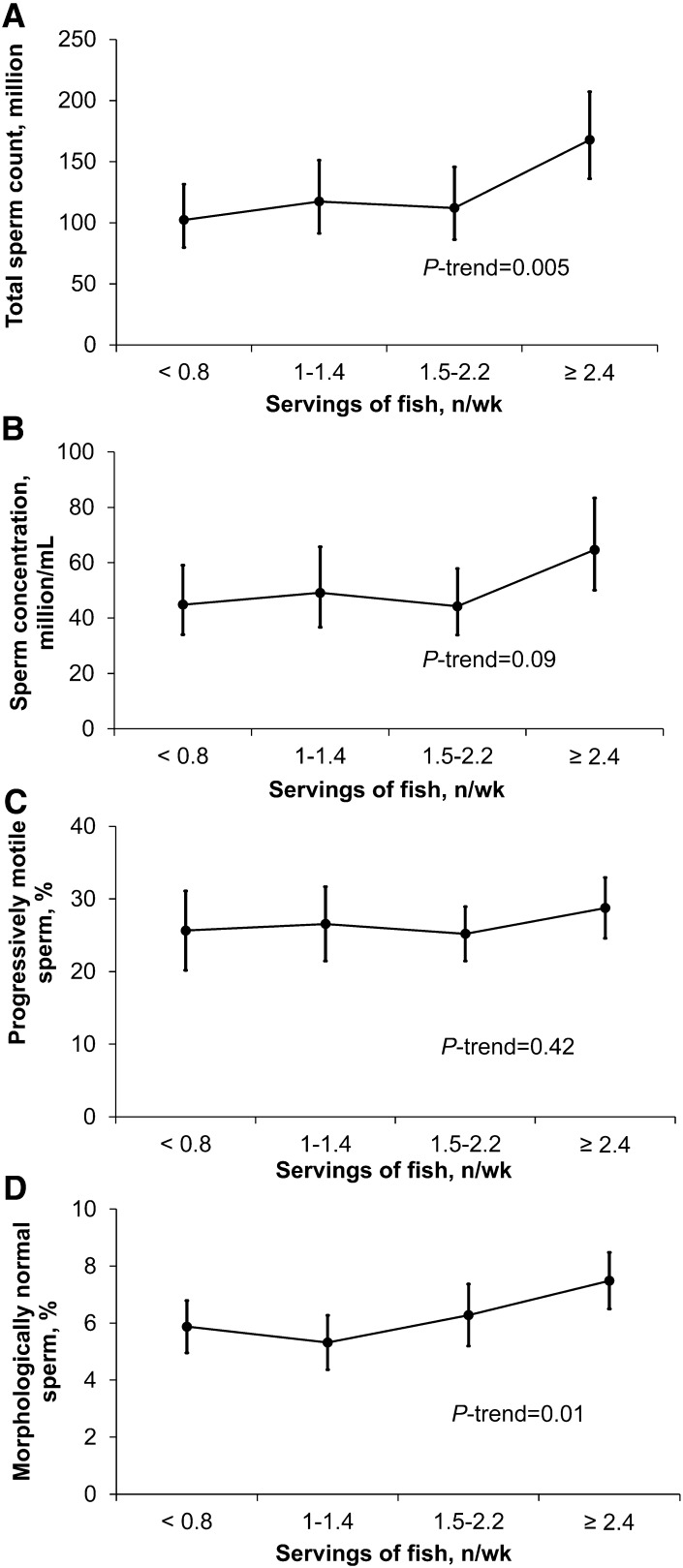
Total fish intake was associated with higher total sperm count (P-trend = 0.005) and percentage of morphologically normal sperm (P-trend = 0.01) (Fig. 1). The association between fish intake and total sperm count was strongest for intake of dark meat fish (e.g., salmon, tuna) (Table 3). Compared with men in the lowest quartile of dark meat fish intake (0.00–0.03 servings/d), total sperm count was 51% (95% CI: 3, 119) higher for men in the highest quartile of intake (0.16–0.86 servings/d). The association with sperm morphology was strongest with intake of white meat fish (e.g., cod, halibut) but was also observed for intake of dark meat fish (Table 3).
FIGURE 1.
Adjusted semen quality indicators in 155 men (338 semen samples) from the Environment and Reproductive Health Study according to total fish intake. Data points and bars represent means and 95% CIs for total sperm count (A), sperm concentration (B), percent progressively motile sperm (C), and percent morphologically normal sperm (D). Adjusted for age, total energy intake, BMI, race, smoking status, abstinence interval, previous infertility diagnosis, and dietary patterns. Total fish was defined as the sum of dark meat fish (including canned tuna and other dark meat fish such as salmon and bluefish), white meat fish (including breaded fish cakes and other white meat fish such as cod, haddock, and halibut), and shellfish (including shrimp, lobster, scallops, and clams as a main dish). Number of participants by increasing quartile of meat intake was n = 40, 35, 43, and 37, respectively.
TABLE 3.
Adjusted semen quality indicators in 155 men (338 semen samples) according to intake of different fish types from the Environment and Reproductive Health Study1
| Meat intake (servings/d) | Participants | Total sperm count | Sperm concentration | Progressive motility | Sperm morphology | Ejaculate volume |
| n | million | million/mL | % motile | % normal | mL | |
| Dark meat fish2 | ||||||
| Quartile 1 [0.00–0.03] | 34 | 96 (72, 127) | 44.0 (32.5, 59.6) | 23.9 (18.4, 29.5) | 5.7 (4.8, 6.7) | 2.4 (2.1, 2.8) |
| Quartile 2 [0.04–0.09] | 38 | 118 (92, 153) | 44.7 (34.2, 58.5) | 25.7 (20.9, 30.4) | 5.7 (4.8, 6.7) | 3.0 (2.6, 3.4)* |
| Quartile 3 [0.10–0.15] | 42 | 126 (103, 155) | 56.4 (45.2, 70.5) | 27.5 (23.5, 31.5) | 6.3 (5.4, 7.2) | 2.6 (2.2, 2.9) |
| Quartile 4 [0.16–0.86] | 41 | 145 (114, 184)* | 52.6 (39.7, 69.7) | 28.2 (23.9, 32.4) | 7.1 (5.9, 8.3) | 3.0 (2.6, 3.3)#x2020 |
| P-trend | 0.04 | 0.24 | 0.20 | 0.06 | 0.39 | |
| White meat fish3 | ||||||
| Tertile 1 [0.00–0.03] | 50 | 112 (88, 143) | 50.8 (39.3, 65.6) | 26.1 (21.4, 30.8) | 5.2 (4.5, 5.9) | 2.6 (2.2, 2.9) |
| Tertile 2 [0.04–0.09] | 55 | 127 (105, 154) | 47.5 (38.2, 59.0) | 27.0 (23.1, 30.9) | 6.6 (5.6, 7.5) | 3.0 (2.7, 3.3) |
| Tertile 3 [0.10–0.51] | 50 | 124 (99, 155) | 50.7 (39.6, 64.9) | 26.0 (22.7, 29.4) | 7.0 (6.1, 7.9) | 2.7 (2.3, 3.0) |
| P-trend | 0.56 | 0.99 | 0.97 | 0.004 | 0.99 | |
| Shellfish4 | ||||||
| Half 1 [0.00–0.07] | 78 | 109 (91, 131) | 45.7 (37.5, 55.7) | 26.2 (22.5, 29.8) | 6.1 (5.4, 6.9) | 2.7 (2.4, 3.0) |
| Half 2 [0.08–0.43] | 77 | 135 (112, 161) | 53.9 (44.6, 65.0) | 26.7 (23.8, 29.6) | 6.3 (5.6, 7.1) | 2.8 (2.5, 3.0) |
| P-trend | 0.13 | 0.26 | 0.83 | 0.73 | 0.77 |
Values are means (95% CIs). *Different from men in the lowest intake category, P < 0.05. †P = 0.06 when compared with men in the lowest intake category. Semen quality indicators were adjusted for age, total energy intake, BMI, race, smoking status, abstinence interval, previous infertility diagnosis, and dietary patterns.
Includes canned tuna and other dark meat fish (e.g., salmon, bluefish, etc.).
Includes breaded fish cakes and other white meat fish (e.g., cod, haddock, halibut).
Includes shrimp, lobster, scallops, and clams as a main dish.
Further adjustment for intakes of niacin, vitamins B-6 and B-12, cholesterol, manganese, zinc, iron, or retinol did not change the associations between fish intake and semen quality indicators. Intake of long-chain ω-3 FAs from food, however, attenuated these associations. After adjustment for long-chain ω-3 FA intake from food, the adjusted total sperm counts (95% CI) in increasing quartiles of fish intake were 111 (85, 146), 123 (95, 159), 109 (84, 141), and 152 (119, 193) million sperm (P-trend = 0.13), and the corresponding values for percentage of normal morphology were 6.3 (5.3, 7.3), 5.5 (4.6, 6.5), 6.1 (5.1, 7.2), and 7.0 (5.8, 8.1) (P-trend = 0.33). Total sperm count or sperm morphology did not differ between men who consumed ω-3 FA supplements (n = 16) and men who did not use these supplements (data not shown). Intake of long-chain ω-3 FAs from foods, however, was positively related to sperm morphology. In separate models not adjusting for fish intake, a 1-g/d increase in ω-3 FA intake was associated with a 2.3-fold (95% CI: 1.0, 5.2) higher total sperm count and 3.8 (95% CI: 1.0, 6.6) percentage unit higher normal sperm morphology.
Because fish and processed red meat intake were related to the same outcomes in opposite directions and were positively related to each other (r = 0.24), we examined the possibility that they were confounding each other’s relations with semen quality indicators. The inverse association between processed meat intake and morphology remained significant after further adjustment for fish intake (Supplemental Table 1). Similarly, the associations of fish intake with total sperm count and sperm morphology also remained essentially unchanged and statistically significant after adjustment for processed meat intake. There were no ostensible differences in results between adjusted models and crude models (Supplemental Table 2 and 3).
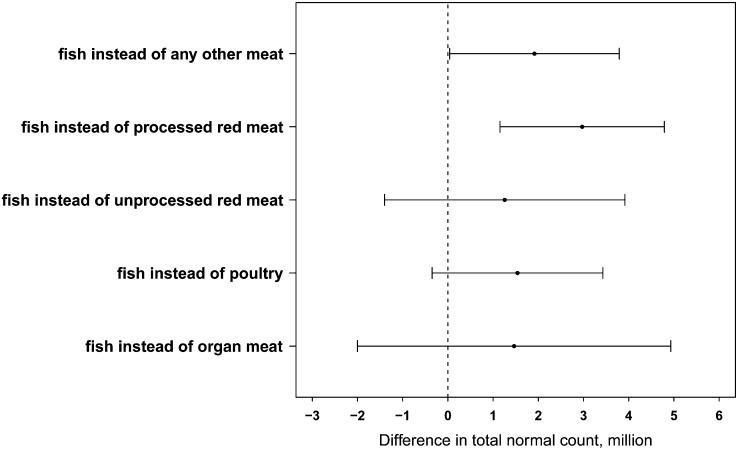
Next, we estimated the effect of consuming fish instead of other meats on total normal count. Replacing processed meats with fish while keeping total meat intake constant was associated with a significantly higher total normal count (Fig. 2). For example, consuming 2 servings/wk of fish in lieu of 2 servings/wk of processed red meats was associated with a 60% higher total normal count (95% CI: 17.9, 117.4). A similar pattern was observed when each individual semen quality indicator was separately examined (Supplemental Fig. 1).
FIGURE 2.
Relative difference in total normal count in 155 men (338 semen samples) from the Environment and Reproductive Health Study associated with consuming 2 servings/wk of fish instead of other meats. Data points and bars represent means and 95% CIs. Adjusted for age, total energy intake, BMI, race, smoking status, abstinence interval, previous infertility diagnosis, and dietary patterns. Keeping total meat intake constant, but switching out processed meats for fish, was associated with significantly higher total normal count. For example, consuming 2 servings/wk of fish in lieu of 2 servings/wk of processed red meats was associated with an ∼60% higher total normal count (95% CI: 17.9, 117.4).
Finally, we observed no modification of the associations of processed red meat and fish intake with semen quality indicators by BMI or smoking (P-heterogeneity > 0.10 in all cases). Exclusion of abstinence time from the models did not change the results.
Discussion
We prospectively examined the relation between meat consumption and semen quality indicators in a cohort of men attending a fertility clinic. We found that higher processed meat intake was associated with a lower percentage of morphologically normal sperm, whereas higher fish intake was related with higher total sperm count and percentage of morphologically normal sperm. We also found an unexpected positive relation between organ meat consumption and sperm morphology that appeared to be explained by copper intake. Our results suggest that consuming fish instead of other meats is related to better semen quality indicators.
Previous studies investigating meat intake and semen quality indicators are scarce but generally consistent with our findings. We previously reported that processed meat intake is associated with lower total sperm count among physically active healthy young men (17). A study in Spain found that intake of processed red meats was ∼31% higher among oligoasthenoteratospermic men than among controls but did not find any difference in fish intake between cases and controls (13). Another study in the Netherlands found that fish and other seafood was associated with higher sperm motility (14). A third study among subfertile men in Iran found that the odds of asthenospermia was higher among men consuming the highest amounts of processed red meat compared with those consuming the lowest amounts, but lower among men in the highest tertile of fish and other seafood intake compared with those in the first tertile of intake (16). Given a previously described association between saturated fat intake and sperm count and concentration (10), we anticipated that the observed relation between processed meat and total sperm count would be explained by saturated fat but this was not the case. Another possibility is that the observed relation could be due to the presence of preservative agents or hormonal residues in processed meat (28, 29). In the United States, anabolic sex steroid hormones are administered to cattle and other animals for growth promotion 60–90 d before slaughter (28, 30). Processed red meats have previously been reported to have higher concentrations of hormone residues compared with other meats (31, 32), raising concerns regarding the potential reproductive health consequences of consuming these foods. Further investigation of the relation between red and processed meats on semen quality or male factor infertility is needed to clarify the potential impact of these foods on male reproductive potential.
Our findings of positive associations of fish intake with sperm counts and morphology are consistent with our previous report of a cross-sectional association between intake of ω-3 FAs and higher sperm morphology among a smaller group of men participating in the EARTH Study (10). They are also consistent with our previous report of a relation between a “prudent” diet pattern, characterized by high intakes of fruits, vegetables, legumes, whole grains, chicken, and fish, and sperm motility among young men (15). These findings are also in line with those of a 32-wk fish oil (DHA + EPA) supplementation randomized trial that observed an increase in sperm concentration and morphology among asthenospermic men (33).
A positive relation between fish intake and semen quality that is mediated through intake of long-chain ω-3 FAs, as suggested by our data, is also consistent with the current understanding of the role of long-chain PUFAs in spermatogenesis. During sperm maturation, DHA concentrations exponentially increase in the sperm membrane (12) as a result of both dietary intake and local metabolism (34, 35). Testes and sperm have higher concentrations of long-chain PUFAs, particularly DHA, than other tissues or cells (34, 36). This suggests that the testes or the epididymides have a very active FA metabolism that preferentially accumulates long-chain PUFAs, metabolizes PUFAs into long-chain metabolites more efficiently than other tissues, or both. In animal models, fish oil supplementation increases testicular concentrations of DHA (35, 37, 38). Further, the expression pattern of enzymes involved in PUFA metabolism in the testes suggests a very active FA metabolism. Δ6-desaturase, the rate-limiting enzyme in the metabolism of PUFAs, and Δ5-desaturase are expressed in Sertoli cells and the epididymis at concentrations comparable with expression in the liver. Moreover, in Sertoli cells, the enzymes involved in this pathway prefer the conversion of ω-3 FAs into its 22 and 24 carbon metabolites over converting ω-6 FAs (39–41), potentially explaining, to some extent, the high concentration of DHA in sperm. Thus, it is plausible that intake of ω-3 FAs is associated with better semen quality indicators.
We found a positive relation between organ meat intake and sperm morphology that appeared to be explained by copper intake. We previously reported a positive association between organ meat intake and sperm concentration, motility, and ejaculate volume among physically active healthy young men (17). Previous work suggests that copper concentrations in seminal plasma or serum leads to decreased sperm motility and morphology (42, 43) because of oxidative damage (44). Clearly, this intriguing association deserves further study.
Although this study has a number of strengths and contributes to the emergent literature on this topic, it has some limitations. First, it is not possible to extrapolate from these findings on semen quality to relations with fertility potential. Although conventional semen quality indicators are used as a proxy measure of male fertility potential, they are not strong predictors of the probability of conceiving (45, 46). Second, we only used a single FFQ to characterize intake. Because spermatogenesis is a relatively rapid process and, in theory, could respond quickly to changes in environmental exposures, like diet, it is possible that a single dietary assessment would lead to misclassification of exposure during the follow-up period when additional semen samples were collected. However, we tried to minimize this issue by limiting the follow-up period to 18 mo. Moreover, said misclassification would most likely lead to attenuation of the observed associations, suggesting that the relations between meat intake and semen quality indicators may be stronger than those observed. Third, as is the case of all observational studies, there is always the possibility of unmeasured confounding. However, we collected data on a large number of known and suspected predictors of semen quality, and adjustment for potential confounders had little impact on the observed relations. Finally, although we observed differences in total sperm count and sperm morphology by quartile of processed meat and fish intake, values of these sperm indicators still fell within the normal range for the sperm evaluation. The strengths of our study include its prospective design, the use of a previously validated FFQ, and the use of repeat semen samples in most men, which allowed us to account for the previously described within-person variability in semen quality indicators (20). In addition, intakes of processed red meat and fish in this study were comparable with intakes among the general U.S. population (47), suggesting that these results may be generalizable.
In summary, in a longitudinal study among men attending a fertility clinic, we found that processed meat intake was negatively associated with sperm morphology, whereas fish intake was positively related to total sperm count and sperm morphology. We also observed an unexpected relation between organ meat intake and sperm morphology. Our data suggest that consuming fish instead of other meats, and particularly instead of processed red meats, could have a beneficial impact on semen quality indicators.
Supplementary Material
Acknowledgments
The authors thank the Harvard School of Public Health research staff, especially research nurses Jennifer Ford and Myra Keller, and senior research assistant Ramace Dadd. M.C.A. and J.E.C. designed the research project, wrote the manuscript, and had primary responsibility for the final content; M.C.A. analyzed the data; A.J.G., P.L.W., and J.E.C. provided statistical expertise; and P.L.W., A.J.G., T.L.T., D.L.W., C.T., R.H., and J.E.C. provided a detailed review and corrected the manuscript. All authors read and approved the final manuscript.
References
- 1.Louis JF, Thoma ME, Sørensen DN, McLain AC, King RB, Sundaram R, Keiding N, Buck Louis GM. The prevalence of couple infertility in the United States from a male perspective: evidence from a nationally representative sample. Andrology 2013;1:741–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thoma ME, McLain AC, Louis JF, King RB, Trumble AC, Sundaram R, Buck Louis GM. Prevalence of infertility in the United States as estimated by the current duration approach and a traditional constructed approach. Fertil Steril 2013;99:1324–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thonneau P, Marchand S, Tallec A, Ferial M-L, Ducot B, Lansac J, Lopes P, Tabaste J-M, Spira A. Incidence and main causes of infertility in a resident population (1 850 000) of three French regions (1988–1989). Hum Reprod 1991;6:811–6. [DOI] [PubMed] [Google Scholar]
- 4.Hammoud AO, Gibson M, Peterson CM, Meikle AW, Carrell DT. Impact of male obesity on infertility: a critical review of the current literature. Fertil Steril 2008;90:897–904. [DOI] [PubMed] [Google Scholar]
- 5.Sermondade N, Faure C, Fezeu L, Lévy R, Czernichow S. Obesity-Fertility Collaborative Group. Obesity and increased risk for oligozoospermia and azoospermia. Arch Intern Med 2012;172:440–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li Y, Lin H, Li Y, Cao J. Association between socio-psycho-behavioral factors and male semen quality: systematic review and meta-analyses. Fertil Steril 2011;95:116–23. [DOI] [PubMed] [Google Scholar]
- 7.Mínguez-Alarcón L, Mendiola J, López-Espín JJ, Sarabia-Cos L, Vivero-Salmerón G, Vioque J, Navarrete-Muñoz EM, Torres-Cantero AM. Dietary intake of antioxidant nutrients is associated with semen quality in young university students. Hum Reprod 2012;27:2807–14. [DOI] [PubMed] [Google Scholar]
- 8.Jensen TK, Swan SH, Skakkebaek NE, Rasmussen S, Jorgensen N. Caffeine intake and semen quality in a population of 2,554 young Danish men. Am J Epidemiol 2010;171:883–91. [DOI] [PubMed] [Google Scholar]
- 9.Chavarro JE, Toth TL, Sadio SM, Hauser R. Soy food and isoflavone intake in relation to semen quality indicators among men from an infertility clinic. Hum Reprod 2008;23:2584–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Attaman JA, Toth TL, Furtado J, Campos H, Hauser R, Chavarro JE. Dietary fat and semen quality among men attending a fertility clinic. Hum Reprod 2012;27:1466–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jensen TK, Heitmann BL, Jensen MB, Halldorsson TI, Andersson AM, Skakkebaek NE, Joensen UN, Lauritsen MP, Christiansen P, Dalgård C, et al. High dietary intake of saturated fat is associated with reduced semen quality among 701 young Danish men from the general population. Am J Clin Nutr 2013;97:411–8. [DOI] [PubMed] [Google Scholar]
- 12.Lenzi A, Gandini L, Maresca V, Rago R, Sgrò P, Dondero F, Picardo M. Fatty acid composition of spermatozoa and immature germ cells. Mol Hum Reprod 2000;6:226–31. [DOI] [PubMed] [Google Scholar]
- 13.Mendiola J, Torres-Cantero AM, Moreno-Grau JM, Ten J, Roca M, Moreno-Grau S, Bernabeu R. Food intake and its relationship with semen quality: a case-control study. Fertil Steril 2009;91:812–8. [DOI] [PubMed] [Google Scholar]
- 14.Vujkovic M, de Vries JH, Dohle GR, Bonsel GJ, Lindemans J, Macklon NS, van der Spek PJ, Steegers EAP, Steegers-Theunissen RPM. Associations between dietary patterns and semen quality in men undergoing IVF/ICSI treatment. Hum Reprod 2009;24:1304–12. [DOI] [PubMed] [Google Scholar]
- 15.Gaskins AJ, Colaci DS, Mendiola J, Swan SH, Chavarro JE. Dietary patterns and semen quality in young men. Hum Reprod 2012;27:2899–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eslamian G, Amirjannati N, Rashidkhani B, Sadeghi M-R, Hekmatdoost A. Intake of food groups and idiopathic asthenozoospermia: a case-control study. Hum Reprod 2012;27:3328–36. [DOI] [PubMed] [Google Scholar]
- 17.Afeiche MC, Williams PL, Mendiola J, Gaskins AJ, Jørgensen N, Swan SH, Chavarro JE. Meat intake and reproductive parameters among physically active young men. Epidemiology 2014;25:323–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chavarro JE, Toth TL, Wright DL, Meeker JD, Hauser R. Body mass index in relation to semen quality, sperm DNA integrity, and serum reproductive hormone levels among men attending an infertility clinic. Fertil Steril 2010;93:2222–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kruger TF, Acosta AA, Simmons KF, Swanson RJ, Matta JF, Oehninger S. Predictive value of abnormal sperm morphology in in vitro fertilization. Fertil Steril 1988;49:112–7. [DOI] [PubMed] [Google Scholar]
- 20.WHO. WHO laboratory manual for the examination and processing of human semen. 5th ed. Geneva, Switzerland: WHO; 2010.
- 21.Rimm EB, Giovannucci EL, Stampfer MJ, Colditz GA, Litin LB, Willett WC. Reproducibility and validity of an expanded self-administered semiquantitative food frequency questionnaire among male health professionals. Am J Epidemiol 1992;135:1114–26. [DOI] [PubMed] [Google Scholar]
- 22.USDA/Agricultural Research Service. USDA National Nutrient Database for Standard Reference, Release 25. Nutrient data laboratory home page [cited 2012]. Available from: http://www.ars.usda.gov/ba/bhnrc/ndl.
- 23.Feskanich D, Rimm EB, Giovannucci EL, Colditz GA, Stampfer MJ, Litin LB, Willett WC. Reproducibility and validity of food intake measurements from a semiquantitative food frequency questionnaire. J Am Diet Assoc 1993;93:790–6. [DOI] [PubMed] [Google Scholar]
- 24.White H. A heteroskedasticity-consistent covariance matrix estimator and a direct test for heteroskedasticity. Econometrica 1980;48:817. [Google Scholar]
- 25.Searle SR, Speed FM, Milliken GA. Population marginal means in the linear model: an alternative to least square means. Am Stat 1980;34:216–21. [Google Scholar]
- 26.Schisterman EF, Cole SR, Platt RW. Overadjustment bias and unnecessary adjustment in epidemiologic studies. Epidemiology 2009;20:488–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Willett WC, Stampfer MJ. Implications of total energy intake for epidemiologic analyses (chapter 11). In: Willett WC, editor. Nutritional epidemiology. 2nd ed. New York: Oxford University Press; 1998. p. 273–301.
- 28.Willingham EJ. Environmental review: trenbolone and other cattle growth promoters: need for a new risk-assessment framework. Environ Pract 2006;8:58–65. [Google Scholar]
- 29.Swan SH, Liu F, Overstreet JW, Brazil C, Skakkebaek NE. Semen quality of fertile US males in relation to their mothers’ beef consumption during pregnancy. Hum Reprod 2007;22:1497–502. [DOI] [PubMed] [Google Scholar]
- 30.Andersson AM, Skakkebaek N. Exposure to exogenous estrogens in food: possible impact on human development and health. Eur J Endocrinol 1999;140:477–85. [DOI] [PubMed] [Google Scholar]
- 31.Daxenberger A, Ibarreta D, Meyer HHD. Possible health impact of animal oestrogens in food. Hum Reprod Update 2001;7:340–55. [DOI] [PubMed] [Google Scholar]
- 32.Henricks DM, Gray SL, Owenby JJ, Lackey BR. Residues from anabolic preparations after good veterinary practice. APMIS 2001;109:273–83. [DOI] [PubMed] [Google Scholar]
- 33.Safarinejad MR. Effect of omega-3 polyunsaturated fatty acid supplementation on semen profile and enzymatic anti-oxidant capacity of seminal plasma in infertile men with idiopathic oligoasthenoteratospermia: a double-blind, placebo-controlled, randomised study. Andrologia 2011;43:38–47. [DOI] [PubMed] [Google Scholar]
- 34.Conquer JA, Martin JB, Tummon I, Watson L, Tekpetey F. Fatty acid analysis of blood serum, seminal plasma, and spermatozoa of normozoospermic vs. asthenozoospermic males. Lipids 1999;34:793–9. [DOI] [PubMed] [Google Scholar]
- 35.Sebokova E, Garg ML, Wierzbicki A, Thomson AB, Clandinin MT. Alteration of the lipid composition of rat testicular plasma membranes by dietary (n-3) fatty acids changes the responsiveness of Leydig cells and testosterone synthesis. J Nutr 1990;120:610–8. [DOI] [PubMed] [Google Scholar]
- 36.Jeong BY, Jeong WG, Moon SK, Ohshima T. Preferential accumulation of fatty acids in the testis and ovary of cultured and wild sweet smelt Plecoglossus altivelis. Comp Biochem Physiol B Biochem Mol Biol 2002;131:251–9. [DOI] [PubMed] [Google Scholar]
- 37.Ayala S, Brenner RR. Effect of polyunsaturated fatty acids of the alpha-linolenic series in the lipid composition of rat testicles during development. Acta Physiol Lat Am 1980;30:147–52. [PubMed] [Google Scholar]
- 38.Ayala S, Brenner RR, Dumm C. Effect of polyunsaturated fatty acids of the α-linolenic series on the development of rat testicles. Lipids 1977;12:1017–24. [DOI] [PubMed] [Google Scholar]
- 39.Retterstøl K, Haugen TB, Woldseth B, Christophersen BO. A comparative study of the metabolism of n-9, n-6 and n-3 fatty acids in testicular cells from immature rat. Biochim Biophys Acta 1998;1392:59–72. [DOI] [PubMed] [Google Scholar]
- 40.Retterstøl K, Haugen TB, Christophersen BO. The pathway from arachidonic to docosapentaenoic acid (20:4n-6 to 22:5n-6) and from eicosapentaenoic to docosahexaenoic acid (20:5n-3 to 22:6n-3) studied in testicular cells from immature rats. Biochim Biophys Acta 2000;1483:119–31. [DOI] [PubMed] [Google Scholar]
- 41.Christophersen BO, Hagve TA, Christensen E, Johansen Y, Tverdal S. Eicosapentaenoic- and arachidonic acid metabolism in isolated liver cells. Scand J Clin Lab Invest Suppl 1986;184:55–60. [PubMed] [Google Scholar]
- 42.Roychoudhury S, Massanyi P, Bulla J, Choudhury MD, Straka L, Lukac N, Formicki G, Dankova M, Bardos L. In vitro copper toxicity on rabbit spermatozoa motility, morphology and cell membrane integrity. J Environ Sci Health A Tox Hazard Subst Environ Eng 2010;45:1482–91. [DOI] [PubMed] [Google Scholar]
- 43.Roychoudhury S, Massanyi P. In vitro copper inhibition of the rabbit spermatozoa motility. J Environ Sci Health A Tox Hazard Subst Environ Eng 2008;43:651–6. [DOI] [PubMed] [Google Scholar]
- 44.Aydemir B, Kiziler A, Onaran I, Alici B, Ozkara H, Akyolcu M. Impact of Cu and Fe concentrations on oxidative damage in male infertility. Biol Trace Elem Res 2006;112:193–203. [DOI] [PubMed] [Google Scholar]
- 45.Jedrzejczak P, Taszarek-Hauke G, Hauke J, Pawelczyk L, Duleba AJ. Prediction of spontaneous conception based on semen quality indicators. Int J Androl 2008;31:499–507. [DOI] [PubMed] [Google Scholar]
- 46.Buck Louis GM, Sundaram R, Schisterman EF, Sweeney A, Lynch CD, Kim S, Maisog JM, Gore-Langton R, Eisenberg ML, Chen Z. Semen quality and time to pregnancy: the Longitudinal Investigation of Fertility and the Environment Study. Fertil Steril 2014;101:453–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Daniel CR, Cross AJ, Koebnick C, Sinha R. Trends in meat consumption in the USA. Public Health Nutr 2011;14:575–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.