Abstract
The Mushroom Council convened the Mushrooms and Health Summit in Washington, DC, on 9–10 September 2013. The proceedings are synthesized in this article. Although mushrooms have long been regarded as health-promoting foods, research specific to their role in a healthful diet and in health promotion has advanced in the past decade. The earliest mushroom cultivation was documented in China, which remains among the top global mushroom producers, along with the United States, Italy, The Netherlands, and Poland. Although considered a vegetable in dietary advice, mushrooms are fungi, set apart by vitamin B-12 in very low quantity but in the same form found in meat, ergosterol converted with UV light to vitamin D2, and conjugated linoleic acid. Mushrooms are a rare source of ergothioneine as well as selenium, fiber, and several other vitamins and minerals. Some preclinical and clinical studies suggest impacts of mushrooms on cognition, weight management, oral health, and cancer risk. Preliminary evidence suggests that mushrooms may support healthy immune and inflammatory responses through interaction with the gut microbiota, enhancing development of adaptive immunity, and improved immune cell functionality. In addition to imparting direct nutritional and health benefits, analysis of U.S. food intake survey data reveals that mushrooms are associated with higher dietary quality. Also, early sensory research suggests that mushrooms blended with meats and lower sodium dishes are well liked and may help to reduce intakes of red meat and salt without compromising taste. As research progresses on the specific health effects of mushrooms, there is a need for effective communication efforts to leverage mushrooms to improve overall dietary quality.
Introduction
Human health concerns such as cancers, diabetes mellitus, and cardiovascular disease are clearly influenced by dietary intake and other lifestyle factors. Both sound science and tools for facilitating dietary change are fundamental to effective global strategies for health promotion and disease risk reduction. Although mushrooms have long been regarded as health-promoting foods, research specific to the role of mushrooms in a healthful diet and the potential for mushrooms in health promotion has advanced in the past decades.
Therefore, the Mushroom Council convened the Mushrooms and Health Summit in Washington, DC, on 9–10 September 2013 to explore the state of relevant science. These proceedings are based on presentations made at the summit by experts from a broad range of disciplines [presentations are archived on the Mushroom Council Web site (1)]. They explored the following topics, focusing primarily on cultivated mushrooms consumed as food in the human diet:
historical and biological context;
market trends;
unique nutritional and bioactive components;
evidence for the impact of mushrooms on health and related biomarkers;
potential mechanisms of action;
consumer consumption trends; and
ways to translate nutrition, health, and sensory science on mushrooms into action to benefit human health.
Not a Plant or a Vegetable—but a Fungus
The role of mushrooms in diets and health has been documented throughout human history. Early Greek, Egyptian, Roman, Chinese, and Mexican civilizations valued mushrooms as culinary delicacies and as medicine. Although dietary recommendations classify mushrooms as vegetables, they are actually fungi. Although in the past fungi were considered plants, some of the characteristics that distinguish them from plants are that they possess cell walls containing chitin rather than cellulose and lack chloroplasts (2, 3).
Fungi are a large and diverse group of eukaryotic organisms that include yeasts, molds, and mushrooms. Most start as microscopic filaments, which grow by elongating threadlike structures called hyphae. Fungi live on dead matter in soil or on living animals, plants, or other fungi. They may also be airborne or aquatic. Although some remain microscopic, others produce large fruiting bodies (4).
Edible fungi include true mushrooms, as well as puffballs, corn smut, jelly fungi, and cup fungi. There are ∼20–30 cultivated edible species, ∼15 wild edible species that are commonly collected for commercial sale, and many more wild noncommercial edibles (5–7). Agaricus bisporus, including white button (WB)32, crimini, and portabella varieties, is especially common in today’s culinary repertoire. The next most common commercial species are shiitake (Lentinus edodes), straw (Volvariella volvacea), oyster (Pleurotus ostreatus), and enoki (Flammulina ostreatus) (8).
Market Trends
In many cultures, mushroom picking is an important tradition and can be a substantial source of income. In the Pacific Northwest of the United States, it is estimated that the value of the yearly mushroom harvest in a forest can equal the value of lumber produced from that same forest in some situations (9). However, most of the mushrooms eaten in the United States are cultivated and sold in stores. The USDA Extension Service is working with small farmers to establish new mushroom businesses in North Carolina (10, 11) and New York (12). The forest farming approach may help to diversify agricultural operations, contribute to rural community health and well-being, and support environmental health (11, 12).
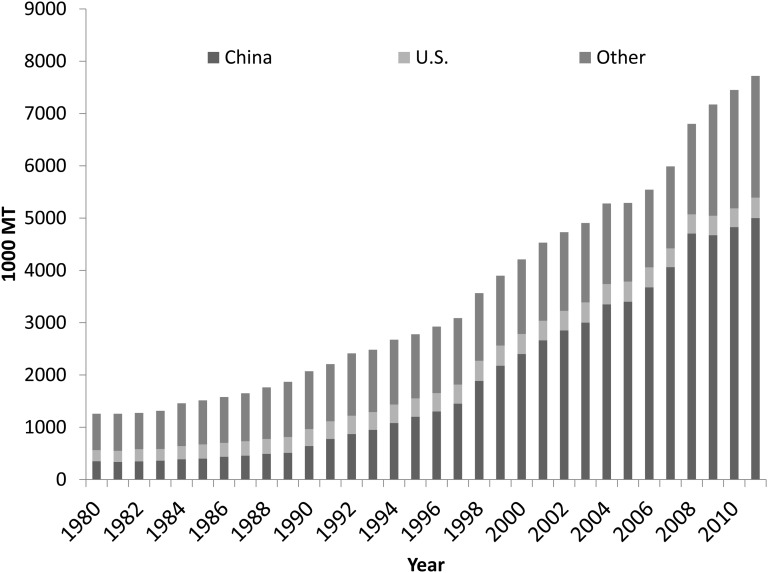
From 1970 to 2011, global mushroom and truffle production grew from <1 million to 7.7 million metric tons (mt) and by >500% between 1980 and 2011 (Fig. 1) (13). The rate of growth has also increased over time, from an average of 5% annually between 1980 and 1995 to nearly 7% annually since 1996. In 2011, China, Italy, the United States, The Netherlands, and Poland were reported as the top 5 mushroom producers in the world. Since 2009, China has produced 65% of global mushrooms and truffles, the European Union 24%, the United States 5%, and Japan, Indonesia, and Canada 1% each.
FIGURE 1.
Growth in world mushroom and truffle production, 1980–2011 (source: reference 19). MT, metric ton.
The U.S. mushroom farm community is small in acreage but an important contributor to the agricultural economy. Pennsylvania is historically the biggest U.S. producer of mushrooms. Collaboration between growers and researchers at The Pennsylvania State University has fostered both mushroom production and processing-company development in the state (14). Notably, supported through USDA National Institute for Food and Agriculture grants, North Carolina A&T University is breeding, screening, and selecting shiitake for indoor cultivation (10).
Sales by U.S. mushroom producers reached $1.1 billion during the 2012–2013 growing season, with increases mainly among fresh vs. processed mushrooms (14). In 2011, cash receipts for mushrooms ranked fifth among vegetables (15). Exports remain a small part of the U.S. mushroom market, with <5% of both fresh mushrooms and processed mushroom supplies exported annually.
In addition to the economic value of mushroom agriculture for growers, mushrooms have a small environmental footprint. Mushrooms grow from agricultural and forest wastes and require relatively little water or land. For example, per hectare of cultivated land, 370 mt of mushrooms were produced globally in 2011, compared with only 44 mt of rice and 56 mt of asparagus (13). Furthermore, spent substrate (i.e., used compost) may be used after harvesting as a soil amendment (i.e., an input to crop production) or processed further for biofuel.
More information is needed regarding the relations between mushroom markets, such as fresh vs. processed, and the growth potential for specialty mushrooms. The complexity of consumer demand patterns, including changing demographic characteristics, changes in healthful eating priorities, and the relation between price swings and purchase patterns will inform the ability to meet the demand of current and future consumers.
Nutritional and Other Bioactive Components
The USDA National Nutrient Database for Standard Reference, release 26, contains 22 mushroom records, including raw and processed forms of WB, crimini, shiitake, enoki, maitake, oyster, portabella, morel, and chanterelle, as well as portabella treated with UV light to enhance the ergocalciferol (vitamin D2) content (16, 17). Mean nutrient values for WB, enoki, maitake, and oyster mushrooms are presented in Table 1, as well as SEs where available. Differences in mean nutrient values were analyzed with ANOVA (Table 1).
TABLE 1.
Mean nutrient content of raw mushrooms per 100 g edible portion1
| Nutrient | White button2 | Enoki3 | Maitake4 | Oyster5 |
| Moisture, g/100 g | 92.2 ± 0.5a | 87.8 ± 0.5b | 90.5a | 90.2 ± 0.5a |
| Energy, kcal/100 g | 24 | 29 | 29 | 31 |
| Protein, g/100 g | 3.00 ± 0.22 | 2.66 ± 0.25 | 1.94 | 2.75 ± 0.24 |
| Fat, g/100 g | 0.34 ± 0.06 | 0.28 ± 0.08 | 0.20 | 0.33 ± 0.04 |
| Ash, g/100 g | 0.79a | 0.91 ± 0.08b | 0.52a | 0.77 ± 0.02a |
| Carbohydrate (by difference), g/100 g | 3.69 | 8.42 | 6.81 | 5.95 |
| Dietary fiber, g/100 g | 1.45 ± 0.46 | 2.80 ± 0.21 | 2.70 | 2.10 |
| Ergosterol, mg/100 g | 59 ± 4 | 37 ± 9 | 59 | 69 |
| β-Glucan, g/100 g | 0.21 ± 0.04a | 0.62b | 0.29a | 0.79 ± 0.06b |
| Calcium, mg/100 g | 4 ± 1a | 0a | 1b | 1b |
| Copper, mg/100 g | 0.30 ± 0.01a | 0.11 ± 0.02a | 0.25b | 0.12a |
| Iron, mg/100 g | 0.22 ± 0.05a | 1.15 ± 0.09b | 0.30a | 0.91b |
| Magnesium, mg/100 g | 10 ± 0a | 16b | 10a | 15b |
| Manganese, mg/100 g | 0.05 ± 0.01a | 0.08b | 0.06b | 0.10c |
| Phosphorus, mg/100 g | 94 ± 8 | 105 | 74 | 98b |
| Potassium, mg/100 g | 358 ± 16a | 359 ± 20a | 204b | 324a |
| Sodium, mg/100 g | 15 ± 4 | 3 ± 0 | 1 | 6 |
| Riboflavin, mg/100 g | 0.22 | 0.20 ± 0.02 | 0.24 | 0.33 |
| Niacin, mg/100 g | 2.80 ± 0.94a | 7.03 ± 0.86b | 6.58b | 5.87 ± 0.97 |
| Pantothenic acid, mg/100 g | 1.36 | 1.35 | 0.27 | 1.30 |
| Vitamin B-6, mg/100 g | 0.05 ± 0.01 | 0.10 ± 0.02 | 0.05 | 0.10 |
| Folic acid, μg/100 g | 19a | 52 ± 2b | 29a | 6c |
Values are means ± SEs unless otherwise indicated. No variance was determined when n < 3. Source: reference 6, except for β-glucan data, which are unpublished (D. B. Haytowitz, Nutrient Data Laboratory, Beltsville Human Nutrition Research Center, Agricultural Research Service, USDA; presented at the Mushrooms and Health Summit on 9 September 2013). Means with the same letter are not significantly different (P ≥ 0.05). Values without SEs (n < 3) were grouped with other values for analysis with ANOVA.
n = 3, except n = 4 for niacin, n = 2 for folic acid, and n = 1 for riboflavin and pantothenic acid.
n = 3, except n = 4 for niacin and vitamin B-6, n = 2 for magnesium and phosphorus, and n = 1 for pantothenic acid.
n = 2, except n = 1 for pantothenic acid.
n = 3, except n = 2 for vitamin B-6 and folic acid and n = 1 for riboflavin and pantothenic acid.
WB mushrooms, the most commonly consumed in the United States, are ∼92% water (16). A 100-g serving contains 3.0 ± 0.22 g protein (Table 1). There are very limited data on mushroom’s Protein Digestibility Corrected Amino Acid Score, a method of evaluating protein quality that is based on the amino acid requirements of humans and a correction for digestibility. One study found a Protein Digestibility Corrected Amino Acid Score for mushroom protein of 0.66, assuming a digestibility of 70% (18), which suggests a moderate-quality protein. Further research on the quality of mushroom protein is warranted.
The carbohydrate fraction includes 1.5 g of mannitol (19), 1.45 ± 0.46 g dietary fiber (Table 1) (16), and 0.21 ± 0.04 g β-glucans (unpublished data presented by D. B. Haytowitz, Nutrient Data Laboratory, Beltsville Human Nutrition Research Center, Agricultural Research Service, USDA, at the Mushrooms and Health Summit, 9 September 2013). β-Glucans are key bioactive compounds in mushrooms (20), with oyster and enoki containing substantially more than WB or maitake varieties. The small amount of fat in mushrooms is predominantly linoleic acid, including conjugated linoleic acid normally found in animal foods (21). WB mushrooms are also a source of several micronutrients, including riboflavin, niacin, pantothenic acid, copper, phosphorus, and selenium (16).
The form of vitamin B-12 in mushrooms was found to be the same form found in beef, liver, and fish, suggesting that it is highly bioavailable (22). However, fresh WB mushrooms contain <2% of the RDA for this nutrient (2.4 μg/d). This low amount of the highly bioavailable form of vitamin B-12 may be important for individuals consuming a vegan diet over a lifetime.
Mushrooms are a natural source of ergosterol, which is converted to vitamin D2 with exposure to UV light. In rats, ergocalciferol-enriched mushroom powders were effective in increasing 25-hydroxyvitamin D [25(OH)D] concentrations and improving bone mineralization, thus demonstrating bioavailability (23). Although simple sun exposure can increase the ergocalciferol amounts in sliced mushrooms, time of day or year, geographic location, and other factors affect the rate of accumulation. Therefore, a more controlled approach is used in commercial mushroom production to provide a 100% Daily Value (400 IU) of vitamin D in 3 oz (85 g) of fresh mushrooms. For the 41–78% of Americans who consume less than the Adequate Intake level (24), ergocalciferol-enriched mushrooms have the potential to provide a safe and effective way to increase 25(OH)D concentrations. Additional studies are warranted to determine effectiveness in young replete or older deficient populations and to assess effects on health outcomes.
The selenium content of mushrooms varies by type and geographic area based on the soil selenium content (25). The compost of cultivated mushrooms can be controlled to increase selenium content (26). Selenium is a cofactor for glutathione peroxidase, an enzyme essential for destroying lipid hydroperoxides and endogenous hydrogen peroxide. It helps prevent oxygen-radical–induced lipid peroxidation and is essential for sperm motility. Marginal selenium deficiency (seen around the world but rarely in the United States) may contribute to reduced immune function, some cancers, viral diseases, and reduced fertility (27, 28).
l-Ergothioneine (ergothioneine) is a unique sulfur-containing amino acid that cannot be synthesized by humans. It is available only from certain dietary sources, specifically fungi, kidney, liver, black and red beans, oat bran, and a few bacteria. Mushrooms are a primary source of ergothioneine, containing from 0.4 to 2.0 mg/g (dry weight) (29). King oyster, maitake, oyster, and shiitake contain the highest amounts of ergothioneine (30).
Ergothioneine bioavailability was documented in a randomized controlled trial (31). There was a significant increase in ergothioneine content of RBCs (mg/dL) with 16 g of mushroom powder (equivalent to 2 servings of fresh mushrooms) vs. placebo after 2, but not 1, 4, or 6 h. There was no effect on several lipid, glycemic, and inflammation biomarkers, although the postprandial TG response was blunted after both 8 and 16 g powdered mushroom doses compared with placebo. Because ergothioneine was shown in humans to be taken up into RBCs (31), and the ergothioneine transporter was noted in animals to be present in fetal liver, bone marrow, kidney, small intestine, cerebellum, and lung (32), where it is stored for 30 d (33), it may prove to be a useful biomarker of mushroom intake compliance in research.
More than a compliance marker, however, ergothioneine is a stable antioxidant in that it does not auto-oxidize at physiologic pH, does not promote generation of hydroxyl radicals from H2O2 and Fe2+ ions (34), and may serve as a final defense against oxidation in cells where glutathione may be depleted (35). It has its own unique transport system in mammals (36), suggesting an advantageous role for long-term human health (34). It has been suggested that ergothioneine should be designated as a vitamin because of its unique role in protecting mitochondria from oxidation (35). Furthermore, ergothioneine may serve as a longevity vitamin on the basis of the triage theory (37). In this theory, nutrients in short supply in the body could be exhausted for use in short-term survival at the expense of long-term disease susceptibility (38).
Effects of Mushrooms on Health and Related Biomarkers
A variety of preclinical and clinical studies suggest that consumption of certain mushroom species, as either food or extracts, or consumption of specific constituents from mushrooms may reduce the risk of certain diseases (18). Although Roupas et al. (18) reviewed evidence for a range of health effects, the following sections touch on evidence pertaining to mushrooms and cognition, cancer, weight management, and oral health, in keeping with the summit presentations.
Cognition.
Very preliminary experimental animal data suggest a protective effect of ergocalciferol-enriched mushrooms on β-amyloid peptide toxicity in the brain and mild cognitive impairment (both precursors to dementia) in mice. The findings warrant replication and further research on the potential role for mushroom consumption in delaying the onset of dementia and Alzheimer disease (39, 40).
Cancer.
WB mushrooms have been proposed as a potential breast cancer risk reduction agent, because they partially suppress aromatase activity and estrogen biosynthesis in vitro and in vivo (21). The association of mushroom intake with breast cancer risk was assessed in 358 Korean female patients with breast cancer and 360 cancer-free Korean control women (41). The highest (11.37 g/d) vs. the lowest (2.61 g/d) mushroom intake was associated with a lower risk of breast cancers among premenopausal women with estrogen receptor–positive/progesterone receptor–positive (ER+/PR+) tumors than those with ER-negative/PR-negative (ER−/PR−) tumors [OR for the highest (11.37 g/d) compared with the lowest quartiles of intake (2.61 g/d): 0.30; 95% CI: 0.11, 0.79].
Weight management.
The substitution of low-calorie foods for high-calorie foods has been proposed as a means of reducing overall caloric intake, therefore reducing risk of obesity. However, individuals tend to compensate for lower-calorie meals or increased physical activity with higher calories at subsequent eating occasions.
One possible dietary substitution for high-fat meats is mushrooms based on their nutritional, satiating, and sensory qualities. In a small (n = 54) 4-d crossover design intervention study in healthy overweight or obese adults, energy intake from mushroom meals was less than half the energy consumed from meat meals consumed in a laboratory setting (42). Energy intake was only partially compensated for over 4 d (11.4 ± 12%), according to self-reported intake. There were no differences in ratings of hunger, satiety, or palatability between the mushroom and meat weeks, but average daily calories and fat were lower during the mushroom vs. meat weeks. These short-term findings of reduced intake without negative effects on appetite warrant further study to determine if such effects could be sustained over time.
A follow-up randomized, controlled, parallel-group 1-y trial was conducted in men and women who were overweight or obese, seeking to lose weight, and willing to substitute mushrooms for meat (n = 73) (43). The control group was advised to eat healthy foods without changing meat intake, and the mushroom group was advised to substitute 8 oz of fresh mushrooms for 8 oz (227 g) of meat 3 times/wk. Both groups were assigned a 500-kcal/d energy-deficit diet with diet counseling for 6 mo, followed by a weight-maintenance diet with no diet counseling for 6 mo. A paired-sample t test was conducted to assess differences within groups (P < 0.05), and multivariate ANOVA was used to assess intervention effects (P < 0.05). During the 6-mo energy restriction phase, those consuming the mushroom diet experienced a significant loss in body weight, BMI, and waist circumference compared with baseline, and the meat diet group had a lower BMI and waist circumference (43). From baseline to 12 mo, there were trends toward greater losses in weight, waist circumference, and BMI in the mushroom vs. meat groups, but the differences were not significant (P = 0.23–0.31). Additional research is warranted to determine if the findings are replicable.
Oral health.
A shiitake mushroom fraction was shown in an in vitro model to strongly inhibit dentin demineralization and induce microbial shifts that could be associated with oral health (44). In a randomized controlled trial (n = 30), participants treated with a shiitake-extract oral mouth rinse (2 rinses with 10 mL mouthwash for 30 s at a 1-min interval twice daily, morning and evening) had a better plaque index than those treated with a water placebo (45). The gingival index was better for mushroom than for placebo or a leading gingivitis mouthwash. Decreases in total and specific oral bacterial pathogen counts were observed for both the mushroom extract and the gingivitis mouthwash vs. placebo. This emerging science is intriguing and, if confirmed, would suggest a potential role for mushrooms in maintaining oral health.
Associations between intakes and disease risk.
Analysis of mushroom consumption data collected in a single 24-h recall with U.S. adults (≥19 y) from 2001 to 2010 in the NHANES revealed associations with disease risk factors. (unpublished data presented by V. L. Fulgoni, III, Nutrition Impact, LLC, at the Mushrooms and Health Summit on 10 September 2013). Mushroom consumers were defined as those with intakes of 281 mushroom-containing food codes. Means and SEs were determined by covariate adjusted regression analyses using appropriate sample weights. Mushroom consumers had a lower risk of being overweight/obese and of having metabolic syndrome, apparently driven by reduced risk of high waist circumference (Table 2).
TABLE 2.
Risk of overweight/obesity (BMI ≥25 kg/m2) and metabolic syndrome for mushroom consumers vs. nonconsumers1
| Health outcome | OR | 95% CI |
| Overweight or obesity | 0.87 | 0.78, 0.98 |
| Metabolic syndrome | 0.85 | 0.73, 0.99 |
Values were adjusted for gender, race/ethnicity, age, poverty-income ratio, physical activity level (categorized as sedentary, moderate, or vigorous), smoking status, and alcohol consumption (P < 0.05). Source: NHANES 2001–2010 (http://www.cdc.gov/nchs/nhanes/nhanes_questionnaires.htm). Mushroom consumers were defined as having reported consuming foods corresponding to 1 of 281 mushroom-containing food codes.
Mechanisms of Action
The mechanisms by which mushrooms may influence health benefits remain an active area of investigation. Undeniably, the chemical composition and total intake of mushrooms determine their potential attributes. Below are some areas that have emerged as potential sites of action of mushrooms.
Gut microbiota.
Interactions between the host and its microbiota are increasingly recognized to be critical for health. A healthy microbiota acts as a barrier to pathogens, supports development and appropriate response of the immune system to threats, and supports tolerance to food antigens and harmless microbes (46, 47).
The effects of adding 1% WB mushrooms (equivalent to 75 g fresh weight in a human diet) to a purified diet (48) resulted in increased gut bacterial diversity, including increases in the Bacteroidetes phyla and decreases in the Firmicutes phyla compared with control-fed mice (47). In 2 different models of gastrointestinal injury (dextran sulfate sodium treatment and Citrobacter rodentium infection), resolution from injury and infection were improved in the WB mushroom–fed mice, and included more rapid recovery of weight loss and colonic length, and reduced hyperplasia and immune infiltrates in the colon histopathology (47, 49). These findings highlight the potential of using diets that contain WB mushrooms to reduce risk of diseases where the microbial flora has been demonstrated to have an impact. Studies with other types of mushrooms and other background diets are warranted.
There are several possible mechanisms that might explain the effects of WB mushrooms on colitis susceptibility. The polysaccharides in mushrooms may mimic bacterial polysaccharides and bind to Toll-like receptors expressed in the host. They may function through immune regulation or by altering inflammatory response. Indeed, a WB mushroom–enriched vs. control diet resulted in higher concentrations of TNF-α in the colon and serum after dextran sulfate sodium treatment (49). After C. rodentium infection, a WB mushroom–enriched vs. control diet resulted in higher concentrations of IFN-γ and IL-17 (47). Several inflammatory responses were higher in the WB mushroom–fed mice than in controls.
WB mushrooms also may affect gastrointestinal tract healing through the action of prebiotics, nondigestible food ingredients that stimulate the growth and/or activity of commensal bacteria in the digestive system. It was hypothesized that the microbiota and the host both use the nutrients that are present in WB mushrooms, and that the microbiota makes bioactive compounds from the polysaccharides in mushrooms. Further experimentation would be necessary to determine which, if any, of these possible mechanisms are most viable.
Immune function.
Dietary WB mushrooms were reported to enhance the activity of NK cells in mice (50). NK cells are an important component of the innate immune system, which is responsible for antiviral and antitumor defense. Increased NK activity may be mediated through increased production of IFN-γ and TNF-α. WB mushroom consumption appears to elicit a shift toward T-helper 1 response, and there is a trend for higher IL-2 production and lymphocyte proliferation.
In vitro, WB mushroom supplementation was shown to promote maturation and function of dendritic cells (DCs), which are the most important antigen-presenting cells that bridge innate and adaptive immunity, suggesting an enhanced development of adaptive immunity (51). Enhanced DC maturation was further evidenced by the changes in DC function, including increased IL-12 production, reduced phagocytosis, and enhanced antigen-presenting function, resulting in an elevated T-cell response to the specific antigen. These results suggest that WB mushrooms may have the potential to improve the development of adaptive immunity after initial exposure to an antigen or pathogen.
The protective effect of mushrooms against Salmonella typhimurium infection was demonstrated in mice (52). Feeding WB mushrooms (2%; equivalent to 150 g/d fresh mushrooms in a human diet) did not improve gastroenteritis or systemic (typhoid) salmonellosis infection with a virulent strain (SL1344) of S. typhimurium. However, when the mice were immunized with an attenuated strain (SL1479 ∆aro A) and then infected with the virulent strain, the vaccination-induced protection (lower weight loss and higher survival) was further enhanced by WB mushroom supplementation. Consistently, mice fed WB mushrooms had higher concentrations of antigen-specific antibody (both serum IgG and fecal IgA) and higher antigen-specific CD4+ T-cell subset Th1 and Th17 response, as well as upregulated number and function of DCs, all of which may contribute to improved vaccine efficacy. These results suggest that WB mushrooms may enhance pathogen exposure–induced adaptive immunity and may reduce the risk of future infections.
One randomized parallel-design study was conducted to determine if consumption of whole shiitake mushrooms (Lentinula edodes) could improve human immune function (53, 54). Fifty-one healthy adults consumed either 5 or 10 g/d of dried shiitake mushrooms for 4 wk. The ability of γδ-T cells and NK-T cells to proliferate ex vivo in autologous serum was enhanced, indicating improved cell functionality after mushroom consumption. Both cell types expressed a greater number of surface molecules, indicating that mushroom consumption improved the cell’s ability to become activated. An increase in the concentration of salivary secretory IgA indicates an improvement in gut immunity. The pattern of cytokine secretion was remarkably altered after mushroom consumption, evoking an anti-inflammatory milieu, with increased secretion of IL-4, IL-10, TNF-α, and IL-1α decreased macrophage inflammatory protein-1α and no change in IL-6, IL-1β, macrophage inflammatory protein-1β, IL-17, or IFN-γ. Although the mechanism is unknown, 1 possibility is that mushrooms prime the immune cells so that they are more responsive to future insults.
Mushroom Safety
Currently available evidence indicates that the consumption of A. bisporus mushrooms poses no toxicologic risk to humans (55). Fresh mushrooms contain 200–500 mg agaritine/kg fresh weight (56), an aromatic hydrazine-derivative mycotoxin. High amounts of chemically synthesized and chemically modified hydrazine compounds, including agaritine, were reported to have carcinogenic effects in mice (57–60). However, these effects could not be replicated in a later study (61). Review of these studies by others concluded that the studies were not performed in accordance with approved protocols for carcinogenicity studies, such as those outlined by the International Conference on Harmonization (62).
Some wild mushrooms are recognized to be poisonous. Amanita phalloides (death cap) contains amatoxins that cause severe gastroenteritis and hepatic necrosis (63). The toxin of Psilocybe semilanceata (liberty cap) is a potent hallucinogen. Anxiety, panic reactions, and peripheral sympathomimetic symptoms may follow ingestion. Amanita pantherina (panther cap) may cause severe anxiety and agitation, hallucinations, and peripheral anticholinergic symptoms. The toxin in Cortinarius speciosissimus has an almost exclusively nephrotoxic action. Schenk-Jaeger et al. (64) recommended that “Inspection of wild mushrooms by a certified mushroom expert or a mycologist prior to consumption seems to be a safe procedure which should be recommended.”
Intersection of Consumer Interests with Mushrooms’ Sensory Properties
In 2011 Americans consumed 1.8 kg mushrooms, including 1.3 kg of fresh mushrooms, per capita (65). A national survey of 12,618 U.S. consumers estimated that 41% of households purchased mushrooms at least once in 2012, spending $553 million (66). In two-thirds of those mushroom-purchasing households, the head of household was younger than 45 y, 31% had children in the home, and 59% were households of 1 to 2 people. Eighty-one percent were white, 7% were African American, and 6% were Asian American. Among all households reporting, 91% were non-Hispanic. Households with income levels >$60,000 generated 47% of the expenditures on mushrooms.
According to the “Menus of Change” initiative of The Culinary Institute of America and the Harvard School of Public Health Department of Nutrition, pairing the evidence for health- and sustainability-linked food choices with flavor, other culinary, and demographic trends and plausible business scenarios allows flavor-rich, largely plant-based food and menu choices to emerge (presented by G. Drescher, The Culinary Institute of America, at the Mushrooms and Health Summit on 9 September 2013). Mushrooms, with their unique sensory and culinary functional properties, may help Americans move toward healthier, plant-based choices. Of particular interest are the high amounts of both glutamates (not as monosodium glutamate) and ribonucleotides in A. bisporus. Glutamate and certain 5′-ribonucleotides are taste-active chemicals responsible for umami, considered by some to be the fifth flavor of food. Calcium diglutamate, in particular, was shown to improve the flavor of low-sodium products (67, 68).
The public is being advised to “Make half your plate fruits and vegetables,” “Skip the salt,” and “Eat plant protein foods more often” (69). These challenges can be met with culinary strategies that center on how to improve both health and flavor. Specifically, rather than tell consumers they must choose between indulgence and health, the emerging trend is to blend health, taste, and functionality through strategic innovation in culinary practice and menu language.
A key emerging contribution of mushrooms appears to be their ability to blend with reduced quantities of meat and still score high with consumers (unpublished data presented by A. Myrdal Miller, The Culinary Institute of America, at the Mushrooms and Health Summit on 10 September 2013). Four mushroom cooking techniques (steaming, sautéing, searing, and oven roasting) were evaluated by a 13-member descriptive analysis panel to determine the cooking technique(s) that provide the most favorable sensory attributes. The sensory attributes and consumer acceptance of 6 taco blends (100% beef, 50% beef/50% WB mushroom, 20% beef/80% WB mushroom, plus the same 3 combinations with 25% less sodium) were then evaluated by the descriptive analysis panel as well as 147 consumers ages 18–65 y (43% male).
Preference mapping analysis, a combination of factor analysis and classification methods designed to assess preference market segmentation and identify drivers of liking for the market segments, uncovered 4 consumer clusters that differed on the basis of their preferences (unpublished data presented by A. Myrdal Miller, The Culinary Institute of America, at the Mushrooms and Health Summit on 10 September 2013). Cluster 1 (n = 35) preferred the 100% beef taco blend and the full-salt recipes overall. Most consumers in cluster 1 consumed an animal-based diet, were in the medium-income bracket ($50,000–100,000), and did not exercise regularly. Cluster 2 (n = 49) liked every recipe and liked the meat-mushroom blends best. They were “foodies” for whom flavor is key. They included younger women and were the most educated and affluent cluster. Cluster 3 (n = 27) disliked the 100% beef blends but liked the mushroom-based blends. Consumers in this cluster consumed a plant-based diet and had a low liking for meat. Few were in the high-income bracket (>$100,000), many were single (74%) or students (44%), and they exercised often. Consumers in cluster 4 (n = 36) only liked the 100% beef recipe with full salt. They consumed a plant-based diet with chicken and demonstrated limited enjoyment of food. This group included more men than women, were older, and were either in the lower (<$50,000) or higher (>$100,000) income brackets.
These results suggest that there is great potential for mushrooms to be used as a partial substitute for meat in a wide variety of mixed dishes without compromising the sensory acceptance of those dishes. To be successful, such a strategy should map out preferences of consumers and market alternative recipes to the proper preference segments on the basis of demographic characteristics, psychographics, usage, and attitudes.
However, research is needed to elucidate how such approaches may work to improve consumers’ eating patterns. Communications research is also needed to address the challenge of presenting new food solutions in a way that is accurate but also compelling.
Exploring the Role of Mushrooms in Dietary Advice
Mushrooms have been mentioned in a range of dietary guidance in recent years. In the 2010 Dietary Guidelines for Americans, mushrooms are noted to be one of the “best” sources of vitamin D (70). The Dietary Guidelines for Americans is the basis for federal nutrition policy, partially based on systematic review of scientific evidence (71). Mushrooms have made their way into nutrition promotion campaigns, such as the Academy of Nutrition and Dietetics’ National Nutrition Month, Health Canada’s Eating Well with Canada Food Guide, and the New Nordic Diet, which touts the value of “plants and mushrooms from the wild countryside” (72). Still, it was noted that white vegetables, such as potatoes, cauliflower, and mushrooms, are “a forgotten source of nutrients” (73).
NHANES analysis (2001–2010) revealed associations between mushroom consumption and nutrient intake and diet quality, as measured by the Healthy Eating Index (HEI)–2005 in U.S. adults (74). Compared with nonconsumers, consumers had higher intakes of energy, protein, thiamin, niacin, folate, copper, selenium, and sodium. Total and added sugars were lower in mushroom consumers. Consumers also had higher total HEI scores than did nonconsumers; specifically, HEI subcomponent scores were higher for total vegetables, dark-green/orange vegetables, total grains, and milk and lower for sodium.
Because some of these data indicate that there are certain foods with which mushrooms are commonly consumed (74), mushrooms may also be used to promote other healthful foods. Such a strategy is consistent with the overall public health and nutrition education movement beyond single foods and toward overall dietary patterns. For example, Americans do not consume many dark-green or orange vegetables, but mushrooms are associated with higher intakes of these vegetables. At the same time, the savory flavor of mushrooms may help consumers who enjoy meat to eat less of it. The flavor profile of mushrooms also could support efforts to reduce sodium in diets, as noted previously.
Another source of dietary guidance for the public is the use of nutrition-related claims in the labeling of food products (75). However, claims regarding ergothioneine in mushrooms are not authorized at this time. Ergothioneine is not a compound for which a Daily Value has been established for a nutrient content claim; likewise, a health claim petition has not been reviewed by the FDA to establish this type of claim.
Overarching Research Needs
Numerous research needs were identified at the Mushrooms and Health Summit, along with a discussion of the importance of responsible communication of research results. Probing studies in humans are needed to understand the implications of the observed effects on immune function, gut microbiota, cognition, periodontitis, cancer mechanisms, body composition, and body weight. Studies are needed to define how much, how often, and perhaps in what pattern specific mushroom species may be consumed to bring about substantial biologic and health responses, as well as to understand the specificity of mushroom impacts on health. Similarly, comparative studies with other mushroom species will likely identify which component(s) are most critical for a biologic response.
On the cusp of an era of personalized nutrition, it is necessary to determine who will benefit most from a given pattern of mushroom consumption, as well as who may not. Biomarkers of susceptibility may be identified. Interaction between diet, the genome, and health is fundamental to reducing the risk of noncommunicable diseases worldwide.
There is a critical need for international public-private collaboration on nutrition in order to address these research needs. Collaboration and coordination allow for shared resources and open science. Already, data.gov is a portal providing G-8 country access to numerous U.S. research platforms.
Finally, society must move closer to sustainable and safe food security solutions. The growing global demands for water, land, and energy pose a serious challenge to meeting food and water needs.
In summary, research on mushrooms and health has emerged rapidly over the past decade, building on thousands of years of culinary and medicinal usage. Promising evidence suggests a positive role for mushrooms and their bioactive components, particularly ergothioneine, vitamin D, β-glucan, and selenium, on immune function, gut function, and weight management. Preliminary evidence suggests a role for mushrooms in reducing the risk of chronic diseases, including cancer, obesity, Alzheimer disease, and periodontitis. The evidence is still in its infancy; therefore, questions remain about how and for whom mushrooms may help support good health. As public health nutrition information increasingly includes guidance on how to shift toward healthier intakes in a manner that allows for continued enjoyment of food, mushrooms appear to be an item that can be leveraged to improve the healthfulness of dietary patterns.
Acknowledgments
The Mushroom Council and authors dedicate the Mushrooms and Health Summit Proceedings to our friend and colleague John A. Milner, PhD, with deep sorrow at his passing, with sincere gratitude for his leadership and insightful contributions to the summit and this manuscript, and for his tireless commitment to the advancement of the profession of nutrition science and research. The authors extend their sincere gratitude to the following individuals who helped them prepare their contributions to the manuscript: Christopher Davis, Market and Trade Economics Division, Economic Research Service (ERS), USDA; Michael Kalaras, Postdoctoral Research Scholar, The Pennsylvania State University; Ilya Rahkovsky, Food Economics Division, ERS, USDA; Peter Roupas, Commonwealth Scientific and Industrial Research Organisation (CSIRO) Animal, Food and Health Sciences; Isabel Walls, Office of the Chief Scientist, USDA; and Hodan Farah Wells, Market and Trade Economics Division, ERS, USDA. M.J.F., J.D., C.M.H.-L., J.A.M., B.M., M.N., S.R., and M.W. formed the Mushrooms and Health Summit agenda; R.B.B., J.C., M.T.C., L.A.C., S.-T.C., L.J.C., R.C., G.D., J.D., V.L.F., C.M.H.-L., D.B.H., V.S.H., D.L., A.M., J.A.M., B.M., M.N., S.S.P., G.R., B.S., S.T., C.E.W., and D.W. presented at the summit and wrote and edited the manuscript; and M.J.F. and C.D.T. wrote and edited the manuscript. All authors read and approved the final manuscript.
Footnotes
Abbreviations used: DC, dendritic cell; HEI, Healthy Eating Index; mt, metric ton; WB, white button; 25(OH)D, 25-hydroxyvitamin D.
References
- 1.The Mushroom Council. Mushrooms and Health Summit [cited 2014 Mar 5]. Available from: http://www.mushroomhealthsummit.com.
- 2.Baldauf SL, Roger AJ, Wenk-Siefert I, Doolittle WF. A kingdom-level phylogeny of eukaryotes based on combined protein data. Science 2000;290:972–7. [DOI] [PubMed] [Google Scholar]
- 3.Katz LA, Grant JR, Parfrey LW, Burleigh JG. Turning the crown upside down: gene tree parsimony roots the eukaryotic tree of life. Syst Biol 2012;61:653–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alexopoulos CJ, Mims CW, Blackwell M. Introductory mycology. New York: John Wiley and Sons; 1996.
- 5.Kuo M. 100 Wild edible mushrooms. Ann Arbor (MI): University of Michigan Press; 2007.
- 6.Chang ST. Training manual on mushroom cultivation technology. Beijing (China): United Nations Asian and Pacific Centre for Agricultural Engineering and Machinery; 2009.
- 7.Lincoff G. The complete mushroom hunter: an illustrated guide to finding, harvesting, and enjoying wild mushrooms. Minneapolis (MN): Quarry Books; 2010.
- 8.Chang ST. Mushroom research and development—equality and mutual benefit. In: Royse DJ, editor. Mushroom biology and mushroom products. University Park (PA): Pennsylvania State University; 1996.
- 9.Alexander SJ, Pilz D, Weber NS, Brown E, Rockwell VA. Mushrooms, trees, and money: value estimates of commercial mushrooms and timber in the Pacific Northwest. Environ Manage 2002;30:129–41. [DOI] [PubMed] [Google Scholar]
- 10.North Carolina Agricultural and Technology, University School of Agriculture and Environmental Sciences, Mushroom Biology and Fungal Biotechnology Laboratory. Mushrooms. Greensboro (NC): North Carolina A&T; 2011 [cited 2013 Dec 20]. Available from: http://www.ag.ncat.edu/OmonMushroom/frameset.htm.
- 11.Davis JM, Harrison J. Producing shiitake mushrooms: a guide for small-scale outdoor cultivation on logs. Raleigh (NC): North Carolina Cooperative Extension; 2011 [cited 2013 Dec 20]. Available from: http://www.ces.ncsu.edu/fletcher/programs/herbs/crops/mushrooms/pdf/2011%20AG-478_Shiitake_Final.pdf.
- 12.Northeast Forest Mushroom Growers Network [homepage on the Internet]. [cited 2013 Dec 20]. Available from: http://blogs.cornell.edu/mushrooms/.
- 13.FAOSTAT [database on the Internet]. Mushrooms and truffles. Rome: Food and Agriculture Organization of the United Nations; 2014 [cited 2013 Nov 25]. Available from: http://faostat3.fao.org/.
- 14.National Agricultural Statistics Service. Mushrooms. Washington: USDA; 2013 [cited 2013 Nov 25]. Available from: http://usda.mannlib.cornell.edu/MannUsda/viewDocumentInfo.do?documentID=1110.
- 15.Economic Research Service. Farm income and wealth statistics. Washington: USDA; 2013 [cited 2013 Nov 25]. Available from: http://www.ers.usda.gov/data-products/farm-income-and-wealth-statistics.aspx#.UtAZJ_RDvAl.
- 16.National Nutrient Database for Standard Reference. Release 26 [database on the Internet]. Washington: USDA; 2013 [cited 2013 Nov 25]. Available from: http://www.ars.usda.gov/ba/bhnrc/ndl.
- 17.Haytowitz DB, Pehrsson PR, Holden JM. The National Food and Nutrient Analysis Program: a decade of progress. J Food Compost Anal 2008;21:S94–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roupas P, Keogh J, Noakes M, Margetts C, Taylor P. The role of edible mushrooms in health: evaluation of the evidence. J Funct Foods 2012;4:687–709. [Google Scholar]
- 19.Parrish GK, Beelman RB, Kneebone LR. Relationship between yield and mannitol content during the crop cycle of cultivated mushrooms. HortScience 1976;11:32–3. [Google Scholar]
- 20.Chang ST, Buswell JA. Medicinal mushrooms—a prominent source of nutraceuticals for the 21 st century. Curr Top Nutraceutical Res. 2003;1:257–80. [Google Scholar]
- 21.Chen S, Oh SR, Phung S, Hur G, Ye JJ, Kwok SL, Shrode GE, Belury M, Adams LS, Williams D. Anti-aromatase activity of phytochemicals in white button mushrooms (Agaricus bisporus). Cancer Res 2006;66:12026–34. [DOI] [PubMed] [Google Scholar]
- 22.Koyyalamudi SR, Jeong SC, Cho KY, Pang G. Vitamin B12 is the active corrinoid produced in cultivated white button mushrooms (Agaricus bisporus). J Agric Food Chem 2009;57:6327–33. [DOI] [PubMed] [Google Scholar]
- 23.Calvo MS, Babu US, Garthoff LH, Woods TO, Dreher M, Hill G, Nagaraja S. Vitamin D2 from light-exposed edible mushrooms is safe, bioavailable and effectively supports bone growth in rats. Osteoporos Int 2013;24:197–207. [DOI] [PubMed] [Google Scholar]
- 24.Bailey RL, Dodd KW, Goldman JA, Gahche JJ, Dwyer JT, Moshfegh AJ, Sempos CT, Picciano MF. Estimation of total usual calcium and vitamin D intakes in the United States. J Nutr 2010;140:817–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Spaulding T, Beelman RB. Survey evaluation of selenium and other minerals in Agaricus mushrooms commercially grown in the United States. Mushroom News 2003;51:6–9. [Google Scholar]
- 26.Werner AW, Beelman RB. Growing high-selenium edible and medicinal button mushroom (Agaricus bisporus (J. Lge) Imbach) as ingredients for functional foods or dietary supplements. Int J Med Mushr 2002;4:194–210. [Google Scholar]
- 27.Beckett GJ, Arthur JR. Selenium and endocrine systems. J Endocrinol 2005;184:455–65. [DOI] [PubMed] [Google Scholar]
- 28.Institute of Medicine, Food and Nutrition Board. Dietary Reference Intakes: vitamin C, vitamin E, selenium, and carotenoids. Washington: National Academies Press; 2000. [PubMed]
- 29.Ey J, Schömig E, Taubert D. Dietary sources and antioxidant effects of ergothioneine. J Agric Food Chem 2007;55:6466–74. [DOI] [PubMed] [Google Scholar]
- 30.Dubost NJ, Beelman R, Peterson D, Royse DJ. Identification and quantification of ergothioneine in cultivated mushrooms by liquid chromatography-mass spectroscopy. Int J Med Mushr 2006;8:215–22. [Google Scholar]
- 31.Weigand-Heller AJ, Kris-Etherton PM, Beelman RB. The bioavailability of ergothioneine from mushrooms (Agaricus bisporus) and the acute effects on antioxidant capacity and biomarkers of inflammation. Prev Med 2012;54:S75–8. [DOI] [PubMed] [Google Scholar]
- 32.Gründemann D. The ergothioneine transporter controls and indicates ergothioneine activity—a review. Prev Med 2012;54:S71–4. [DOI] [PubMed] [Google Scholar]
- 33.Kawano H, Otani M, Takeyama K, Kawai Y, Mayumi T, Hama T. Studies on ergothioneine. VI. Distribution and fluctuations of ergothioneine in rats. Chem Pharm Bull (Tokyo) 1982;30:1760–5. [DOI] [PubMed] [Google Scholar]
- 34.Grigat S, Harlfinger S, Pal S, Striebinger R, Golz S, Geerts A, Lazar A, Schömig E, Gründemann D. Probing the substrate specificity of the ergothioneine transporter with methimazole, hercynine, and organic cations. Biochem Pharmacol 2007;74:309–16. [DOI] [PubMed] [Google Scholar]
- 35.Paul BD, Snyder SH. The unusual amino acid L-ergothioneine is a physiologic cytoprotectant. Cell Death Differ 2010;17:1134–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gründemann D, Harlfinger S, Golz S, Geerts A, Lazar A, Berkels R, Jung N, Rubbert A, Schömig E. Discovery of the ergothioneine transporter. Proc Natl Acad Sci USA 2005;102:5256–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ames BN. Low micronutrient intake may accelerate the degenerative diseases of aging through allocation of scarce micronutrients by triage. Proc Natl Acad Sci USA 2006;103:17589–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McCann JC, Ames BN. Adaptive dysfunction of selenoproteins from the perspective of the triage theory: why modest selenium deficiency may increase risk of diseases of aging. FASEB J 2011;25:1793–814. [DOI] [PubMed] [Google Scholar]
- 39.Bennett L, Sheean P, Zabaras D, Head R. Heat-stable components of wood ear mushroom, Auricularia polytricha (higher Basidiomycetes), inhibit in vitro activity of beta secretase (BACE1). Int J Med Mushr 2013;15:233–49. [DOI] [PubMed] [Google Scholar]
- 40.Wang LC, Wang SE, Wang JJ, Tsai TY, Lin CH, Pan TM, Lee CL. In vitro and in vivo comparisons of the effects of the fruiting body and mycelium of Antrodia camphorata against amyloid β-protein-induced neurotoxicity and memory impairment. Appl Microbiol Biotechnol 2012;94:1505–19. [DOI] [PubMed] [Google Scholar]
- 41.Shin A, Kim J, Lim SY, Kim G, Sung MK, Lee ES, Ro J. Dietary mushroom intake and the risk of breast cancer based on hormone receptor status. Nutr Cancer 2010;62:476–83. [DOI] [PubMed] [Google Scholar]
- 42.Cheskin LJ, Davis LM, Lipsky LM, Mitola AH, Lycan T, Mitchell V, Mickle B, Adkins E. Lack of energy compensation over 4 days when white button mushrooms are substituted for beef. Appetite 2008;51:50–7. [DOI] [PubMed] [Google Scholar]
- 43.Poddar KH, Ames M, Hsin-Jen C, Feeney MJ, Wang Y, Cheskin LJ. Positive effect of mushrooms substituted for meat on body weight, body composition, and health parameters: a 1-year randomized clinical trial. Appetite 2013;71:379–87. [DOI] [PubMed] [Google Scholar]
- 44.Zaura E, Buijs MJ, Hoogenkamp MA, Ciric L, Papetti A, Signoretto C, Stauder M, Lingström P, Pratten J, Spratt DA, Wilson M. The effects of fractions from shiitake mushroom on composition and cariogenicity of dental plaque microcosms in an in vitro caries model. J Biomed Biotechnol 2011;2011:135034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Signoretto C, Burlacchini G, Marchi A, Grillenzoni M, Cavalleri G, Ciric L, Lingström P, Pezzati E, Daglia M, Zaura E, et al. Testing a low molecular mass fraction of a mushroom (Lentinus edodes) extract formulated as an oral rinse in a cohort of volunteers. J Biomed Biotechnol 2011;2011:857987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Guarner F. Enteric flora in health and disease. Digestion 2006;73 Suppl 1:5–12. [DOI] [PubMed] [Google Scholar]
- 47.Varshney J, Ooi JH, Jayarao BM, Albert I, Fisher J, Smith RL, Patterson AD, Cantorna MT. White button mushrooms increase microbial diversity and accelerate the resolution of Citrobacter rodentium infection in mice. J Nutr 2013;143:526–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cantorna MT, Hayes CE, DeLuca HF. 1,25-Dihydroxycholecalciferol inhibits the progression of arthritis in murine models of human arthritis. J Nutr 1998;128:68–72. [DOI] [PubMed] [Google Scholar]
- 49.Yu S, Weaver V, Martin K, Cantorna MT. The effects of whole mushrooms during inflammation. BMC Immunol 2009;10:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wu D, Pae M, Ren Z, Guo Z, Smith D, Meydani SN. Dietary supplementation with white button mushroom enhances natural killer cell activity in C57BL/6 mice. J Nutr 2007;137:1472–7. [DOI] [PubMed] [Google Scholar]
- 51.Ren Z, Guo Z, Meydani SN, Wu D. White button mushroom enhances maturation of bone marrow-derived dendritic cells and their antigen presenting function in mice. J Nutr 2008;138:544–50. [DOI] [PubMed] [Google Scholar]
- 52.Wang J, Niu X, Du X, Smith D, Meydani SN, Wu D. Dietary supplementation with white button mushroom augments the protective immune response to Salmonella vaccine in mice. J Nutr 2014;144:98–105. [DOI] [PubMed] [Google Scholar]
- 53.Dai X, Stanilka JM, Rowe CA, Creasy RA, Percival SS. Consumption of Lentinula edodes modulates human immune function by altering cytokine secretion of PBMC ex vivo. FASEB J 2013;27:643.15. [Google Scholar]
- 54.Stanilka JM, Rowe CA, Creasy RA, Dai X, Percival SS. Lentinula edodes consumption: proliferation, activation and modification of memory and naive innate immune cell populations. FASEB J 2013;27:643.17. [Google Scholar]
- 55.Roupas P, Keogh J, Noakes M, Margetts C, Taylor P. Mushrooms and agaritine: a mini-review. J Funct Foods 2010;2:91–8. [Google Scholar]
- 56.Schulzová V, Hajslová J, Peroutka R, Gry J, Andersson HC. Influence of storage and household processing on the agaritine content of the cultivated Agaricus mushroom. Food Addit Contam 2002;19:853–62. [DOI] [PubMed] [Google Scholar]
- 57.Toth B. Carcinogenesis by N2-[gamma-L(+)-glutamyl]-4-carboxyphenylhydrazine of Agaricus bisporus in mice. Anticancer Res 1986;6:917–20. [PubMed] [Google Scholar]
- 58.Toth B, Gannett P. Carcinogenesis study in mice by 3-methylbutanal methylformylhydrazone of Gyromitra esculenta. In Vivo 1990;4:283–8. [PubMed] [Google Scholar]
- 59.Toth B, Nagel D. Studies of the tumorigenic potential of 4-substituted phenylhydrazines by the subcutaneous route. J Toxicol Environ Health 1981;8:1–9. [DOI] [PubMed] [Google Scholar]
- 60.Toth B, Patil K. Carcinogenesis by a single dose of Nmethyl- N-formylhydrazine. J Toxicol Environ Health 1980;6:577–84. [DOI] [PubMed] [Google Scholar]
- 61.Toth B, Erickson J, Gannett P. Lack of carcinogenesis by the baked mushroom Agaricus bisporus in mice: different feeding regimen. In Vivo 1997;11:227–31. [PubMed] [Google Scholar]
- 62.Andersson HC, Gry J. Phenylhydrazines in the cultivated mushroom (Agaricus bisporus)—occurrence, biological properties, risk assessment and recommendations. TemaNord 558. Copenhagen: Nordic Council of Ministers; 2004.
- 63.Persson H. Mushrooms. Medicine 2012;40:135–8. [Google Scholar]
- 64.Schenk-Jaeger KM, Rauber-Lüthy C, Bodmer M, Kupferschmidt H, Kullak-Ublick GA, Ceschi A. Mushroom poisoning: a study on circumstances of exposure and patterns of toxicity. Eur J Intern Med 2012;23:e85–91. [DOI] [PubMed] [Google Scholar]
- 65.Thornsbury S, Wells HF, Bond J. Vegetable and pulses yearbook data. Washington: USDA; 2013 [cited 2013 Nov 25]. Available from: http://www.ers.usda.gov/data-products/vegetables-and-pulses-data/yearbook-tables.aspx#.UnqIzEoo670.
- 66.IRI (Information Resouces, Inc.). Consumer Panel Survey, 2012. Chicago: Information Resources, Inc.; 2014 [cited 2014 Jan 7]. Available from: http://www.iriworldwide.com/SolutionsandServices/Detail.aspx?ProductID=180.
- 67.Ball P, Woodward D, Beard T, Shoobridge A, Ferrier M. Calcium diglutamate improves taste characteristics of lower-salt soup. Eur J Clin Nutr 2002;56:519–23. [DOI] [PubMed] [Google Scholar]
- 68.Woodward DR, Lewis PA, Ball PJ, Beard TC. Calcium glutamate enhances acceptability of reduced-salt sausages. Asia Pac J Clin Nutr 2003;12:S35.15023641 [Google Scholar]
- 69.Choose My Plate. Washington: USDA [cited 2014 Mar 5]. Available from: http://www.choosemyplate.gov.
- 70.USDA; U.S. Department of Health and Human Services. Dietary guidelines for Americans, 2010. 7th ed. Washington: U.S. Government Printing Office; 2010. [DOI] [PMC free article] [PubMed]
- 71.DietaryGuidelines.gov [homepage on the Internet]. Washington: USDA [cited 5 Mar 2014]. Available from: http://www.health.gov/dietaryguidelines.
- 72.Mithril C, Dragsted LO, Meyer C, Tetens I, Biltoft-Jensen A, Astrup A. Dietary composition and nutrient content of the New Nordic Diet. Public Health Nutr 2013;16:777–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Weaver C, Marr ET. White vegetables: a forgotten source of nutrients: Purdue roundtable executive summary. Adv Nutr 2013;4(suppl):318S–26S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.O’Neil CE, Nicklas TA, Fulgoni VL. Mushroom intake is associated with better nutrient intake and diet quality: 2001–2010 National Health and Nutrition Examination Survey. Nutr Food Sci 2013;3:5. [Google Scholar]
- 75.U.S. Food and Drug Administration. Guidance for industry: evidence-based review system for the scientific evaluation of health claims—final. Silver Spring (MD): U.S. Food and Drug Administration [last updated 25 Feb 2014; cited 8 March 2014]. Available from: http://www.fda.gov/Food/GuidanceRegulation/GuidanceDocumentsRegulatoryInformation/LabelingNutrition/ucm073332.htm.