Abstract
Objective
Optogenetics promises exciting neuroscience research by offering optical stimulation of neurons with unprecedented temporal resolution, cell-type specificity and the ability to excite as well as to silence neurons. This work provides the technical solution to deliver light to local neurons and record neural potentials, facilitating local circuit analysis and bridging the gap between optogenetics and neurophysiology research.
Approach
We have designed and obtained the first in vivo validation of a neural probe with monolithically integrated electrodes and waveguide. High spatial precision enables optical excitation of targeted neurons with minimal power and recording of single-units in dense cortical and subcortical regions.
Main results
The total coupling and transmission loss through the dielectric waveguide at 473 nm was 10.5 ± 1.9 dB, corresponding to an average output intensity of 9400 mW mm−2 when coupled to a 7 mW optical fiber. Spontaneous field potentials and spiking activities of multiple Channelrhodopsin-2 expressing neurons were recorded in the hippocampus CA1 region of an anesthetized rat. Blue light stimulation at intensity of 51 mW mm−2 induced robust spiking activities in the physiologically identified local populations.
Significance
This minimally invasive, complete monolithic integration provides unmatched spatial precision and scalability for future optogenetics studies at deep brain regions with high neuronal density.
1. Introduction
In order to advance the understanding of brain function and behavior, it is critical to monitor how neural circuits work together and perform computational processing. Because neural circuits are made of interacting cells of diverse types, selective activation/silencing of single neurons of specific types is required to identify the computational mechanisms of underlying functions and perturb the local circuits of a cortical system in a controlled manner [1]. For example, by activating (depolarizing) specific neurons within a region, it is possible to assess the processes that contribute to a specific behavior. Similarly, silencing (hyperpolarizing) specific neurons within a region can provide information about their roles in both network functions and behavior. This goal cannot be achieved effectively by electrical stimulation since it indiscriminately stimulates neuronal processes, including somata, dendrites and axons in a complex manner [2].
Recent advances in optogenetics provide a new approach to neural circuit analysis [3]. Optogenetics can introduce photo-sensitive proteins called opsins into specific cell types and achieve optical control of defined action potential patterns in specific targeted neuronal populations. Neurons that express these opsins can be selectively stimulated by visible light at an appropriate wavelength and cell-type specificity can be achieved with well-controlled spatial and temporal resolution (order of milliseconds) [4]. For example, Channelrhodopsin-2 (ChR2), when expressed in neurons, reacts rapidly to blue light (~473 nm) with large depolarizing photocurrents to induce light-driven action potentials [4–7]. Halorhodopsins [8, 9] and archaerhodopsin [10, 11], when illuminated with yellow light (~590 nm), mediate hyperpolarization, enabling the silencing of neural activity [10, 12, 13]. Multiple opsins can be expressed in the same cell so that it can be either depolarized or hyperpolarized by the corresponding wavelengths [9]. Likewise, different opsins can be expressed in intermingled neuronal populations, enabling independent temporal control of those populations [14]. This specific targeting allows sophisticated manipulations of neural activity, testing spike timing during specific neural computations and behaviors at the resolution of individual subcircuits within the brain.
Although optogenetics promises exciting new possibilities for neuroscience research, to date there is still an unmet need for reliable implantable tools to precisely deliver light to the targeted neurons and to simultaneously record the electrical signals from the individual neurons. Typically, optical stimulation has been achieved by placing a single light source on the surface of the brain [15] or a thick fiber in the brain parenchyma a few hundred microns away from the recording sites [16–22]. This approach inevitably activates many unmonitored neurons, making the separation between direct and population-mediated effects impossible. The high intensity used to activate deep neurons may generate multiple superimposed spike waveforms [23] and considerable light artifacts [18, 20, 21].
Current state-of-the-art implantable optical probes include the assembly of four tetrodes (25 μm in diameter each) symmetrically attached about the perimeter of a 200 μm diameter optical fiber [17, 22]. Although the components of such systems are readily available, the distance between the tetrodes and the fiber cannot be accurately controlled by manual alignment. In addition, the assembly displaces a relatively large neuronal volume and may cause tissue damage along its insertion path [24], limiting its potential for scaling up toward the large number of sites. In another innovation, a complete multi-site/multi-color optical stimulation and electrical recording system was demonstrated by using diode-coupled optical fibers attached to commercial multi-shank silicon recording probes [25]. The manual attachment of fibers glued to each probe shank is very labor-intensive, resulting in potential alignment inaccuracy and contamination of the recording sites by misplaced glue [25]. Fabrication of waveguide array integrated onto a single silicon shank with dimensions similar to a 200 μm diameter optical fiber was reported [1, 26], but recording electrodes were not integrated onto the probe [26]. Other recent work demonstrated a polymer-based neural probe integrated with SU-8 waveguide, electrodes and a microfluidic channel [27]. Although in vivo experiment was performed, spontaneous neural activities were only recorded in the tip-most electrodes (two out of nine) that were farthest away from the bulky waveguide and microfluidic channel measuring over 200 μm thick and 190 μm wide. In addition, only a single neuron responded indirectly to blue light stimulation with excessively high power (1 to 2 mW) [27]. Another SU-8 design demonstrated the coupling of light from a bare laser diode chip [28]. Although the compact diode assembly presents significant advantage for chronic experiments with moving animals, no in vivo validation was reported, and the probe shanks were still too bulky for applications that require concurrent optical targeting and electrophysiological monitoring of dense neural population regions such as the hippocampus.
In the current application, we have designed, fabricated and tested a monolithically integrated optical waveguide in a multi-electrode array silicon probe. This novel approach provides spatially-confined stimulation (activation and silencing) of simultaneously monitored neurons by enabling local light delivery precisely above the recording sites at specific wavelengths of choice. The lithographically defined probe shank was designed with minimal dimensions to contain all necessary optical and electrical components, in order to minimize insertion-induced tissue damage and foreign body reactions [24], as well as to reduce alignment tolerance between the optical stimulation site and the electrical recording sites. We validated device feasibility by recording data from an anesthetized rat using the simplest possible design: a single optical stimulation source and eight recording channels, all on the same silicon shank. This technology can be easily expanded to complex waveguiding configurations and recording electrode arrays to meet the requirements of specific neurobiological applications [29, 30]. This class of devices will have a significant impact in capturing the full potential of optogenetics technology and accelerate our understanding of the role of specific neurons in behavior and complex circuits of the central nervous system such as neocortex and hippocampus.
2. Results
2.1. Design
To determine whether the integrated device can be used in a densely packed brain region, we chose the hippocampus CA1 pyramidal. We thus designed a probe with eight nearby recording electrodes and a single waveguide, all integrated on a single silicon shank as shown by the schematics in figure 1(a). The overall shank dimensions were designed to minimize tissue damage while maintaining sufficient mechanical strength for insertion and having enough space to contain all optical and electrical components. The shank thickness was defined by the top silicon layer of our starting silicon-on-insulator (SOI) wafer, which can be commercially made with 15 μm in thickness and an accuracy of ± 0.5 μm. Combined with a lithographically defined sharp tip, the thin probe can easily penetrate the pia mater with minimal tissue damage along its insertion path. For this particular probe design, the shank width was 70 μm, the minimum dimension to carry eight interconnection lines and a waveguide given the fabrication process constraints. The interconnection lines carried electrical signals from the eight recording electrodes to the bonding pads which were wirebonded to a custom-made printed circuit board (PCB). The waveguide delivered light from an aligned optical fiber to the stimulation site. We created a groove in the silicon body for butt-coupling between an optical fiber and the integrated waveguide by the self-alignment between them. The groove was designed to perfectly fit a 125 μm diameter optical fiber, having the fiber core (50 μm in diameter) in an aligned position with the integrated waveguide as shown in figure 1(b). The optical fiber provided a convenient coupling intermediate between an external diode-pumped solid-state (DPSS) laser source and the waveguide.
Figure 1.
Design of single shank, monolithically integrated probe: (a) 3D schematics of overall probe design. (b) Coupling junction between the optical fiber and the integrated waveguide. (c) A–A′ cross-section showing the waveguide with oxynitride core (purple) and oxide cladding (blue). (d) Simulation results of light intensity distribution as light propagates through the brain tissue.
The optical waveguide was composed of a 5 μm thick oxynitride core (index of refraction: 1.51) surrounded on each side by a 3 μm thick oxide cladding (index of refraction: 1.46). The waveguide width tapers from 28 μm at the end proximal to optical fiber, to 14 μm at the distal end. The larger cross-sectional area at the proximal end allowed higher coupling efficiency to the optical fiber core while the smaller cross-sectional area at the distal end (figure 1(c)) allowed spatial confinement of light exiting from the waveguide and minimized tissue damage. Assuming tissue isotropy and light scattering, we estimated the distribution of light coming out of the waveguide in brain tissue [25, 31]. Given a horizontal pitch of 40 μm between the top two recording sites and a light output of 0.5 mW at the waveguide tip, positioning the tip of the waveguide 50 μm above the top recording site placed all recording sites within a light cone with intensity above 7 mW mm−2 (figure 1(d)), which was sufficient to activate/silence single-units in vivo [25].
Monolithic integration of both electrical and optical components of the neural probe allowed precise definition of the optical stimulation site and the recording electrodes in terms of position, size and alignment in a high density array. For example, to enable single-unit recordings in structures with dense cell body layer, such as neocortex and hippocampus, recording sites span 140 μm in depth with each electrode separated by a pitch of 20 μm [32]. Sizing the electrode area has a tradeoff between low impedance, high probability of coming into proximity of an active neuron (large recording area) and the ability to distinguish single-unit activity (small recording area) [33]. We have designed each electrode with an area of 143 μm2 to achieve low impedance and to record from dense populations of individual neurons (soma diameter 10–20 μm) in the neocortex and hippocampus. The accurate alignment between the waveguide and electrodes ensures precise delivery of light to the neurons monitored by the electrodes with minimal power. The spatially-confined, low power stimulation generates less heat and minimizes electromagnetic interference to the recording channels, as well as unintended excitation of nearby neurons.
2.2. Fabrication
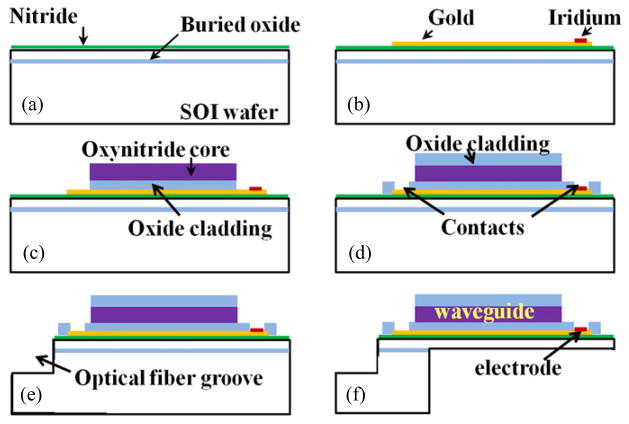
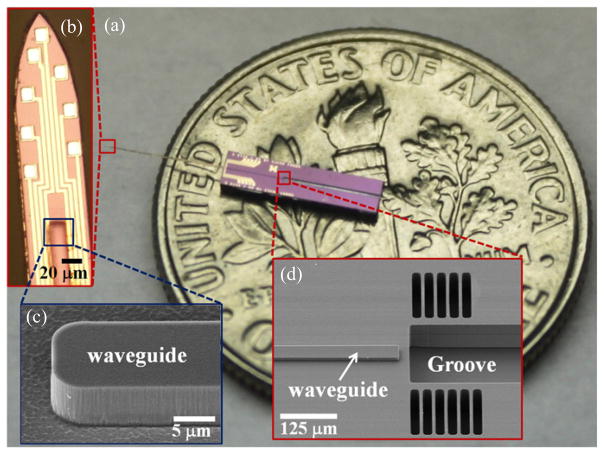
Figure 2 shows the probe fabrication steps (detailed process is described in section 4). Briefly, our probes were fabricated with SOI wafers for precise control of the shank thickness, which was defined by the top silicon layer (15 μm in our probes). We began by depositing a nitride layer for the electrical insulation from the silicon substrate. This nitride layer in tensile stress compensated compressive stresses of much thicker oxide cladding layers deposited in later processes. Electrical interconnections and recording sites were patterned by lift-off, after evaporation of gold and sputtering of iridium respectively. The waveguide was composed of a 5 μm thick oxynitride core layer patterned by plasma etching, and 3 μm thick oxide cladding layers deposited to surround each side of the core. Deep reactive ion etching (DRIE) of the fiber groove was performed after waveguide patterning steps. Finally, the probes were released by a double-sided DRIE process with the final structure shown sitting on top of a US quarter in figure 3(a). The recording electrodes and the waveguide are shown in the microscope image in figure 3(b). SEM images of the waveguide at the distal and proximal ends (fiber coupling junction) are shown in figures 3(c) and (d), respectively.
Figure 2.
Outline of fabrication steps with cross-sections along the long axis of the probe. (a) Deposition of bottom insulation layer on SOI wafer. (b) Patterning of electrical interconnections and electrodes. (c) Defining waveguide bottom cladding and core layers. (d) Deposition of top cladding layer and formation of electrical contacts. (e) Silicon DRIE for the optical fiber groove. (f) Final release of the complete probe.
Figure 3.
Images of the released probe. (a) Relative size in contrast with a US quarter. (b) Microscope image of probe tip showing the lithographically defined electrode array and the waveguide. (c) SEM image of the waveguide magnified at the distal end. (d) SEM image of the waveguide at the proximal end and the optical fiber groove.
2.3. Device performance characterization
The released probes were wirebonded to a custom-made PCB which has an Omnetics connector (A79038, Omnetics Connector Corporation, Minneapolis, MN) for electrical interface with an external amplifier. Impedances of recording sites were analyzed in saline solution with an impedance analyzer (HP 4194A Impedance/Gain-Phase Analyzer, Test Equipment Depot, Melrose, MA). The average impedance of the recording sites was 1.37 MΩ read at 1 kHz, which is sufficiently low to record neural signals with high signal-to-noise ratio [34].
We coupled a multi-mode optical fiber (GIF50, Thorlabs, Newton, NJ) to the integrated waveguide by direct (butt-) coupling. The entire optical assembly is illustrated in figure 4 showing light transmitted from the 473 nm DPSS laser (Dream-Lasers), advancing through the optical fiber, and exiting at the distal end of the integrated waveguide. The overall transmission loss from the fiber to the stimulation site was 10.5 ± 1.9 dB (mean, SD; n = 3) for blue light. The optical loss predominately occurred at the coupling junction due to the cross-sectional area mismatch between the multimode fiber (core diameter = 50 μm) and the waveguide (area = 140 μm2 proximal to the fiber). Nonetheless, coupling to a multi-mode fiber with 7 mW blue light output produced a total power of 660 μW at the end of the waveguide. Given the small aperture size of our waveguide (70 μm2) at the distal end, this corresponded to an optical intensity of more than 9400 mW mm−2. It is critical to obtain a much higher intensity at the waveguide than the minimal intensity (1 mW mm−2) reported for ChR2 excitation [25] considering the optical attenuation when light propagates through the neural tissue as shown in figure 1(d). In general, we observed an increase in transmission efficiency through the oxynitride waveguide as wavelength increased: roughly doubled efficiency when switching from 473to 593 nm laser source and tripled efficiency upon switching from 405 nm (Sony KES-410ACA) to 639 nm laser diode (Opnext HL6359MG).
Figure 4.
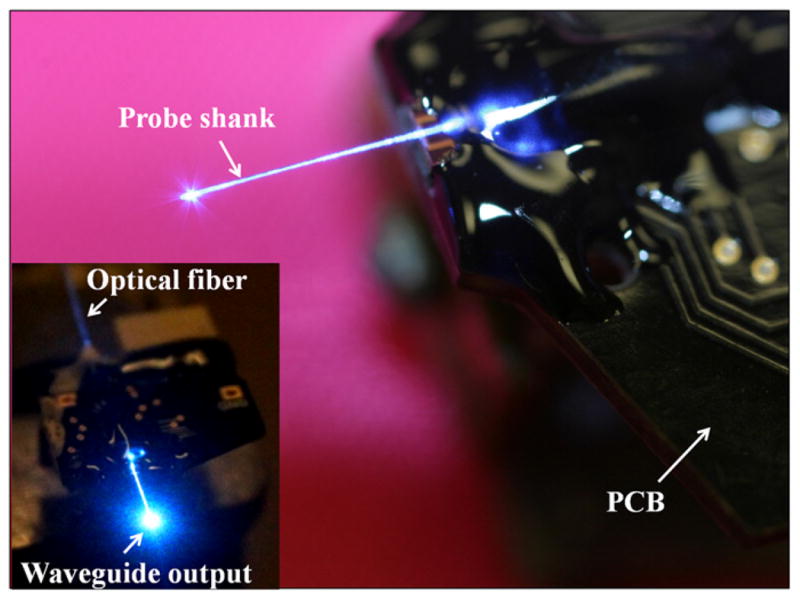
Fully packaged system with silicon probe bonded to the PCB and optical fiber aligned to the integrated waveguide. Light has been successfully guided from the laser source to the stimulation site.
2.4. In vivo experiments
We implanted the eight-site single-shank oxynitride waveguide probe into the neocortex and hippocampus of a wild-type rat expressing ChR2 under the CAG promoter. In the CA1 pyramidal layer, a brain region in which cells are tightly packed, the electrodes recorded spontaneous local field potentials and spiking activities of multiple single-units (figure 5(a)), indicating that the electrode configuration was adequate for recording from multiple singe cells and that the additional volume of the waveguide did not compromise the quality of the recordings. We then used a 473 nm DPSS laser (Dream-Lasers) with a sinusoidal light output and adjusted the light output to 120 μW at the probe tip, corresponding to an intensity of ~51 mW mm−2 at the center of the electrode array given the attenuation of optical intensity through neural tissue. This resulted in robust spiking of multiple single-units; notably, different units exhibited distinct firing patterns during the light stimulus (figure 5(b)). For instance, the ‘red’ unit occasionally fired one or two spikes during a given stimulus cycle. In contrast, the unit depicted in pink emitted multiple spikes per stimulus cycle.
Figure 5.
In vivo recording from the CA1 pyramidal layer of a Long-Evans rat across eight recording channels (sites 1 and 8 correspond to the deepest and shallowest recording sites respectively). (a) Spontaneous local field potential and spiking activity. Each spiking event is marked and color-coded to represent a distinguishable single-unit. (b) Spiking activity recorded during a 25 Hz sinusoidal optical stimulation pattern. Two distinct single-units (pink and red) spiked robustly following the optical stimulation cycles. (c) Optical stimulation with square pulse waveforms (50 ms, 40 μW max power) showing distinct temporal relations between the light stimulus and spiking of the two units.
To quantify the temporal relations between light stimulation and single-unit spiking as well as the influence on extracellular spike waveform, we also used square light pulses at weaker intensities (40 μW, corresponding to 17 mW mm−2 at the shank center). The first (red) unit described above tended to spike following most light pulses (71%) at short latencies (median latency, 4 ms), suggesting a direct light effect (figure 5(c), top). In contrast, the second (pink) fired only following some light pulses (31%) and at longer latencies (center of mass, 41 ms), suggesting a network-driven effect (figure 5(c), bottom). For both cells, the spontaneous and light-induced waveforms were indistinguishable (correlation coefficient >0.99). Thus, the monolithic silicon waveguide/recording platform enables monitoring and control of multiple single-units in dense brain structures in vivo.
3. Discussion
It has been seven years since the publication that demonstrated the first neuron firing a precise action potential in response to blue light stimulation [4]. Although many investigators have realized the unparalleled advantages that optical stimulation of neurons had offered as compared to the conventional electric stimulation, only few laboratories had the capability to couple optogenetics with extracellular recordings for in vivo neuroscience research due to the lack of reliable optical stimulation probes [17–25].
In this study, we have designed a neural probe with monolithically integrated optical waveguide and recording electrodes. The key innovations of this work are the precise geometric definition of all electrical and optical components as well as the overall miniaturization of probe dimensions for biocompatibility and scalability. The recording electrodes and the optical stimulation sites can be custom-designed into any configuration to target a specific application. Here we described a probe with eight recording electrodes and one waveguide integrated on a single silicon shank. The electrode array has been carefully designed in terms of recording site area, pitch and total recording span, in order to maximize the probability to record from single-unit potential given the anatomy of the hippocampus CA1 region [32]. Because the stimulation site was positioned very close to the recording sites (60 μm from the closest), only a small amount of light (<20 mW mm−2) was required to illuminate all the neurons being monitored by the electrode array, thus preventing spike superposition and local field potential (LFP) offset which can be the result of strong photo-induced currents [23]. Yet the current design also enables emitting higher light intensities, on the order that may be required to target large neuronal populations and influence certain behaviors [15, 31]. The shank dimensions were also reduced to the bare minimum to avoid displacing large volume of tissue during implantation. This is critical especially for scaling toward simultaneous stimulation and recording from large number of neurons with multiple probe shanks without serious insertion damage or increased foreign body reactions [24].
One of the biggest challenges of this technology was to obtain high optical transmission efficiency from the optical fiber to the stimulation site via the integrated waveguide. The dielectric waveguide was more appropriate than SU-8 waveguides [27–30, 35] for applications that involve the stimulation of ChR2 expressed neurons since absorption loss of SU-8 is very high near 473 nm [27, 36]. In addition, SU-8 absorption of water is well known and could significantly affect long term optical property of the waveguide [37]. The two drawbacks of the dielectric waveguide were the surface roughness created during plasma etching of the waveguide sidewall and the limitation of waveguide thickness constrained by the fabrication equipments (up to 5 μm) such as deposition and etching tools. The surface roughness can lead to propagation loss through the waveguide surface but has been alleviated by optimizing the lithography and plasma etching conditions to create smooth sidewalls [38] as shown in figure 3(c). Because it was difficult to fabricate thick dielectric layers due to stress and extended plasma etching time, a thin waveguide limited our ability to match mode-shape between the large multi-mode optical fiber core and the smaller waveguide. This resulted in relatively large coupling loss at the fiber–waveguide junction. To reduce coupling loss, we doubled the width of the waveguide at the fiber junction from 14 to 28 μm, which allowed the waveguide to overlap 7.13% of the total cross-sectional area of the 50 μm diameter optical fiber core. In previous report [26], very low coupling loss (0.4 ± 0.3 dB) was achieved due to their fabrication capability of depositing very thick (9 μm) waveguide core, which matched closely with the cross-sectional area of their single-mode optical fiber with 8 μm in diameter. In our experience, we have been able to achieve higher coupling efficiency between single-mode fiber (3 μm core diameter; 460 HP, Thorlabs, Newton, NJ) and our oxynitride waveguide. However, the single-mode fiber suffers more than 60% loss in comparison to the multi-mode fiber when coupled to our laser source. Therefore, to obtain highest output power at the end of the waveguide, it was more appropriate to use multi-mode fiber despite larger coupling loss to the waveguide. Nonetheless, the total loss through our entire assembly has been measured to be 10.5 ± 1.9 dB, which is comparable to recent report using SU-8 (>12.4 dB) with significantly larger cross-sectional area (150 μm × 150 μm) [27]. Besides reducing insertion damage, another advantage of the small waveguide aperture is the confinement of high optical intensity (9400 mW mm−2) into a small region (14 μm × 5 μm) with minimal input power (7 mW from optical fiber). This not only provides stimulation with high spatial resolution but also minimizes electromagnetic interference from the optical stimulation site to the recording channels.
When coupled to the yellow (593 nm) laser, the overall transmission loss was roughly 7.45 dB, which included coupling and propagation losses through the waveguide. The overall efficiency (18%) was higher than the cross-sectional area ratio after we applied index-matching epoxy at the fiber/waveguide junction, which helped convergence of skew rays toward the waveguide and reduction of reflective loss at the waveguide interface. Previous studies have observed optimized coupling efficiencies at around twice the ratio between the fiber and the waveguide cross-sectional areas [25]. Our lithographically defined waveguide and fiber groove have allowed precise alignment to obtain coupling efficiency close to the theoretical value (more than twice the fiber-to-waveguide cross-sectional area ratio), given the current waveguide design. Because the coupling loss dominated the total transmission loss at 593 nm (total measured transmission efficiency was close to the maximum theoretical coupling efficiency), we can deduce that the propagation loss through the oxynitride waveguide at 593 nm was negligible. However, we have observed a dramatic increase in propagation loss as we coupled to light sources with shorter wavelengths. Our measurements have indicated that the efficiency of yellow (593 nm) light was roughly two times that of the blue (473 nm) light and at least three times that of the violet (405 nm) light. For applications involving excitation of ChR2 expressed neurons, it is sometimes advantageous to use violet (405 nm) rather than blue (473 nm) light source because of lower source cost, smaller spatial spread, and greater spectral separation from the longer wavelengths that could be used to activate other types of opsins [3]. From our preliminary analysis, we estimated that the transmission efficiency of violet light by the dielectric waveguide is roughly 67% of the blue light. In addition, the sensitivity of ChR2 to violet versus blue is approximately 60% [39]. The maximum output power of the probe we used for the in vivo experiment was 1.1 mW when connected to a 17 mW blue laser source. Therefore, since the effective stimulation power of the violet light was around 40% of the blue light, it would still be more than enough to excite ChR2 as we have shown robust photo-induced spiking in response to 40 μW stimulation power.
Our acute in vivo experiment demonstrated the feasibility of the monolithically integrated optical stimulation probe by recording single-unit activity in response to a variety of optical stimulation patterns in the rat hippocampus CA1 region. However, concurrent LFP analysis (in addition to spike detection) was not possible during optical stimulation because it was necessary to filter out the low-frequency light artifact, which also removes other low-frequency features of the LFP. We believe the light artifact was mainly caused by a large amount of light (>80%) leaking at the fiber–waveguide junction which was only 1 mm away from the nearest wire bond (rather than photons hitting the recording sites). In a simple modification to the current design, the bonding pads would be moved farther away from the fiber–waveguide junction, allowing sufficient amount of opaque epoxy to be applied on the pads, thus blocking the electrical signal lines from the leaked light.
In the future, we will scale up the number of recording channels and optical stimulation sites without compromising the intrinsic advantages of the monolithic integration technology. We also have the flexibility to design alternative waveguiding structures with the same fabrication process for other applications, such as implementing an optical mixer configuration to deliver different wavelengths from multiple light sources to the same stimulation site [29]. Recent work [25] demonstrated the advantages of directly integrating multiple light sources, such as light emitting diodes (LED) or laser diodes (LD), on the animal head stage to enable free animal movement while minimizing mechanical damage to the implanted probe by removing tethered optical fibers altogether. Combining this technology with our monolithically integrated probe design will be the next step toward applications involving chronic large-scale extra-cellular recordings, optical stimulation, and animal behavior. Although challenging, as there will be two optical coupling junctions (LED or LD to fiber and fiber to waveguide), the high transmission from fiber to waveguide obtained in this work (~ 9%) is likely to suffice given the previously reported 5% transmission efficiency from LD to fiber [25]. At the minimum and without any modifications to the present system, commercially-available 10 mW LD will deliver ~45 μW at the tip of the waveguide, similar to the power used to activate single-units in the current study (figure 5(c)). Therefore, we believe that our monolithically integrated optical probe, having precisely defined geometries through lithography, high optical transmission efficiency obtained by enhanced fabrication/packaging technologies and the flexibility for alternative configurations as well as scaling, will be able to provide the key component bringing optogenetics to its full in vivo potential.
4. Materials and methods
4.1. Fabrication
Fabrication of the optical stimulation probe started with P-type, 0.001 Ω cm, SOI wafers with 15 μm thick top silicon layer that defines the shank thickness. We first deposited 0.6 μm of nitride by low pressure chemical vapor deposition. This nitride layer was designed to insulate the active electrical components from the low resistive silicon shank. In addition to insulation, the nitride layer, which has high tensile stress of ~900 MPa, compensated the compressive stresses from the thick oxide layers (~−70 MPa) that will be deposited later for the waveguide claddings. For straight probe shanks, stress compensation was carefully controlled by monitoring stress conditions of films from all deposition tools; because curved probes can be broken during implantation or become misguided from the intended insertion path.
Next, we evaporated Cr/Au/Cr (50 nm/200 nm/100 nm) composite layers and patterned these metal layers by lift-off to form the interconnection lines that carry the electrical signals from the recording sites to the bonding pads. The Cr layers were necessary for good adhesion between Au and other materials. We used Au to form the interconnection lines instead of doped poly-silicon as used in the traditional Michigan probe process [29, 30] because it simplified the process by reducing one masking layer and lowered the sheet resistance from 10 to 0.1–0.2 Ω/□ (data not shown). Low impedance recording electrodes were then sputtered and patterned by lift-off of Ti/Ir/Cr (50 nm/150 nm/100 nm). In order to protect the interconnection lines from the plasma later at the contact etching step, a 0.5 μm oxide layer was deposited by plasma enhanced chemical vapor deposition (PECVD) and a Cr layer (100 nm) was evaporated. This Cr layer served as an etch stop against C4F8 plasma during the contact etch step; and the oxide layer served as an etch stop against Cr etchant when removing the Cr etch stop layer.
We then deposited 3 μm oxide and 5 μm oxynitride by PECVD for bottom cladding and waveguide core layers, respectively. Both layers were then etched together by C4F8 plasma, forming a rectangular waveguide structure. Additional 3 μm oxide layer was then deposited by PECVD to conformally cover all the surfaces of the waveguide core, completing the waveguide fabrication. The top cladding layer also covered the entire shank region, insulating the interconnection lines from the top side. We used another mask to etch away the top oxide layer in the field (outside of the shank) as well as the contact regions to expose both the bonding pads and the recording sites.
The optical fiber groove was created by a triple etch process which etched the 15 μm top silicon, 2 μm buried oxide and additional 39.5 μm of the bottom silicon of the SOI wafer. Considering the combined thicknesses of all deposited layers, a 125 μm diameter optical fiber should be vertically aligned with the fabricated waveguide when sitting at the groove bottom. In terms of the horizontal direction, the etched groove was made with a width of 128 μm enabling a tight fit for a 125 μm diameter fiber with center-to-center alignment with the waveguide. Then, front side DRIE was followed to etch away the top silicon layer around the perimeter of the probe shank; this etch step was accurately and automatically stopped by the buried oxide. Finally, backside DRIE was performed to completely etch away the bottom silicon layer underneath the shanks and the buried oxide was removed, releasing the probe shanks in the form of cantilevers.
4.2. Optical fiber alignment with the waveguide
The optical fiber was held with a micromanipulator above the DRIE-defined groove and a power meter was positioned near the waveguide tip. We applied opaque epoxy (EPO-TEK OG147, Epoxy Technology, Inc., Billerica, MA) to block the scattered light from the fiber–waveguide coupling junction in order to only measure the optical power that was transmitted through the waveguide. The laser source was turned on and a straight-cleaved multi-mode optical fiber (GIF50, Thorlabs, Newton, NJ) was lowered into the groove with a micromanipulator (ULTRAlign Precision Fiber Optic Alignment Stages, 561/562 Series, Newport) for precise alignment. The fiber was aligned with the waveguide when the power meter read the maximum value. After alignment, a small amount of index-matching epoxy (NOA-61, Norland 260 Products) was applied to fill the gap between the optical fiber and the waveguide. This typically increased the output power by two folds as the epoxy increased the acceptance angle of the waveguide to skew rays and reduced reflective losses between interfaces of different refractive indices [25]. When the epoxy was cured, it mechanically fixed the aligned fiber in the aligned position. Finally, opaque epoxy was applied to cover the entire probe backend in order to shield the electrical signal lines from any scattered light as well as to provide additional mechanical strength to hold the aligned fiber in place.
4.3. Acute in vivo recording
A Long-Evans rat (300 g) was injected with CAG-ChR2-GFP (produced by the University of North Carolina viral core facility and packaged in AAV5; courtesy of Dr. Ed Boyden) at nine sites in the right CA1, 35 nl per site (1012 IU ml−1): PA 3.3 ± 0.5; ML 3.0 ± 0.5; DV 2.2 ± 0.2 as described [25]. 12 months later, the animal was anesthetized using 1% isoflurane and placed in a stereotactic apparatus (Kopf). Anesthesia, monitored by breathing rate, rectal temperature, and response to paw pinching, was kept at the minimal level possible. Ground and reference screws were inserted into the occipital bone, a 1 mm craniotomy was made above the central injection site, the dura mater was excised, and the probe was lowered into place using a stereotactic arm (Kopf). Starting at a depth of 600 μm, neuronal activity was recorded (filtered 1–5000 Hz and amplified 1000 ×; Plexon headstage and DataMax system, RC electronics) at 100 μm intervals and spiking activity was tested for optical responses. The pyramidal cell layer of the CA1 region was identified physiologically by the sudden appearance of multiple large amplitude bursting single-units a few hundred micron after cessation of cortical activity. Optical stimuli were given by a 50 mW, 473 nm DPSS laser (Dream-Lasers, China) driven by custom software written in MATLAB via the analog output of a PCI-6229 card (National Instruments). The laser output was collimated (PAF-X11, Thorlabs), coupled into a 5 m jacketed single-mode fiber (460HP, Thorlabs), and connected to the multi-mode fiber previously aligned and fixed to the probe via an LC connector. Just before insertion, the maximal light power at the end of the fiber was measured at 17 mW; the power at the probe tip was 1.1 mW corresponding to ~15700 mW mm−2. For the recording experiment, we adjusted the laser power so that only 120 μW was provided at the tip of the waveguide. This stimulation power roughly corresponded to 1700 mW mm−2 and 51 mW mm−2 at the tip of the waveguide and at the center of the electrode array (considering attenuation through tissue), respectively.
Acknowledgments
This work has been funded in part by NSF with the Graduate Research Fellowship awarded to Fan Wu and NSF-ECCS-1102067, and in part by KIST Institutional Program. Fabrication of the probes has been done at the Lurie Nanofabrication Facility at the University of Michigan.
References
- 1.Bernstein JG, Boyden ES. Optogenetic tools for analyzing the neural circuits of behavior. Trends Cogn Sci. 2011;15:592–600. doi: 10.1016/j.tics.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Butovas S, Schwarz C. Spatiotemporal effects of microstimulation in rat neocortex: a parametric study using multielectrode recordings. J Neurophysiol. 2003;90:3024–39. doi: 10.1152/jn.00245.2003. [DOI] [PubMed] [Google Scholar]
- 3.Yizhar O, Fenno EL, Davidson JT, Mogri M, Deisseroth K. Optogenetics in neural systems. Neuron. 2011;71:9–34. doi: 10.1016/j.neuron.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 4.Boyden ES, Zhang F, Bamberg B, Nagel G, Deisseroth K. Millisecond-timescale, genetically targeted optical control of neural activity. Nature Neurosci. 2005;8:1263–8. doi: 10.1038/nn1525. [DOI] [PubMed] [Google Scholar]
- 5.Nagel G, Brauner M, Liewald JF, Adeishvili N, Bamberg E, Gottschalk A. Light activation of Channelrhodopsin-2 in excitable cells of Caenorhabditis elegans triggers rapid behavioral responses. Curr Biol. 2005;15:2279–84. doi: 10.1016/j.cub.2005.11.032. [DOI] [PubMed] [Google Scholar]
- 6.Li X, Gutierrez DV, Hanson MG, Han J, Mark MD, Chiel H, Hegemann P, Landmesser LT, Herlitze S. Fast noninvasive activation and inhibition of neural and network activity by vertebrate rhodopsin and green algae Channelrhodopsin. Proc Natl Acad Sci. 2005;102:17816–21. doi: 10.1073/pnas.0509030102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ishizuka T, Kakuda M, Araki R, Yawo H. Kinetic evaluation of photosensitivity in genetically engineered neurons expressing green algae light-gated channels. Neurosci Res. 2006;54:85–94. doi: 10.1016/j.neures.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 8.Zhang F, et al. Multimodal fast optical interrogation of neural circuitry. Nature. 2007;446:633–9. doi: 10.1038/nature05744. [DOI] [PubMed] [Google Scholar]
- 9.Gradinaru V, Zhang F, Ramakrishnan C, Mattis J, Prakash R, Diester I, Goshen I, Thompson KR, Deisseroth K. Molecular and cellular approaches for diversifying and extending optogenetics. Cell. 2010;141:154–65. doi: 10.1016/j.cell.2010.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chow BY, et al. High-performance genetically targetable optical neural silencing by light-driven proton pumps. Nature. 2010;463:98–102. doi: 10.1038/nature08652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Han X, et al. A high-light sensitivity optical neural silencer: development and application to optogenetic control of non-human primate cortex. Front Syst Neurosci. 2011;5:1–8. doi: 10.3389/fnsys.2011.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang F, Prigge M, Beyriere F, Tsunoda SP, Mattis J, Yizhar O, Hegemann P, Deisseroth K. Red-shifted optogenetic excitation: a tool for fast neural control derived from. Volvox carteri Nature Neurosci. 2008;11:631–3. doi: 10.1038/nn.2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Royer S, Zemelman BV, Losonczy A, Kim J, Chance F, Magee JC, Buzsáki G. Control of timing, rate and bursts of hippocampal place cells by dendritic and somatic inhibition. Nature Neurosci. 2012;15:769–75. doi: 10.1038/nn.3077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sohal VS, Zhang F, Yizhar O, Deisseroth K. Parvalbumin neurons and gamma rhythms enhance cortical circuit performance. Nature. 2009;459:698–702. doi: 10.1038/nature07991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huber D, Petreanu L, Ghitani N, Ranade S, Hromadka T, Mainen Z, Svoboda K. Sparse optical microstimulation in barrel cortex drives learned behaviour in freely moving mice. Nature. 2008;451:61–64. doi: 10.1038/nature06445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Airan RD, Thompson KR, Fenno LE, Bernstein H, Deisseroth K. Temporally precise in vivo control of intracellular signaling. Nature. 2009;458:1025–9. doi: 10.1038/nature07926. [DOI] [PubMed] [Google Scholar]
- 17.Anikeeva P, Andalman AS, Witten I, Warden M, Goshen I, Grosenick L, Gunaydin LA, Frank LM, Deisseroth K. Optetrode: a multichannel readout for optogenetic control in freely moving mice. Nature Neurosci. 2012;15:163–70. doi: 10.1038/nn.2992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cardin JA, Carlen M, Meletis K, Knoblich U, Zhang F, Deisseroth K, Tsai L-H, Moore CI. Targeted optogenetic stimulation and recording of neurons in vivo using cell-type-specific expression of Channelrhodopsin-2. Nature Protoc. 2010;5:247–54. doi: 10.1038/nprot.2009.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Halassa MM, Siegle JH, Ritt JT, Ting JT, Feng G, Moore CI. Selective optical drive of thalamic reticular nucleus generates thalamic bursts and cortical spindles. Nature Neurosci. 2011;14:1118–20. doi: 10.1038/nn.2880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Han X, Qian X, Bernstein JG, Zhou H-H, Franzesi GT, Stern P, Bronson RT, Graybiel AM, Desimone R, Boyden ES. Millisecond-timescale optical control of neural dynamics in the nonhuman primate brain. Neuron. 2009;62:191–8. doi: 10.1016/j.neuron.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kravitz AV, Freeze BS, Parker PRL, Kay K, Thwin MT, Deisseroth K, Kreitzer AC. Regulation of parkinsonian motor behaviours by optogenetic control of basal ganglia circuitry. Nature. 2010;466:622–6. doi: 10.1038/nature09159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.English DF, Ibanez-Sandoval O, Stark E, Tecuapetia F, Buzsáki G, Deisseroth K, Tepper JM, Koos T. GABAergic circuits mediate the reinforcement-related signals of striatal cholinergic interneurons. Nature Neurosci. 2011;15:123–30. doi: 10.1038/nn.2984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Royer S, Zemelman BV, Barbic M, Losonczy A, Buzsáki G, Magee JC. Multi-array silicon probes with integrated optical fibers: light-assisted perturbation and recording of local neural circuits in the behaving animal. Eur J Neurosci. 2010;31:2279–91. doi: 10.1111/j.1460-9568.2010.07250.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Polikov VS, Tresco PA, Reichert WM. Response of brain tissue to chronically implanted neural electrodes. J Neurosci Methods. 2005;148:1–18. doi: 10.1016/j.jneumeth.2005.08.015. [DOI] [PubMed] [Google Scholar]
- 25.Stark E, Koos E, Buzsáki G. Diode-probes for spatiotemporal optical control of multiple neurons in freely-moving animals. J Neurophysiol. 2012;108:349–63. doi: 10.1152/jn.00153.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zorzos AN, Boyden ES, Fonstad CG. Multiwaveguide implantable probe for light delivery to sets of distributed brain targets. Opt Lett. 2010;35:4133–5. doi: 10.1364/OL.35.004133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rubehn B, Wolff SBE, Tovote P, Schuettler M, Luthi A, Stieglitz T. Polymer-based shaft microelectrodes with optical and fluidic capabilities as a tool for optogenetics. Conf Proc IEEE Engineering in Medicine and Biology Society. 2011:2969–72. doi: 10.1109/IEMBS.2011.6090815. [DOI] [PubMed] [Google Scholar]
- 28.Schwaerzle M, Seidl K, Schwarz UT, Paul O, Ruther P. Ultracompact optrode with integrated laser diode chips and SU-8 waveguides for optogenetic applications. Conf Proc IEEE MEMS. 2013:1029–32. [Google Scholar]
- 29.Im M, Cho I-J, Wu F, Wise KD, Yoon E. Neural probes integrated with optical mixer/splitter waveguides and multiple stimulation sites. Conf Proc IEEE MEMS. 2011:1051–4. [Google Scholar]
- 30.Im M, Cho I-J, Wu F, Wise KD, Yoon E. Dual-shank neural probe integrated with double waveguides on each shank for optogenetic applications. Conf Proc IEEE Engineering in Medicine and Biology Society. 2011:5480–83. doi: 10.1109/IEMBS.2011.6091398. [DOI] [PubMed] [Google Scholar]
- 31.Aravanis AM, Wang LP, Zhang F, Meltzer LA, Mogri MZ, Schneider MB, Deisseroth K. An optical neural interface: in vivo control of rodent motor cortex with integrated fiberoptic and optogenetic technology. J Neural Eng. 2007;4:143–56. doi: 10.1088/1741-2560/4/3/S02. [DOI] [PubMed] [Google Scholar]
- 32.Csicsvari J, Henze DA, Jamieson B, Harris KD, Sirota A, Barthó P, Wise KD, Buzsáki G. Massively parallel recording of unit and local field potentials with silicon-based electrodes. J Neurophysiol. 2003;90:1314–23. doi: 10.1152/jn.00116.2003. [DOI] [PubMed] [Google Scholar]
- 33.Kindlundh M, Norlin P, Hofmann UG. A neural probe process enabling variable electrode configurations. Sensors Actuators B. 2004;102:51–58. [Google Scholar]
- 34.Marriam ME. PhD Thesis. University of Michigan; Ann Arbor, MI, USA: 2010. A three-dimensional bidirectional interface for neural mapping studies. [Google Scholar]
- 35.Cho I-J, Baac HW, Yoon E. A 16-site neural probe integrated with a waveguide for optical stimulation. Conf Proc IEEE MEMS. 2010:1051–4. [Google Scholar]
- 36.Eldada L, Shacklette LW. Advances in polymer integrated optics. Sel Top Quantum Electron. 2000;6:54–68. [Google Scholar]
- 37.Liu C, Liu Y, Sokuler M, Fell D, Keller S, Boisen A, Butt H-J, Auernhammer GK, Bonaccurso E. Diffusion of water into SU-8 microcantilevers. Phys Chem Chem Phys. 2010;12:10577–83. doi: 10.1039/c002478c. [DOI] [PubMed] [Google Scholar]
- 38.Fadel M, Bulters M, Niemand M, Voges E, Krummrich PM. Low-loss and low-birefringence high-contrast silicon-oxynitride waveguides for optical communication. J Lightwave Technol. 2009;27:698–705. [Google Scholar]
- 39.Nagel G, Szellas T, Huhn W, Kateriya S, Adeishvili N, Berthold P, Ollig D, Hegemann P, Bamberg E. Channelrhodopsin-2, a directly light-gated cation-selective membrane channel. Proc Natl Acad Sci. 2003;100:13940–5. doi: 10.1073/pnas.1936192100. [DOI] [PMC free article] [PubMed] [Google Scholar]