Abstract
Meiotic recombination in budding yeast requires two RecA-related proteins, Rad51 and Dmc1, both of which form filaments on DNA capable of directing homology search and catalyzing formation of homologous joint molecules (JMs) and strand exchange. Using a separation-of-function mutant form of Rad51, that retains filament-forming but not JM forming activity, we show that the JM activity of Rad51 is fully dispensable for meiotic recombination. The corresponding mutation in Dmc1 causes a profound recombination defect, demonstrating Dmc1’s JM activity alone is responsible for meiotic recombination. We further provide biochemical evidence that Rad51 acts with Mei5-Sae3 as a Dmc1 accessory factor. Thus, Rad51 is a multifunctional protein that catalyzes recombination directly in mitosis and indirectly, via Dmc1, during meiosis.
Meiosis reduces chromosome number as required for bi-parental reproduction. The meiotic program evolved by modification of the mitotic cell cycle, via duplication and specialization of key proteins including the RecA family members Rad51 and Dmc1 (1, 2). Rad51 and Dmc1 form nucleoprotein filaments on ssDNA tracts that flank double-strand break (DSB) sites. These filaments search for, and swap strands with, homologous dsDNA segments on unbroken chromatids to form homologous JMs. Rad51 is the only protein that acts directly in JM formation during mitotic recombination. Dmc1 is a meiosis-specific protein. Normal meiotic recombination depends on both Rad51 and Dmc1. Previous results reveal that both Rad51 and Dmc1 are capable of carrying out homology search and catalyzing the formation of JMs (3-5), but they do not reveal if one, the other, or both proteins contribute these activities during wild type (WT) meiosis.
The E. coli RecA protein has two DNA binding sites, a high affinity site (site I) sufficient for polymerization of proteins on ssDNA tracts, and a low affinity DNA binding site (site II) specifically required for interaction of the ssDNA-protein filament with a second DNA during homology search and JM formation (6) (Fig. S1A). Site II in RecA includes positively charged residues R243 and K245 (7); a third residue, R227, completes a basic patch on the groove of the helical filament. This patch corresponds to a patch of three residues in Rad51 protein (R188, K361, and K371; Fig. S1B). We mutated these three residues in Rad51 to alanine to form Rad51-II3A. This protein was then purified (Fig. S2).
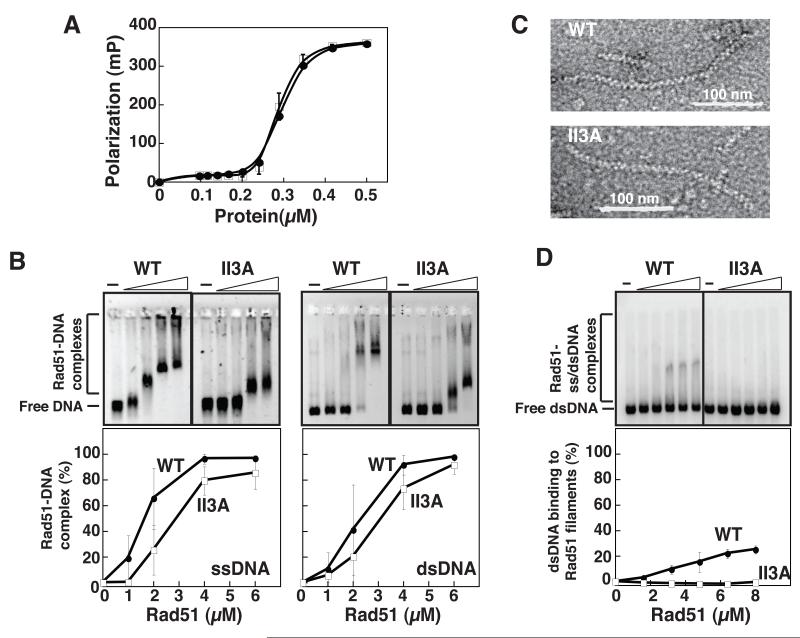
To test Rad51-II3A for site I binding activity we used fluorescence polarization (FP) and electrophoretic mobility shift assays (EMSA). FP detected no difference in apparent binding affinity between the wild type protein (Rad51-WT) and Rad51-II3A (Fig. 1A). EMSA analysis showed that both Rad51-WT and Rad51-II3A shift the mobility of both ss and dsDNA under similar conditions (Fig. 1B). However, the extent of mobility shift by Rad51-II3A was less that by Rad51-WT, possibly reflecting a greater tendency of Rad51-II3A to dissociate during electrophoresis and/or a difference in site II binding. In some experiments, Rad51-WT, but not Rad51-II3A, caused a substantial fraction of DNA to be retained in the well. EM analysis showed Rad51-II3A forms filaments on ssDNA that do not differ from those formed by Rad51-WT with respect to average length, width, or pitch (Fig. 1C and Fig. S3). Together these observations indicate that Rad51-II3A retains substantial site I binding activity.
Fig. 1.
Rad51-II3A binds DNA to form nucleoprotein filaments. (A) Rad51-WT (closed circle) and Rad51-II3A (open-square) bind oligo-dT as revealed by fluorescence polarization. Error bars represent SEM, N = 4. The apparent affinity of both proteins is about 300 nM. (B) EMSA analysis of DNA binding. Protein (at 0, 1, 2, 4, and 6 μM) binds M13mp18 ssDNA (22 μM nucleotides) and linear pBluescript dsDNA (8 μM base pairs). The inverted images of agarose gels are presented. The percentage of DNA in Rad51-DNA complexes is plotted. SEM, N = 6 for ssDNA, N = 3 for dsDNA. (C) Rad51-WT and Rad51-II3A proteins form filaments on a 1000-nucleotide fragment of ØX174 ssDNA. (D) ssDNA-Rad51-WT but not ssDNA-Rad51-II3A filaments bind 32P-pBluescript dsDNA (18 μM base pairs). Filament concentrations = 0, 1.5, 2.9, 4.4, 5.8, and 7.3 μM protomer (at 3nt/protomer). Phorphorimage of radioactive EMSA gel is presented. For plotted data, N= 3.
To assay for site II binding, we first saturated site I with unlabeled ssDNA (3nt/Rad51 protomer) in the presence of AMPPNP to block filament dissociation (8). We then added heterologous 32P-labeled dsDNA. EMSA analysis showed that ssDNA-Rad51-WT filaments were able to alter the mobility of up to 25% of input dsDNA (Fig. 1D). The shifted species was not detected with ssDNA-Rad51-II3A filaments under the same conditions, consistent with a site II binding defect.
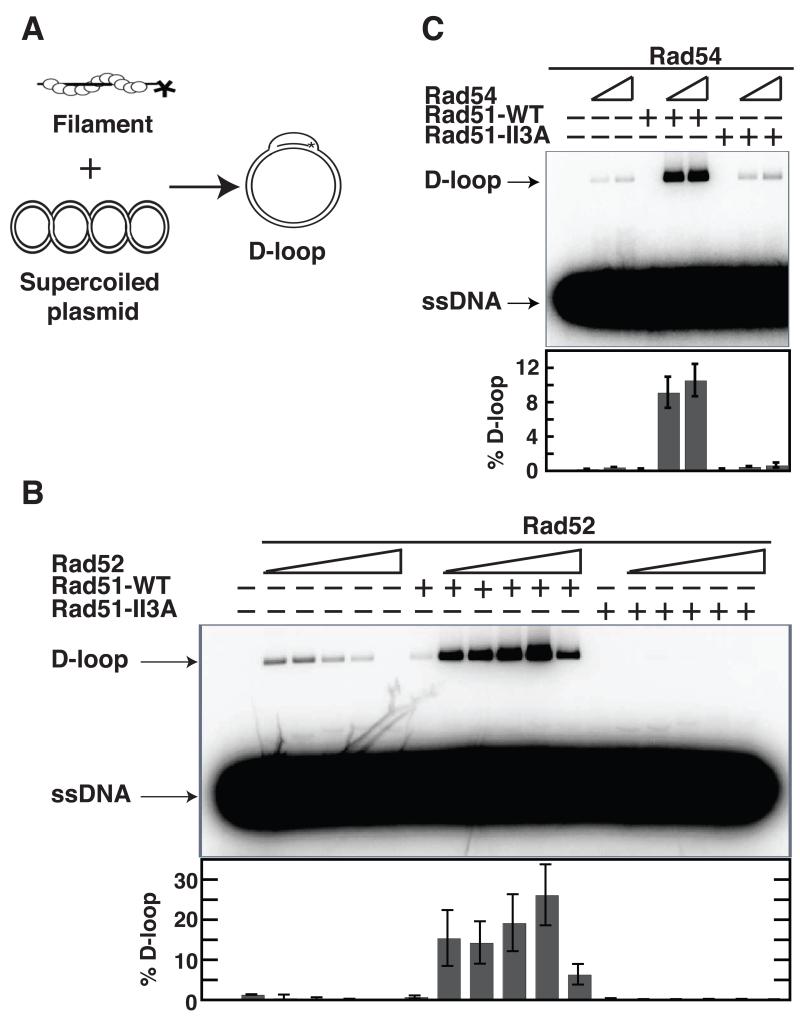
We then measured the ability of Rad51-II3A to form JMs via a D-loop assay. 32P-labeled oligonucleotide was pre-incubated with protein and mixed with homologous supercoiled plasmid DNA (Fig. 2A). Reactions mixtures are incubated for 30 minutes at which time D-loop levels plateau (data not shown). D-loops were detected by co-migration of oligonucleotide and plasmid on gels. The Rad51 mediator Rad52 or the DNA translocase Rad54 was added to enhance Rad51 D-loop activity (9). Rad51-II3A had no detectable activity in Rad52-stimulated reactions, whereas 26 ± 8% of duplex DNA was converted to D-loops by Rad51-WT (Fig. 2B). Results were similar with Rad54-stimulated assays, although a small amount of activity was detected for Rad51-II3A (0.7 ± 0.3% compared to 11 ± 2% for Rad51-WT; Fig. 2C). These results demonstrate Rad51-II3A is profoundly defective in the ability to form D-loops despite retaining site I DNA binding activity.
Fig. 2.
ScRad51-II3A mutant protein is defective in D-loop activity. (A) Schematic of D-loop assay. (B,C) D-loop formation by Rad51-WT or Rad51-II3A (1.2 μM) was examined with or without ScRad52 (0.3, 0.6, 1.2, 2.4 and 4.8 μM) in (B); with or without ScRad54 (0.2 and 0.4 μM) in (C). Rad51 was pre-incubated with either Rad52 or Rad54, followed by incubation with 32P-labeled 90-mer ssDNA (3.6 μM nucleotides) at 37 °C. D-loops were generated upon the addition of plasmid (22 μM base pairs) to the mixture. Error bars represent SEM, N=3.
To test for DNA binding activity in vivo we examined the effect of the rad51-II3A mutation on immunostaining Rad51 foci, which are formed by binding at tracts of DSB-dependent ssDNA (10). The rad51-II3A strain (Table S1) formed the same number of Rad51 foci as wild type (33 ± 3 in WT and 32 ± 2 in rad51-II3A; Fig. S4) indicating the protein retains DNA binding activity in cells. We also stained meiotic nuclei for Dmc1 (Fig. S5). The foci formed by Dmc1 in rad51Δ are about 3-fold fainter in average staining intensity than those in WT, implying a role for Rad51 in normal assembly of Dmc1 at DSBs (11, 12). In contrast, rad51-II3A formed Dmc1 foci of WT staining intensity. This result suggests that Rad51-II3A retains the ability to enhance assembly of Dmc1 at sites of recombination.
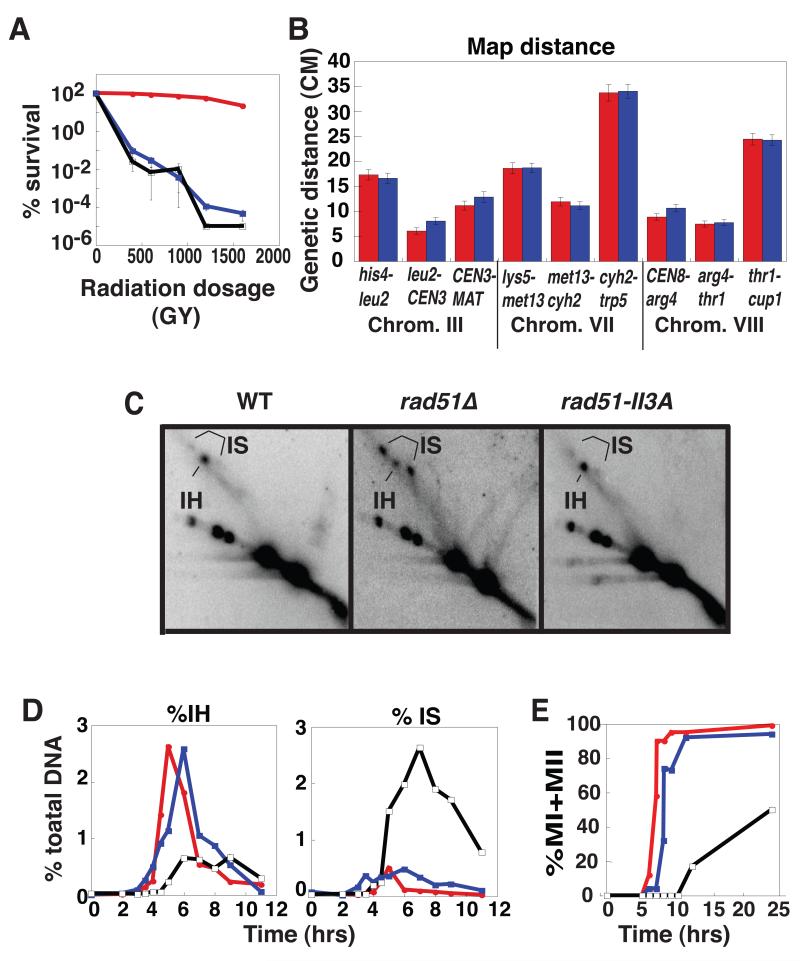
To test for JM activity in vivo, we examined mitotic yeast cells where Rad51 is the only RecA homolog expressed. Rad51’s JM activity promotes recombinational repair of lethal DSBs induced by ionizing radiation. We therefore compared the γ ray sensitivity of rad51-II3A to that of WT and rad51Δ. A rad51Δ and a rad51-II3A mutant showed the same high sensitivity to γ rays (D0 = 360 Gy compared to D0 = 1410 Gy in WT; Fig. 3A) indicating that rad51-II3A cells are severely defective in repair of mitotic DNA breaks.
Fig. 3.
Phenoptypic analysis of rad51-II3A mutants. (A) Cell survival as a function of γ ray dose for WT (DKB3507, red), rad51-II3A (DKB3690, blue), and rad51Δ (DKB3048, black). (B) Genetic map distances (with standard error) from diploids WT (NHY1848, red) and rad51-II3A (DKB4005, blue). (C) 2-D gel analysis of IH JMs at HIS4::LEU2. Representative images showing peak time points are presented. The positions IH and IS JM species are shown (21). (D) IH and IS as a percentage of total DNA: WT (red), rad51-II3A (blue), rad51Δ (black). (E) Timing of meiotic divisions % MI ± MII = number of cells with >1 DAPI staining body over the total # of cells X100.
We next used the rad51-II3A mutant to ask if Rad51’s JM activity is required for interhomolog recombination. We measured map distance (Fig. 3B), gene conversion frequency (Table S2A), and crossover interference (Table S2B) using strains with twelve markers (13). No significant differences were detected between WT and rad51-II3A indicating interhomolog recombination occurs normally in a strain that is severely deficient in Rad51’s JM activity.
Previous analysis of the rad51Δ mutant using 2-D gels to detect interhomolog (IH) JMs and intersister (IS) JMs in meiosis showed that the null mutant is severely defective in interhomolog bias (Fig. 3C); the 4.8 ± 0.2 to 1 (SEM, N=6) bias towards IH JMs over IS JMs observed in WT is altered to 1 to 3.9 ± 0.3 (N=3) in rad51Δ (3). In contrast to rad51Δ, rad51-II3A has the same IH/IS ratio as WT (4.7 ± 0.3 to 1, N=6; Fig. 3D), providing further evidence that Rad51’s JM activity does not contribute to its role in meiotic recombination. The only significant phenotypic differences detected between WT and rad51-II3A were a 1 hour delay in formation of JMs and a reduction in spore viability from 99 to 83%
As an additional control to ensure that Rad51-II3A lacks recombinogenic activity in meiotic cells, we examined the effect of the mutation in a hed1 dmc1 background where Rad51 contributes the only strand exchange activity. In this background, RAD51+ promotes high levels of JM formation, but rad51-II3A confers a strong block to Rad51-dependent formation of homologous joint molecules (JM) in meiosis (Fig. S6).
Thus, Dmc1’s JM forming activity is sufficient for normal meiotic interhomolog recombination. To confirm that Dmc1’s JM activity is necessary for meiotic recombination, we constructed and analyzed a dmc1-II3A mutant strain. The dmc1-II3A strain showed a uniform meiotic arrest in prophase and a complete block to JM formation identical to that observed in the dmc1Δ mutant (Fig. S7). This result indicates that, in contrast to Rad51, Dmc1’s ability to form nucleoprotein filaments is not sufficient for its role in interhomolog recombination. Biochemical experiments showed that, like Rad51-II3A, Dmc1-II3A retains DNA binding, but not D-loop activity (Fig. S8).
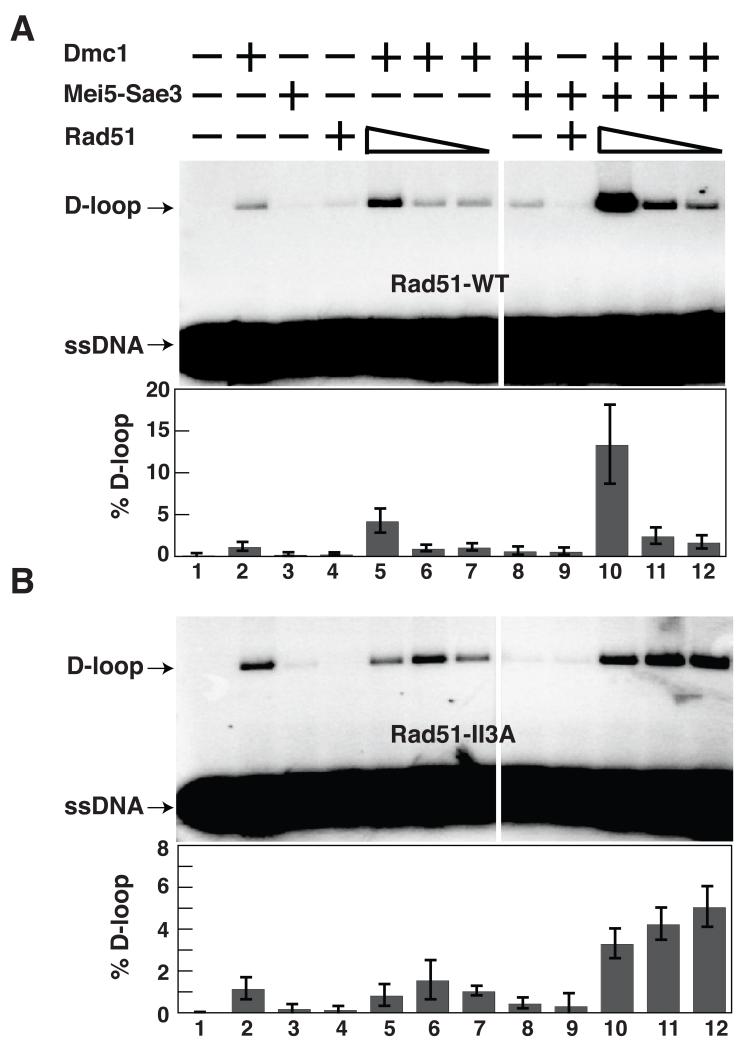
The observation that Rad51 contributes to homolog bias of JM formation independently of its strand exchange activity, together with the observation that Rad51 promotes formation of Dmc1 foci, implies that Rad51 regulates Dmc1’s strand exchange activity. The heterodimeric mediator Mei5-Sae3 stimulates Dmc1 function in vivo and in vitro (14, 15). We therefore asked if we could identify conditions under which Rad51 could function with Mei5-Sae3 to promote Dmc1’s D-loop activity in a purified system. In the presence of magnesium, and ATP about 1.2 ± 0.5% of duplex plasmid is converted to D-loop in reactions containing 1 μM Dmc1 as the only protein (Fig. 4A, lane 2). Addition of 0.5 μM Mei5-Sae3 reduced the activity of Dmc1 under these conditions such that D-loop yield was 0.5 ± 0.3% (Fig. 4A, lane 8). However, addition of 0.5 μM Rad51-WT protein converted Mei5-Sae3’s inhibitory activity to a stimulatory activity, enhancing the level of D-loops to 13 ± 5% a level 26 ± 17 fold higher than adding Mei5-Sae3 alone (Fig. 4A, lane 10). Rad51-II3A protein also stimulated the reaction with Mei5-Sae3 and Dmc1 11 ± 4 fold, indicating that Rad51’s D-loop activity is not required for Dmc1 stimulation (Fig. 4B, Lanes 10-12). Interestingly, the stimulatory activity of Rad51-II3A was less sensitive to dilution than that of the WT protein. Although the reason for this difference remains to be determined, it is possible that removal of 3 positively charged amino acids from the Rad51 filament groove enhances the direct interaction of Rad51 with Mei5-Sae3 (16). Consistent with this idea, modeling studies have suggested that Mei5-Sae3 binds in the filament groove (17).
Fig. 4.
Rad51 stimulates Dmc1’s D-loop activity. Dmc1 (1 μM) was pre-incubated with or without Rad51-WT (A) or Rad51-II3A (B) (0.5, 0.1, and 0.02 μM), Mei5-Sae3 (0.5 μM), and 32P-labeled 90-mer ssDNA (3.6 μM nucleotides). Plasmid pRS306 (22 μM base pairs) was then added to initiate D-loop formation. Error bars represent SEM, N=4.
Overall, the results show Rad51 can function with Mei5-Sae3 as a mediator of Dmc1’s strand exchange activity, consistent with the finding that Mei5, Sae3, and Rad51 are required for Dmc1 focus formation during meiosis. It remains to be determined if and how Rad51’s role in Mei5-Sae3-dependent stimulation of Dmc1’s D-loop activity is related to its role in homolog bias.
Our results suggest that, following the duplication that created Rad51 and Dmc1, Dmc1 evolved to serve the specialized mechanisms of meiotic recombination. These mechanisms ensure generation of chiasmata connecting every homologous chromosome pair. Rad51 instead has evolved to be an accessory for Dmc1, while retaining its JM-forming activity, as required for mitotic DNA repair. Rad51’s JM-forming activity may also be important as a fail-safe for rare occasions when the Dmc1-dependent interhomolog JM mechanism fails. Such a fail-safe function for Rad51 would account for the modest reduction of spore viability observed in rad51-II3A. Similar modes of evolution may have formed other functional partnerships between meiosis-specific proteins and their mitotically-active paralogues.
Supplementary Material
Acknowledgments
We are grateful to Andrianna Zhang and Phoebe Rice for structural alignments. We thank Neil Hunter for critical reading of the manuscript, and strains. Patrick Sung provided the pET11d-ScRad52 vector, and Wolf Heyer provided Rad54 protein. This work was supported by NIGMS grant GM50936.
Footnotes
Methods and materials are available as supplemental material on Science online.
References and Notes
- 1.Bishop DK, Park D, Xu L, Kleckner N. Cell. 1992;69:439. doi: 10.1016/0092-8674(92)90446-j. [DOI] [PubMed] [Google Scholar]
- 2.Shinohara A, Ogawa H, Ogawa T. Cell. 1992;69:457. doi: 10.1016/0092-8674(92)90447-k. [DOI] [PubMed] [Google Scholar]
- 3.Schwacha A, Kleckner N. Cell. 1997 Sep 19;90:1123. doi: 10.1016/s0092-8674(00)80378-5. [DOI] [PubMed] [Google Scholar]
- 4.Sheridan S, Bishop DK. Genes Dev. 2006 Jul 1;20:1685. doi: 10.1101/gad.1447606. [DOI] [PubMed] [Google Scholar]
- 5.Tsubouchi H, Roeder GS. Genes Dev. 2006 Jul 1;20:1766. doi: 10.1101/gad.1422506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Muller B, Koller T, Stasiak A. J Mol Biol. 1990 Mar 5;212:97. doi: 10.1016/0022-2836(90)90307-8. [DOI] [PubMed] [Google Scholar]
- 7.Kurumizaka H, Ikawa S, Sarai A, Shibata T. Arch Biochem Biophys. 1999 May 1;365:83. doi: 10.1006/abbi.1999.1166. [DOI] [PubMed] [Google Scholar]
- 8.Chi P, Van Komen S, Sehorn MG, Sigurdsson S, Sung P. DNA Repair (Amst) 2006 Mar 7;5:381. doi: 10.1016/j.dnarep.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 9.San Filippo J, Sung P, Klein H. Annu Rev Biochem. 2008;77:229. doi: 10.1146/annurev.biochem.77.061306.125255. [DOI] [PubMed] [Google Scholar]
- 10.Gasior SL, Wong AK, Kora Y, Shinohara A, Bishop DK. Genes Dev. 1998 Jul 15;12:2208. doi: 10.1101/gad.12.14.2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bishop DK. Cell. 1994 Dec 16;79:1081. doi: 10.1016/0092-8674(94)90038-8. [DOI] [PubMed] [Google Scholar]
- 12.Shinohara A, Gasior S, Ogawa T, Kleckner N, Bishop DK. Genes Cells. 1997 Oct;2:615. doi: 10.1046/j.1365-2443.1997.1480347.x. [DOI] [PubMed] [Google Scholar]
- 13.Oh SD, et al. Cell. 2007 Jul 27;130:259. doi: 10.1016/j.cell.2007.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ferrari SR, Grubb J, Bishop DK. J Biol Chem. 2009 May 1;284:11766. doi: 10.1074/jbc.C900023200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hayase A, et al. Cell. 2004 Dec 29;119:927. doi: 10.1016/j.cell.2004.10.031. [DOI] [PubMed] [Google Scholar]
- 16.Say AF, et al. DNA Repair (Amst) 2011 Jun 10;10:586. doi: 10.1016/j.dnarep.2011.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kokabu Y, et al. J Biol Chem. 2011 Dec 16;286:43569. doi: 10.1074/jbc.M111.303339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hong EL, Shinohara A, Bishop DK. J Biol Chem. 2001 Nov 9;276:41906. doi: 10.1074/jbc.M105563200. [DOI] [PubMed] [Google Scholar]
- 19.Zeng G. Biotechniques. 1998 Aug;25:206. doi: 10.2144/98252bm05. [DOI] [PubMed] [Google Scholar]
- 20.Jayathilaka K, et al. Proc Natl Acad Sci U S A. 2008 Oct 14;105:15848. doi: 10.1073/pnas.0808046105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sheridan SD, et al. Nucleic Acids Res. 2008 Jul;36:4057. doi: 10.1093/nar/gkn352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hunter N, Kleckner N. Cell. 2001 Jul 13;106:59. doi: 10.1016/s0092-8674(01)00430-5. [DOI] [PubMed] [Google Scholar]
- 23.Schwacha A, Kleckner N. Cell. 1994 Jan 14;76:51. doi: 10.1016/0092-8674(94)90172-4. [DOI] [PubMed] [Google Scholar]
- 24.Perkins DD. Genetics. 1949;34:607. doi: 10.1093/genetics/34.5.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Papazian HP. Genetics. 1952;37:178. doi: 10.1093/genetics/37.2.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stahl FW. Genetics. 2008 May;179:701. doi: 10.1534/genetics.108.086918. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.