Abstract
Introduction
Cerebral glucose metabolism and energy production are affected by serum glucose levels. Systemic glucose variability has been shown to be associated with poor outcome in critically ill patients. The objective of this study was to assess whether glucose variability is associated with cerebral metabolic distress and outcome after subarachnoid hemorrhage.
Methods
A total of 28 consecutive comatose patients with subarachnoid hemorrhage, who underwent cerebral microdialysis and intracranial pressure monitoring, were studied. Metabolic distress was defined as lactate/pyruvate ratio (LPR) >40. The relationship between daily glucose variability, the development of cerebral metabolic distress and hospital outcome was analyzed using a multivariable general linear model with a logistic link function for dichotomized outcomes.
Results
Daily serum glucose variability was expressed as the standard deviation (SD) of all serum glucose measurements. General linear models were used to relate this predictor variable to cerebral metabolic distress and mortality at hospital discharge. A total of 3,139 neuromonitoring hours and 181 days were analyzed. After adjustment for Glasgow Coma Scale (GCS) scores and brain glucose, SD was independently associated with higher risk of cerebral metabolic distress (adjusted odds ratio = 1.5 (1.1 to 2.1), P = 0.02). Increased variability was also independently associated with in hospital mortality after adjusting for age, Hunt Hess, daily GCS and symptomatic vasospasm (P = 0.03).
Conclusions
Increased systemic glucose variability is associated with cerebral metabolic distress and increased hospital mortality. Therapeutic approaches that reduce glucose variability may impact on brain metabolism and outcome after subarachnoid hemorrhage.
Introduction
Hyperglycemia has been associated with morbidity and poor outcome in patients with subarachnoid hemorrhage [1-4]. Tight glucose control with intravenous insulin has been shown to reduce mortality among surgical ICU patients [5,6], but not in mixed populations of critically ill patients [7-10]. The impact of tight glycemic control in neurological critically ill patients remains controversial. While some data suggest that intensive insulin therapy fails to improve the outcome of neurologic patients and may be deleterious due to an increased incidence of hypoglycemia and low brain tissue glucose levels, some authors have shown that tighter glycemic control may avoid neurological complications in the ICU [1-5,11-13]. Microdialysis studies of cerebral metabolism indicate that tight glucose control is associated with an increased risk of metabolic distress, which is defined as an elevation of the lactate/pyruvate ratio [1,14-22].
Cerebral energy production depends on an adequate supply of glucose. Systemic glucose levels affect glucose availability to the brain and can impact cellular metabolism and energy production after subarachnoid hemorrhage (SAH). Because of impaired glucose transport, systemic glucose levels considered to be normal may be relatively insufficient to meet the increased cerebral metabolic demand seen in patients with SAH [1,16,20].
Both hypoglycemia and hyperglycemia have been shown to exacerbate secondary brain injury [2-4,16,20,21,23] after SAH. Acute fluctuations of systemic glucose have also been associated with oxidative stress in diabetic outpatients [24,25], with increased mortality in critically ill patients and with worse functional outcome and mortality in neurological patients [26-32]. Patients with SAH may be more vulnerable to glycemic variability if these acute fluctuations trigger cerebral metabolic distress and lead to secondary brain injury.
In this study, we sought to understand better the potential role of increased systemic glucose variability in cerebral oxidative metabolism and potentially secondary brain injury. Specifically, we hypothesized that increased glycemic variability is associated with cerebral metabolic distress and increased mortality in patients with SAH.
Materials and methods
Patients
We retrospectively reviewed 28 consecutive patients admitted to the neurological ICU at Columbia University Medical Center between May 2006 and January 2009 after SAH who underwent multimodality neuromonitoring with intracranial pressure (ICP), cerebral microdialysis and brain tissue oxygen pressure (PbtO2) as part of their clinical care. This study was approved by the Columbia University Institutional Review Board (IRB). Written informed consent was obtained from all patients or person responsible.
Clinical management
Patient care for SAH conformed to guidelines established by the American Heart Association [11]. Hemodynamic and fluid management were targeted to maintain cerebral perfusion pressure (CPP) >60 mm Hg and ICP <20 mm Hg. Hemoglobin cutoff for blood transfusions was 8 g/dL unless there was clinical, imaging or laboratory evidence of active cerebral or myocardial ischemia. Fever was aggressively treated using intravascular (Celsius Control System®, Innercool Therapies, Inc, San Diego, CA, USA) or surface (Arctic Sun Cooling System®, Medivance Inc, Louisville, CO, USA) cooling devices. Shivering was treated with buspirone, skin counterwarming, magnesium infusion and analog-sedation (dexmedetomidine, fentanyl or meperidine) according to a stepwise protocol [33,34].
Systemic glucose control
Systemic glucose was measured with the Sure Step Flexx system (Lifescan, Milpitas, CA, USA) using arterial blood and the target range was between 4.4 and 8.3 mmol/L (80 to 150 mg/dL) as part of a glucose control protocol using intravenous insulin infusion Humulin (©Lilly, Indianapolis, IN, USA). Hypoglycemia with systemic glucose below 3.3 mmol/L (60 mg/dL) was managed with a bolus of 20 to 25 g of glucose in D50 solution. Enteral nutrition (Osmolite, Ross Nutrition, Abbott Laboratories, Columbus, OH, USA) was provided via a naso-duodenal tube starting within the first 24 hours of admission, aiming to 25 kcal/kg/day of ideal body weight. No parenteral nutrition was given. Almost all of the systemic glucose measurements while patients underwent neuromonitoring were performed hourly. The median number of systemic glucose measurements per patient was 105 (interquartile range (IQR), 69 to 144).
Multimodality neuromonitoring
ICP, PbtO2 and microdialysis probes were placed via a triple lumen bolt at the bedside using full sterile technique. ICP was measured using an intraparenchymal fiberoptic catheter (Camino System, Integra Neurosciences®, Plainsboro, NJ, USA). Hourly microdialysis samples were obtained with a 10 mm membrane length CMA-70 microdialysis catheter (CMA Microdialysis®, Stockholm, Sweden). The probes were placed via a frontal approach into the hemisphere deemed at greatest risk for secondary injury (that is, perihematomal or pericontusional tissue, or the ipsilateral anterior watershed zone in lateralized SAH) or in the right frontal lobe in patients with diffuse injury. Immediately after the procedure, a brain CT scan was performed in each patient to confirm the location of the microdialysis catheter.
Cerebral microdialysis
A CMA 106 microdialysis perfusion pump (CMA Microdialysis®) was used to perfuse the interior of the catheter with sterile artificial cerebrospinal fluid (Na+ 148 mmol/L, Ca2+ 1.2 mmol/L, Mg2+ 0.9 mmol/L, K+ 2.7 mmol/L, Cl- 155 mmol/L) at a rate of 0.3 μl/minute. Samples were collected every 60 minutes into microvials, and immediately analyzed at the bedside for glucose, lactate and pyruvate (mmol/L) with the CMA 600 analyzer (CMA Microdialysis®). At least one hour passed between the insertion of the probe and the start of the sampling, to allow for normalization of changes due to probe insertion. The analyzer was automatically calibrated on initiation and every six hours using standard calibration solutions from the manufacturer. Quality controls at three different concentrations for each marker were performed daily.
Physiologic variables
Physiological variables including heart rate (HR), arterial blood pressure, respiratory rate (RR), fraction of inspired oxygen (FiO2) and oxygen saturation (SpO2) were continuously monitored in all patients. Hourly ICP and mean arterial pressure (MAP) were prospectively recorded as part of the standard of care. CPP was calculated as CPP = MAP – ICP, with both MAP and ICP referenced to the level of the foramen of Monroe. FiO2 was routinely maintained at 40%. Symptomatic vasospasm was defined as neurologic worsening and/or cerebral infarction attributed to vasospasm.
Glycemic variability and metabolic distress
Daily glycemic variability was assessed using standard deviation (SD) [29,35-37]. SD is calculated as the squared root of the average of the squared differences between individual glucose values and the mean. SD was calculated daily to test for associations with metabolic distress and calculated for the entire monitoring period to test for associations with mortality.
Metabolic distress was defined as a lactate/pyruvate ratio (LPR) above 40. This threshold was defined based on previous reports demonstrating associations with cerebral metabolic disarray, cerebral ischemia, or poor clinical outcome in patients with SAH [38,39].
Data acquisition
A Solar 8000i utilizing a General Electric Medical Systems Information Technologies’ Unity Network® was used as the patient physiologic monitor. A high resolution data acquisition system (BedmasterEX, Excel Medical Electronics, Jupiter, FL, USA) using an open architecture of the Unity Network® automatically acquired vital signs, alarm and waveform data from all the patient monitoring devices in the NICU. Digital data were acquired every five seconds and recorded in an SQL database. Waveform data were stored at a resolution of 240 Hz in binary files. LICOX® (Integra Neuroscience, Plainsboro, NJ, USA) and brain metabolism data were incorporated into the data acquisition system utilizing the communications (COM) port on the device which was plugged into a serial-to-TCP/IP interface device (Equinox ESP-8, Avocent, Sunrise, FL, USA).
Statistical analysis
Due to the small sample of patients and large number of measurements the data were analyzed using generalized estimating equations (GEE). Univariate analyses were used to test for associations between predictor and outcome variables. Variables with significant associations (P <0.1) were considered candidates for the multivariable analyses. Multivariable models were constructed using a general linear model (GLM) with a logistic link function (logistic regression), extended by generalized estimating equations (GEE) to account for within-subject variation. The within-subject correlation structure was modeled using the auto-regressor of the first order (AR-1) [40-42]. Model building was performed with a stepwise procedure starting with the variable of interest. The relationship between serum glucose variability (SD) and cerebral metabolic distress was assessed using a multivariable model. The occurrence of at least one episode of metabolic distress (LPR >40) in each day of monitoring was considered a binary outcome variable. SD was tested as the main predictor variable and adjusted for significant covariates. We reported the final multivariable model. The model building procedure used the corrected quasi-likelihood under independence model criterion (QICC) for model selection [40].
Finally, in order to identify independent associations between SD and outcome we fitted a multivariable logistic regression model with hospital mortality as the binary outcome. Serum glucose variability averaged over the period of monitoring was entered as the predictor variable and adjusted for other significant covariates and clinically important variables. Goodness of fit was assessed with the Hosmer-Lemeshow test.
Adjusted odds ratios (OR) and 95% confidence intervals (CI) were reported for all significant predictor variables. All statistical analyses were performed using SPSS 16 software (SPSS Inc., Chicago, IL, USA). A P value <0.05 was considered statistically significant.
Results
Clinical characteristics and systemic parameters
Patients’ baseline characteristics are listed in Table 1. All 28 patients included in the study were mechanically ventilated and had a GCS less than or equal to 8 at the time of monitoring. During the study period, 3,139 hourly microdialysate samples and serum glucose measurements were collected (median per patient 105 hourly samples (IQR, 69 to 144). Serum glucose variability was calculated for each of the 181 days of neuromonitoring. The median duration from admission to the start of neuromonitoring was two days and the median duration of monitoring was six days. Values for multimodality monitoring including CPP, PbtO2, systemic glucose and hemoglobin concentrations, as well as SD are presented in Table 2. We did not record other systemic parameters that may influence brain metabolism such as pCO2 and temperature.
Table 1.
Clinical characteristics (number = 28)
| Variable | Median or number | IQR or% |
|---|---|---|
| Age |
54 |
41 to 61 |
| Gender (female) |
19 |
68 |
| Diabetes mellitus |
3 |
11 |
| Hunt Hess |
|
|
| 2 |
1 |
4 |
| 3 |
5 |
18 |
| 4 |
8 |
29 |
| 5 |
14 |
50 |
| Modified Fisher |
|
|
| 2 |
4 |
14 |
| 3 |
14 |
50 |
| 4 |
10 |
36 |
| APACHE II |
23 |
19 to 29 |
| Admission Glasgow Coma Scale (GCS) |
6 |
5 to 9 |
| Days from admission to neuromonitoring |
2 |
1 to 4 |
| Days with neuromonitoring |
6 |
4 to9 |
| Delayed cerebral ischemia (DCI) |
10 |
36 |
| Symptomatic vasospasm |
7 |
25 |
| Hospital mortality | 7 | 25 |
Data are reported as number (%) or median (interquartile range) unless otherwise indicated. APACHE II, Acute Physiology and Chronic Health Evaluation II; IQR, interquartile range.
Table 2.
Multimodality monitoring
| Variable | Median | IQR |
|---|---|---|
| Cerebral perfusion pressure (mmHg) |
95 |
78 to 105 |
| Hemoglobin (g/dL) |
9.7 |
9 to 10.5 |
| Serum glucose (mmol/L) |
7.7 |
6.9 to 8.3 |
| Serum glucose variability |
|
|
| Standard deviation (SD) per day |
1.4 |
1.2 to 1.8 |
| Microdialysis |
|
|
| Lactate (mmol/L) |
4.0 |
3.1 to 4.8 |
| Pyruvate (mmol/L) |
121 |
87 to 162 |
| Glucose (mmol/L) |
0.98 |
0.68 to 1.48 |
| LPR |
30 |
27 to 50 |
| PbtO2 (mmHg) | 28 | 20 to 40 |
IQR, interquartile range; LPR, lactate/pyruvate ratio; PbtO2, partial pressure of brain tissue oxygen.
Hypoglycemia
No episodes of severe hypoglycemia (<2.3 mmol/L) occurred during the study period. Sixteen patients (57%) presented with at least one episode of moderate hypoglycemia (<3.9 mmol/L). Ten of these patients had one or two episodes and the maximum number of episodes occurred in one patient – five episodes. There was no difference in the number of episodes of moderate hypoglycemia sustained by patients with increased SD (above the median) as compared to those with lower SD (1 IQR (0 to 3) versus 0 IQR (0 to 1.5)); P = NS, respectively). The development of moderate hypoglycemia was also tested in the multivariate models for metabolic distress and hospital mortality but no association was found.
Glycemic variability and metabolic distress
SD was treated as a continuous variable in the multivariable model with a binary outcome variable: at least one episode of metabolic distress per day. The proportion of days with at least one episode of metabolic distress progressively increased with SD (Figure 1). After adjusting for GCS and brain glucose, SD was independently associated with an increased risk of developing at least one episode of metabolic distress per day (Table 3).
Figure 1.
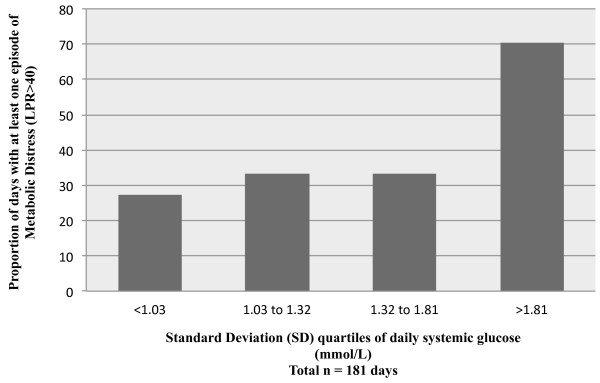
Relative frequency of at least one episode of metabolic distress (LPR >40) per day monitored across the quartiles of daily standard deviation (SD). The multivariable general linear model (GLM) with a logistic link function using GEE showed an independent association between SD and metabolic distress. GEE, generalized estimating equations.
Table 3.
Predictors of at least one episode per day of metabolic distress (LPR >40)
| |
|
Univariate analysis |
Multivariate analysis |
|||
|---|---|---|---|---|---|---|
| Variable | Threshold | Unadjusted OR | CI | Adjusted OR | CI | P value |
| Glucose variability (SD) |
NA |
1.3 |
0.9 to 1.6 |
1.5 |
1.1 to 2.1 |
0.02 |
| Brain glucose |
NA |
0.4 |
0.2 to 0.8 |
0.3 |
0.1 to 0.8 |
0.02 |
| Glasgow Coma Scale | NA | 0.8 | 0.7 to 0.9 | 0.7 | 0.6 to 0.9 | <.001 |
Multivariable logistic regression model accounting for between-subject and within-subject variations over time using generalized estimating equations (GEE) adjusted for the variables listed. All variables, including SD were entered as continuous variables. CI, confidence interval; NA, not applicable; OR, odds ratio; SD, standard deviation.
Glycemic variability and outcome
Hospital mortality was higher for patients with increased variability (SD above the median) (Figure 2). After adjusting for age, worst Hunt Hess on admission, daily GCS and the development of delayed cerebral ischemia (DCI), SD was independently associated with increased hospital mortality in a multivariable logistic regression model (Table 4).
Figure 2.
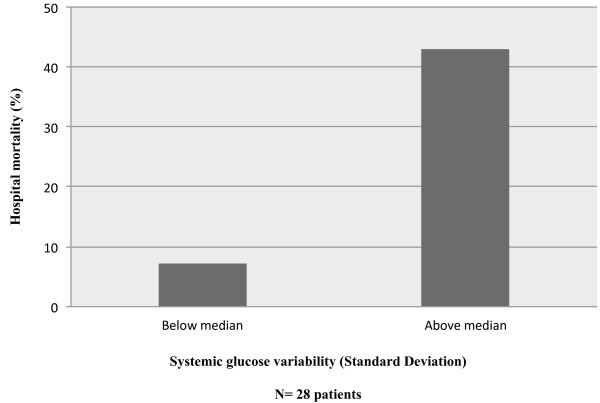
Hospital mortality of patients with serum glucose variability below and above the median (median = 1.4) for standard deviation (SD). Multivariable logistic regression demonstrated independent associations between SD and hospital mortality.
Table 4.
Predictors of hospital mortality
| |
|
Univariate analysis |
Multivariate analysis |
|||
|---|---|---|---|---|---|---|
| Variable | Threshold | Unadjusted OR | CI | Adjusted OR | CI | P value |
| Glucose variability (SD) |
NA |
5.9 |
0.9 to 37 |
10.4 |
1.3 to 86 |
.03 |
| GCS | Every 1 point | 1.1 | 0.8 to 1.4 | 0.5 | 0.2 to 0.99 | .04 |
Multivariable logistic regression model with hospital mortality as the binary outcome adjusted for the variables listed, age, Hunt Hess, and DCI. GCS and SD were entered as continuous variables. CI, confidence interval; DCI, delayed cerebral ischemic; GCS, Glasgow Coma Scale; NA, not applicable; OR, odds ratio; SD, standard deviation.
We tested for interactions between DCI, diabetes status and systemic glucose and SD and no interaction was found. Furthermore, although our cohort had only three diabetic patients we compared systemic glucose and SD between patients with and without DM and no difference was found (median systemic glucose 7.7 IQR (7.2 to 8.7) versus 7.6 IQR (6.9 to 8.2); P = NS and median SD 1.9 (IQR 1.3 to 1.9) versus 1.6 (IQR 1.4 to 2.0); P = NS, respectively).
Discussion
In this study we demonstrated an association between increased systemic glucose variability with cerebral metabolic distress and mortality after SAH.
In our study we used metabolic distress to evaluate energy failure. Metabolic distress, defined as an elevated LPR above 40, has been reported in the absence of ischemia, possibly caused by mitochondrial dysfunction, seizures or reduced substrate availability. Moreover, elevated LPR is a well-studied complication and has been shown to be associated with poor outcome [16,34,38,39,43-48].
Systemic glucose variability has been associated with mortality in mixed populations of critically ill patients [29-31] and after traumatic brain injury [26,49]. Although variability has been shown to affect diabetic and non-diabetic patients differently [50], we found no effect in our cohort. Recently glucose variability has been associated with the development of cerebral infarction in a cohort of SAH patients [49]. In our study, daily acute fluctuation of systemic glucose was a predictor of the development of cerebral metabolic distress after adjusting for the presence of DCI. This finding suggests that increased glycemic variability and oxidative metabolism may be associated with, and contribute to, poor outcome. Interestingly, the occurrence of hypoglycemia was not associated with increased SD or mortality in our model. This may be explained by the absence of severe hypoglycemia during the study and the very low number of episodes of moderate hypoglycemia.
The potential mechanisms involved in our findings range from the well described morbidity of hypoglycemia and hyperglycemia to oxidative stress triggered by acute fluctuations of glucose levels [3,4,24]. In a case-control study of diabetic outpatients serum glucose variability showed a strong correlation with 8-iso prostaglandin F2, a marker of oxidative stress [24]. The pathophysiology behind this relationship is not clearly defined but potentially involves mitochondrial dysfunction caused by overproduction of superoxide by the mitochondrial electron-transport chain [51-53].
Our study has a number of important limitations. First, we were not able to analyze the temporal relation between the development of metabolic distress and glycemic variability, which limits any inference of causality. Second, we did not evaluate factors that might be related to variability and may influence its effect on outcome, such as intensive insulin therapy, sepsis and organ dysfunction. Third, brain glucose, lactate and pyruvate are involved in multiple biochemical pathways, being produced and consumed. This limits straightforward interpretation of their concentrations, especially as microdialysis only measures the extracellular pool. Fourth, we cannot assess mitochondrial dysfunction directly, which can cause abnormal oxidative metabolism in the presence of adequate oxygen and substrate delivery. Fifth, a glucometer was the method used in the study, which may add inaccuracy to systemic glucose measurement and potentially affect variability. Sixth, we were not able to provide some physiological parameters that may affect brain metabolism, such as body temperature and pCO2 levels. Finally, although we found an association with hospital mortality, we did not prospectively evaluate functional short and long-term outcomes, which will be critical for future studies in patients with SAH.
Conclusions
We showed that glycemic variability is associated with cerebral metabolic distress and hospital mortality in SAH patients. Our findings are hypothesis generating but may have important clinical implications. With increasing evidence that systemic glucose variability is deleterious to critically ill neurological patients, strategies aimed at minimizing acute fluctuations may play a role in glycemic control protocols in the Neurological ICU. Further studies are needed in order to determine the effect of taking into account glycemic variability in glucose control protocols. Moreover, as multimodality monitoring becomes increasingly integrated into clinical practice, randomized clinical trials are needed to assess the effect of goal-directed interventions aimed at improving cerebral metabolic profiles on long-term outcomes of patients with SAH.
Key messages
•Increased systemic glucose variability was independently associated with cerebral metabolic distress, as measured by microdialysis, in patients after poor-grade SAH.
•Glycemic variability was an independent predictor of mortality in patients with severe SAH.
•These findings suggest that glucose variability may impact cerebral oxidative metabolism and contribute to secondary brain injury.
Abbreviations
AR-1: auto-regressor of the first order; CPP: cerebral perfusion pressure; DCI: delayed cerebral ischemia; GCS: Glasgow Coma Score; GEE: generalized estimating equations; GLM: general linear model; HR: heart rate; ICP: intracranial pressure; LPR: lactate pyruvate ratio; MAP: mean arterial pressure; PbtO2: partial pressure of brain tissue oxygen; SAH: subarachnoid hemorrhage; SD: standard deviation.
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
PK, JC, RH, NB, KL, ESC and SAM conceived of the study, participated in its design and coordination and helped to draft the manuscript. PK wrote the manuscript. PK, JC, RH, JMS, LF, MP and RMS collected the data and performed the statistical analysis. All authors critically reviewed, drafted and approved the manuscript for publication. All authors read and approved the final manuscript.
Contributor Information
Pedro Kurtz, Email: kurtzpedro@mac.com.
Jan Claassen, Email: jc1439@mail.cumc.columbia.edu.
Raimund Helbok, Email: raimund.helbok@uki.at.
J Michael Schmidt, Email: mjs2134@mail.cumc.columbia.edu.
Luis Fernandez, Email: luisfernandezmd@gmail.com.
Mary Presciutti, Email: presciu@nyp.org.
R Morgan Stuart, Email: rms2130@gmail.com.
E Sander Connolly, Email: sconnolly@neuro.columbia.edu.
Kiwon Lee, Email: kl2356@mail.cumc.columbia.edu.
Neeraj Badjatia, Email: nb2217@mail.cumc.columbia.edu.
Stephan A Mayer, Email: sam14@columbia.edu.
Acknowledgements
We thank the attending physicians, fellows and nurses of the Division of Neurocritical Care, Department of Neurology/Neurosurgery, Columbia University Medical Center for their overall support of this project.
References
- Schlenk F, Nagel A, Graetz D, Sarrafzadeh AS. Hyperglycemia and cerebral glucose in aneurysmal subarachnoid hemorrhage. Intensive Care Med. 2008;34:1200–1207. doi: 10.1007/s00134-008-1044-5. [DOI] [PubMed] [Google Scholar]
- Wartenberg KE, Schmidt JM, Claassen J, Temes RE, Frontera JA, Ostapkovich N, Parra A, Connolly ES, Mayer SA. Impact of medical complications on outcome after subarachnoid hemorrhage. Crit Care Med. 2006;34:617–623. doi: 10.1097/01.ccm.0000201903.46435.35. quiz 624. [DOI] [PubMed] [Google Scholar]
- Frontera JA, Fernandez A, Claassen J, Schmidt M, Schumacher HC, Wartenberg K, Temes R, Parra A, Ostapkovich ND, Mayer SA. Hyperglycemia after SAH: predictors, associated complications, and impact on outcome. Stroke. 2006;37:199–203. doi: 10.1161/01.STR.0000194960.73883.0f. [DOI] [PubMed] [Google Scholar]
- Badjatia N, Topcuoglu MA, Buonanno FS, Smith EE, Nogueira RG, Rordorf GA, Carter BS, Ogilvy CS, Singhal AB. Relationship between hyperglycemia and symptomatic vasospasm after subarachnoid hemorrhage. Crit Care Med. 2005;33:1603–1609. doi: 10.1097/01.CCM.0000168054.60538.2B. quiz 1623. [DOI] [PubMed] [Google Scholar]
- Van den Berghe G, Schoonheydt K, Becx P, Bruyninckx F, Wouters PJ. Insulin therapy protects the central and peripheral nervous system of intensive care patients. Neurology. 2005;64:1348–1353. doi: 10.1212/01.WNL.0000158442.08857.FC. [DOI] [PubMed] [Google Scholar]
- van den Berghe G, Wouters P, Weekers F, Verwaest C, Bruyninckx F, Schetz M, Vlasselaers D, Ferdinande P, Lauwers P, Bouillon R. Intensive insulin therapy in critically ill patients. N Engl J Med. 2001;345:1359–1367. doi: 10.1056/NEJMoa011300. [DOI] [PubMed] [Google Scholar]
- Finfer S, Chittock DR, Su SY, Blair D, Foster D, Dhingra V, Bellomo R, Cook D, Dodek P, Henderson WR, Hébert PC, Heritier S, Heyland DK, McArthur C, McDonald E, Mitchell I, Myburgh JA, Norton R, Potter J, Robinson BG, Ronco JJ. NICE-SUGAR Study Investigators. Intensive versus conventional glucose control in critically ill patients. N Engl J Med. 2009;360:1283–1297. doi: 10.1056/NEJMoa0810625. [DOI] [PubMed] [Google Scholar]
- Preiser JC, Devos P, Ruiz-Santana S, Melot C, Annane D, Groeneveld J, Iapichino G, Leverve X, Nitenberg G, Singer P, Wernerman J, Joannidis M, Stecher A, Chioléro R. A prospective randomised multi-centre controlled trial on tight glucose control by intensive insulin therapy in adult intensive care units: the Glucontrol study. Intensive Care Med. 2009;35:1738–1748. doi: 10.1007/s00134-009-1585-2. [DOI] [PubMed] [Google Scholar]
- Wiener RS, Wiener DC, Larson RJ. Benefits and risks of tight glucose control in critically ill adults: a meta-analysis. JAMA. 2008;300:933–944. doi: 10.1001/jama.300.8.933. [DOI] [PubMed] [Google Scholar]
- Brunkhorst FM, Engel C, Bloos F, Meier-Hellmann A, Ragaller M, Weiler N, Moerer O, Gruendling M, Oppert M, Grond S, Olthoff D, Jaschinski U, John S, Rossaint R, Welte T, Schaefer M, Kern P, Kuhnt E, Kiehntopf M, Hartog C, Natanson C, Loeffler M, Reinhart K. German Competence Network Sepsis (SepNet) Intensive insulin therapy and pentastarch resuscitation in severe sepsis. N Engl J Med. 2008;358:125–139. doi: 10.1056/NEJMoa070716. [DOI] [PubMed] [Google Scholar]
- Bederson JB, Connolly ES Jr, Batjer HH, Dacey RG, Dion JE, Diringer MN, Duldner JE Jr, Harbaugh RE, Patel AB, Rosenwasser RH. American Heart Association. Guidelines for the management of aneurysmal subarachnoid hemorrhage: a statement for healthcare professionals from a special writing group of the Stroke Council, American Heart Association. Stroke. 2009;40:994–1025. doi: 10.1161/STROKEAHA.108.191395. [DOI] [PubMed] [Google Scholar]
- Vespa PM. The implications of cerebral ischemia and metabolic dysfunction for treatment strategies in neurointensive care. Curr Opin Crit Care. 2006;12:119–123. doi: 10.1097/01.ccx.0000216577.57180.bd. [DOI] [PubMed] [Google Scholar]
- Kramer AH, Roberts DJ, Zygun DA. Optimal glycemic control in neurocritical care patients: a systematic review and meta-analysis. Crit Care. 2012;16:R203. doi: 10.1186/cc11812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zazulia AR, Videen TO, Powers WJ. Transient focal increase in perihematomal glucose metabolism after acute human intracerebral hemorrhage. Stroke. 2009;40:1638–1643. doi: 10.1161/STROKEAHA.108.536037. [DOI] [PubMed] [Google Scholar]
- Schlenk F, Graetz D, Nagel A, Schmidt M, Sarrafzadeh AS. Insulin-related decrease in cerebral glucose despite normoglycemia in aneurysmal subarachnoid hemorrhage. Crit Care. 2008;12:R9. doi: 10.1186/cc6776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oddo M, Schmidt JM, Carrera E, Badjatia N, Connolly ES, Presciutti M, Ostapkovich ND, Levine JM, Le Roux P, Mayer SA. Impact of tight glycemic control on cerebral glucose metabolism after severe brain injury: a microdialysis study. Crit Care Med. 2008;36:3233–3238. doi: 10.1097/CCM.0b013e31818f4026. [DOI] [PubMed] [Google Scholar]
- Marcoux J, McArthur DA, Miller C, Glenn TC, Villablanca P, Martin NA, Hovda DA, Alger JR, Vespa PM. Persistent metabolic crisis as measured by elevated cerebral microdialysis lactate-pyruvate ratio predicts chronic frontal lobe brain atrophy after traumatic brain injury. Crit Care Med. 2008;36:2871–2877. doi: 10.1097/CCM.0b013e318186a4a0. [DOI] [PubMed] [Google Scholar]
- Belli A, Sen J, Petzold A, Russo S, Kitchen N, Smith M. Metabolic failure precedes intracranial pressure rises in traumatic brain injury: a microdialysis study. Acta Neurochir (Wien) 2008;150:461–469. doi: 10.1007/s00701-008-1580-3. discussion 470. [DOI] [PubMed] [Google Scholar]
- Vespa PM, O'Phelan K, McArthur D, Miller C, Eliseo M, Hirt D, Glenn T, Hovda DA. Pericontusional brain tissue exhibits persistent elevation of lactate/pyruvate ratio independent of cerebral perfusion pressure. Crit Care Med. 2007;35:1153–1160. doi: 10.1097/01.CCM.0000259466.66310.4F. [DOI] [PubMed] [Google Scholar]
- Vespa P, Boonyaputthikul R, McArthur DL, Miller C, Etchepare M, Bergsneider M, Glenn T, Martin N, Hovda D. Intensive insulin therapy reduces microdialysis glucose values without altering glucose utilization or improving the lactate/pyruvate ratio after traumatic brain injury. Crit Care Med. 2006;34:850–856. doi: 10.1097/01.CCM.0000201875.12245.6F. [DOI] [PubMed] [Google Scholar]
- Vespa PM, McArthur D, O'Phelan K, Glenn T, Etchepare M, Kelly D, Bergsneider M, Martin NA, Hovda DA. Persistently low extracellular glucose correlates with poor outcome 6 months after human traumatic brain injury despite a lack of increased lactate: a microdialysis study. J Cereb Blood Flow Metab. 2003;23:865–877. doi: 10.1097/01.WCB.0000076701.45782.EF. [DOI] [PubMed] [Google Scholar]
- Bergsneider M, Hovda DA, Shalmon E, Kelly DF, Vespa PM, Martin NA, Phelps ME, McArthur DL, Caron MJ, Kraus JF, Becker DP. Cerebral hyperglycolysis following severe traumatic brain injury in humans: a positron emission tomography study. J Neurosurg. 1997;86:241–251. doi: 10.3171/jns.1997.86.2.0241. [DOI] [PubMed] [Google Scholar]
- Kruyt ND, Biessels GJ, de Haan RJ, Vermeulen M, Rinkel GJ, Coert B, Roos YB. Hyperglycemia and clinical outcome in aneurysmal subarachnoid hemorrhage: a meta-analysis. Stroke. 2009;40:e424–e430. doi: 10.1161/STROKEAHA.108.529974. [DOI] [PubMed] [Google Scholar]
- Monnier L, Mas E, Ginet C, Michel F, Villon L, Cristol JP, Colette C. Activation of oxidative stress by acute glucose fluctuations compared with sustained chronic hyperglycemia in patients with type 2 diabetes. JAMA. 2006;295:1681–1687. doi: 10.1001/jama.295.14.1681. [DOI] [PubMed] [Google Scholar]
- Ryan EA, Shandro T, Green K, Paty BW, Senior PA, Bigam D, Shapiro AM, Vantyghem MC. Assessment of the severity of hypoglycemia and glycemic lability in type 1 diabetic subjects undergoing islet transplantation. Diabetes. 2004;53:955–962. doi: 10.2337/diabetes.53.4.955. [DOI] [PubMed] [Google Scholar]
- Brunner R, Adelsmayr G, Herkner H, Madl C, Holzinger U. Glycemic variability and glucose complexity in critically ill patients: a retrospective analysis of continuous glucose monitoring data. Crit Care. 2012;16:R175. doi: 10.1186/cc11657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermanides J, Vriesendorp TM, Bosman RJ, Zandstra DF, Hoekstra JB, Devries JH. Glucose variability is associated with intensive care unit mortality. Crit Care Med. 2010;38:838–842. doi: 10.1097/CCM.0b013e3181cc4be9. [DOI] [PubMed] [Google Scholar]
- Bagshaw SM, Bellomo R, Jacka MJ, Egi M, Hart GK, George C. The impact of early hypoglycemia and blood glucose variability on outcome in critical illness. Crit Care. 2009;13:R91. doi: 10.1186/cc7921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krinsley JS. Glycemic variability: a strong independent predictor of mortality in critically ill patients. Crit Care Med. 2008;36:3008–3013. doi: 10.1097/CCM.0b013e31818b38d2. [DOI] [PubMed] [Google Scholar]
- Dossett LA, Cao H, Mowery NT, Dortch MJ, Morris JM Jr, May AK. Blood glucose variability is associated with mortality in the surgical intensive care unit. Am Surg. 2008;74:679–685. doi: 10.1177/000313480807400802. discussion 685. [DOI] [PubMed] [Google Scholar]
- Ali NA, O'Brien JM Jr, Dungan K, Phillips G, Marsh CB, Lemeshow S, Connors AF Jr, Preiser JC. Glucose variability and mortality in patients with sepsis. Crit Care Med. 2008;36:2316–2321. doi: 10.1097/CCM.0b013e3181810378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egi M, Bellomo R, Stachowski E, French CJ, Hart G. Variability of blood glucose concentration and short-term mortality in critically ill patients. Anesthesiology. 2006;105:244–252. doi: 10.1097/00000542-200608000-00006. [DOI] [PubMed] [Google Scholar]
- Badjatia N, Strongilis E, Prescutti M, Fernandez L, Fernandez A, Buitrago M, Schmidt JM, Mayer SA. Metabolic benefits of surface counter warming during therapeutic temperature modulation. Crit Care Med. 2009;37:1893–1897. doi: 10.1097/CCM.0b013e31819fffd3. [DOI] [PubMed] [Google Scholar]
- Badjatia N, Strongilis E, Gordon E, Prescutti M, Fernandez L, Fernandez A, Buitrago M, Schmidt JM, Ostapkovich ND, Mayer SA. Metabolic impact of shivering during therapeutic temperature modulation: the Bedside Shivering Assessment Scale. Stroke. 2008;39:3242–3247. doi: 10.1161/STROKEAHA.108.523654. [DOI] [PubMed] [Google Scholar]
- Mackenzie IM, Whitehouse T, Nightingale PG. The metrics of glycaemic control in critical care. Intensive Care Med. 2011;37:435–443. doi: 10.1007/s00134-010-2103-2. [DOI] [PubMed] [Google Scholar]
- Krinsley JS. Glycemic control in the critically ill - 3 domains and diabetic status means one size does not fit all! Crit Care. 2013;17:131. doi: 10.1186/cc12584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krinsley JS. Glycemic variability in critical illness and the end of Chapter 1. Crit Care Med. 2010;38:1206–1208. doi: 10.1097/CCM.0b013e3181d3aba5. [DOI] [PubMed] [Google Scholar]
- Sarrafzadeh A, Haux D, Kuchler I, Lanksch WR, Unterberg AW. Poor-grade aneurysmal subarachnoid hemorrhage: relationship of cerebral metabolism to outcome. J Neurosurg. 2004;100:400–406. doi: 10.3171/jns.2004.100.3.0400. [DOI] [PubMed] [Google Scholar]
- Nagel A, Graetz D, Schink T, Frieler K, Sakowitz O, Vajkoczy P, Sarrafzadeh A. Relevance of intracranial hypertension for cerebral metabolism in aneurysmal subarachnoid hemorrhage. Clinical article. J Neurosurg. 2009;111:94–101. doi: 10.3171/2009.1.JNS08587. [DOI] [PubMed] [Google Scholar]
- Pan W. Akaike's information criterion in generalized estimating equations. Biometrics. 2001;57:120–125. doi: 10.1111/j.0006-341X.2001.00120.x. [DOI] [PubMed] [Google Scholar]
- Pan W. Model selection in estimating equations. Biometrics. 2001;57:529–534. doi: 10.1111/j.0006-341X.2001.00529.x. [DOI] [PubMed] [Google Scholar]
- Prentice RL, Zhao LP. Estimating equations for parameters in means and covariances of multivariate discrete and continuous responses. Biometrics. 1991;47:825–839. doi: 10.2307/2532642. [DOI] [PubMed] [Google Scholar]
- Sarrafzadeh AS, Nagel A, Czabanka M, Denecke T, Vajkoczy P, Plotkin M. Imaging of hypoxic-ischemic penumbra with (18)F-fluoromisonidazole PET/CT and measurement of related cerebral metabolism in aneurysmal subarachnoid hemorrhage. J Cereb Blood Flow Metab. 2010;30:36–45. doi: 10.1038/jcbfm.2009.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helbok R, Schmidt JM, Kurtz P, Hanafy KA, Fernandez L, Stuart RM, Presciutti M, Ostapkovich ND, Connolly ES, Lee K, Badjatia N, Mayer SA, Claassen J. Systemic glucose and brain energy metabolism after subarachnoid hemorrhage. Neurocrit Care. 2010;12:317–323. doi: 10.1007/s12028-009-9327-4. [DOI] [PubMed] [Google Scholar]
- Vespa PM, Miller C, McArthur D, Eliseo M, Etchepare M, Hirt D, Glenn TC, Martin N, Hovda D. Nonconvulsive electrographic seizures after traumatic brain injury result in a delayed, prolonged increase in intracranial pressure and metabolic crisis. Crit Care Med. 2007;35:2830–2836. doi: 10.1097/01.CCM.0000295667.66853.BC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vespa P, Bergsneider M, Hattori N, Wu HM, Huang SC, Martin NA, Glenn TC, McArthur DL, Hovda DA. Metabolic crisis without brain ischemia is common after traumatic brain injury: a combined microdialysis and positron emission tomography study. J Cereb Blood Flow Metab. 2005;25:763–774. doi: 10.1038/sj.jcbfm.9600073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarrafzadeh AS, Haux D, Ludemann L, Amthauer H, Plotkin M, Kuchler I, Unterberg AW. Cerebral ischemia in aneurysmal subarachnoid hemorrhage: a correlative microdialysis-PET study. Stroke. 2004;35:638–643. doi: 10.1161/01.STR.0000116101.66624.F1. [DOI] [PubMed] [Google Scholar]
- Hlatky R, Valadka AB, Goodman JC, Contant CF, Robertson CS. Patterns of energy substrates during ischemia measured in the brain by microdialysis. J Neurotrauma. 2004;21:894–906. doi: 10.1089/0897715041526195. [DOI] [PubMed] [Google Scholar]
- Barletta JF, Figueroa BE, Deshane R, Blau SA, McAllen KJ. High glucose variability increases cerebral infarction in patients with spontaneous subarachnoid hemorrhage. J Crit Care. 2013;28:798–803. doi: 10.1016/j.jcrc.2012.12.012. [DOI] [PubMed] [Google Scholar]
- Krinsley JS. Glycemic variability and mortality in critically ill patients: the impact of diabetes. J Diabetes Sci Technol. 2009;3:1292–1301. doi: 10.1177/193229680900300609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownlee M. The pathobiology of diabetic complications: a unifying mechanism. Diabetes. 2005;54:1615–1625. doi: 10.2337/diabetes.54.6.1615. [DOI] [PubMed] [Google Scholar]
- Suh SW, Gum ET, Hamby AM, Chan PH, Swanson RA. Hypoglycemic neuronal death is triggered by glucose reperfusion and activation of neuronal NADPH oxidase. J Clin Invest. 2007;117:910–918. doi: 10.1172/JCI30077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh SW, Hamby AM, Swanson RA. Hypoglycemia, brain energetics, and hypoglycemic neuronal death. Glia. 2007;55:1280–1286. doi: 10.1002/glia.20440. [DOI] [PubMed] [Google Scholar]


