Abstract
The microbiota plays a fundamental role on the induction, training and function of the host immune system. In return, the immune system has largely evolved as a means to maintain the symbiotic relationship of the host with these highly diverse and evolving microbes. When operating optimally this immune system–microbiota alliance allows the induction of protective responses to pathogens and the maintenance of regulatory pathways involved in the maintenance of tolerance to innocuous antigens. However, in high-income countries overuse of antibiotics, changes in diet, and elimination of constitutive partners such as nematodes has selected for a microbiota that lack the resilience and diversity required to establish balanced immune responses. This phenomenon is proposed to account for some of the dramatic rise in autoimmune and inflammatory disorders in parts of the world where our symbiotic relationship with the microbiota has been the most affected.
Introduction
“The states of health or disease are the expressions of the success or failure experienced by the organism in its efforts to respond adaptively to environmental challenges”
(Rene Dubos, 1965).
Multicellular organisms exist as meta-organisms comprised of both the macroscopic host and its symbiotic commensal microbiota. With an estimated composition of 100 trillion cells, human symbionts outnumber host cells by at least a factor of 10 and express at least 10 fold more unique genes than their host’s genome (Ley et al., 2006a). These complex communities of microbes that include bacteria, fungi, viruses and other microbial and eukaryotic species, provide a tremendous enzymatic capability and play a fundamental role in controlling most aspects of host physiology. Over the past few years, the field of immunology has been revolutionized by the growing understanding of the fundamental role of the microbiota in the induction, education and function of the mammalian immune system.
The immune system is composed of a complex network of innate and adaptive components endowed with an extraordinary capacity to adapt and respond to highly diverse challenges. Collectively this cellular network acts as a formidable regulator of host homeostasis allowing to sustain and restore tissue function in the context of microbial and environmental encounters. The development of defined arms of the immune system and more particularly the ones associated with adaptive immunity has coincided with the acquisition of a complex microbiota supporting the concept that a large fraction of this machinery has evolved as a means to maintain a symbiotic relationships with these highly diverse microbial communities. In turn the microbiota promote and calibrate all aspects of the immune system.
When operating optimally, the immune system - microbiota alliance interweaves the innate and adaptive arms of immunity in a dialogue that selects, calibrates and terminates responses in the most appropriate manner. However, both the acquisition of a complex immune system and its reliance on the microbiota came at a price. Pathologies that increasingly affect humans such as allergies, autoimmune and inflammatory disorders all arise from a failure to control misdirected immune responses against self, microbiota derived or environmental antigens. Further, alteration of the composition and function of the microbiota as a result of antibiotic use, diet evolution and recent elimination of constitutive partners such as helminth worms has transformed our microbial allies into potential liabilities. Although members of the microbiota are often referred to as commensals the symbiosis - persistent interaction- between the microbiota and its mammalian host encompasses various forms of relationship including mutualistic, parasitic or commensal. However, how defined members of the microbiota interact with their host can be highly contextual with the same microbe developing as mutualist or parasite according to the nutritional, co-infection or genetic landscape of its host. Over the past decade, exploration of optimal and dysregulated partnerships between the microbiota and its mammalian host has taken center stage in the field of immunology and led to the re-discovey of a more holistic view of host physiology. Indeed, the notion that microbial partners can promote human health is not a recent concept and was originally proposed by the seminal work of Döderlein (1892) and his understanding of the role of lactobacilli as gatekeepers of the vaginal ecosystem as well as the observation of Metchnikoff associating prolonged life with fermented milk products. Recent sequencing efforts of the human meta-genome have changed our understanding of the microbiome and how variations in these populations can contribute to disease states. In this review we will discuss some of the major concepts that have emerged from the recent dialogue between immunologists, geneticists, microbiologists and clinicians that highlight the complex role of the microbiota on the immune system in health and diseases.
Microbiota-immune system interaction during development
Under normal conditions, the fetal gastrointestinal tract is believed to be sterile with the first exposure of the immune system to commensals occurring during the passage through the birth canal. These early interactions are considered to set the tone of the mucosal and systemic immune system for the long term. The mechanism by which neonate tissues adapt to the formidable challenge of microbial colonization remains incompletely understood but factors contained in maternal milk are believed to define some of these early responses to commensals. Indeed, colostrum and breast milk contain live microbes, metabolites, IgA, immune cells as well as cytokines. These factors synergize to shape the breast-fed infant microbiota and the response of the host to these microbes. For instance, maternal IgA restricts immune activation and microbial attachment by binding nutritional and microbial antigens and the presence of metabolites including oligosaccharides in mother’s milk promote the expansion of defined constituents of the microbiota such as Bifidobacterium (Marcobal et al., 2010; Marcobal and Sonnenburg, 2012). Bacterial translocation from the mouse gut is increased during pregnancy and lactation, and bacterially loaded dendritic cells in the milk have been proposed to contribute to neonatal immune imprinting by influencing the nature of the immune response toward commensal antigens (Perez et al., 2007).
The capacity to accept the microbiota can also be explained by the relative immaturity of the neonate immune system at birth and the tolerogenic environment that defines early mammalian life. Indeed, the developing immune system is characterized by blunted inflammatory cytokine production and skewed T and B cell development in favor of regulatory responses (PrabhuDas et al., 2011; Siegrist, 2001). While a consequence of this blunted immune response is high susceptibility to infections, this regulatory environment ensures that the establishment of the microbiota occurs without overt inflammation. Recent report reveal that defined population of erythroid cells enriched in neonates contribute to the maintenance of this immunoregulatory environment and limit mucosal inflammation following colonization with the microbiota (Elahi et al., 2013). Early exposure of the host to commensals can also repress cells involved in the induction of inflammatory responses such as invariant natural killer T (iNKT) cells, an effect that has long-term consequences for the host capacity to develop inflammatory diseases (Olszak et al., 2012). A recent report proposed that this control can be mediated by the direct interaction, early in life, of unique inhibitory commensal derived sphingolipids with iNKT cells (An et al., 2014).
One of the primary modes of dialogue between the host and the microbiota is mediated by the recognition of conserved microbial associated molecular patterns (MAMPs). The neonate innate immune system integrates these signals in a unique way to promote healthy microbial colonization. For instance, although neonate innate cells express Toll Like Receptors (TLR) ligands, their response to microbial ligands is distinct from the ones of adult cells with notable impairment in the production of inflammatory mediators such as oxygen radicals and heightened production of regulatory cytokines such as IL-10 (Kollmann et al., 2012). Part of this phenomenon results from the action of the microbiota itself. Indeed, early responses to microbial ligands such as LPS, the endotoxin found in the outer membrane of gram negative bacterial walls, condition gut epithelial cells to become hypo-responsive to subsequent TLR stimulation (Chassin et al., 2010; Lotz et al., 2006). How the innate immune system integrates microbial derived signals remains unclear but recent findings support the idea that expression of epigenome modifying enzymes by epithelial cells may be required for the coordination of commensal dependent intestinal homeostasis (Alenghat et al., 2013).
Commensals also contribute to the post-natal development of the immune system that in turn contributes to their containment. Studies performed in animals raised in the absence of live microbes referred to as germ-free (GF), revealed that the microbiota plays a critical role in secondary and lymphoid structure development. This effect which is particularly evident in the gastrointestinal tract with smaller Peyer’s patch size and a reduced number of CD4+T cells and IgA producing plasma cells (Bauer et al., 1963; Hamada et al., 2002; Macpherson et al., 2001; Mazmanian et al., 2005; Smith et al., 2007; Talham et al., 1999). In the intestine, tertiary lymphoid structures such as isolated lymphoid follicle or crytopatches are induced after birth as a result of commensal exposure (Bouskra et al., 2008; Ohnmacht et al., 2011). As further discussed below, commensals can also contribute to the fortification of the intestinal barrier by various mechanisms including the promotion of epithelial cell maturation and angiogenesis (Hooper et al., 2001; Stappenbeck et al., 2002).
When operating properly, the highly regulatory tone of the neonate immune system and the action of commensals in the development and training of this system lead to the establishment of a durable and homeostatic host/commensal relationship. These primary encounters between the host immune system and the microbiota have profound and long-term implications for human health. Indeed, epidemiological observations revealed that alteration of the microbiota in mothers or in neonates may predisposes to diseases associated with dysregulated barrier responses such as asthma (Ege et al., 2011)
Containing the microbiota
An important point to consider when exploring the role of the microbiota on the immune system is that pathogenicity is, in most cases, a contextual state. Indeed, the capacity of a given microbe, including the ones composing the microbiota, to trigger or promote disease is highly dependent on the state of activation of the host, the host’s genetic predisposition and the localization of the particular microbe. As such, the mechanisms utilized by the immune system to maintain its relationship with the microbiota are highly analogous to the ones that are used to constrain organisms with pathogenic potential.
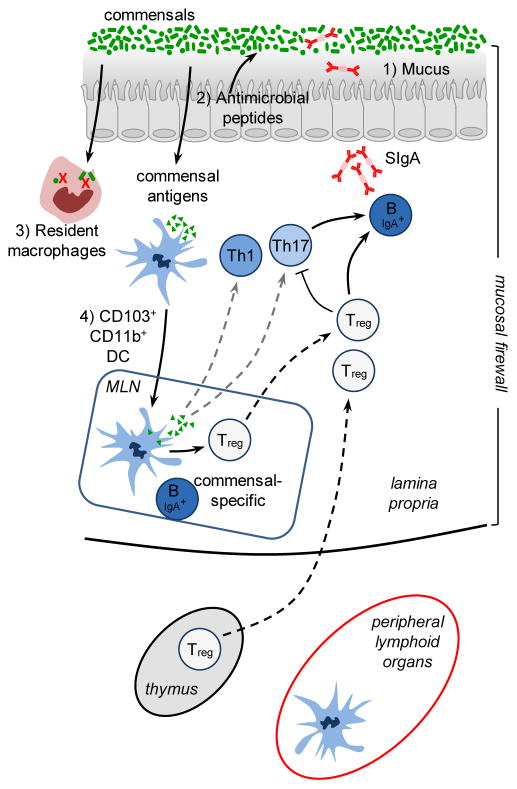
An enormous fraction of the immune system’s constitutive function is aimed at controlling our relationship with the microbiota. As such the highest number of immune cells in the body are resident at sites colonized by commensals such as the skin or the GI tract. In turn, in order to protect their ecological niche, a dominant action of the healthy microbiota on the immune system is aimed at reinforcing barrier immunity and therefore their own containment. A central strategy utilized by the host to maintain its homeostatic relationship with the microbiota is to minimize contact between microorganisms and the epithelial cell surface thereby limiting tissue inflammation and microbial translocation. In the gastrointestinal tract, home to the largest density of commensals, this segregation is accomplished by the combined action of epithelial cells, mucus, IgA, antimicrobial peptides and immune cells. Collectively these structural and immunological components have been referred to as the “mucosal firewall” (Figure 1) (Macpherson et al., 2009).
Figure 1. The mucosal firewall.
1) The mucus represents the primary barrier limiting contact between the microbiota and host tissue and preventing microbial translocation. 2) Epithelial cells produce antimicrobial peptides that also play a significant role in limiting exposure to the commensal microbiota. 3) Translocating commensals are rapidly eliminated by tissue resident macrophages. 4) Commensals can also be captured by CD103+ CD11b+ DCs that traffic to the mLN from the lamina propria but do not penetrate further. Presentation of commensal antigens by these DCs leads to the differentiation of commensal specific regulatory cells (Treg) Th17 cells and IgA producing B cells. Commensal specific lymphocytes traffic to the lamina propria and Peyer’s Patches. In the Peyer’s patches Treg can further promote class switching and IgA generation against commensals. The combination of the epithelial barrier, mucus layer, IgA and DCs and T cells comprises the ‘mucosal firewall’, which limits the passage and exposure of commensals to the Gut-Associated Lymphoid tissue preventing untoward activation and pathology.
The mucus represents the primary shield limiting contact between the microbiota and host tissue and preventing microbial translocation (McGuckin et al., 2011). In addition to the production of mucus by goblet cells, all intestinal epithelial cell lineages can produce antimicrobial peptides that play a significant role in limiting exposure to the commensal microbiota (Hooper and Macpherson, 2010). These proteins can exert antimicrobial functions resulting from enzymatic attack of the bacterial cell wall or by disrupting the bacterial inner membrane (Hooper and Macpherson, 2010). Some of these molecules such as α-defensins are constitutively expressed by epithelial cells while in other cases, engagement of pattern recognition receptors (PRRs) by commensally derived products is required (Hooper and Macpherson, 2010). One of the best-characterized mucosal antimicrobial peptides is RegIIIγ, which is expressed soon after birth or following colonization of germ-free mice (Cash et al., 2006). Production of this lectin is tightly controlled by the flora in a MyD88 dependent manner and has a direct microbicidal effect on gram-positive bacteria (Brandl et al., 2007; Cash et al., 2006; Ismail et al., 2011; Mukherjee et al., 2009). Accumulation of antimicrobial peptides such as RegIIIγ in the mucus contributes to the maintenance of the segregation between the microbiota and the host intestine creating a physical separation referred to as the “demilitarized zone” (Vaishnava et al., 2011).
Compartmentalization of intestinal bacteria also depends on secreted immunoglobulin A (IgA). IgA specific for commensals is produced with the help of intestinal dendritic cells that sample commensals associated with the epithelium and interact with B and T cells in the Peyer’s patches to produce IgA specific for commensal derived antigens (Macpherson and Uhr, 2004). Further, commensals that translocate across the intestinal epithelial cell barrier can be rapidly engulfed and eliminated by macrophages that reside in the lamina propria or carried alive by dendritic cells (DC) (Kelsall, 2008; Macpherson and Uhr, 2004). The bacteria loaded DC traffic to the mesenteric lymph node via the intestinal lymphatics but do not penetrate further allowing the induction of a mucosal compartmentalized IgA response (Macpherson and Uhr, 2004). IgA+B cells migrate to the intestinal lamina propria and secrete IgA that are subsequently transcytosed across epithelial cells. These transcytosed IgAs control host commensal interaction by both impacting commensal gene expression (Peterson et al., 2007) and preventing adhesion of commensal bacteria to the epithelial surfaces (Figure 1) (Boullier et al., 2009; Fernandez et al., 2003; Hornquist and Lycke, 1995; Martinoli et al., 2007; Wade and Wade, 2008; Wei et al., 2011). Mucosa IgA responses lack classical memory characteristics and are able to respond to flux in commensal microbiota composition. Indeed, established IgA producing clones are outcompeted by novel anti-bacterial responses allowing the mucosal immune system to respond to a constantly changing microbiota (Hapfelmeier et al., 2010).
Most activated or memory T cells reside in tissues that are constitutively colonized by commensals such as the skin and the GI tract. Notably, at steady state most IL-17 (Th17) and IFNγ (Th1) T cells are found in the GI tract and develop from signals derived from the microbiota (Gaboriau-Routhiau et al., 2009; Ivanov et al., 2008; Macpherson and Harris, 2004). The current view is that constitutive sensing of commensals plays an important homeostatic role while active responses against the flora is believed to be associated with pathogenesis. However, this distinction is clearly not absolute and needs to be revisited in light of the observation that healthy human serum normally contains antibodies and T cells specific to commensals (Ergin et al., 2011; Macpherson et al., 1996) suggesting that a certain degree of commensal recognition is a common occurrence and in most circumstances, is not associated with pathogenic responses. Although tissue derived cues can dictate the induction and maintenance of Th17 cells irrespective of antigen-specificity, we could speculate that in a similar manner to that proposed for Treg cells, antigenic specificities of tissue resident effector T cells are highly enriched for commensal antigens. Notably, Th17 cells produce cytokines such as IL-17 and more particularly IL-22 that contribute to the homeostatic dialogue with commensals via the capacity of these cytokines to act on epithelial cell function. Failure to maintain the Th17 lineage in the gut, as observed during HIV or SIV infection is associated with microbial translocation that contributes to dissemination of the virus (Klatt et al., 2013). The action of IL-22 on the mucosal immune system is highly pleiotropic and promotes the production of antimicrobial peptides, enhances of epithelial regeneration, increases mucus production and regulates of wound repair (Wolk and Sabat, 2006; Zenewicz and Flavell, 2011). This cytokine can also be produced by other cell lineages and more particularly a population of gut resident innate cells referred to as group 3 innate lymphoid cells (ILCs). Although some reports propose that the development of these cells is independent of signals derived from the microbiota, their phenotype, and functional capacity can evolve to accommodate physiologic alterations in the intestinal environment following microbial colonization at birth (Satoh-Takayama et al., 2008). Production of IL-22 by ILC promotes the containment of specific members of the microbiota community and more particularly microbes that reside in mucosal lymphoid structures, such as bacteria of the Alcaligenes genus (Qiu et al., 2013; Sonnenberg et al., 2012). Thus, in addition of broad and non-specific modes of commensal containment, discrete pathways may have evolved to promote the selective containment of communities of microbes residing in unique ecological niches.
Induction of regulatory responses by the microbiota
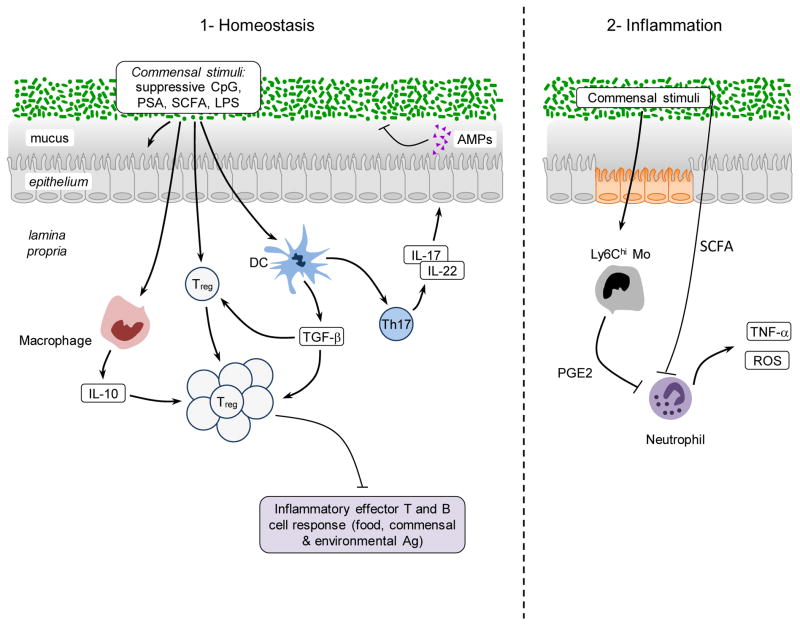
Maintenance of tissue homeostasis is an imperative to host survival. This fundamental process relies on a complex and coordinated set of innate and adaptive responses that selects and calibrates responses against self, food, commensals and pathogens in the most appropriate manner. To this end, specialized populations of cells have to integrate local cues such as defined metabolites, cytokines, or hormones allowing the induction of responses in a way that preserve the physiological and functional requirements of each tissue. As such, the regulatory pathways that are involved in the maintenance of a homeostatic relationship with the microbiota are likely to be tissue specific. However, most of our current understanding of commensal dependent regulatory pathways relates to the gastrointestinal environment. In the gut, the formidable challenge represented by the exposure to the microbiota, food derived antigens, metabolites and pathogens requires a highly complex network of regulatory pathway which is only beginning to be understood (Figure 2). Failure to regulate these responses can lead to severe pathological outcomes ranging from Inflammatory Bowel Diseases (IBD), allergies or, as further discussed, metabolic syndromes.
Figure 2. Promotion of immune regulation by the microbiota during steady state and inflammation.
1) Commensals promote the induction of regulatory T cells via direct sensing of microbial products or metabolites by T cells or dendritic cells. Further commensals promote the induction of Th17 cells that can regulate the function and homeostasis of epithelial cells. In the context of inflammation similar mechanisms may account for the regulatory role of the microbiota. 2) Commensal derived metabolites can have a systemic effect on inflammatory cells. For example, SCFA can inhibit neutrophil activation. Upon entrance in the tissue inflammatory monocytes can also respond to microbial derived ligands by producing mediators such as PGE2 that limit neutrophil activation and tissue damage.
Commensals are a critical and active inducer of regulatory responses. Notably, the establishment of tolerance - the active suppression of inflammatory responses to food and other orally ingested antigens - could not be induced in the absence of gut flora derived signals (Kiyono et al., 1982; Sudo et al., 1997; Wannemuehler et al., 1982; Weiner et al., 2011). Although immunological tolerance is likely to be achieved via multiple and redundant mechanisms (Weiner et al., 2011), over the past few years, Foxp3 regulatory T (Treg) cells have taken central stage in our understanding of this process. These cells maintain both peripheral and mucosal homeostasis throughout the lifespan of the host and disruption of the homeostasis of these cells results in loss of oral tolerance and development of aberrant effector responses in the gut (Josefowicz et al., 2012b; Mucida et al., 2005; Worbs et al., 2006). Although Foxp3+ Treg can arise as differentiated cells in the thymus, the gastrointestinal tract environment is a privileged site for the induction of Treg cells in response to oral antigens (Coombes et al., 2007; Mucida et al., 2005; Sun et al., 2007). A current consensus is that optimal maintenance of tolerance to commensal and environmental antigens requires the combined effect of both thymically and GI induced Treg (Cebula et al., 2013; Josefowicz et al., 2012a; Lathrop et al., 2011). This specialized property of the gut to induce Treg can be at least in part explained by the presence of defined populations of antigen-presenting cells such as the CD103+CD11b+ DC endowed with the capacity to produce factors involved in the induction of Treg such as the cytokine TGF-β and the vitamin A metabolite retinoic acid (RA)(Coombes et al., 2007; Mucida et al., 2007; Sun et al., 2007). Tissue-specific factors such as Vitamin A and MUC2, a mucus glycoprotein produced by intestinal goblet cells, contribute to the regulatory specialization of mucosal dendritic cells (Klebanoff et al., 2013; Shan et al., 2013). The importance of this pathway for the control of mucosal homeostasis is highlighted by the finding that a proportion of induced Treg in the colonic tissue are specific for antigens derived from the commensal microbiota (Lathrop et al., 2011). Induction of Treg cells is proposed as one of the mechanisms of action of probiotics - defined bacteria known to confer a health benefit to the host. Indeed, some of the regulatory effects of probiotics in the context of inflammatory diseases and atopic eczema in neonates and infants is believed to be associated with the induction or expansion of Treg (Di Giacinto et al., 2005; Feleszko et al., 2007; Karimi et al., 2009; Zoumpopoulou et al., 2008) and the manipulation of mucosal DCs towards a pro-regulatory function (Foligne et al., 2007; Smits et al., 2005). Commensals can also control oral antigen sampling by mucosal DCs and promote the induction of lamina propria resident macrophages associated with local expansion of Treg cells (Chieppa et al., 2006) (Niess and Adler, 2010). Aside from the direct influence of the microbiota on the immune machinery associated with the induction of oral tolerance, commensal-specific Treg can promote class-switching to IgA in an antigen-specific manner (Cong et al., 2009; Tsuji et al., 2009) thereby controlling the host relationship with the microbiota via multiple mechanisms (Peterson et al., 2007; Suzuki et al., 2004) (Figure 2).
The first demonstration that a unique symbiont molecule could promote regulatory responses was provided by the identification of the polysaccharide A (PSA) which is produced by a prominent human symbiont Bacteroides fragilis (Mazmanian et al., 2005). B. fragilis, via PSA expression, can protect mice from experimental colitis induced by Helicobacter hepaticus, a commensal bacterium with pathogenic potential (Mazmanian et al., 2008). This protective activity was associated with the capacity of PSA to induce and expand IL-10 producing Treg cells (Mazmanian et al., 2008; O’Mahony et al., 2008; Ochoa-Reparaz et al., 2010a). B. Fragilis was able to promote Treg cell function and induction via engagement of the microbial derived PSA with TLR2 expressed by T cells, a phenomenon associated with the capacity of this bacterium to also limit Th17 responses (Round et al., 2011). The discovery of a link between defined members of the microbiota and the induction of regulatory cells able to limit mucosal inflammation and promote tolerance led to a rational approach for the identification of the next generation of probiotics with superior capacity to induce Treg cells. Induction of Treg cells is not restricted to B. fragilis as the presence of an indigenous Clostridium species also promotes Treg cell accumulation via, at least in part, its capacity to create a TGF-β rich environment (Atarashi et al., 2011). Of note, optimal induction of Treg in the colonic environment relies on the synergistic effect of a consortium of Clostridia strains while individual species have a modest effect on the immune system (Atarashi et al., 2013). Based on the fundamental role of Treg in maintaining mucosal homeostasis, it is likely that rather than being restricted to defined commensals, a large fraction of any given microbiota or microbiota metabolism may have evolved to favor this aspect of the regulatory network. Indeed, recent findings have shown that many organisms can increase the frequency of Tregs in the colon (Faith et al., 2014; Geuking et al., 2011).
Mammals rely on bacteria to break down undigestible dietary components such as fibers (Ley et al., 2006a). One dominant metabolite resulting from this process are short chain fatty acids (SCFA), ubiquitous bacterial fermentation products highly enriched in the colonic environment (Cummings et al., 1987). Although a role for SCFA in controlling various aspects of immune responses has been long recognized (Meijer et al., 2010), their link to lymphocyte function has only recently been appreciated. Notably, SCFA and in particular butyrate regulate the size and function of the regulatory T cell network by promoting the induction and fitness of regulatory T cells in the colonic environment (Arpaia et al., 2013; Furusawa et al., 2013; Smith et al., 2013). Butyrate is well known to regulate gene expression epigenetically by inhibiting histone deacetylases (HDACs) (Davie, 2003) and this property is currently proposed as an underlying mechanism for enhanced Treg generation in the gut. The action of SCFA likely results from the manipulation of various cells involved in the induction of regulatory responses and indeed the effect of SCFA on both T cell and dendritic cells have been linked to this process (Arpaia et al., 2013; Furusawa et al., 2013; Smith et al., 2013).
Altogether these results reveal a major role for the microbiota in shaping the repertoire, number and activation of tissue resident Treg cells and in the maintenance of host-microbe mutualism at barrier sites. Based on the abundance and complexity of the flora, one could also speculate that opportunity for cross reactivity between commensals, pathogenic and environmental antigens is high. Thus, microbial pressure in the gut could lead to the induction and maintenance of a pool of activated Treg cells that may not only contribute to the maintenance of a mutualistic relationship with the microbiota, but also to the systemic control of immune responses.
One of the first demonstrations of the protective role of commensals during acute injury was provided by the observation that, in the gut, TLR activation by commensals was required to promote tissue repair and host survival (Rakoff-Nahoum et al., 2004). Part of the protective effect of the microbiota in the context of inflammation relates to its capacity to sustain the aforementioned regulatory network (Arpaia et al., 2013; Bouladoux et al., 2012; Furusawa et al., 2013; Smith et al., 2013). Commensal derived products can also act by controlling directly or indirectly the function of inflammatory cells. For instance recognition of the commensal derived metabolites SCFA by innate immune cells is critical for the regulation of inflammation in response not only to intestinal injury but also in models of arthritis and allergy (Maslowski et al., 2009). Further commensals can also tune the function of inflammatory monocytes a population of cells involved in the control of pathogens (Figure 2). During acute mucosal infection, encounter of inflammatory monocytes with the microbiota in the gastrointestinal tract promotes their production of the lipid mediator PGE2 that in turn limits the level of activation of tissue damaging neutrophils (Grainger et al., 2013). Although most of what is known today about the regulatory properties of the microbiota arise from the exploration of the bacterial component of the flora, other microbes such as fungi and virus are likely to promote similar or complementary aspects of the regularly network. In the gastrointestinal tract, experimentally, interaction of commensal fungi with the C type lectin receptor Dectin 1 was able to prevent inflammation in the context of acute mucosal injury (Iliev et al., 2012).
Induction of protective responses by commensals
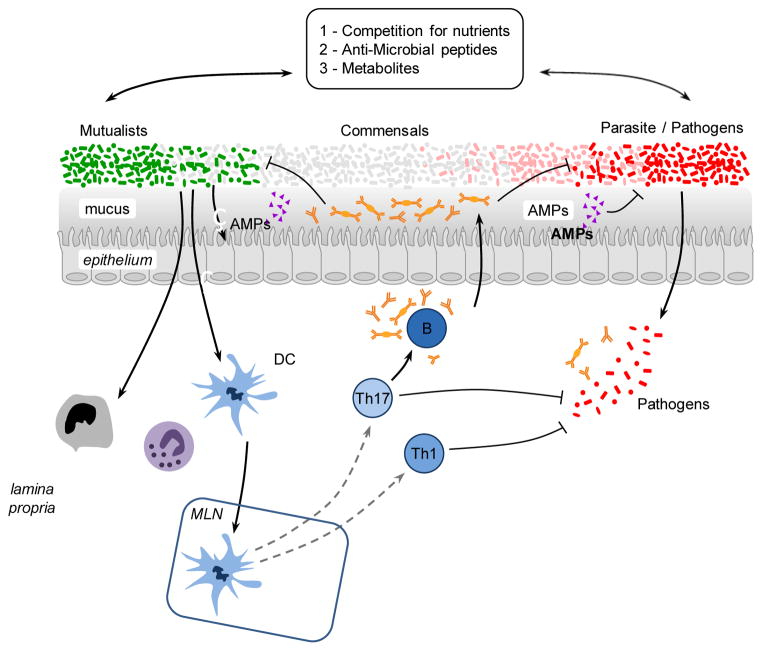
Tissues that are natural habitats of the microbiota such as the skin, the GI tract or the lung are also the portals by which pathogens access the host and often the primary site of infections. This implies that the initial encounter of pathogens with the immune system occurs in an environment conditioned and regulated by its endogenous microbiota. As such the fate of commensals and pathogens (as well as their classification) is highly interdependent (Figure 3). Notably, commensals can directly and dynamically interact with pathogens and immune cells and the results of this interaction can define the pathogenesis and outcome of a given infection.
Figure 3. Promotion of protective immunity by the microbiota.
The symbiosis between the microbiota and its mammalian host encompasses multiple of relationships including mutualistic, parasitic and commensal. The capacity of a given microbe, including the ones composing the microbiota, to trigger or promote disease is highly contextual and most microbes can shift from mutualist to commensal to parasite according to the state of activation of the host, co-infection or localization. Commensals can control microbes with pathogenic potential (as normal constituent of the microbiota or acquired) via distinct mechanisms. Commensals can compete for nutrients, produce antimicrobial molecules and metabolites that affect the survival and virulence of pathogens. Commensals can promote the production of antimicrobial peptides by epithelial cells and reinforce tight junctions. Commensals can modulate the function of dendritic cells and other innate cells both locally and systemically in a manner that promotes the induction of effector T and B cells responses against pathogens. When uncontrolled, this adjuvant property of the microbiota can promote inflammatory and autoimmune disorders.
Protection of the host from exogenous pathogens by commensal bacteria, a phenomenon referred to as colonization resistance was described more than five decades ago (Buffie and Pamer, 2013; van der Waaij et al., 1971). One of the major forms of interaction between the microbiota and invading microbes relates to the need for both forms of organisms to compete for the same ecological niche. Consequently, commensals have been shown to limit pathogen colonization though competition for defined metabolites in a process referred to as colonization resistance (Kamada et al., 2013). Alteration of nutrient availability by the host microbiota can also have profound consequences on the expression of virulence genes and growth rate of pathogens such as enterohemorragic E. coli, or Clostridia difficile (Kamada et al., 2013; Ng et al., 2013; Pacheco et al., 2012). Manipulation of microbial virulence can also occur as a consequence of commensal metabolism. In some instances, the very same metabolites involved in the manipulation of the immune system such as SCFA can also directly act on pathogens by down regulating the expression of virulence genes such as those encoding the type 3 secretion system in Salmonella enterica and typhimurium (Gantois et al., 2006). Commensals can also promote the establishment of an environment hostile for pathogen establishment with the best illustration in the vaginal environment in which lactobacilli can protect from pathogenic colonization via reduction of the local pH (Turovskiy et al., 2011). Finally, commensals can also produce antimicrobial peptides that directly affect pathogen growth or survival (Hammami et al., 2013). For instance, E coli produces bacteriocins -proteinaceous toxins that specifically inhibit the growth of the same or similar bacterial specie- therefore, impairing the growth of the related pathogen enterohemorrhagic E. coli. Additionally the dominant skin commensal Staphylococcus epidermidis produces several antimicrobial proteins and proteases that can limit the biofilm formation of Staphylococcus aureus (Iwase et al., 2010; Schamberger and Diez-Gonzalez, 2002) (Figure 3).
In tandem of its direct action on invasive microbes, the microbiota’s capacity to control infection is also associated to its remarkable ability to promote and calibrate both innate and adaptive immunity (Molloy et al., 2012). Indeed, commensals play a fundamental role in both the training of the immune system and its functional tuning thereby acting as adjuvants to the immune system as a whole. As previously discussed, commensals can reinforce defined components of barrier immunity, an effect that has the dual function of promoting their own containment and limiting pathogen invasion. For example, the commensal-dependent antimicrobial peptide RegIIIγ not only contributes to maintenance of segregation between the microbiota and host epithelial cells but also promotes protection against vancomycin resistant Enterococcus faecalis (Brandl et al., 2008; Vaishnava et al., 2008; Vaishnava et al., 2011). An important role of the microbiota is associated with its capacity to condition cells to respond to infectious challenge both systemically and locally. For instance, commensals can tune innate cells in a way that allow them to rapidly respond to pathogen encounters. An example of this control is provided by the capacity of the gut microbiota to control the production of IL-1β, a cytokine involved in host defense. The microbiota contributes to the homeostatic production of pro-IL-1β by intestinal resident macrophages in a MyD88 dependent manner thereby priming these cells to respond rapidly to enteric infections by conversion of pro-IL-1β to mature active IL1β (Franchi et al., 2012).
Some of the stimulatory effects of the microbiota can be attributed to dominant microbial derived signals. Indeed, commensals and pathogenic microbes interact with the host immune system through conserved ligands that are immutable features of microorganisms (Sansonetti and Di Santo, 2007). Many of these ligands signal through the Toll-like and Nod-like families of receptors (Sansonetti and Di Santo, 2007; Takeda et al., 2003). One example of this is flagellin, a structural protein that forms the main portion of flagella and promotes bacterial chemotaxis, adhesion and invasion of host tissue by pathogenic bacteria. Flagellin is also expressed by a large number of commensal bacteria and several lines of evidence indicate that interaction of commensal flagellin with Toll-like receptor 5, in particular in the context of barrier breach, plays an important role in the promotion of mucosal immunity. Notably, dendritic cells that reside in the lamina propria of the intestine are poised to respond to flagellin by rapidly expressing chemokines, antimicrobial peptides as well as cytokines involved in the initiation of immune responses (Kinnebrew et al., 2012; Uematsu et al., 2008; Uematsu et al., 2006). In response to flagellin, CD103+ DC produce IL-23 in part to induce IL-22 by innate lymphoid cells (ILCs), thereby promoting epithelial mediated host defense (Kinnebrew et al., 2012). Unmethylated cytosine phosphate guanosine (CpG) dinucleotides, which are abundant within the prokaryotic DNA of intestinal flora, can contribute to intestinal homeostasis under steady state conditions (Hall et al., 2008) and constitutive interaction between CpG expressing commensal DNA and TLR9 can act as a local adjuvant of immune responses (Hall et al., 2008). Nonetheless, under homeostatic conditions both inflammatory and regulatory signals are constantly integrated the sum of these signals leading to the establishment of an immune tone compatible with tissue immunity.
The capacity of the microbiota to stimulate innate responses translates into its important role in the induction of adaptive immunity (Figure 3). Indeed, early studies have identified significant impaired host immune responses to pathogens in mice treated with antibiotics or raised under germ free conditions (Cebra, 1999; Hall et al., 2008; Ivanov et al., 2009; Mazmanian et al., 2005). One of the first demonstrations of this ‘adjuvant’ effect was revealed in a parasitic model of small intestine infection with Encephalitozoon cuniculi in which protective Th1 and Th17 responses were severely compromised in the absence of commensals (Hall et al., 2008). Similarly, protective Th17 responses failed to develop in response to Citrobacter rodentium, a model of attaching and effacing infection that primarily affects the large intestine (Ivanov et al., 2009). The adjuvant property of the microbiota has also been revealed in a model of oral vaccination (Hall et al., 2008), an observation that may help to explain some of the failures of oral vaccination in developing countries in which malnutrition and infection have profoundly affected the microbiota (Korpe and Petri, 2012).
In addition to the pleiotropic effects of conserved microbial ligands or metabolites in the education and function of the immune system, it is now becoming clear that unique microbes or groups of bacteria can dominantly influence immune system development and function under steady state and inflammatory conditions. In the language of ecological systems these organisms of paramount importance are termed ‘keystone species’. The prototype of a keystone species in the GI tract is represented by the interaction between Segmented Filamentous Bacteria (SFB) and the immune system. Under steady state conditions, this pore forming Gram-positive anaerobic bacteria colonizes the terminal ileum of mice. SFB has a dominant effect on the mucosal immune system by promoting the accumulation of both Th17 and Th1 cells in the small intestine and driving the production of IgA (Gaboriau-Routhiau et al., 2009; Ivanov et al., 2009; Umesaki et al., 1999). In contrast to most commensals that reside outside of the “demilitarized zone”, SFB interacts closely with the mucosal tissue via tight adhesion to Peyer’s Patches and epithelial cells, inducing cytoskeletal reorganization in these cells at the site of contact. (Ivanov et al., 2008; Sczesnak et al., 2011). This intimate contact with epithelial cells, a property shared by a select minority of commensal organisms, is believed to account for the capacity of SFB to heighten tissue immunity and promote protective responses to pathogens (Ivanov et al., 2009). The presence of bacteria with a keystone role on the immune system raises an intriguing possibility. Although optimal control of host metabolism and physiology may rely on complex and redundant populations of microbes we could speculate that a more limited number of microbes may act as adjuvants of immune responses. Indeed, over-population of the GI tract with bacteria that have enhanced inflammatory potential can have local and systemic pathological consequences. Under steady state conditions these ambivalent members of the microbiota such as E. coli or SFB are maintained in check by the immune system but coopted by the host to control invasive microbes.
This protective effect of the microbiota has been revealed in clinical and experimental settings in which broad antibiotic treatment can allow the domination of intestinal microbiota with drug resistant microbes such as vancomycin resistant enterococcus (VRE), a pathogen that causes bloodstream infections in immunocompromised patients (Buffie and Pamer, 2013; Murray, 2000). Infections caused by multidrug-resistant organisms are on the rise and have developed into endemic and epidemic situations worldwide (Gupta et al., 2011). Harnessing the microbiota to combat these infections represents an important therapeutic avenue with the most spectacular results obtained thus far in the context of Clostridium difficile colitis (van Nood et al., 2013). During this recurrent infection, transfer of a microbiota from a healthy donor eradicated the infection with a remarkable efficiency (van Nood et al., 2013). Again highlighting the concept of defined microbes endowed with superior adjuvant capacity, the protective effect of the microbiota in VRE infected patient was highly dependent on the presence of the commensal Barnesiella (Ubeda et al., 2013).
Thus, the microbiota is a required component of the effector response of the host. Although currents studies are attempting to link defined microbes to unique immunological states in human, the microbiota is a highly dynamic and complex composite of microbes all expressing a large number of potential ligand and metabolites. Under homeostatic conditions both inflammatory and regulatory signals are constantly integrated, the sum of these signals leading to the establishment of a controlled inflammation compatible with tissue immunity. Therefore, under most settings changes in inflammatory state associated with the microbiota are unlikely due to a single microbial product or metabolite but may result from a shift in the balance of signals.
Systemic control of protective immunity
While it is readily accepted that shifts in the gut microbiota composition and density can affect local immune responses, it is becoming apparent that these changes can also alter immunity and inflammation in organs distal from the intestine(Belkaid and Naik, 2013). For instance, reduction of gut commensals via broad-spectrum antibiotic treatment results in blunted T and B cell response against intranasal infection with influenza (Ichinohe et al., 2011). This effect of the microbiota is linked to its capacity to promote the inflammasome-mediated induction of IL-1β and IL-18 secretion (Ichinohe et al., 2011). In this setting rectal administration of TLR agonists restored protective immune responses indicating that either the microbial products are capable of diffusing systemically, or that inflammasome activation does not need to occur at the site of infection (Ichinohe et al., 2011). Antibiotic treatment also impaired adaptive and innate antiviral responses following exposure to systemic Lymphocytic Choriomeningitis Virus (LCMV) and musosal influenza virus (Abt et al., 2012). Genome wide transcriptional profiling of macrophages from antibiotic treated mice revealed a broad decrease of genes associated with antiviral immunity (Abt et al., 2012). This bystander control of peripheral responses can be, at least in part, explained by the unique requirement of the GI tract for absorption resulting in the constant diffusion of low-level microbial products such as TLR or NOD ligands and metabolites into the bloodstream. For instance, commensal derived peptidoglycan can be found in the serum and have been shown to improve the killing of Streptococcus pneumonia and Staphylococcus aureus by bone-marrow derived neutrophils in a NOD1 dependent manner (Clarke et al., 2010). Experimental evidence suggests that the tonic sensing of commensal products or metabolites present in the blood stream also contributes to steady-state hematopoiesis and monocyte egress from the bone marrow (Maslowski et al., 2009; Shi et al., 2011).
Recent evidence implies that the capacity of commensals to calibrate systemic immunity has profound consequences in the context of immunotherapy. Total body irradiation, used in defined settings of immunotherapy and bone marrow transplantation, is associated with gut damage and microbial translocation, providing an adjuvant effect to the transferred anti-tumoral T cells (Paulos et al., 2007). A similar mechanism is proposed to explain the protective role of the microbiota in the context of chemotherapy. Cyclophosphamide, a clinically important cancer drug, also leads to intestinal damage, bacterial dysbiosis and translocation and induction of anti-commensal Th17 responses that, collectively, contribute to the anti-tumoral response (Viaud et al., 2013). Some of the effect of the microbiota can also occur independently of gut damage. Indeed, disruption of the gut flora via antibiotic treatment or in germ free mice impairs the capacity of the host to control subcutaneous tumors during immunotherapy (Iida et al., 2013). In these experimental settings, the protective effect results from the capacity of the commensal derived ligands to control the status of activation of tumor myeloid cells and more particularly their level of TNF-α and reactive oxygen species both associated with optimal tumor control (Iida et al., 2013). Remarkably tumor control was associated with the presence of defined commensal species such as Alistipes shahii (Iida et al., 2013). Thus, commensals, and more particularly defined member of the microbiota, can control various aspects of immunity associated with anti-tumoral responses, an effect that has profound clinical implications.
Altogether, these data reveal that exposure to microbial ligands shape systemic immunity at both the steady state and in the context of inflammation. The basal tuning of the immune system associated with constant sensing of microbial products or ligands implies that subtle changes in this conditioning may have long-term consequences on the capacity of the host to mount systemic immune responses and develop inflammatory diseases. The mechanism underlying this phenomenon remains incompletely understood but we could speculate that commensal bacteria-derived signals can influence gene expression profiles of immune cells via epigenetic modifications of genes involved in innate responses thus creating a transcriptional state that enables basal expression of host-defense factors and rapid responses upon encounter with a pathogens. How permanent the effects of microbial sensing are and to what extent tissue and hematopoetic stem cells are permanently influenced by this tonic sensing remains to be explored.
Compartmentalized control of tissue immunity
Although the above mentioned observations propose that gut commensals can control the systemic threshold of activation of innate and adaptive cells, these studies do not exclude a direct role for commensals residing in the lung, skin or other barrier sites in the control of local immunity. Indeed, microbial surveys unveiled the remarkable partitioning of commensals within the human body with each tissue and microenvironment hosting unique microbial communities (Belkaid and Naik, 2013). Thus, each barrier tissue is a complex and in some cases, unstable composite of microbes and host structural, hormonal, nervous and immunological networks, with each of these systems potentially controlled by resident microbiota.
The skin, the largest organ of the body, represents a critical interface between the host and the environment. Unbiased microbial sequencing has shown the presence of highly diverse and specific commensal niches along distinct topographical sites of the skin (Costello et al., 2009; Grice et al., 2009). Although the skin is a rather inhospitable environment, poor in nutrients and moisture, up to 1 billion bacteria inhabit a typical square centimeter of human skin, covering the surface and extending down into the sebaceous glands and hair follicles (Grice et al., 2008). In contrast to the known role of the gut microbiota in promoting the gastrointestinal associated lymphoid tissue (GALT) development, skin commensals are not required for the development of associated lymphoid tissue (Naik et al., 2012). The skin resident bacteria, such as Staphylococcus epidermidis, can control fundamental aspects of local immunity and tissue repair (Lai et al., 2009) (Naik et al., 2012). Skin commensals do not affect the capacity of T cells to be primed or to migrate to the skin, but modulate dermal T cell function by tuning the cutaneous inflammatory milieu and more particularly the production of IL-1α that in turn directly controls the capacity of dermal resident T cells to produce inflammatory cytokines such as IFN-γ and IL-17A (Naik et al., 2012). Thus in contrast to the role of the gut microbiota, the action of skin commensals on the local immune system is discrete and highly compartmentalized.
The oral cavity also harbors a unique and complex microbial community accumulating on both the hard and soft oral tissues in sessile biofilms (Avila et al., 2009). One of the proposed roles of the oral microbiome on the immune system is associated with its capacity to promote inflammasome activity leading to the local increase of the inflammatory cytokine IL1β (Dixon et al., 2004). At other sites such as the lung or vaginal mucosa the role of commensals on tissue immunity remains largely unknown. In the absence of commensals, the number of infiltrating Th2 lymphocytes and eosinophils was elevated and the composition and status of activation of lung dendritic cells was altered during airway inflammation (Herbst et al., 2011). Additionally, intranasal priming of mice with live or heat-inactivated Lactobacillus can dampen local responses thereby protecting against lethal sequelae infection with the virulent pathogen, pneumonia virus of mice (PVM) (Gabryszewski et al., 2011; Garcia-Crespo et al., 2012). These results are consistent with the notion that commensal bacteria, in most tissues, can establish a threshold of activation and regulation required for immune fitness. Nonetheless and despite the growing number of studies that associate commensal dysbiosis at diverse tissue sites with specific pathologies such as psoriasis, atopic dermatitis and asthma, (Abreu et al., 2012; Belda-Ferre et al., 2012; Hilty et al., 2010; Kong et al., 2012; Srinivasan et al., 2010) little is known about the effect of these unique microbial communities in the control of tissue specific immunity. Based on our understanding of tissue specialization we could postulate that these unique microbial communities have coevolved with their host to finely tune the unique environment of each tissue site.
Role of the microbiota in the pathogenesis of infection: The accidental pathogen
As previously discussed, pathogenicity of most microbes is a contextual state. Owing to the adjuvant capacity of the microbiota, infections that occur at sites colonized by commensals can be, in some cases, considered as co-infections with normal constituents of the microbiota as a major culprit of tissue damage and pathogen transmission. Although tissue resident symbionts can provide an immunological and ecological shield against pathogen invasion, these microbes can in some instances be coopted by pathogens for optimal transmission. One of the first illustrations of the positive effect of the flora on pathogen development and survival was revealed in a model of Trichuris muris nematode infection (Hayes et al., 2011). In this model of infection, egg hatching in the large intestine only occurred upon tight contact with bacteria suggesting that the microflora provides critical cues for the appropriate establishment of the life cycle of gut tropic nematodes (Hayes et al., 2011). This pro-infection role of the microbiota represents a novel paradigm for the transmission of various pathogens including viruses. Poliovirus relies on the microbiota for efficient replication, an effect that can be at least in part associated with the capacity of the virus to bind to cardinal microbial products such as LPS (Kuss et al., 2011). Similarly, the capacity of MMTV to bind to commensally derived LPS favors mucosal transmission of the virus via the induction of the regulatory cytokine IL-10. Such effects lead to a state of immunological unresponsiveness toward viral antigen that favors transmission of the virus (Kane et al., 2011). In these studies the elements accounting for the pathogen transmission and/or virulence promoting effect of the flora are highly represented microbial products such as LPS, suggesting that both virus and nematodes may have evolved to bypass commensal population shifts by thriving on ubiquitous microbial derived components. This would suggest that although manipulating the flora may represent an efficient way of altering immunity to pathogens, this strategy is unlikely to have global consequences on pathogen transmission. Based on the pleitropic effect of the flora in induction of regulatory pathways, host metabolism and tissue resident function, one would expect that a high number of pathogens transmitted via mucosa or using commensal rich habitats have evolved to benefit from the complex interaction of the host with its microbiota.
Infections represent highly volatile situations for barrier tissues, with pathogens, commensals and environmental antigens transiently sharing the same inflamed environment. In westernized countries, it is estimated that a child will suffer ten diarrheal episodes on average before the age of 5, a number that can be dramatically increased in the developing world (Kosek et al., 2003; Vernacchio et al., 2006). Thus, when added together with skin and lung infections, the immune system has ample opportunity to be exposed to commensals during inflammation, a situation that has the potential to disrupt our homeostatic relationship with these microbes. In the gastrointestinal tract, acute mucosal infections are characterized by dysbiosis associated with significant shifts in the microbiota and dominance of bacteria with enhanced invasive and inflammatory properties that can directly exacerbate inflammation and tissue damage (Egan et al., 2011; Heimesaat et al., 2006; Lupp et al., 2007; Stecher et al., 2007). In particular, γ-proteobacteria dominance has emerged as a hallmark of acute mucosal infections and enhanced pathology (Benson et al., 2009; Craven et al., 2012; Egan et al., 2011; Heimesaat et al., 2006; Lupp et al., 2007; Molloy et al., 2013; Raetz et al., 2012; Stecher et al., 2007). Various mechanisms could contribute to proteobacterial ‘blooms’, including the capacity of these commensals to thrive on metabolites derived from inflammatory setting such as nitrates and the selective death of paneth cells (Raetz et al., 2012; Winter et al., 2013). Because of the pathogenic role of these bacteria, the immune system may have evolved specific mode of control. Indeed, in the context of acute mucosal infection both neutrophils and monocytes can exit the lamina propria and enter the gut lumen thereby creating a containment structure referred to as intraluminal cast that limit epithelial contact with these invasive microbes and translocation(Molloy et al., 2013).
In a number of models of gastrointestinal infections, such as Toxoplasma gondii and Yersinia pseudotuberculosis, immunopathology can also induce the translocation of commensal bacteria (Brenchley and Douek; Brenchley et al., 2006; Estes et al.; Hand et al., 2012; Heimesaat et al., 2006; Jung et al., 2012; Meinzer et al., 2012). A consequence of infection that results from the induction of inflammation and enhanced microbial translocation is that in contrast to steady-state responses, commensal-specific T cells, much like pathogen-specific cells, become activated and differentiate to an inflammatory phenotype (Hand et al., 2012). In the GI tract these commensal-specific T cells form memory cells that are phenotypically and functionally indistinguishable from pathogen-specific T cells (Hand et al., 2012). Because of the extraordinary number of potential antigens expressed by the host microbiota, this implies that a significant fraction of memory cells are expected to be commensal specific and will develop over time in response to successive infections and/or various barrier breaches. Thus, primary exposure to a pathogen in the skin, lung and GI tract is likely to occur in the context of a much broader recall response against commensal bacteria. Much like pathogen-specific CD4 T cells and in contrast to CD8 memory T cells, commensal-specific memory T cells declined steadily over time (Hand et al., 2012; Homann et al., 2001; Pepper et al., 2009). As CD4 T cells carry out the complex task of remembering and responding to pathogenic and commensal organisms in a constantly changing environment, perhaps development of CD4 memory reflects this necessity for flexibility. An evolving pool of specificities within the regulatory and effector CD4 T cell compartment may allow for the maintenance of tolerance and barrier function in the face of variable commensal populations and intermittent infection.
The physiological consequence of long-term T cell memory against commensals remains to be addressed. One possible consequence may be the induction of heterologous memory wherein antigen-specific responses against commensal bacteria could drive the rapid production of inflammatory cytokines upon secondary infection, leading to increased protection against both pathogens and commensal bacteria. In support of this hypothesis, healthy human serum contains antibodies specific to the skin and intestinal microbiota (Haas et al., 2011). In addition, recent studies suggest that CD4 T cell clones that are cross-reactive to commensals and viruses are common in healthy individuals (Su et al., 2013). Conversely, aberrant accumulation of commensal-specific T cells may lead to the development of inflammation and IBD (Sartor, 2006). A further exploration of antigen-specific memory T cells residing at all barrier sites and their regulation would inform us of the potential of impact of effector responses against commensals at steady state or in the context of inflammation.
Role of the microbiota in chronic diseases
Optimal microbiota host interaction implies that balance between stimulatory and regulatory signals would allow the development of immunity without compromising the capacity of the host to maintain tolerance to innocuous antigens. Yet, in westernized countries, the overuse of antibiotics, changes in the diet and elimination of chronic parasitic infections, such as those caused by helminth worms such as roundworms, hookworms or whipworms may has selected for a microbiota that may lack the resilience required for the establishment of balanced immune responses. For instance, as recently as 1940, the prevalence of helminthic worm infection in children in some rural areas of the United States was as high as 70% (Johnston et al., 2014; Weinstock et al., 2004). As well, the use of antibiotics and changes in diet have caused the chronic and sometimes severe disappearance of potentially critical components of the human microbiota (Blaser and Falkow, 2009). These profound changes in the microbiota and as a direct result, the immune system, are now believed to contribute to the dramatic and rapid increase in chronic inflammatory and autoimmune disorders seen in high-income countries. Indeed, while each inflammatory disease is associated with unique genetic and biological mechanisms, a unifying trend seems to be that many inflammatory diseases are associated with significant shifts in the resident microbiota from a ‘healthy’ to a ‘diseased’ state. One tantalizing hypothesis is that the partially penetrant nature of genetic polymorphisms associated with many complex diseases is due to the necessity of changes in the microbiota for the onset of pathology. An alarming consequence of this hypothesis is that susceptibility to some diseases is partly due to the stochastic acquisition of specific commensal organisms. Interestingly, similar ‘opportunists’ are associated with multiple disease states. Therefore, some bacteria may be particular adept at surviving in, and contributing to, inflammation and the recent acquisition of a particular set of inflammatory clades of bacteria may represent an be an important contributor to the etiology of inflammatory and autoimmune diseases. Below, we will discuss a number of diseases and their associations with the microbiota.
Role of the microbiota in IBD and autoimmune disease
The gastrointestinal tract is home to the largest population of commensal organisms in the human body and as such is home to a unique set of immunoregulatory mechanisms that prevent the unnecessary activation of the immune system against innocuous antigens including those expressed by the microbiota. A breakdown in these overlapping regulatory mechanisms results in a set of chronic inflammatory conditions that are collectively known as Inflammatory Bowel Disease (IBD). The relationship between mucosal immune dysfunction and IBD is illustrated by the fact that both Crohn’s Disease (CD) and Ulcerative Colitis (UC) are associated with genes that are critical in maintenance of the epithelial barrier and the regulation of innate and adaptive immune responses (Rivas et al., 2011). The etiology of IBD is complex and is believe to be the consequence of genetic factors, the host immune system, and environmental factors such as the microbiota (Maloy and Powrie, 2011). Stressors such as defined infections have been also proposed to contribute to the induction of these disorders (Cadwell et al., 2010).
IBD is associated with profound changes in the composition of the intestinal microbiota highlighting the importance of the microbiota in disease etiology. Notably, numerous studies have shown that both CD and UC are associated with a reduced complexity of the commensal microbiota and consistent shifts in the consortia to a dysbiotic state. For example, in a similar manner to what is observed during acute mucosal infections, both CD and UC are characterized by the outgrowth of the phyla proteobacteria and in particular the Enterobacteriaceae family (Frank et al., 2007). Notably Crohn’s Disease has been associated with commensals that are intrinsically inflammatory and blur the line between commensal and pathogen. For example, Adherent-Invasive E. coli, Yersinia and Clostridium difficile are much more common in patients suffering from Crohn’s disease than healthy individuals and in some mouse models have been shown to be key contibutors to IBD (Issa et al., 2008; Lamps et al., 2003; Rolhion and Darfeuille-Michaud, 2007). In mice deficient in IL-10 or in IL-10 and TGF-β and therefore prone to develop colitis, defined commensals such as Helicobacter hepaticus or commensal Bacteroides are sufficient to induce disease (Bloom et al., 2011; Cahill et al., 1997; Kullberg et al., 2002). It is hypothesized that these commensal bacteria with enhanced inflammatory potential contribute to disease via their capacity to invade and promote innate and adaptive immune responses to otherwise benign food antigens and commensal organisms (Elson and Cong, 2012; Packey and Sartor, 2008). Indeed, increased serum antibody responses against antigens derived from the microbiota is characteristic of Crohn’s Disease patients (Dubinsky et al., 2006; Lodes et al., 2004). One simple explanation as to why particular commensal organisms are able to ‘bloom’ during inflammation is that these organisms are closely related to obligate pathogens and therefore possess modules that allow for survival under the harsh conditions of immune activation. Indeed, commensal E. coli, which is benign under homeostatic conditions, is capable of using inflammatory nitric oxides generated by the immune response as an energy source, conferring a significant growth advantage (Winter et al., 2013). Other organisms such as Bilophila wadsworthia can take advantage of dietary induced bile acids to dominate the intestine, contributing to disease in mouse strains prone to colitis providing a glimpse at how diet may trigger IBD via its profound impact on the microbiota (Devkota et al., 2012a). An intriguing hypothesis that can be derived from these studies is that IBD is caused or exacerbated by a positive feedback loop, where host mutation leads to dysregulated immune responses in the GI tract, which drive the outgrowth of inflammatory bacteria that in turn promote more inflammation. One striking experimental example of the influence of the flora on IBD is that of mice deficient in both T-bet and adaptive immunity that develop a spontaneous and transferrable form of ulcerative colitis (TRUC mice) (Garrett et al., 2007). Deficiency of T-bet in the innate immune system leads to exaggerated tumor necrosis factor alpha (TNF-α) production by dendritic cells, which together with the absence of Treg creates a chronic inflammatory state that modulates the composition of the microflora and eventually leads to the development of colorectal cancer (Garrett et al., 2007; Garrett et al., 2009). Interestingly, transfer of the microbiota from TRUC mice into wild-type recipients also transfers the colitis. Defined species of commensals such as Proteus mirabilis, Klebsiella pneumoniae or Helicobacter typhlonius are found at increased frequency in TRUC mice and can transfer disease in wild-type mice (Garrett et al., 2010; Powell et al., 2012). Further demonstration that colitis can, at least in experimental settings, become “contagious” was revealed in a number of mouse models where innate immune defects in components of the inflammasome or IL-22 led to aberrant responses to commensals (Elinav et al., 2011). Deficiency of the NLRP6 component of the inflammasome from colonic cells results in reduced IL-18 levels and shifts in microbiota composition characterized by expansion of the bacterial phyla Bacteroidetes (in particular the family Prevotellaceae) and TM7. Consequent to the microbial shift, these mice spontaneously develop intestinal hyperplasia and are more susceptible to chemically induced colitis and colonic cancer (Elinav et al., 2011; Hu et al., 2013). As for the TRUC mice, the colitic phenotype can be transferred by the microbiota to neonatal or adult wild-type mice that have no innate immune defect, though it should be noted that colitis is less severe in these animals (Garrett et al., 2007). How these infectious and dysbiotic microbiota contribute to human disorders remains to be addressed but is of critical importance if we are to understand the recent rise of IBD incidence.
Perhaps equally important to the outgrowth of inflammatory components of the microbiota in the induction of IBD is the loss of symbiotic commensal organisms that rely upon fermentation as their energy source. Sequencing studies have shown that the bacteria of the phylum Firmicutes and in particular the class Clostridia are reduced in patients suffering IBD (Frank et al., 2007). This is potentially important because of the effect that bacteria within this class can have on GI resident Treg cells. Clostridia, has been shown to directly induce colonic Tregs that can oppose colitis induction (Atarashi et al., 2013; Atarashi et al., 2011). Additionally, and as previously discussed, single chain fatty acids (SCFA), have been shown to limit GI inflammation both by the induction of Treg and the direct inhibition of macrophage and neutrophil activation (Arpaia et al., 2013; Furusawa et al., 2013; Maslowski et al., 2009; Smith et al., 2013). The enzymatic processes necessary for the fermentation of fiber to SCFA are largely dependent upon bacteria within the Clostridia class(Pryde et al., 2002). Thus, the mucosa-associated immune system is shaped by commensal dysbiosis in at least two distinct ways. First, the outgrowth of opportunistic clades of bacteria drives increased inflammation and secondly, the loss of benign fermenting bacteria that produce ‘keystone’ metabolites results in reduced immunoregulation.
The microbiome may also contribute to autoimmune disease at other barrier and systemic sites. While it was initially thought to be largely sterile, the resident microbiota of the respiratory tract has recently been described, (Beck et al., 2012) and is a likely contributor to the etiology of asthma and other airway inflammatory disorders, such as cystic fibrosis (CF). Cystic fibrosis is caused by inactivating mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) gene required for the homeostatic control of chloride ions in the lung. The expression of mutated CFTR proteins leads to the build-up of thick mucus on the surface of the airway that cannot be easily removed by the action of the cilia. The build up of mucus on the surface of the lung allows inflammatory constituents of the lung microbiota to gain a foothold and grow out, leading to an influx of neutrophils and macrophages and immune-mediated damage to the airway that severely limits pulmonary function. The bacteria that are most commonly associated with the disease, Pseudomonas and Staphylococcus are often constituents of the healthy lung microbiota (Blainey et al., 2012) and therefore CF is perhaps the simplest example of a genetic disease whose mechanism is the induction of dysbiosis within the microbiota and subsequent activation of the immune system leading to uncontrolled inflammation.
Given the microbiota’s role in setting the systemic immune tone, a number of groups have investigated the role of the commensal microbiota in animal models of autoimmune disease. Rheumatoid Arthritis is an autoimmune disease characterized by the invasion of joints by immune cells and in particular immune complexes formed from autoreactive antibodies. In a murine model of rheumatoid arthritis, disease is significantly reduced in the absence of commensal bacteria with diminished disease associated with a reduction in the pro-inflammatory Th17 response (Wu et al., 2010). Arthritic phenotypes were completely restored when germ-free animals were monocolonized with SFB, which drives the differentiation and or dissemination of both intestinal and splenic Th17 cells (Wu et al., 2010). Analysis of the microbiome of patients suffering RA revealed a surprising relationship between the disease and a potentially inflammatory clade of Prevotellaceae but how the microbiota contributes to immune activation and dissemination of T cells and antibodies to the joints in RA is unknown (Scher et al., 2013).
Multiple Sclerosis is an autoimmune disease characterized by the invasion of the Central Nervous System by immune cells resulting in the demyelination of neurons and subsequent pathology. The incidence of disease in the mouse model of Multiple Sclerosis: Experimental Autoimmune Encephalitis (EAE), is also dependent upon the commensal microbiota (Berer et al., 2011; Lee et al., 2011; Ochoa-Reparaz et al., 2010a; Ochoa-Reparaz et al., 2009; Ochoa-Reparaz et al., 2010b). This data is in agreement with what had been known anecdotally for many years, that disease varied amongst genetically identical animals housed in different settings. Multiple studies have now shown that the activation of myelin-specific B cells and the invasion of the neurons by effector T cells requires the commensal microbiota (Berer et al., 2011; Ochoa-Reparaz et al., 2009). It should be noted that the role of the microbiota in inducing EAE is contextual. For example the commensal microbiota may be protective in this disease as PSA of B. fragilis can ameliorate symptoms via the induction of IL-10 producing regulatory T cells (Lee et al., 2011).
How in these models the microbiota is acting to drive disease is not completely understood. It does seem likely that in individuals prone to autoimmunity the intrinsic inflammatory nature of defined commensal bacteria could lead to an increase in the systemic threshold of activation. Additionally the average microbiota possesses millions of potential antigens and as recently highlighted the possibility of dangerous cross-reactivity between commensals, pathogens and self is higher than previously understood (Su et al., 2013).
As alluded to previously, the commensal microbiota can provide protection against some inflammatory disorders. For instance, incidence of disease in the Non-Obese Diabetic (NOD) mouse model of Type 1 Diabetes (T1D) is maximal in germ-free animals and the presence of SFB within the microbiota segregates with protection from the disease (Kriegel et al., 2011; Pozzilli et al., 1993). Moreover, MyD88 knockout mice bred to the pro-diabetic background are also protected from disease due to the possession of a ‘protective’ microbiota that can be transmitted from mother to child (Wen et al., 2008). The mouse model of diabetes, but not the human disease has a significant preference for females, which is in concert with most other autoimmune disorders. Interestingly, this sexual dimorphism is also dependent upon the microbiota, as it is not seen in germ-free mice, and the transfer of a male microbiota to females equalizes the incidence of the disease as a result of changes in the expression of sex hormones (Markle et al., 2013; Yurkovetskiy et al., 2013). How the microbiota shapes the immune system and which bacterial clades are important in T1D is still not known but this research may lead to an understanding of factors contributing to the initiation of T1D, a disease that has been increasing in prevalence worldwide without a known stressor. Additionally, how the microbiota may shape host physiology, such as sex hormones, resulting in modification of the immune response adds an additional layer of complexity that will be an important component of future studies.
Role of the microbiota in cancer
Perhaps the first indication that the commensal microbiota could drive cancer was provided by the observation that stomach ulcers and subsequent stomach cancer were caused by the presence of a single type of bacteria Helicobacter pylori (Marshall and Warren, 1984). The vast majority of people carrying H. pylori are asymptomatic and thus as for most host-microbe interactions, the host response to the colonization determines whether chronic inflammation and carcinogenesis occurs. Similar phenomena seem to be important to the etiology of cancers at other barrier surfaces, most notably the intestine. On the other hand, H. pylori is associated with protection from other types of cancer (Atherton and Blaser, 2009) further highlighting the functional contextuality of commensal.
Intestinal cancer is inextricably linked to inflammation in the GI tract and commensal-driven immune activation is an important contributing factor to the etiology of these diseases (Grivennikov and Karin, 2010). Notably, IBD and in particular CD, is associated with the development of colon cancer (Elinav et al., 2013). Liver cancer has also been linked to the microbiota via induction of specific bile acids that damage DNA within the liver (Yoshimoto et al., 2013). A number of studies have attempted to link signaling via innate immune receptors in the GI tract and disease in mouse models of colorectal cancer, however the results have been disparate, perhaps due to the confounding factor of the microbiota (Elinav et al., 2013; Fukata et al., 2007; Lowe et al., 2010; Rakoff-Nahoum and Medzhitov, 2007; Salcedo et al., 2010). Indeed, dysbiosis of the microbiota seems to be an important factor in determining whether the immune system contributes or protects against the initiation of cancer. For example in the NLRP6 deficient mice, that lack a key component of the inflammasome and TRUC mice, susceptibility to colorectal cancer is associated with dysbiosis that is directly related to immune dysfunction (Garrett et al., 2009; Hu et al., 2013). Moreover, as a direct illustration of the importance of the microbiota in colon cancer, disease susceptibility can be transferred to healthy mice via the commensal microbiota (Garrett et al., 2009; Hu et al., 2013). The microbiome of human patients suffering from colorectal cancer is also modestly disrupted with an increased representation of Prevotella (Sobhani et al., 2011). Additionally, mouse models of colorectal cancer have been shown to be exacerbated by specific strains of bacteria. For example, E. coli that expresses the polyketide synthase genotoxic island can promote tumorigenesis in defined models of colon cancer and E. coli that express the same gene module are found in patients afflicted with colon cancer (Arthur et al., 2012). Bacteria of the phylum Fusobacteria are also found at a high frequency residing within the tumor tissue and are important in inducing the traffic of tumor promoting myeloid cells (Kostic et al., 2013; Kostic et al., 2012; Rubinstein et al., 2013). In general, commensal-driven infiltration of intestinal tumors by immune cells is believed to be an important driving factor in tumorigenesis. Studies have shown that colonic tumors have a reduced mucus layer and are susceptible to bacterial translocation and the influx of Th17 cells (Grivennikov et al., 2012). Related to this, in the Multiple Intestinal Neoplasia mouse model, chronic colonization with the Th17 inducing bacteria enterotoxigenic Bacteroides fragilis leads to increased tumorigenesis (Wu et al., 2009). Therefore, colon cancer, much like IBD and stomach cancer, may be caused or exacerbated by an over-exuberant immune response against a dysbiotic commensal microbiota. However, it should be noted that commensal-induced inflammation is a ‘double-edged sword’ for the host. As previously discussed the microbiota is also an integral part of the capacity of the host to control tumor in the context of immunotherapy and chemotherapy. Thus, the role of the microbiota in tumor development and control is highly contextual and very much dependent upon the exact constituents of the microbiota and the state of the host immune system.
Role of the microbiota in Type II Diabetes and metabolic syndrome
Metabolic syndrome is a set of risk factors for heart disease, stroke and diabetes that are related to increased adiposity and sedentary lifestyles. Type II diabetes (T2D) is an inflammatory condition where increased adiposity and metabolic defects drive chronic production of inflammatory cytokines such as TNF-α and IL-1 (Gregor and Hotamisligil, 2011). These inflammatory cytokines inhibit insulin signaling leading to insulin resistance and increased blood sugar (Osborn and Olefsky, 2012). The microbiota has been proposed to play a prominent role in this process. Indeed, the commensal microbiota of obese individuals is significantly altered such that the ratio of firmicutes is increased in relation to bacteroidetes, a shift associated with increased nutrient production for the host (Turnbaugh et al., 2006). Importantly these changes are transferrable as the microbiota of genetically obese mice leads to increased adiposity amongst healthy cage mates (Turnbaugh et al., 2006). Significant changes to the commensal microbiota have been observed in T2D as well and interestingly are associated with a reduction in SCFA producing strains of Clostridia and increases in E. coli (Karlsson et al., 2013; Qin et al., 2012). Serum LPS levels are also increased as a result of T2D, indicating that increased translocation of bacterial products may be taking place and in mouse models, high fat diets have been associated with increased translocation of commensal E. coli (Amar et al., 2011; Jayashree et al., 2013). Finally, there is some evidence that susceptibility to obesity itself is regulated by the immune system. Mice deficient in TLR5 develop exacerbated metabolic syndrome and grow obese faster than wild-type controls (Vijay-Kumar et al., 2010). The transfer of the microbiota alone is sufficient to transfer the susceptibility to metabolic syndrome and tellingly, these mice are particularly susceptible to the outgrowth of E. coli (Carvalho et al., 2012; Vijay-Kumar et al., 2010). Therefore in T2D, as in IBD, the disease can be exacerbated by complex interactions between the immune system and the microbiota, leading to chronic inflammation.
Interdependence of diet, commensal and the immune system
The immune system is not only controlled by its symbiotic relationship with the microbiota but is also exquisitely sensitive to the nutritional status of the host. Evidence now exists for a multi-directional interaction between the diet, immune system and commensal microflora. The intestine serves as the primary site of nutrient absorption in the body. As such, the immune system and commensal microbiota are sensitive to changes in diet. Dietary control of immune cells is mediated both by metabolic requirements as well as direct sensing of food- derived metabolites. For instance, metabolite of vitamin A, retinoic acid, that is highly enriched in the gut, can directly control the capacity of lymphocytes to respond to antigen and to migrate to the gastrointestinal tract (Hall et al., 2011a; Hall et al., 2011b). In addition, dietary derived AHR ligands from cruciferous vegetables are important signals for intestinal immune development and immune responses (Kiss et al., 2011; Li et al., 2011). The concept that defined metabolites could control unique aspects of the immune system is believed to be a highly generalizable observation extending to all micronutrients explored thus far, including iron, folate, zinc, selenium and Vitamin C, D and E (Mora et al., 2008; Savy et al., 2009; von Essen et al., 2010). However, the diet has a profound and highly dynamic impact on the composition and function of the microbiota. Therefore, an understanding of the role of diet in immune responses requires integration of the commensal microbiota as well (Figure 4). As an example, mice fed a high fat diet have altered commensal communities that not only effect energy harvest, but that can also alter immunity and increase the severity of disease states such as colitis (Devkota et al., 2012b; Gregor and Hotamisligil, 2011; Ley et al., 2006b; Monira et al., 2011). Defined dietary deficiency can also alter commensal communities. For example, the essential micronutrient Vitamin A that is required for the induction of protective immunity has also profound effects on the composition of the microbiota (Cha et al., 2010). Recent findings have also shown that the immune system may also be able to directly affect dietary intake. For instance, mice lacking B cells have deficiencies in dietary absorption (Shulzhenko et al., 2011). Conversely and as mentioned mice deficient in the receptor for bacterial flagellin, TLR5, have a dysbiotic commensal microflora that leads to increased weight gain and the development of metabolic syndrome (Vijay-Kumar et al., 2010). Thus immunodeficiency can alter the gut flora’s composition and thereby the metabolic capacity of both the flora and the host. This idea may have important consequences for human health not only in the developed world where metabolic syndrome is epidemic but also in the developing world. Children with severe malnutrition represent an example of both immunodeficiency and dietary deficiency coinciding in a clinically relevant way. Malnourished children have dramatically altered commensal communities likely with diminished metabolic capacity and impaired ability to promote host energy absorption; a problem that can persist even after dietary intervention (Gordon et al., 2012; Monira et al., 2011; Yatsunenko et al., 2012).
Figure 4. Interdependence of diet, immune and microbiota interactions.
Evidence now exists for bidirectional communication between the three key factors in the GI tract: Diet, Immune and commensal microflora. Diet can have profound influence on the immune system (e.g. Vitamin A, Vitamin D, AHR), while the immune system can also affect dietary intake. Diet also has dominant influence on the composition and metabolic capacity of commensal bacteria, while this, in return, influences nutrient absorption. The immune system is able to exert control over both commensal composition and localization, while commensal signals are critical for development and function of the immune system.
Fundamental changes in cultural traditions and socio-economic status have affected the nutritional status of humans worldwide, altering microbial communities in unpredictable and possibly harmful ways (Lozupone et al., 2012). Considering the interdependency of the diet, immune system and microbiota an important challenge over the next few years will be to develop experimental strategies aimed at defining critical dietary regulators of this complex and interdependent network.
Concluding remarks
Autoimmune and inflammatory diseases, all associated with dysregulated immune responses have been rising dramatically over the past few decades. The field of immunology is at a fascinating crossroad. In recent years, immunological research has evolved from a lymphoid tissue-centric view of the immune system to the understanding of tissue microenvironments as a fundamental determinant of immune responses. This area of research has led to the integration of the microbiota as an intrinsic regulator of all immune responses. There is today an explosion of discoveries associated with the growing understanding of the role of communities of microbes, keystone bacterial species, commensal derived products or metabolites and more particularly of the link between some of these components and disease states in humans. This provides scientists and clinicians with a unique opportunity to develop an integrated exploration of human health that includes ecologists, nutritionists, geneticists, microbiologists, biochemists and immunologists. In this multidisciplinary area of research lies the key to fundamental discoveries aimed at manipulating or restoring defined aspects of the immune system-microbiota dialogue in order to promote or restore the health of the human meta-organism.
Acknowledgments
This work was supported by the Division of Intramural Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health. We would like to thank the members of the Belkaid laboratory for helpful discussions and more particularly John Grainger and Sean Spencer for critical reading of the manuscript. We would like to also apologize to our colleagues for not having cited all papers relevant to this expanding field because of space constrain.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abreu NA, Nagalingam NA, Song Y, Roediger FC, Pletcher SD, Goldberg AN, Lynch SV. Sinus microbiome diversity depletion and Corynebacterium tuberculostearicum enrichment mediates rhinosinusitis. Sci Transl Med. 2012;4:151ra124. doi: 10.1126/scitranslmed.3003783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abt MC, Osborne LC, Monticelli LA, Doering TA, Alenghat T, Sonnenberg GF, Paley MA, Antenus M, Williams KL, Erikson J, et al. Commensal bacteria calibrate the activation threshold of innate antiviral immunity. Immunity. 2012;37:158–170. doi: 10.1016/j.immuni.2012.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alenghat T, Osborne LC, Saenz SA, Kobuley D, Ziegler CG, Mullican SE, Choi I, Grunberg S, Sinha R, Wynosky-Dolfi M, et al. Histone deacetylase 3 coordinates commensal-bacteria-dependent intestinal homeostasis. Nature. 2013;504:153–157. doi: 10.1038/nature12687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amar J, Serino M, Lange C, Chabo C, Iacovoni J, Mondot S, Lepage P, Klopp C, Mariette J, Bouchez O, et al. Involvement of tissue bacteria in the onset of diabetes in humans: evidence for a concept. Diabetologia. 2011;54:3055–3061. doi: 10.1007/s00125-011-2329-8. [DOI] [PubMed] [Google Scholar]
- An D, Oh SF, Olszak T, Neves JF, Avci FY, Erturk-Hasdemir D, Lu X, Zeissig S, Blumberg RS, Kasper DL. Sphingolipids from a symbiotic microbe regulate homeostasis of host intestinal natural killer T cells. Cell. 2014;156:123–133. doi: 10.1016/j.cell.2013.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arpaia N, Campbell C, Fan X, Dikiy S, van der Veeken J, Deroos P, Liu H, Cross JR, Pfeffer K, Coffer PJ, et al. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature. 2013 doi: 10.1038/nature12726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arthur JC, Perez-Chanona E, Muhlbauer M, Tomkovich S, Uronis JM, Fan TJ, Campbell BJ, Abujamel T, Dogan B, Rogers AB, et al. Intestinal inflammation targets cancer-inducing activity of the microbiota. Science. 2012;338:120–123. doi: 10.1126/science.1224820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atarashi K, Tanoue T, Oshima K, Suda W, Nagano Y, Nishikawa H, Fukuda S, Saito T, Narushima S, Hase K, et al. Treg induction by a rationally selected mixture of Clostridia strains from the human microbiota. Nature. 2013;500:232–236. doi: 10.1038/nature12331. [DOI] [PubMed] [Google Scholar]
- Atarashi K, Tanoue T, Shima T, Imaoka A, Kuwahara T, Momose Y, Cheng G, Yamasaki S, Saito T, Ohba Y, et al. Induction of colonic regulatory T cells by indigenous Clostridium species. Science. 2011;331:337–341. doi: 10.1126/science.1198469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atherton JC, Blaser MJ. Coadaptation of Helicobacter pylori and humans: ancient history, modern implications. The Journal of clinical investigation. 2009;119:2475–2487. doi: 10.1172/JCI38605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avila M, Ojcius DM, Yilmaz O. The oral microbiota: living with a permanent guest. DNA Cell Biol. 2009;28:405–411. doi: 10.1089/dna.2009.0874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer H, Horowitz RE, Levenson SM, Popper H. The response of the lymphatic tissue to the microbial flora. Studies on germfree mice. The American journal of pathology. 1963;42:471–483. [PMC free article] [PubMed] [Google Scholar]
- Beck JM, Young VB, Huffnagle GB. The microbiome of the lung. Transl Res. 2012;160:258–266. doi: 10.1016/j.trsl.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belda-Ferre P, Alcaraz LD, Cabrera-Rubio R, Romero H, Simon-Soro A, Pignatelli M, Mira A. The oral metagenome in health and disease. Isme J. 2012;6:46–56. doi: 10.1038/ismej.2011.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belkaid Y, Naik S. Compartmentalized and systemic control of tissue immunity by commensals. Nature immunology. 2013;14:646–653. doi: 10.1038/ni.2604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson A, Pifer R, Behrendt CL, Hooper LV, Yarovinsky F. Gut commensal bacteria direct a protective immune response against Toxoplasma gondii. Cell host & microbe. 2009;6:187–196. doi: 10.1016/j.chom.2009.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berer K, Mues M, Koutrolos M, Rasbi ZA, Boziki M, Johner C, Wekerle H, Krishnamoorthy G. Commensal microbiota and myelin autoantigen cooperate to trigger autoimmune demyelination. Nature. 2011;479:538–541. doi: 10.1038/nature10554. [DOI] [PubMed] [Google Scholar]
- Blainey PC, Milla CE, Cornfield DN, Quake SR. Quantitative analysis of the human airway microbial ecology reveals a pervasive signature for cystic fibrosis. Sci Transl Med. 2012;4:153ra130. doi: 10.1126/scitranslmed.3004458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaser MJ, Falkow S. What are the consequences of the disappearing human microbiota? Nat Rev Microbiol. 2009;7:887–894. doi: 10.1038/nrmicro2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom SM, Bijanki VN, Nava GM, Sun L, Malvin NP, Donermeyer DL, Dunne WM, Jr, Allen PM, Stappenbeck TS. Commensal Bacteroides species induce colitis in host-genotype-specific fashion in a mouse model of inflammatory bowel disease. Cell host & microbe. 2011;9:390–403. doi: 10.1016/j.chom.2011.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouladoux N, Hall JA, Grainger JR, dos Santos LM, Kann MG, Nagarajan V, Verthelyi D, Belkaid Y. Regulatory role of suppressive motifs from commensal DNA. Mucosal immunology. 2012;5:623–634. doi: 10.1038/mi.2012.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boullier S, Tanguy M, Kadaoui KA, Caubet C, Sansonetti P, Corthesy B, Phalipon A. Secretory IgA-mediated neutralization of Shigella flexneri prevents intestinal tissue destruction by down-regulating inflammatory circuits. Journal of Immunology. 2009;183:5879–5885. doi: 10.4049/jimmunol.0901838. [DOI] [PubMed] [Google Scholar]
- Bouskra D, Brezillon C, Berard M, Werts C, Varona R, Boneca IG, Eberl G. Lymphoid tissue genesis induced by commensals through NOD1 regulates intestinal homeostasis. Nature. 2008;456:507–510. doi: 10.1038/nature07450. [DOI] [PubMed] [Google Scholar]
- Brandl K, Plitas G, Mihu CN, Ubeda C, Jia T, Fleisher M, Schnabl B, DeMatteo RP, Pamer EG. Vancomycin-resistant enterococci exploit antibiotic-induced innate immune deficits. Nature. 2008;455:804–807. doi: 10.1038/nature07250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandl K, Plitas G, Schnabl B, DeMatteo RP, Pamer EG. MyD88-mediated signals induce the bactericidal lectin RegIII gamma and protect mice against intestinal Listeria monocytogenes infection. The Journal of experimental medicine. 2007;204:1891–1900. doi: 10.1084/jem.20070563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenchley JM, Douek DC. Microbial translocation across the GI tract. Annual review of immunology. 30:149–173. doi: 10.1146/annurev-immunol-020711-075001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenchley JM, Price DA, Schacker TW, Asher TE, Silvestri G, Rao S, Kazzaz Z, Bornstein E, Lambotte O, Altmann D, et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nature medicine. 2006;12:1365–1371. doi: 10.1038/nm1511. [DOI] [PubMed] [Google Scholar]
- Buffie CG, Pamer EG. Microbiota-mediated colonization resistance against intestinal pathogens. Nature reviews Immunology. 2013;13:790–801. doi: 10.1038/nri3535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadwell K, Patel KK, Maloney NS, Liu TC, Ng AC, Storer CE, Head RD, Xavier R, Stappenbeck TS, Virgin HW. Virus-plus-susceptibility gene interaction determines Crohn’s disease gene Atg16L1 phenotypes in intestine. Cell. 2010;141:1135–1145. doi: 10.1016/j.cell.2010.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill RJ, Foltz CJ, Fox JG, Dangler CA, Powrie F, Schauer DB. Inflammatory bowel disease: an immunity-mediated condition triggered by bacterial infection with Helicobacter hepaticus. Infect Immun. 1997;65:3126–3131. doi: 10.1128/iai.65.8.3126-3131.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho FA, Koren O, Goodrich JK, Johansson ME, Nalbantoglu I, Aitken JD, Su Y, Chassaing B, Walters WA, Gonzalez A, et al. Transient Inability to Manage Proteobacteria Promotes Chronic Gut Inflammation in TLR5-Deficient Mice. Cell host & microbe. 2012;12:139–152. doi: 10.1016/j.chom.2012.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cash HL, Whitham CV, Behrendt CL, Hooper LV. Symbiotic bacteria direct expression of an intestinal bactericidal lectin. Science. 2006;313:1126–1130. doi: 10.1126/science.1127119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cebra JJ. Influences of microbiota on intestinal immune system development. The American journal of clinical nutrition. 1999;69:1046S–1051S. doi: 10.1093/ajcn/69.5.1046s. [DOI] [PubMed] [Google Scholar]
- Cebula A, Seweryn M, Rempala GA, Pabla SS, McIndoe RA, Denning TL, Bry L, Kraj P, Kisielow P, Ignatowicz L. Thymus-derived regulatory T cells contribute to tolerance to commensal microbiota. Nature. 2013;497:258–262. doi: 10.1038/nature12079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha HR, Chang SY, Chang JH, Kim JO, Yang JY, Kim CH, Kweon MN. Downregulation of Th17 cells in the small intestine by disruption of gut flora in the absence of retinoic acid. J Immunol. 2010;184:6799–6806. doi: 10.4049/jimmunol.0902944. [DOI] [PubMed] [Google Scholar]
- Chassin C, Kocur M, Pott J, Duerr CU, Gutle D, Lotz M, Hornef MW. miR-146a mediates protective innate immune tolerance in the neonate intestine. Cell host & microbe. 2010;8:358–368. doi: 10.1016/j.chom.2010.09.005. [DOI] [PubMed] [Google Scholar]
- Chieppa M, Rescigno M, Huang AY, Germain RN. Dynamic imaging of dendritic cell extension into the small bowel lumen in response to epithelial cell TLR engagement. The Journal of experimental medicine. 2006;203:2841–2852. doi: 10.1084/jem.20061884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke TB, Davis KM, Lysenko ES, Zhou AY, Yu Y, Weiser JN. Recognition of peptidoglycan from the microbiota by Nod1 enhances systemic innate immunity. Nature medicine. 2010;16:228–231. doi: 10.1038/nm.2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cong Y, Feng T, Fujihashi K, Schoeb TR, Elson CO. A dominant, coordinated T regulatory cell-IgA response to the intestinal microbiota. Proc Natl Acad Sci U S A. 2009;106:19256–19261. doi: 10.1073/pnas.0812681106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coombes JL, Siddiqui KR, Arancibia-Carcamo CV, Hall J, Sun CM, Belkaid Y, Powrie F. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-{beta}- and retinoic acid-dependent mechanism. The Journal of experimental medicine. 2007 doi: 10.1084/jem.20070590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costello EK, Lauber CL, Hamady M, Fierer N, Gordon JI, Knight R. Bacterial community variation in human body habitats across space and time. Science. 2009;326:1694–1697. doi: 10.1126/science.1177486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craven M, Egan CE, Dowd SE, McDonough SP, Dogan B, Denkers EY, Bowman D, Scherl EJ, Simpson KW. Inflammation drives dysbiosis and bacterial invasion in murine models of ileal Crohn’s disease. PLoS ONE. 2012;7:e41594. doi: 10.1371/journal.pone.0041594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings JH, Pomare EW, Branch WJ, Naylor CP, Macfarlane GT. Short chain fatty acids in human large intestine, portal, hepatic and venous blood. Gut. 1987;28:1221–1227. doi: 10.1136/gut.28.10.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davie JR. Inhibition of histone deacetylase activity by butyrate. The Journal of nutrition. 2003;133:2485S–2493S. doi: 10.1093/jn/133.7.2485S. [DOI] [PubMed] [Google Scholar]
- Devkota S, Wang Y, Musch MW, Leone V, Fehlner-Peach H, Nadimpalli A, Antonopoulos DA, Jabri B, Chang EB. Dietary-fat-induced taurocholic acid promotes pathobiont expansion and colitis in Il10−/− mice. Nature. 2012a;487:104–108. doi: 10.1038/nature11225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devkota S, Wang Y, Musch MW, Leone V, Fehlner-Peach H, Nadimpalli A, Antonopoulos DA, Jabri B, Chang EB. Dietary-fat-induced taurocholic acid promotes pathobiont expansion and colitis in Il10(−/−) mice. Nature. 2012b doi: 10.1038/nature11225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Giacinto C, Marinaro M, Sanchez M, Strober W, Boirivant M. Probiotics ameliorate recurrent Th1-mediated murine colitis by inducing IL-10 and IL-10-dependent TGF-b-bearing regulatory cells. J Immunol. 2005;174:3237–3246. doi: 10.4049/jimmunol.174.6.3237. [DOI] [PubMed] [Google Scholar]
- Dixon DR, Reife RA, Cebra JJ, Darveau RP. Commensal bacteria influence innate status within gingival tissues: a pilot study. J Periodontol. 2004;75:1486–1492. doi: 10.1902/jop.2004.75.11.1486. [DOI] [PubMed] [Google Scholar]
- Dubinsky MC, Lin YC, Dutridge D, Picornell Y, Landers CJ, Farrior S, Wrobel I, Quiros A, Vasiliauskas EA, Grill B, et al. Serum immune responses predict rapid disease progression among children with Crohn’s disease: immune responses predict disease progression. The American journal of gastroenterology. 2006;101:360–367. doi: 10.1111/j.1572-0241.2006.00456.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan CE, Cohen SB, Denkers EY. Insights into inflammatory bowel disease using Toxoplasma gondii as an infectious trigger. Immunol Cell Biol. 2011 doi: 10.1038/icb.2011.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ege MJ, Mayer M, Normand AC, Genuneit J, Cookson WO, Braun-Fahrlander C, Heederik D, Piarroux R, von Mutius E. Exposure to environmental microorganisms and childhood asthma. The New England journal of medicine. 2011;364:701–709. doi: 10.1056/NEJMoa1007302. [DOI] [PubMed] [Google Scholar]
- Elahi S, Ertelt JM, Kinder JM, Jiang TT, Zhang X, Xin L, Chaturvedi V, Strong BS, Qualls JE, Steinbrecher KA, et al. Immunosuppressive CD71+ erythroid cells compromise neonatal host defence against infection. Nature. 2013;504:158–162. doi: 10.1038/nature12675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elinav E, Nowarski R, Thaiss CA, Hu B, Jin C, Flavell RA. Inflammation-induced cancer: crosstalk between tumours, immune cells and microorganisms. Nature reviews Cancer. 2013;13:759–771. doi: 10.1038/nrc3611. [DOI] [PubMed] [Google Scholar]
- Elinav E, Strowig T, Kau AL, Henao-Mejia J, Thaiss CA, Booth CJ, Peaper DR, Bertin J, Eisenbarth SC, Gordon JI, et al. NLRP6 inflammasome regulates colonic microbial ecology and risk for colitis. Cell. 2011;145:745–757. doi: 10.1016/j.cell.2011.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elson CO, Cong Y. Host-microbiota interactions in inflammatory bowel disease. Gut microbes. 2012;3:332–344. doi: 10.4161/gmic.20228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ergin A, Syrbe U, Scheer R, Thiel A, Adam T, Bussow K, Duchmann R, Zeitz M, Sieper J. Impaired peripheral Th1 CD4+ T cell response to Escherichia coli proteins in patients with Crohn’s disease and ankylosing spondylitis. Journal of clinical immunology. 2011;31:998–1009. doi: 10.1007/s10875-011-9575-x. [DOI] [PubMed] [Google Scholar]
- Estes JD, Harris LD, Klatt NR, Tabb B, Pittaluga S, Paiardini M, Barclay GR, Smedley J, Pung R, Oliveira KM, et al. Damaged intestinal epithelial integrity linked to microbial translocation in pathogenic simian immunodeficiency virus infections. PLoS Pathog. 6:e1001052. doi: 10.1371/journal.ppat.1001052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faith JJ, Ahern PP, Ridaura VK, Cheng J, Gordon JI. Identifying gut microbe-host phenotype relationships using combinatorial communities in gnotobiotic mice. Sci Transl Med. 2014;6:220ra211. doi: 10.1126/scitranslmed.3008051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feleszko W, Jaworska J, Rha RD, Steinhausen S, Avagyan A, Jaudszus A, Ahrens B, Groneberg DA, Wahn U, Hamelmann E. Probiotic-induced suppression of allergic sensitization and airway inflammation is associated with an increase of T regulatory-dependent mechanisms in a murine model of asthma. Clin Exp Allergy. 2007;37:498–505. doi: 10.1111/j.1365-2222.2006.02629.x. [DOI] [PubMed] [Google Scholar]
- Fernandez MI, Pedron T, Tournebize R, Olivo-Marin JC, Sansonetti PJ, Phalipon A. Anti-inflammatory role for intracellular dimeric immunoglobulin a by neutralization of lipopolysaccharide in epithelial cells. Immunity. 2003;18:739–749. doi: 10.1016/s1074-7613(03)00122-5. [DOI] [PubMed] [Google Scholar]
- Foligne B, Zoumpopoulou G, Dewulf J, Ben Younes A, Chareyre F, Sirard JC, Pot B, Grangette C. A key role of dendritic cells in probiotic functionality. PLoS ONE. 2007;2:e313. doi: 10.1371/journal.pone.0000313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franchi L, Kamada N, Nakamura Y, Burberry A, Kuffa P, Suzuki S, Shaw MH, Kim YG, Nunez G. NLRC4-driven production of IL-1beta discriminates between pathogenic and commensal bacteria and promotes host intestinal defense. Nature immunology. 2012;13:449–456. doi: 10.1038/ni.2263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank DN, St Amand AL, Feldman RA, Boedeker EC, Harpaz N, Pace NR. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc Natl Acad Sci U S A. 2007;104:13780–13785. doi: 10.1073/pnas.0706625104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukata M, Chen A, Vamadevan AS, Cohen J, Breglio K, Krishnareddy S, Hsu D, Xu R, Harpaz N, Dannenberg AJ, et al. Toll-like receptor-4 promotes the development of colitis-associated colorectal tumors. Gastroenterology. 2007;133:1869–1881. doi: 10.1053/j.gastro.2007.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furusawa Y, Obata Y, Fukuda S, Endo TA, Nakato G, Takahashi D, Nakanishi Y, Uetake C, Kato K, Kato T, et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature. 2013 doi: 10.1038/nature12721. [DOI] [PubMed] [Google Scholar]
- Gaboriau-Routhiau V, Rakotobe S, Lecuyer E, Mulder I, Lan A, Bridonneau C, Rochet V, Pisi A, De Paepe M, Brandi G, et al. The key role of segmented filamentous bacteria in the coordinated maturation of gut helper T cell responses. Immunity. 2009;31:677–689. doi: 10.1016/j.immuni.2009.08.020. [DOI] [PubMed] [Google Scholar]
- Gabryszewski SJ, Bachar O, Dyer KD, Percopo CM, Killoran KE, Domachowske JB, Rosenberg HF. Lactobacillus-mediated priming of the respiratory mucosa protects against lethal pneumovirus infection. J Immunol. 2011;186:1151–1161. doi: 10.4049/jimmunol.1001751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gantois I, Ducatelle R, Pasmans F, Haesebrouck F, Hautefort I, Thompson A, Hinton JC, Van Immerseel F. Butyrate specifically down-regulates salmonella pathogenicity island 1 gene expression. Applied and environmental microbiology. 2006;72:946–949. doi: 10.1128/AEM.72.1.946-949.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Crespo KE, Chan CC, Gabryszewski SJ, Percopo CM, Rigaux P, Dyer KD, Domachowske JB, Rosenberg HF. Lactobacillus priming of the respiratory tract: Heterologous immunity and protection against lethal pneumovirus infection. Antiviral Res. 2012 doi: 10.1016/j.antiviral.2012.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett WS, Gallini CA, Yatsunenko T, Michaud M, DuBois A, Delaney ML, Punit S, Karlsson M, Bry L, Glickman JN, et al. Enterobacteriaceae act in concert with the gut microbiota to induce spontaneous and maternally transmitted colitis. Cell host & microbe. 2010;8:292–300. doi: 10.1016/j.chom.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett WS, Lord GM, Punit S, Lugo-Villarino G, Mazmanian SK, Ito S, Glickman JN, Glimcher LH. Communicable ulcerative colitis induced by T-bet deficiency in the innate immune system. Cell. 2007;131:33–45. doi: 10.1016/j.cell.2007.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett WS, Punit S, Gallini CA, Michaud M, Zhang D, Sigrist KS, Lord GM, Glickman JN, Glimcher LH. Colitis-associated colorectal cancer driven by T-bet deficiency in dendritic cells. Cancer Cell. 2009;16:208–219. doi: 10.1016/j.ccr.2009.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geuking MB, Cahenzli J, Lawson MA, Ng DC, Slack E, Hapfelmeier S, McCoy KD, Macpherson AJ. Intestinal bacterial colonization induces mutualistic regulatory T cell responses. Immunity. 2011;34:794–806. doi: 10.1016/j.immuni.2011.03.021. [DOI] [PubMed] [Google Scholar]
- Gordon JI, Dewey KG, Mills DA, Medzhitov RM. The human gut microbiota and undernutrition. Sci Transl Med. 2012;4:137ps112. doi: 10.1126/scitranslmed.3004347. [DOI] [PubMed] [Google Scholar]
- Grainger JR, Wohlfert EA, Fuss IJ, Bouladoux N, Askenase MH, Legrand F, Koo LY, Brenchley JM, Fraser ID, Belkaid Y. Inflammatory monocytes regulate pathologic responses to commensals during acute gastrointestinal infection. Nature medicine. 2013;19:713–721. doi: 10.1038/nm.3189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregor MF, Hotamisligil GS. Inflammatory mechanisms in obesity. Annual review of immunology. 2011;29:415–445. doi: 10.1146/annurev-immunol-031210-101322. [DOI] [PubMed] [Google Scholar]
- Grice EA, Kong HH, Conlan S, Deming CB, Davis J, Young AC, Bouffard GG, Blakesley RW, Murray PR, Green ED, et al. Topographical and temporal diversity of the human skin microbiome. Science. 2009;324:1190–1192. doi: 10.1126/science.1171700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grice EA, Kong HH, Renaud G, Young AC, Bouffard GG, Blakesley RW, Wolfsberg TG, Turner ML, Segre JA. A diversity profile of the human skin microbiota. Genome research. 2008;18:1043–1050. doi: 10.1101/gr.075549.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grivennikov SI, Karin M. Dangerous liaisons: STAT3 and NF-kappaB collaboration and crosstalk in cancer. Cytokine & growth factor reviews. 2010;21:11–19. doi: 10.1016/j.cytogfr.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grivennikov SI, Wang K, Mucida D, Stewart CA, Schnabl B, Jauch D, Taniguchi K, Yu GY, Osterreicher CH, Hung KE, et al. Adenoma-linked barrier defects and microbial products drive IL-23/IL-17-mediated tumour growth. Nature. 2012;491:254–258. doi: 10.1038/nature11465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta N, Limbago BM, Patel JB, Kallen AJ. Carbapenem-resistant Enterobacteriaceae: epidemiology and prevention. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2011;53:60–67. doi: 10.1093/cid/cir202. [DOI] [PubMed] [Google Scholar]
- Haas A, Zimmermann K, Graw F, Slack E, Rusert P, Ledergerber B, Bossart W, Weber R, Thurnheer MC, Battegay M, et al. Systemic antibody responses to gut commensal bacteria during chronic HIV-1 infection. Gut. 2011;60:1506–1519. doi: 10.1136/gut.2010.224774. [DOI] [PubMed] [Google Scholar]
- Hall JA, Bouladoux N, Sun CM, Wohlfert EA, Blank RB, Zhu Q, Grigg ME, Berzofsky JA, Belkaid Y. Commensal DNA Limits Regulatory T Cell Conversion and Is a Natural Adjuvant of Intestinal Immune Responses. Immunity. 2008 doi: 10.1016/j.immuni.2008.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall JA, Cannons JL, Grainger JR, Dos Santos LM, Hand TW, Naik S, Wohlfert EA, Chou DB, Oldenhove G, Robinson M, et al. Essential role for retinoic acid in the promotion of CD4(+) T cell effector responses via retinoic acid receptor alpha. Immunity. 2011a;34:435–447. doi: 10.1016/j.immuni.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall JA, Grainger JR, Spencer SP, Belkaid Y. The role of retinoic acid in tolerance and immunity. Immunity. 2011b;35:13–22. doi: 10.1016/j.immuni.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada H, Hiroi T, Nishiyama Y, Takahashi H, Masunaga Y, Hachimura S, Kaminogawa S, Takahashi-Iwanaga H, Iwanaga T, Kiyono H, et al. Identification of multiple isolated lymphoid follicles on the antimesenteric wall of the mouse small intestine. J Immunol. 2002;168:57–64. doi: 10.4049/jimmunol.168.1.57. [DOI] [PubMed] [Google Scholar]
- Hammami R, Fernandez B, Lacroix C, Fliss I. Anti-infective properties of bacteriocins: an update. Cellular and molecular life sciences: CMLS. 2013;70:2947–2967. doi: 10.1007/s00018-012-1202-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hand TW, Dos Santos LM, Bouladoux N, Molloy MJ, Pagan AJ, Pepper M, Maynard CL, Elson CO, 3rd, Belkaid Y. Acute gastrointestinal infection induces long-lived microbiota-specific T cell responses. Science. 2012;337:1553–1556. doi: 10.1126/science.1220961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hapfelmeier S, Lawson MA, Slack E, Kirundi JK, Stoel M, Heikenwalder M, Cahenzli J, Velykoredko Y, Balmer ML, Endt K, et al. Reversible microbial colonization of germ-free mice reveals the dynamics of IgA immune responses. Science. 2010;328:1705–1709. doi: 10.1126/science.1188454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes KS, Bancroft AJ, Goldrick M, Portsmouth C, Roberts IS, Grencis RK. Exploitation of the intestinal microflora by the parasitic nematode Trichuris muris. Science. 2011;328:1391–1394. doi: 10.1126/science.1187703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heimesaat MM, Bereswill S, Fischer A, Fuchs D, Struck D, Niebergall J, Jahn HK, Dunay IR, Moter A, Gescher DM, et al. Gram-negative bacteria aggravate murine small intestinal Th1-type immunopathology following oral infection with Toxoplasma gondii. Journal of immunology. 2006;177:8785–8795. doi: 10.4049/jimmunol.177.12.8785. [DOI] [PubMed] [Google Scholar]
- Herbst T, Sichelstiel A, Schar C, Yadava K, Burki K, Cahenzli J, McCoy K, Marsland BJ, Harris NL. Dysregulation of allergic airway inflammation in the absence of microbial colonization. Am J Respir Crit Care Med. 2011;184:198–205. doi: 10.1164/rccm.201010-1574OC. [DOI] [PubMed] [Google Scholar]
- Hilty M, Burke C, Pedro H, Cardenas P, Bush A, Bossley C, Davies J, Ervine A, Poulter L, Pachter L, et al. Disordered microbial communities in asthmatic airways. PLoS ONE. 2010;5:e8578. doi: 10.1371/journal.pone.0008578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homann D, Teyton L, Oldstone MB. Differential regulation of antiviral T-cell immunity results in stable CD8+ but declining CD4+ T-cell memory. Nature medicine. 2001;7:913–919. doi: 10.1038/90950. [DOI] [PubMed] [Google Scholar]
- Hooper LV, Macpherson AJ. Immune adaptations that maintain homeostasis with the intestinal microbiota. Nature reviews Immunology. 2010;10:159–169. doi: 10.1038/nri2710. [DOI] [PubMed] [Google Scholar]
- Hooper LV, Wong MH, Thelin A, Hansson L, Falk PG, Gordon JI. Molecular analysis of commensal host-microbial relationships in the intestine. Science. 2001;291:881–884. doi: 10.1126/science.291.5505.881. [DOI] [PubMed] [Google Scholar]
- Hornquist E, Lycke N. Cholera toxin increases T lymphocyte responses to unrelated antigens. Advances in experimental medicine and biology. 1995;371B:1507–1512. [PubMed] [Google Scholar]
- Hu B, Elinav E, Huber S, Strowig T, Hao L, Hafemann A, Jin C, Wunderlich C, Wunderlich T, Eisenbarth SC, et al. Microbiota-induced activation of epithelial IL-6 signaling links inflammasome-driven inflammation with transmissible cancer. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:9862–9867. doi: 10.1073/pnas.1307575110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichinohe T, Pang IK, Kumamoto Y, Peaper DR, Ho JH, Murray TS, Iwasaki A. Microbiota regulates immune defense against respiratory tract influenza A virus infection. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:5354–5359. doi: 10.1073/pnas.1019378108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iida N, Dzutsev A, Stewart CA, Smith L, Bouladoux N, Weingarten RA, Molina DA, Salcedo R, Back T, Cramer S, et al. Commensal bacteria control cancer response to therapy by modulating the tumor microenvironment. Science. 2013;342:967–970. doi: 10.1126/science.1240527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iliev ID, Funari VA, Taylor KD, Nguyen Q, Reyes CN, Strom SP, Brown J, Becker CA, Fleshner PR, Dubinsky M, et al. Interactions between commensal fungi and the C-type lectin receptor Dectin-1 influence colitis. Science. 2012;336:1314–1317. doi: 10.1126/science.1221789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ismail AS, Severson KM, Vaishnava S, Behrendt CL, Yu X, Benjamin JL, Ruhn KA, Hou B, DeFranco AL, Yarovinsky F, et al. Gammadelta intraepithelial lymphocytes are essential mediators of host-microbial homeostasis at the intestinal mucosal surface. Proc Natl Acad Sci U S A. 2011;108:8743–8748. doi: 10.1073/pnas.1019574108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Issa M, Ananthakrishnan AN, Binion DG. Clostridium difficile and inflammatory bowel disease. Inflammatory bowel diseases. 2008;14:1432–1442. doi: 10.1002/ibd.20500. [DOI] [PubMed] [Google Scholar]
- Ivanov II, Atarashi K, Manel N, Brodie EL, Shima T, Karaoz U, Wei D, Goldfarb KC, Santee CA, Lynch SV, et al. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell. 2009;139:485–498. doi: 10.1016/j.cell.2009.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov II, de Frutos RL, Manel N, Yoshinaga K, Rifkin DB, Sartor RB, Finlay BB, Littman DR. Specific microbiota direct the differentiation of IL-17-producing T-helper cells in the mucosa of the small intestine. Cell host & microbe. 2008;4:337–349. doi: 10.1016/j.chom.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwase T, Uehara Y, Shinji H, Tajima A, Seo H, Takada K, Agata T, Mizunoe Y. Staphylococcus epidermidis Esp inhibits Staphylococcus aureus biofilm formation and nasal colonization. Nature. 2010;465:346–349. doi: 10.1038/nature09074. [DOI] [PubMed] [Google Scholar]
- Jayashree B, Bibin YS, Prabhu D, Shanthirani CS, Gokulakrishnan K, Lakshmi BS, Mohan V, Balasubramanyam M. Increased circulatory levels of lipopolysaccharide (LPS) and zonulin signify novel biomarkers of proinflammation in patients with type 2 diabetes. Molecular and cellular biochemistry. 2013 doi: 10.1007/s11010-013-1911-4. [DOI] [PubMed] [Google Scholar]
- Johnston CJ, McSorley HJ, Anderton SM, Wigmore SJ, Maizels RM. Helminths and immunological tolerance. Transplantation. 2014;97:127–132. doi: 10.1097/TP.0b013e3182a53f59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josefowicz SZ, Niec RE, Kim HY, Treuting P, Chinen T, Zheng Y, Umetsu DT, Rudensky AY. Extrathymically generated regulatory T cells control mucosal TH2 inflammation. Nature. 2012a;482:395–399. doi: 10.1038/nature10772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josefowicz SZ, Niec RE, Kim HY, Treuting P, Chinen T, Zheng Y, Umetsu DT, Rudensky AY. Extrathymically generated regulatory T cells control mucosal TH2 inflammation. Nature. 2012b;482:395–399. doi: 10.1038/nature10772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung C, Meinzer U, Montcuquet N, Thachil E, Chateau D, Thiebaut R, Roy M, Alnabhani Z, Berrebi D, Dussaillant M, et al. Yersinia pseudotuberculosis disrupts intestinal barrier integrity through hematopoietic TLR-2 signaling. J Clin Invest. 2012;122:2239–2251. doi: 10.1172/JCI58147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamada N, Chen GY, Inohara N, Nunez G. Control of pathogens and pathobionts by the gut microbiota. Nature immunology. 2013;14:685–690. doi: 10.1038/ni.2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane M, Case LK, Kopaskie K, Kozlova A, MacDearmid C, Chervonsky AV, Golovkina TV. Succesful transmission of a retrovirus depends on the commensal microbiota. Science. 2011 doi: 10.1126/science.1210718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karimi K, Inman MD, Bienenstock J, Forsythe P. Lactobacillus reuteri-induced regulatory T cells protect against an allergic airway response in mice. Am J Respir Crit Care Med. 2009;179:186–193. doi: 10.1164/rccm.200806-951OC. [DOI] [PubMed] [Google Scholar]
- Karlsson FH, Tremaroli V, Nookaew I, Bergstrom G, Behre CJ, Fagerberg B, Nielsen J, Backhed F. Gut metagenome in European women with normal, impaired and diabetic glucose control. Nature. 2013;498:99–103. doi: 10.1038/nature12198. [DOI] [PubMed] [Google Scholar]
- Kelsall B. Recent progress in understanding the phenotype and function of intestinal dendritic cells and macrophages. Mucosal immunology. 2008;1:460–469. doi: 10.1038/mi.2008.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinnebrew MA, Buffie CG, Diehl GE, Zenewicz LA, Leiner I, Hohl TM, Flavell RA, Littman DR, Pamer EG. Interleukin 23 production by intestinal CD103(+)CD11b(+) dendritic cells in response to bacterial flagellin enhances mucosal innate immune defense. Immunity. 2012;36:276–287. doi: 10.1016/j.immuni.2011.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiss EA, Vonarbourg C, Kopfmann S, Hobeika E, Finke D, Esser C, Diefenbach A. Natural aryl hydrocarbon receptor ligands control organogenesis of intestinal lymphoid follicles. Science. 2011;334:1561–1565. doi: 10.1126/science.1214914. [DOI] [PubMed] [Google Scholar]
- Kiyono H, McGhee JR, Wannemuehler MJ, Michalek SM. Lack of oral tolerance in C3H/HeJ mice. The Journal of experimental medicine. 1982;155:605–610. doi: 10.1084/jem.155.2.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klatt NR, Funderburg NT, Brenchley JM. Microbial translocation, immune activation, and HIV disease. Trends in microbiology. 2013;21:6–13. doi: 10.1016/j.tim.2012.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klebanoff CA, Spencer SP, Torabi-Parizi P, Grainger JR, Roychoudhuri R, Ji Y, Sukumar M, Muranski P, Scott CD, Hall JA, et al. Retinoic acid controls the homeostasis of pre-cDC-derived splenic and intestinal dendritic cells. Journal of Experimental Medicine. 2013;43:1608. doi: 10.1084/jem.20122508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollmann TR, Levy O, Montgomery RR, Goriely S. Innate immune function by Toll-like receptors: distinct responses in newborns and the elderly. Immunity. 2012;37:771–783. doi: 10.1016/j.immuni.2012.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong HH, Oh J, Deming C, Conlan S, Grice EA, Beatson MA, Nomicos E, Polley EC, Komarow HD, Murray PR, et al. Temporal shifts in the skin microbiome associated with disease flares and treatment in children with atopic dermatitis. Genome research. 2012;22:850–859. doi: 10.1101/gr.131029.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korpe PS, Petri WA., Jr Environmental enteropathy: critical implications of a poorly understood condition. Trends in molecular medicine. 2012;18:328–336. doi: 10.1016/j.molmed.2012.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosek M, Bern C, Guerrant RL. The global burden of diarrhoeal disease, as estimated from studies published between 1992 and 2000. Bull World Health Organ. 2003;81:197–204. [PMC free article] [PubMed] [Google Scholar]
- Kostic AD, Chun E, Robertson L, Glickman JN, Gallini CA, Michaud M, Clancy TE, Chung DC, Lochhead P, Hold GL, et al. Fusobacterium nucleatum potentiates intestinal tumorigenesis and modulates the tumor-immune microenvironment. Cell host & microbe. 2013;14:207–215. doi: 10.1016/j.chom.2013.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostic AD, Gevers D, Pedamallu CS, Michaud M, Duke F, Earl AM, Ojesina AI, Jung J, Bass AJ, Tabernero J, et al. Genomic analysis identifies association of Fusobacterium with colorectal carcinoma. Genome research. 2012;22:292–298. doi: 10.1101/gr.126573.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriegel MA, Sefik E, Hill JA, Wu HJ, Benoist C, Mathis D. Naturally transmitted segmented filamentous bacteria segregate with diabetes protection in nonobese diabetic mice. Proc Natl Acad Sci U S A. 2011;108:11548–11553. doi: 10.1073/pnas.1108924108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kullberg MC, Jankovic D, Gorelick PL, Caspar P, Letterio JJ, Cheever AW, Sher A. Bacteria-triggered CD4(+) T regulatory cells suppress Helicobacter hepaticus-induced colitis. The Journal of experimental medicine. 2002;196:505–515. doi: 10.1084/jem.20020556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuss SK, Best GT, Etheredge CA, Pruijssers AJ, Frierson JM, Hooper LV, Dermody TS, Pfeiffer JK. Intestinal Microbiota promote enteric virus replication and systemic pathogenesis. Science. 2011 doi: 10.1126/science.1211057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai Y, Di Nardo A, Nakatsuji T, Leichtle A, Yang Y, Cogen AL, Wu ZR, Hooper LV, Schmidt RR, von Aulock S, et al. Commensal bacteria regulate Toll-like receptor 3-dependent inflammation after skin injury. Nature medicine. 2009;15:1377–1382. doi: 10.1038/nm.2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamps LW, Madhusudhan KT, Havens JM, Greenson JK, Bronner MP, Chiles MC, Dean PJ, Scott MA. Pathogenic Yersinia DNA is detected in bowel and mesenteric lymph nodes from patients with Crohn’s disease. The American journal of surgical pathology. 2003;27:220–227. doi: 10.1097/00000478-200302000-00011. [DOI] [PubMed] [Google Scholar]
- Lathrop SK, Bloom SM, Rao SM, Nutsch K, Lio CW, Santacruz N, Peterson DA, Stappenbeck TS, Hsieh CS. Peripheral education of the immune system by colonic commensal microbiota. Nature. 2011;478:250–254. doi: 10.1038/nature10434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YK, Menezes JS, Umesaki Y, Mazmanian SK. Proinflammatory T-cell responses to gut microbiota promote experimental autoimmune encephalomyelitis. Proc Natl Acad Sci U S A. 2011;108(Suppl 1):4615–4622. doi: 10.1073/pnas.1000082107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ley RE, Peterson DA, Gordon JI. Ecological and evolutionary forces shaping microbial diversity in the human intestine. Cell. 2006a;124:837–848. doi: 10.1016/j.cell.2006.02.017. [DOI] [PubMed] [Google Scholar]
- Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Microbial ecology: human gut microbes associated with obesity. Nature. 2006b;444:1022–1023. doi: 10.1038/4441022a. [DOI] [PubMed] [Google Scholar]
- Li Y, Innocentin S, Withers DR, Roberts NA, Gallagher AR, Grigorieva EF, Wilhelm C, Veldhoen M. Exogenous stimuli maintain intraepithelial lymphocytes via aryl hydrocarbon receptor activation. Cell. 2011;147:629–640. doi: 10.1016/j.cell.2011.09.025. [DOI] [PubMed] [Google Scholar]
- Lodes MJ, Cong Y, Elson CO, Mohamath R, Landers CJ, Targan SR, Fort M, Hershberg RM. Bacterial flagellin is a dominant antigen in Crohn disease. J Clin Invest. 2004;113:1296–1306. doi: 10.1172/JCI20295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotz M, Gutle D, Walther S, Menard S, Bogdan C, Hornef MW. Postnatal acquisition of endotoxin tolerance in intestinal epithelial cells. The Journal of experimental medicine. 2006;203:973–984. doi: 10.1084/jem.20050625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe EL, Crother TR, Rabizadeh S, Hu B, Wang H, Chen S, Shimada K, Wong MH, Michelsen KS, Arditi M. Toll-like receptor 2 signaling protects mice from tumor development in a mouse model of colitis-induced cancer. PLoS ONE. 2010;5:e13027. doi: 10.1371/journal.pone.0013027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozupone CA, Stombaugh JI, Gordon JI, Jansson JK, Knight R. Diversity, stability and resilience of the human gut microbiota. Nature. 2012;489:220–230. doi: 10.1038/nature11550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupp C, Robertson ML, Wickham ME, Sekirov I, Champion OL, Gaynor EC, Finlay BB. Host-mediated inflammation disrupts the intestinal microbiota and promotes the overgrowth of Enterobacteriaceae. Cell host & microbe. 2007;2:204. doi: 10.1016/j.chom.2007.08.002. [DOI] [PubMed] [Google Scholar]
- Macpherson A, Khoo UY, Forgacs I, Philpott-Howard J, Bjarnason I. Mucosal antibodies in inflammatory bowel disease are directed against intestinal bacteria. Gut. 1996;38:365–375. doi: 10.1136/gut.38.3.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macpherson AJ, Harris NL. Interactions between commensal intestinal bacteria and the immune system. Nature reviews Immunology. 2004;4:478–485. doi: 10.1038/nri1373. [DOI] [PubMed] [Google Scholar]
- Macpherson AJ, Hunziker L, McCoy K, Lamarre A. IgA responses in the intestinal mucosa against pathogenic and non-pathogenic microorganisms. Microbes and infection/Institut Pasteur. 2001;3:1021–1035. doi: 10.1016/s1286-4579(01)01460-5. [DOI] [PubMed] [Google Scholar]
- Macpherson AJ, Slack E, Geuking MB, McCoy KD. The mucosal firewalls against commensal intestinal microbes. Seminars in immunopathology. 2009;31:145–149. doi: 10.1007/s00281-009-0174-3. [DOI] [PubMed] [Google Scholar]
- Macpherson AJ, Uhr T. Induction of protective IgA by intestinal dendritic cells carrying commensal bacteria. Science. 2004;303:1662–1665. doi: 10.1126/science.1091334. [DOI] [PubMed] [Google Scholar]
- Maloy KJ, Powrie F. Intestinal homeostasis and its breakdown in inflammatory bowel disease. Nature. 2011;474:298–306. doi: 10.1038/nature10208. [DOI] [PubMed] [Google Scholar]
- Marcobal A, Barboza M, Froehlich JW, Block DE, German JB, Lebrilla CB, Mills DA. Consumption of human milk oligosaccharides by gut-related microbes. Journal of agricultural and food chemistry. 2010;58:5334–5340. doi: 10.1021/jf9044205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcobal A, Sonnenburg JL. Human milk oligosaccharide consumption by intestinal microbiota. Clinical microbiology and infection: the official publication of the European Society of Clinical Microbiology and Infectious Diseases. 2012;18(Suppl 4):12–15. doi: 10.1111/j.1469-0691.2012.03863.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markle JG, Frank DN, Mortin-Toth S, Robertson CE, Feazel LM, Rolle-Kampczyk U, von Bergen M, McCoy KD, Macpherson AJ, Danska JS. Sex differences in the gut microbiome drive hormone-dependent regulation of autoimmunity. Science. 2013;339:1084–1088. doi: 10.1126/science.1233521. [DOI] [PubMed] [Google Scholar]
- Marshall BJ, Warren JR. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet. 1984;1:1311–1315. doi: 10.1016/s0140-6736(84)91816-6. [DOI] [PubMed] [Google Scholar]
- Martinoli C, Chiavelli A, Rescigno M. Entry route of Salmonella typhimurium directs the type of induced immune response. Immunity. 2007;27:975–984. doi: 10.1016/j.immuni.2007.10.011. [DOI] [PubMed] [Google Scholar]
- Maslowski KM, Vieira AT, Ng A, Kranich J, Sierro F, Yu D, Schilter HC, Rolph MS, Mackay F, Artis D, et al. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature. 2009;461:1282–1286. doi: 10.1038/nature08530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazmanian SK, Liu CH, Tzianabos AO, Kasper DL. An immunomodulatory molecule of symbiotic bacteria directs maturation of the host immune system. Cell. 2005;122:107–118. doi: 10.1016/j.cell.2005.05.007. [DOI] [PubMed] [Google Scholar]
- Mazmanian SK, Round JL, Kasper DL. A microbial symbiosis factor prevents intestinal inflammatory disease. Nature. 2008;453:620–625. doi: 10.1038/nature07008. [DOI] [PubMed] [Google Scholar]
- McGuckin MA, Linden SK, Sutton P, Florin TH. Mucin dynamics and enteric pathogens. Nature reviews Microbiology. 2011;9:265–278. doi: 10.1038/nrmicro2538. [DOI] [PubMed] [Google Scholar]
- Meijer K, de Vos P, Priebe MG. Butyrate and other short-chain fatty acids as modulators of immunity: what relevance for health? Current opinion in clinical nutrition and metabolic care. 2010;13:715–721. doi: 10.1097/MCO.0b013e32833eebe5. [DOI] [PubMed] [Google Scholar]
- Meinzer U, Barreau F, Esmiol-Welterlin S, Jung C, Villard C, Leger T, Ben-Mkaddem S, Berrebi D, Dussaillant M, Alnabhani Z, et al. Yersinia pseudotuberculosis effector YopJ subverts the Nod2/RICK/TAK1 pathway and activates caspase-1 to induce intestinal barrier dysfunction. Cell host & microbe. 2012;11:337–351. doi: 10.1016/j.chom.2012.02.009. [DOI] [PubMed] [Google Scholar]
- Molloy MJ, Bouladoux N, Belkaid Y. Intestinal microbiota: shaping local and systemic immune responses. Seminars in immunology. 2012;24:58–66. doi: 10.1016/j.smim.2011.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molloy MJ, Grainger JR, Bouladoux N, Hand TW, Koo LY, Naik S, Quinones M, Dzutsev AK, Gao JL, Trinchieri G, et al. Intraluminal containment of commensal outgrowth in the gut during infection-induced dysbiosis. Cell host & microbe. 2013;14:318–328. doi: 10.1016/j.chom.2013.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monira S, Nakamura S, Gotoh K, Izutsu K, Watanabe H, Alam NH, Endtz HP, Cravioto A, Ali SI, Nakaya T, et al. Gut microbiota of healthy and malnourished children in bangladesh. Frontiers in microbiology. 2011;2:228. doi: 10.3389/fmicb.2011.00228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mora JR, Iwata M, von Andrian UH. Vitamin effects on the immune system: vitamins A and D take centre stage. Nat Rev Immunol. 2008;8:685–698. doi: 10.1038/nri2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mucida D, Kutchukhidze N, Erazo A, Russo M, Lafaille JJ, Curotto de Lafaille MA. Oral tolerance in the absence of naturally occurring Tregs. The Journal of clinical investigation. 2005;115:1923–1933. doi: 10.1172/JCI24487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mucida D, Park Y, Kim G, Turovskaya O, Scott I, Kronenberg M, Cheroutre H. Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science. 2007;317:256–260. doi: 10.1126/science.1145697. [DOI] [PubMed] [Google Scholar]
- Mukherjee S, Partch CL, Lehotzky RE, Whitham CV, Chu H, Bevins CL, Gardner KH, Hooper LV. Regulation of C-type lectin antimicrobial activity by a flexible N-terminal prosegment. J Biol Chem. 2009;284:4881–4888. doi: 10.1074/jbc.M808077200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray BE. Vancomycin-resistant enterococcal infections. The New England journal of medicine. 2000;342:710–721. doi: 10.1056/NEJM200003093421007. [DOI] [PubMed] [Google Scholar]
- Naik S, Bouladoux N, Wilhelm C, Molloy MJ, Salcedo R, Kastenmuller W, Deming C, Quinones M, Koo L, Conlan S, et al. Compartmentalized control of skin immunity by resident commensals. Science. 2012;337:1115–1119. doi: 10.1126/science.1225152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng KM, Ferreyra JA, Higginbottom SK, Lynch JB, Kashyap PC, Gopinath S, Naidu N, Choudhury B, Weimer BC, Monack DM, et al. Microbiota-liberated host sugars facilitate post-antibiotic expansion of enteric pathogens. Nature. 2013;502:96–99. doi: 10.1038/nature12503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niess JH, Adler G. Enteric flora expands gut lamina propria CX3CR1+ dendritic cells supporting inflammatory immune responses under normal and inflammatory conditions. J Immunol. 2010;184:2026–2037. doi: 10.4049/jimmunol.0901936. [DOI] [PubMed] [Google Scholar]
- O’Mahony C, Scully P, O’Mahony D, Murphy S, O’Brien F, Lyons A, Sherlock G, MacSharry J, Kiely B, Shanahan F, et al. Commensal-induced regulatory T cells mediate protection against pathogen-stimulated NF-kappaB activation. PLoS Pathog. 2008;4:e1000112. doi: 10.1371/journal.ppat.1000112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochoa-Reparaz J, Mielcarz DW, Ditrio LE, Burroughs AR, Begum-Haque S, Dasgupta S, Kasper DL, Kasper LH. Central nervous system demyelinating disease protection by the human commensal Bacteroides fragilis depends on polysaccharide A expression. J Immunol. 2010a;185:4101–4108. doi: 10.4049/jimmunol.1001443. [DOI] [PubMed] [Google Scholar]
- Ochoa-Reparaz J, Mielcarz DW, Ditrio LE, Burroughs AR, Foureau DM, Haque-Begum S, Kasper LH. Role of gut commensal microflora in the development of experimental autoimmune encephalomyelitis. J Immunol. 2009;183:6041–6050. doi: 10.4049/jimmunol.0900747. [DOI] [PubMed] [Google Scholar]
- Ochoa-Reparaz J, Mielcarz DW, Wang Y, Begum-Haque S, Dasgupta S, Kasper DL, Kasper LH. A polysaccharide from the human commensal Bacteroides fragilis protects against CNS demyelinating disease. Mucosal immunology. 2010b;3:487–495. doi: 10.1038/mi.2010.29. [DOI] [PubMed] [Google Scholar]
- Ohnmacht C, Marques R, Presley L, Sawa S, Lochner M, Eberl G. Intestinal microbiota, evolution of the immune system and the bad reputation of pro-inflammatory immunity. Cellular microbiology. 2011;13:653–659. doi: 10.1111/j.1462-5822.2011.01577.x. [DOI] [PubMed] [Google Scholar]
- Olszak T, An D, Zeissig S, Vera MP, Richter J, Franke A, Glickman JN, Siebert R, Baron RM, Kasper DL, et al. Microbial exposure during early life has persistent effects on natural killer T cell function. Science. 2012;336:489–493. doi: 10.1126/science.1219328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborn O, Olefsky JM. The cellular and signaling networks linking the immune system and metabolism in disease. Nature medicine. 2012;18:363–374. doi: 10.1038/nm.2627. [DOI] [PubMed] [Google Scholar]
- Pacheco AR, Curtis MM, Ritchie JM, Munera D, Waldor MK, Moreira CG, Sperandio V. Fucose sensing regulates bacterial intestinal colonization. Nature. 2012;492:113–117. doi: 10.1038/nature11623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packey CD, Sartor RB. Interplay of commensal and pathogenic bacteria, genetic mutations, and immunoregulatory defects in the pathogenesis of inflammatory bowel diseases. Journal of internal medicine. 2008;263:597–606. doi: 10.1111/j.1365-2796.2008.01962.x. [DOI] [PubMed] [Google Scholar]
- Paulos CM, Wrzesinski C, Kaiser A, Hinrichs CS, Chieppa M, Cassard L, Palmer DC, Boni A, Muranski P, Yu Z, et al. Microbial translocation augments the function of adoptively transferred self/tumor-specific CD8+ T cells via TLR4 signaling. The Journal of clinical investigation. 2007;117:2197–2204. doi: 10.1172/JCI32205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepper M, Linehan JL, Pagan AJ, Zell T, Dileepan T, Cleary PP, Jenkins MK. Different routes of bacterial infection induce long-lived TH1 memory cells and short-lived TH17 cells. Nature immunology. 2009;11:83–89. doi: 10.1038/ni.1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez PF, Dore J, Leclerc M, Levenez F, Benyacoub J, Serrant P, Segura-Roggero I, Schiffrin EJ, Donnet-Hughes A. Bacterial imprinting of the neonatal immune system: lessons from maternal cells? Pediatrics. 2007;119:e724–732. doi: 10.1542/peds.2006-1649. [DOI] [PubMed] [Google Scholar]
- Peterson DA, McNulty NP, Guruge JL, Gordon JI. IgA response to symbiotic bacteria as a mediator of gut homeostasis. Cell host & microbe. 2007;2:328–339. doi: 10.1016/j.chom.2007.09.013. [DOI] [PubMed] [Google Scholar]
- Powell N, Walker AW, Stolarczyk E, Canavan JB, Gokmen MR, Marks E, Jackson I, Hashim A, Curtis MA, Jenner RG, et al. The transcription factor T-bet regulates intestinal inflammation mediated by interleukin-7 receptor+ innate lymphoid cells. Immunity. 2012;37:674–684. doi: 10.1016/j.immuni.2012.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozzilli P, Signore A, Williams AJ, Beales PE. NOD mouse colonies around the world--recent facts and figures. Immunology today. 1993;14:193–196. doi: 10.1016/0167-5699(93)90160-M. [DOI] [PubMed] [Google Scholar]
- PrabhuDas M, Adkins B, Gans H, King C, Levy O, Ramilo O, Siegrist CA. Challenges in infant immunity: implications for responses to infection and vaccines. Nature immunology. 2011;12:189–194. doi: 10.1038/ni0311-189. [DOI] [PubMed] [Google Scholar]
- Pryde SE, Duncan SH, Hold GL, Stewart CS, Flint HJ. The microbiology of butyrate formation in the human colon. FEMS microbiology letters. 2002;217:133–139. doi: 10.1111/j.1574-6968.2002.tb11467.x. [DOI] [PubMed] [Google Scholar]
- Qin J, Li Y, Cai Z, Li S, Zhu J, Zhang F, Liang S, Zhang W, Guan Y, Shen D, et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature. 2012;490:55–60. doi: 10.1038/nature11450. [DOI] [PubMed] [Google Scholar]
- Qiu J, Guo X, Chen ZM, He L, Sonnenberg GF, Artis D, Fu YX, Zhou L. Group 3 innate lymphoid cells inhibit T-cell-mediated intestinal inflammation through aryl hydrocarbon receptor signaling and regulation of microflora. Immunity. 2013;39:386–399. doi: 10.1016/j.immuni.2013.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raetz M, Hwang SH, Wilhelm CL, Kirkland D, Benson A, Sturge CR, Mirpuri J, Vaishnava S, Hou B, Defranco AL, et al. Parasite-induced T(H)1 cells and intestinal dysbiosis cooperate in IFN-gamma-dependent elimination of Paneth cells. Nature immunology. 2012 doi: 10.1038/ni.2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakoff-Nahoum S, Medzhitov R. Regulation of spontaneous intestinal tumorigenesis through the adaptor protein MyD88. Science. 2007;317:124–127. doi: 10.1126/science.1140488. [DOI] [PubMed] [Google Scholar]
- Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell. 2004;118:229–241. doi: 10.1016/j.cell.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Rivas MA, Beaudoin M, Gardet A, Stevens C, Sharma Y, Zhang CK, Boucher G, Ripke S, Ellinghaus D, Burtt N, et al. Deep resequencing of GWAS loci identifies independent rare variants associated with inflammatory bowel disease. Nature genetics. 2011;43:1066–1073. doi: 10.1038/ng.952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolhion N, Darfeuille-Michaud A. Adherent-invasive Escherichia coli in inflammatory bowel disease. Inflammatory bowel diseases. 2007;13:1277–1283. doi: 10.1002/ibd.20176. [DOI] [PubMed] [Google Scholar]
- Round JL, Lee SM, Li J, Tran G, Jabri B, Chatila TA, Mazmanian SK. The Toll-like receptor 2 pathway establishes colonization by a commensal of the human microbiota. Science. 2011;332:974–977. doi: 10.1126/science.1206095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinstein MR, Wang X, Liu W, Hao Y, Cai G, Han YW. Fusobacterium nucleatum promotes colorectal carcinogenesis by modulating E-cadherin/beta-catenin signaling via its FadA adhesin. Cell host & microbe. 2013;14:195–206. doi: 10.1016/j.chom.2013.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salcedo R, Worschech A, Cardone M, Jones Y, Gyulai Z, Dai RM, Wang E, Ma W, Haines D, O’HUigin C, et al. MyD88-mediated signaling prevents development of adenocarcinomas of the colon: role of interleukin 18. The Journal of experimental medicine. 2010;207:1625–1636. doi: 10.1084/jem.20100199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sansonetti PJ, Di Santo JP. Debugging how bacteria manipulate the immune response. Immunity. 2007;26:149–161. doi: 10.1016/j.immuni.2007.02.004. [DOI] [PubMed] [Google Scholar]
- Sartor RB. Mechanisms of disease: pathogenesis of Crohn’s disease and ulcerative colitis. Nature clinical practice Gastroenterology & hepatology. 2006;3:390–407. doi: 10.1038/ncpgasthep0528. [DOI] [PubMed] [Google Scholar]
- Satoh-Takayama N, Vosshenrich CA, Lesjean-Pottier S, Sawa S, Lochner M, Rattis F, Mention JJ, Thiam K, Cerf-Bensussan N, Mandelboim O, et al. Microbial flora drives interleukin 22 production in intestinal NKp46+ cells that provide innate mucosal immune defense. Immunity. 2008;29:958–970. doi: 10.1016/j.immuni.2008.11.001. [DOI] [PubMed] [Google Scholar]
- Savy M, Edmond K, Fine PE, Hall A, Hennig BJ, Moore SE, Mulholland K, Schaible U, Prentice AM. Landscape analysis of interactions between nutrition and vaccine responses in children. The Journal of nutrition. 2009;139:2154S–2218S. doi: 10.3945/jn.109.105312. [DOI] [PubMed] [Google Scholar]
- Schamberger GP, Diez-Gonzalez F. Selection of recently isolated colicinogenic Escherichia coli strains inhibitory to Escherichia coli O157:H7. Journal of food protection. 2002;65:1381–1387. doi: 10.4315/0362-028x-65.9.1381. [DOI] [PubMed] [Google Scholar]
- Scher JU, Sczesnak A, Longman RS, Segata N, Ubeda C, Bielski C, Rostron T, Cerundolo V, Pamer EG, Abramson SB, et al. Expansion of intestinal Prevotella copri correlates with enhanced susceptibility to arthritis. eLife. 2013;2:e01202. doi: 10.7554/eLife.01202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sczesnak A, Segata N, Qin X, Gevers D, Petrosino JF, Huttenhower C, Littman DR, Ivanov II. The genome of th17 cell-inducing segmented filamentous bacteria reveals extensive auxotrophy and adaptations to the intestinal environment. Cell host & microbe. 2011;10:260–272. doi: 10.1016/j.chom.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan M, Gentile M, Yeiser JR, Walland AC, Bornstein VU, Chen K, He B, Cassis L, Bigas A, Cols M, et al. Mucus Enhances Gut Homeostasis and Oral Tolerance by Delivering Immunoregulatory Signals. Science (New York, NY) 2013;342:447–453. doi: 10.1126/science.1237910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi C, Jia T, Mendez-Ferrer S, Hohl TM, Serbina NV, Lipuma L, Leiner I, Li MO, Frenette PS, Pamer EG. Bone marrow mesenchymal stem and progenitor cells induce monocyte emigration in response to circulating toll-like receptor ligands. Immunity. 2011;34:590–601. doi: 10.1016/j.immuni.2011.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulzhenko N, Morgun A, Hsiao W, Battle M, Yao M, Gavrilova O, Orandle M, Mayer L, Macpherson AJ, McCoy KD, et al. Crosstalk between B lymphocytes, microbiota and the intestinal epithelium governs immunity versus metabolism in the gut. Nature medicine. 2011;17:1585–1593. doi: 10.1038/nm.2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegrist CA. Neonatal and early life vaccinology. Vaccine. 2001;19:3331–3346. doi: 10.1016/s0264-410x(01)00028-7. [DOI] [PubMed] [Google Scholar]
- Smith K, McCoy KD, Macpherson AJ. Use of axenic animals in studying the adaptation of mammals to their commensal intestinal microbiota. Seminars in immunology. 2007;19:59–69. doi: 10.1016/j.smim.2006.10.002. [DOI] [PubMed] [Google Scholar]
- Smith PM, Howitt MR, Panikov N, Michaud M, Gallini CA, Bohlooly YM, Glickman JN, Garrett WS. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science. 2013;341:569–573. doi: 10.1126/science.1241165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smits HH, Engering A, van der Kleij D, de Jong EC, Schipper K, van Capel TM, Zaat BA, Yazdanbakhsh M, Wierenga EA, van Kooyk Y, et al. Selective probiotic bacteria induce IL-10-producing regulatory T cells in vitro by modulating dendritic cell function through dendritic cell-specific intercellular adhesion molecule 3-grabbing nonintegrin. J Allergy Clin Immunol. 2005;115:1260–1267. doi: 10.1016/j.jaci.2005.03.036. [DOI] [PubMed] [Google Scholar]
- Sobhani I, Tap J, Roudot-Thoraval F, Roperch JP, Letulle S, Langella P, Corthier G, Tran Van Nhieu J, Furet JP. Microbial dysbiosis in colorectal cancer (CRC) patients. PLoS ONE. 2011;6:e16393. doi: 10.1371/journal.pone.0016393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnenberg GF, Monticelli LA, Alenghat T, Fung TC, Hutnick NA, Kunisawa J, Shibata N, Grunberg S, Sinha R, Zahm AM, et al. Innate lymphoid cells promote anatomical containment of lymphoid-resident commensal bacteria. Science. 2012;336:1321–1325. doi: 10.1126/science.1222551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan S, Liu C, Mitchell CM, Fiedler TL, Thomas KK, Agnew KJ, Marrazzo JM, Fredricks DN. Temporal variability of human vaginal bacteria and relationship with bacterial vaginosis. PLoS ONE. 2010;5:e10197. doi: 10.1371/journal.pone.0010197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stappenbeck TS, Hooper LV, Gordon JI. Developmental regulation of intestinal angiogenesis by indigenous microbes via Paneth cells. Proc Natl Acad Sci U S A. 2002;99:15451–15455. doi: 10.1073/pnas.202604299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stecher B, Robbiani R, Walker AW, Westendorf AM, Barthel M, Kremer M, Chaffron S, Macpherson AJ, Buer J, Parkhill J, et al. Salmonella enterica serovar typhimurium exploits inflammation to compete with the intestinal microbiota. PLoS Biol. 2007;5:2177–2189. doi: 10.1371/journal.pbio.0050244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su LF, Kidd BA, Han A, Kotzin JJ, Davis MM. Virus-Specific CD4(+) Memory-Phenotype T Cells Are Abundant in Unexposed Adults. Immunity. 2013 doi: 10.1016/j.immuni.2012.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudo N, Sawamura S, Tanaka K, Aiba Y, Kubo C, Koga Y. The requirement of intestinal bacterial flora for the development of an IgE production system fully susceptible to oral tolerance induction. J Immunol. 1997;159:1739–1745. [PubMed] [Google Scholar]
- Sun CM, Hall JA, Blank RB, Bouladoux N, Oukka M, Mora JR, Belkaid Y. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. The Journal of experimental medicine. 2007;204:1775–1785. doi: 10.1084/jem.20070602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki K, Meek B, Doi Y, Muramatsu M, Chiba T, Honjo T, Fagarasan S. Aberrant expansion of segmented filamentous bacteria in IgA-deficient gut. Proc Natl Acad Sci U S A. 2004;101:1981–1986. doi: 10.1073/pnas.0307317101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda K, Kaisho T, Akira S. Toll-like receptors. Annual review of immunology. 2003;21:335–376. doi: 10.1146/annurev.immunol.21.120601.141126. [DOI] [PubMed] [Google Scholar]
- Talham GL, Jiang HQ, Bos NA, Cebra JJ. Segmented filamentous bacteria are potent stimuli of a physiologically normal state of the murine gut mucosal immune system. Infect Immun. 1999;67:1992–2000. doi: 10.1128/iai.67.4.1992-2000.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuji M, Komatsu N, Kawamoto S, Suzuki K, Kanagawa O, Honjo T, Hori S, Fagarasan S. Preferential generation of follicular B helper T cells from Foxp3+ T cells in gut Peyer’s patches. Science. 2009;323:1488–1492. doi: 10.1126/science.1169152. [DOI] [PubMed] [Google Scholar]
- Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444:1027–1031. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- Turovskiy Y, Sutyak Noll K, Chikindas ML. The aetiology of bacterial vaginosis. Journal of applied microbiology. 2011;110:1105–1128. doi: 10.1111/j.1365-2672.2011.04977.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ubeda C, Bucci V, Caballero S, Djukovic A, Toussaint NC, Equinda M, Lipuma L, Ling L, Gobourne A, No D, et al. Intestinal microbiota containing Barnesiella species cures vancomycin-resistant Enterococcus faecium colonization. Infection and immunity. 2013;81:965–973. doi: 10.1128/IAI.01197-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uematsu S, Fujimoto K, Jang MH, Yang BG, Jung YJ, Nishiyama M, Sato S, Tsujimura T, Yamamoto M, Yokota Y, et al. Regulation of humoral and cellular gut immunity by lamina propria dendritic cells expressing Toll-like receptor 5. Nature immunology. 2008;9:769–776. doi: 10.1038/ni.1622. [DOI] [PubMed] [Google Scholar]
- Uematsu S, Jang MH, Chevrier N, Guo Z, Kumagai Y, Yamamoto M, Kato H, Sougawa N, Matsui H, Kuwata H, et al. Detection of pathogenic intestinal bacteria by Toll-like receptor 5 on intestinal CD11c+ lamina propria cells. Nature immunology. 2006;7:868–874. doi: 10.1038/ni1362. [DOI] [PubMed] [Google Scholar]
- Umesaki Y, Setoyama H, Matsumoto S, Imaoka A, Itoh K. Differential roles of segmented filamentous bacteria and clostridia in development of the intestinal immune system. Infect Immun. 1999;67:3504–3511. doi: 10.1128/iai.67.7.3504-3511.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaishnava S, Behrendt CL, Ismail AS, Eckmann L, Hooper LV. Paneth cells directly sense gut commensals and maintain homeostasis at the intestinal host-microbial interface. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:20858–20863. doi: 10.1073/pnas.0808723105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaishnava S, Yamamoto M, Severson KM, Ruhn KA, Yu X, Koren O, Ley R, Wakeland EK, Hooper LV. The antibacterial lectin RegIIIgamma promotes the spatial segregation of microbiota and host in the intestine. Science. 2011;334:255–258. doi: 10.1126/science.1209791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Waaij D, Berghuis-de Vries JM, Lekkerkerk L-v. Colonization resistance of the digestive tract in conventional and antibiotic-treated mice. The Journal of hygiene. 1971;69:405–411. doi: 10.1017/s0022172400021653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Nood E, Vrieze A, Nieuwdorp M, Fuentes S, Zoetendal EG, de Vos WM, Visser CE, Kuijper EJ, Bartelsman JF, Tijssen JG, et al. Duodenal infusion of donor feces for recurrent Clostridium difficile. The New England journal of medicine. 2013;368:407–415. doi: 10.1056/NEJMoa1205037. [DOI] [PubMed] [Google Scholar]
- Vernacchio L, Vezina RM, Mitchell AA, Lesko SM, Plaut AG, Acheson DW. Diarrhea in American infants and young children in the community setting: incidence, clinical presentation and microbiology. Pediatr Infect Dis J. 2006;25:2–7. doi: 10.1097/01.inf.0000195623.57945.87. [DOI] [PubMed] [Google Scholar]
- Viaud S, Saccheri F, Mignot G, Yamazaki T, Daillere R, Hannani D, Enot DP, Pfirschke C, Engblom C, Pittet MJ, et al. The intestinal microbiota modulates the anticancer immune effects of cyclophosphamide. Science. 2013;342:971–976. doi: 10.1126/science.1240537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijay-Kumar M, Aitken JD, Carvalho FA, Cullender TC, Mwangi S, Srinivasan S, Sitaraman SV, Knight R, Ley RE, Gewirtz AT. Metabolic syndrome and altered gut microbiota in mice lacking Toll-like receptor 5. Science. 2010;328:228–231. doi: 10.1126/science.1179721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Essen MR, Kongsbak M, Schjerling P, Olgaard K, Odum N, Geisler C. Vitamin D controls T cell antigen receptor signaling and activation of human T cells. Nature immunology. 2010;11:344–349. doi: 10.1038/ni.1851. [DOI] [PubMed] [Google Scholar]
- Wade TK, Wade WF. Variable gene family usage of protective and non-protective anti-Vibrio cholerae O1 LPS antibody heavy chains. Microbiology and immunology. 2008;52:611–620. doi: 10.1111/j.1348-0421.2008.00078.x. [DOI] [PubMed] [Google Scholar]
- Wannemuehler MJ, Kiyono H, Babb JL, Michalek SM, McGhee JR. Lipopolysaccharide (LPS) regulation of the immune response: LPS converts germfree mice to sensitivity to oral tolerance induction. J Immunol. 1982;129:959–965. [PubMed] [Google Scholar]
- Wei M, Shinkura R, Doi Y, Maruya M, Fagarasan S, Honjo T. Mice carrying a knock-in mutation of Aicda resulting in a defect in somatic hypermutation have impaired gut homeostasis and compromised mucosal defense. Nature immunology. 2011;12:264–270. doi: 10.1038/ni.1991. [DOI] [PubMed] [Google Scholar]
- Weiner HL, da Cunha AP, Quintana F, Wu H. Oral tolerance. Immunological reviews. 2011;241:241–259. doi: 10.1111/j.1600-065X.2011.01017.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstock JV, Summers R, Elliott DE. Helminths and harmony. Gut. 2004;53:7–9. doi: 10.1136/gut.53.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen L, Ley RE, Volchkov PY, Stranges PB, Avanesyan L, Stonebraker AC, Hu C, Wong FS, Szot GL, Bluestone JA, et al. Innate immunity and intestinal microbiota in the development of Type 1 diabetes. Nature. 2008;455:1109–1113. doi: 10.1038/nature07336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter SE, Winter MG, Xavier MN, Thiennimitr P, Poon V, Keestra AM, Laughlin RC, Gomez G, Wu J, Lawhon SD, et al. Host-derived nitrate boosts growth of E. coli in the inflamed gut. Science. 2013;339:708–711. doi: 10.1126/science.1232467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolk K, Sabat R. Interleukin-22: a novel T- and NK-cell derived cytokine that regulates the biology of tissue cells. Cytokine & growth factor reviews. 2006;17:367–380. doi: 10.1016/j.cytogfr.2006.09.001. [DOI] [PubMed] [Google Scholar]
- Worbs T, Bode U, Yan S, Hoffmann MW, Hintzen G, Bernhardt G, Forster R, Pabst O. Oral tolerance originates in the intestinal immune system and relies on antigen carriage by dendritic cells. The Journal of experimental medicine. 2006;203:519–527. doi: 10.1084/jem.20052016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu HJ, Ivanov II, Darce J, Hattori K, Shima T, Umesaki Y, Littman DR, Benoist C, Mathis D. Gut-residing segmented filamentous bacteria drive autoimmune arthritis via T helper 17 cells. Immunity. 2010;32:815–827. doi: 10.1016/j.immuni.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S, Rhee KJ, Albesiano E, Rabizadeh S, Wu X, Yen HR, Huso DL, Brancati FL, Wick E, McAllister F, et al. A human colonic commensal promotes colon tumorigenesis via activation of T helper type 17 T cell responses. Nature medicine. 2009;15:1016–1022. doi: 10.1038/nm.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yatsunenko T, Rey FE, Manary MJ, Trehan I, Dominguez-Bello MG, Contreras M, Magris M, Hidalgo G, Baldassano RN, Anokhin AP, et al. Human gut microbiome viewed across age and geography. Nature. 2012;486:222–227. doi: 10.1038/nature11053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimoto S, Loo TM, Atarashi K, Kanda H, Sato S, Oyadomari S, Iwakura Y, Oshima K, Morita H, Hattori M, et al. Obesity-induced gut microbial metabolite promotes liver cancer through senescence secretome. Nature. 2013;499:97–101. doi: 10.1038/nature12347. [DOI] [PubMed] [Google Scholar]
- Yurkovetskiy L, Burrows M, Khan AA, Graham L, Volchkov P, Becker L, Antonopoulos D, Umesaki Y, Chervonsky AV. Gender bias in autoimmunity is influenced by microbiota. Immunity. 2013;39:400–412. doi: 10.1016/j.immuni.2013.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zenewicz LA, Flavell RA. Recent advances in IL-22 biology. International immunology. 2011;23:159–163. doi: 10.1093/intimm/dxr001. [DOI] [PubMed] [Google Scholar]
- Zoumpopoulou G, Foligne B, Christodoulou K, Grangette C, Pot B, Tsakalidou E. Lactobacillus fermentum ACA-DC 179 displays probiotic potential in vitro and protects against trinitrobenzene sulfonic acid (TNBS)-induced colitis and Salmonella infection in murine models. International journal of food microbiology. 2008;121:18–26. doi: 10.1016/j.ijfoodmicro.2007.10.013. [DOI] [PubMed] [Google Scholar]