Abstract
Background
The relationships of circulating concentrations of oestrogens, progesterone and androgens with breast cancer and related risk factors in premenopausal women are not well understood.
Methods
Individual data on prediagnostic sex hormone and sex hormone binding globulin (SHBG) concentrations were contributed by 7 prospective studies. Analyses were restricted to women who were premenopausal and under age 50 at blood collection, and to breast cancer cases diagnosed before age 50. The odds ratios (ORs) with 95% confidence intervals (95% CIs) for breast cancer associated with hormone concentrations were estimated by conditional logistic regression in up to 767 cases and 1699 controls matched for age, date of blood collection, and day of cycle, with stratification by study and further adjustment for cycle phase. The associations of hormones with risk factors for breast cancer in control women were examined by comparing geometric mean hormone concentrations in categories of these risk factors, adjusted for study, age, phase of menstrual cycle and body mass index (BMI). All statistical tests were two-sided.
Findings
ORs for breast cancer associated with a doubling in hormone concentration were 1.19 (95% CI 1.06–1.35) for oestradiol, 1.17 (1.03–1.33) for calculated free oestradiol, 1.27 (1.05–1.54) for oestrone, 1.30 (1.10–1.55) for androstenedione, 1.17 (1.04–1.32) for dehydroepiandrosterone sulphate, 1.18 (1.03–1.35) for testosterone and 1.08 (0.97–1.21) for calculated free testosterone. Breast cancer risk was not associated with luteal phase progesterone (for a doubling in concentration OR=1.00 (0.92–1.09)), and adjustment for other factors had little effect on any of these ORs. The cross-sectional analyses in control women showed several associations of sex hormones with breast cancer risk factors.
Interpretation
Circulating oestrogens and androgens are positively associated with the risk for breast cancer in premenopausal women.
Keywords: Breast cancer, sex hormones, premenopausal
Introduction
Breast cancer risk is affected by several reproductive and hormonal factors and it has long been hypothesized that endogenous sex hormones influence risk.1 There are now sufficient data from studies of hormones and breast cancer risk in postmenopausal women to show that risk is positively associated with circulating concentrations of oestrogens and androgens,2–4 but in premenopausal women fewer data are available and hormone measurements are complicated by the substantial variation in hormone concentrations across the menstrual cycle.
The Endogenous Hormones and Breast Cancer Collaborative Group was established to conduct pooled analyses of individual data from prospective studies in order to increase the precision of the estimated associations of endogenous hormones with breast cancer risk.2 We report here a collaborative analysis of data from seven studies. We describe the associations of circulating sex hormones with breast cancer risk, including examination of consistency between studies, associations in subgroups, and the effects of adjustment for other risk factors. We also describe cross-sectional analyses of the associations of circulating sex hormones and sex hormone binding globulin (SHBG) with risk factors for breast cancer. Our aim was to improve understanding of the role of premenopausal sex hormones in the aetiology of breast cancer developing before menopause, because it is likely that hormonal changes after menopause would influence the relationship of premenopausal hormone levels with the risk for postmenopausal breast cancer. We therefore restricted all analyses to women who were premenopausal and aged under 50 years at blood collection, and to case-control sets where the case was diagnosed with breast cancer before age 50.
Methods
Data collection
Published studies were eligible for the collaborative re-analysis if they included data on endogenous hormones and breast cancer risk using prospectively collected blood samples from premenopausal women. Studies were identified by computer aided literature searches and through discussions with colleagues. The studies included were: CLUE I, Washington County, MD, USA;5,6 Columbia, MO, USA;7 European Prospective Investigation into Cancer and Nutrition (EPIC), Europe;8 Guernsey, UK;9 Nurses’ Health Study II (NHS-II), USA;10–12 New York University Women’s Health Study (NYU WHS), USA;13 Study of Hormones and Diet in the Etiology of Breast Tumors (ORDET), Italy.14 The majority of the women in these studies were of white European ethnic origin. Two further studies in the Collaborative Group had prospective hormone data but were not included in the analyses reported here because data on day of menstrual cycle at blood collection were not available: Melbourne Collaborative Cohort Study (MCCS), Australia,15 and Radiation Effects Research Foundation (RERF) study phases 1 and 2, Japan.16,17 One further study was identified but the data could not be retrieved; this study included 17 cases of breast cancer and 67 matched controls among women who were premenopausal at blood collection.18
Table 1 summarizes the study designs. Participants in NHS-II were volunteers who were nurses, in the other cohorts the participants were volunteers living in areas near the recruitment centres. Details of the recruitment of participants, informed consent, ethics approvals and definitions of reproductive variables are in the original publications. The current analyses did not need further ethics approval. The majority of cases were of invasive breast cancer, but five of the studies (Columbia, EPIC, NHS-II, NYU WHS, ORDET) also included some in situ cases. Cases were individually matched to between 2 and 4 controls: all studies matched on age and date of blood sample (or follow-up time for EPIC), and on the day or phase of menstrual cycle at blood collection. Collaborators were asked to provide data on concentrations of the hormones oestradiol (total), oestrone, progesterone, androstenedione, dehydroepiandrosterone sulphate (DHEAS), testosterone, and SHBG, as well as data on reproductive, anthropometric and lifestyle factors for each woman in their study, where available. Women who were using hormonal contraceptives or other exogenous sex hormones at the time of blood collection were excluded, as were women missing information for date of birth, date at blood collection, day of menstrual cycle at blood collection, or date of diagnosis (cases).
Table 1.
Description of study methods in prospective cohort studies combined in the collaborative re-analysis
| Study | Recruitment population | Recruitment period | Fasting status | Storage temperature | Matching criteria | ||||
|---|---|---|---|---|---|---|---|---|---|
| Controls per case | Age at blood collection | Date of blood sample | Day of cycle | Other matching criteria | |||||
| CLUE I, USA | Residents of Washington County, MD, USA | 1974 | Non-fasting | −70 C | 2 | ± 1 year | ± 14 days | ± 1 day | Time of day, fasting status, ethnic group, freeze/thaw history of serum sample |
| Columbia, USA | Residents of Columbia, MO, USA | 1977–1989 | Non-fasting | −70 C | 2 | ± 2 years | ± 1 year | ± 2 days | Time of day at blood collection |
| EPIC, Europe | Volunteers in Denmark, France, Germany, Greece, Italy, Netherlands, Spain, Sweden, United Kingdom | 1992–1998 | Matched | Mostly −196 C1 | 2 | ± 6 months | No (incidence density sampling) | 5 phases | Time of day at blood collection, fasting status, subcohort |
| Guernsey, UK | Residents on the island of Guernsey, UK | 1977–1990 | Non-fasting | −20 C | 3 | ± 2 years | ± 1 year | ± 1 day | |
| Nurses’ Health Study II phases 1 (1999–2003 follow- up cycles) and 2 (2005–2009 follow- up cycles), USA | Registered nurses in the USA | 1996–1999 | Matched | −130 C | 2 | ± 2 years | ± 2 months | ± 1 day for luteal blood sample (asked to provide follicular sample at days 3 to 5 and luteal sample at 7- 9 days before anticipated start of next cycle) | Time of day, fasting status |
| NYU WHS, USA | Women attending breast cancer screening centre, New York, USA | 1985–1991 | Non-fasting | −80 C | 2 | ± 6 months | ± 3 months | 5 phases and day | Number of subsequent samples |
| ORDET, Italy | Residents of Varese province, Italy | 1987–1992 | 12 hour fast prior to collection. Samples taken 07.30–09.00 | −80 C | 4 | ± 5 years | ± 89 days | All days 20 to 24 | Daylight saving period, recruitment centre |
Stored in liquid nitrogen at −196 C, except in Denmark in nitrogen vapour at −150 C.
CLUE I = Washington County, MD Study “Give us a clue to cancer and heart disease”; EPIC = European Prospective Investigation into Cancer and Nutrition; NYU WHS = New York University Women’s Health Study; ORDET = Study of Hormones and Diet in the Etiology of Breast Tumors.
Brief details of the assays are in Web Table 1, with further details in the original publications. Six studies measured hormone concentrations in serum whereas one (NHS-II) used heparin plasma; for convenience we refer to serum concentrations for the pooled analyses. Circulating concentrations of free oestradiol and free testosterone were calculated from the concentrations of oestradiol and testosterone respectively and of SHBG, with albumin assumed to be constant (40 g/L), according to the law of mass action.19,20
Statistical analysis
Day of cycle at blood collection was grouped into six categories, using days until next period if available (backward dating), otherwise using days since last period (forward dating). The six categories were: early follicular (days 24+ backwards, or days 1–5 forwards), late follicular (19–23 backwards, 6–10 forwards), mid-cycle (15–18 backwards, 11–14 forwards), early luteal (11–14 backwards, 15–18 forwards), mid luteal (5–10 backwards, 19–24 forwards), late luteal (0–4 backwards, 25+ forwards). For CLUE I and Columbia, day of cycle was determined using forward dating for all participants. For Guernsey and NHS-II, day of cycle was determined backwards for all participants (except one case in NHS-II). For the other three studies, the percentages determined using backward dating (otherwise forward dating) were: 54.4% and 51.0% in cases and controls respectively in EPIC; 75.2% and 82.9% in cases and controls in NYU WHS; and 94.0% and 96.1% in cases and controls in ORDET.
In NHS-II participants provided two blood samples at baseline, one collected in the follicular phase and one in the luteal phase. For most of the analyses reported here we use values for oestradiol and oestrone from the follicular phase, and progesterone, androstenedione, DHEAS, testosterone and SHBG from the luteal phase; in the analyses of oestradiol and free oestradiol subdivided by phase of cycle (Figure 1 and Web Figures 12 and 13) we used both the follicular and the luteal measures, as appropriate. In the other studies only one blood sample was collected from each participant.
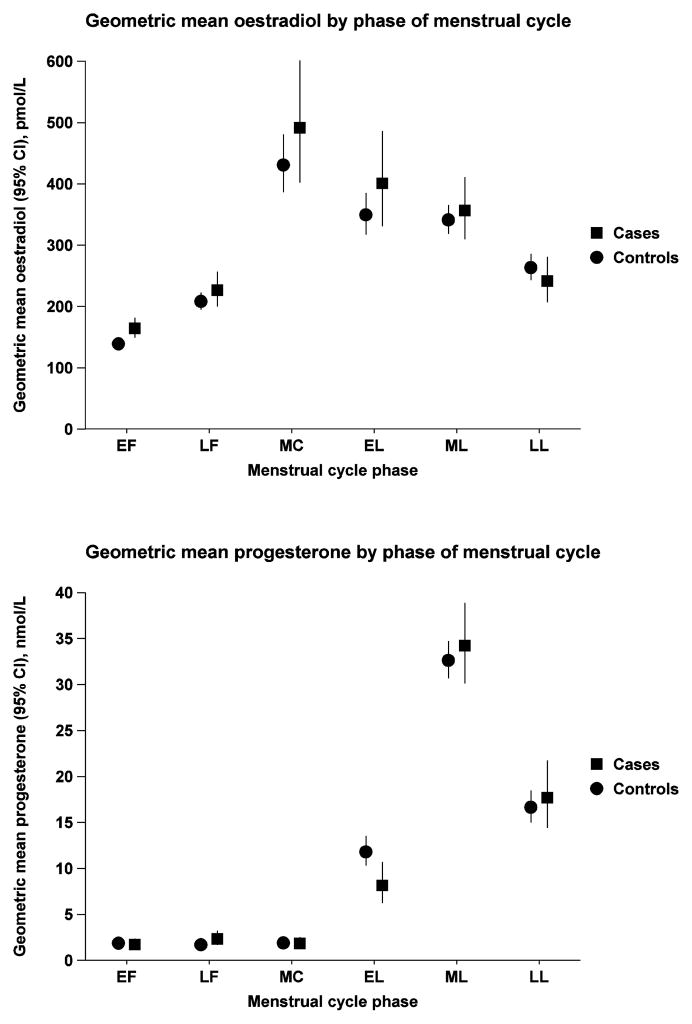
Fig. 1.
Geometric mean oestradiol and progesterone concentrations (pmol/L and nmol/L, respectively, with 95% confidence intervals) in cases and controls by phase of menstrual cycle. Adjusted for study and age at blood collection. EF=early follicular; LF=late follicular; MC=mid cycle; EL=early luteal; ML=mid luteal; LL=late luteal.
All women were classified as premenopausal in the contributed datasets, with the criteria for this based on questionnaire information, as described in the original studies; three studies additionally measured serum FSH concentration and excluded women with FSH values higher than the cut-off recommended by their laboratory (Guernsey, NYU WHS and ORDET). We restricted the analyses to cases diagnosed before age 50 (and their matched controls), so that most cases would have been diagnosed when premenopausal; this restriction further served to reduce the possibility that some participants were perimenopausal at blood collection.
All the studies used a nested case-control design, with assays arranged so that case-control sets were generally measured in the same batch, thus eliminating inter-assay variation from the case-control comparisons. We retained the original matched sets in the analyses. Some studies used density sampling, meaning that an individual participant could appear more than once in a data file.
Conditional logistic regression was used to estimate the odds ratio (OR) for breast cancer in relation to the serum concentrations of hormones and SHBG, categorizing women in each study according to the quintiles of hormone concentration for the controls in that study, after standardizing for phase of menstrual cycle using residuals from the study-specific mean for each cycle phase; for progesterone we restricted the analysis to samples collected in the luteal phase. For each study we fit the simple linear regression model: log(hormone) = A + B*(phase of cycle), where phase of cycle is a categorical variable. The residuals from this model are then added to the mean log(hormone) value and the result exponentiated to give the standardized value. Study-specific quintile cut-points were used because the absolute concentrations of hormones and SHBG vary between studies due to laboratory variation; further explanation of this approach is provided in previous publications.2,21 To test for the significance of the association and to provide a summary measure of risk we also estimated the odds ratio associated with a unit increase in a continuous variable equal to the logarithm to the base 2 of the hormone concentration. A unit increase in this variable is equivalent to a doubling in hormone concentration. Heterogeneity in linear trends between studies was tested by comparing the chi-squared values for models with and without a (study) x (linear trend) interaction term. We also used chi-squared tests to examine whether there was evidence of heterogeneity in the associations of hormones with breast cancer risk according to subgroups defined by years between blood collection and diagnosis, stage of disease, receptor status and other characteristics. We also investigated the associations of hormones with breast cancer risk after adjusting for reproductive and hormonal risk factors for breast cancer: age at menarche (<12, 12–13, 14+ years, unknown); parity (zero, 1, 2, 3, 4+ full-term pregnancies, unknown); age at first full-term pregnancy (<20, 20–24, 25–29, 30+ years, unknown); body mass index (BMI; <22.5, 22.5–24.9, 25.0–27.4, 27.5–29.9, 30.0+ kg/m2, unknown).
Concentrations of the hormones and SHBG were positively skewed, therefore log-transformed concentrations were used for all parametric analyses. Correlations between hormones were calculated using standardised log-transformed concentrations within each study, the standardised values being calculated by subtracting the mean log concentration and dividing by the standard deviation of the log concentration. The associations of hormones with risk factors for breast cancer were examined in the controls. Geometric means and 95% confidence intervals were calculated according to categories of these factors, adjusting for study, age (<40, 40–44, 45–49), cycle phase, and body mass index (BMI), as appropriate. F-tests were used to test for heterogeneity in the geometric mean hormone concentrations between the categories of risk factors, and where appropriate to test for trends across the categories, with the ordered categories scored from 1 to the maximum number of categories. The heterogeneity between studies in the associations of hormones with risk factors was assessed by adding a (study) x (factor) interaction term to the model and using the F-test to calculate its significance.
All statistical tests were two-sided and statistical significance was set at the 5% level. All analyses were performed using Stata version 12.0 (Stata Corp., College Station, TX).
Role of the funding source
The funding source had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The members of the writing team had full access to all data in the study. The corresponding author had the final responsibility for the decision to submit for publication.
Results
Characteristics of cases and controls
Mean age at blood collection ranged from 35.6 years for cases in CLUE to 42.2 years for cases and controls in EPIC (Web Table 2). The median time between blood collection and diagnosis ranged from 2 years in EPIC to 9 years in CLUE. Geometric mean concentrations of sex hormones and SHBG in controls ranged from 164 to 316 pmol/L for oestradiol, 1.85 to 4.25 pmol/L for calculated free oestradiol, 145 to 360 pmol/L for oestrone, 9.62 to 43.5 nmol/L for luteal phase progesterone, 2.88 to 5.79 nmol/L for androstenedione, 2208 to 3921 nmol/L for DHEAS, 0.84 to 1.56 nmol/L for testosterone, 10.1 to 23.3 pmol/L for calculated free testosterone, and 43.0 to 74.3 nmol/L for SHBG (Table 2).
Table 2.
Geometric mean hormone concentrations (95% CI) by study and case-control status
| Study | Number1 | Oestradiol, pmol/L |
Calculated free oestradiol, pmol/L |
Oestrone, pmol/L |
Luteal phase progesterone, nmol/L |
Androstenedio ne, nmol/L |
DHEAS, nmol/L |
Testosterone, nmol/L |
Calculated free testosterone, pmol/L |
SHBG, nmol/L | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| CLUE I, USA | Cases | 21 | 172 (134–222) | 2.03 (1.55–2.64) | 252 (211–301) | 5.32 (1.73–16.3) | 2.98 (2.31–3.85) | 3903 (2787–5465) | - | - | 69.9 (58.0–84.2) |
| Controls | 42 | 168 (137–206) | 1.85 (1.51–2.26) | 239 (207–275) | 9.62 (5.47–16.9) | 2.88 (2.42–3.43) | 3853 (3023–4910) | - | - | 74.3 (65.3–84.4) | |
| Columbia, USA | Cases | 13 | 239 (165–347) | 3.26 (2.24–4.75) | - | - | - | - | 1.00 (0.79–1.28) | 13.7 (9.86–19.1) | 48.2 (34.3–67.7) |
| Controls | 24 | 316 (257–387) | 4.05 (3.34–4.92) | - | - | - | - | 0.86 (0.73–1.02) | 10.7 (9.10–12.7) | 56.6 (48.3–66.4) | |
| EPIC, Europe | Cases | 206 | 318 (285–355) | 4.60 (4.13–5.12) | 384 (354–416) | 8.42 (6.30–11.3) | 5.59 (5.22–5.98) | 3712 (3469–3972) | 1.70 (1.60–1.81) | 25.2 (23.2–27.3) | 43.5 (40.6–46.6) |
| Controls | 408 | 296 (275–318) | 4.25 (3.94–4.60) | 360 (339–383) | 12.3 (9.84–15.4) | 4.92 (4.68–5.18) | 3341 (3169–3522) | 1.56 (1.49–1.63) | 23.3 (21.8–24.8) | 43.0 (40.9–45.3) | |
| Guernsey, UK | Cases | 32 | 323 (253–412) | 3.16 (2.39–4.17) | - | 10.7 (5.84–19.5) | - | 2253 (1410–3599) | 1.17 (0.97–1.40) | 13.2 (11.3–15.5) | 68.6 (59.5–79.1) |
| Controls | 94 | 282 (246–323) | 3.02 (2.52–3.62) | - | 10.6 (7.25–15.4) | - | 2548 (1924–3375) | 1.12 (1.02–1.23) | 13.4 (11.6–15.5) | 61.5 (55.8–67.7) | |
| Nurses’ Health Study II phase 1, USA | Cases | 139 | 182 (166–199) | 2.30 (2.12–2.49) | 150 (142–159) | 45.7 (41.1–50.8) | 3.91 (3.68–4.16) | 2302 (2129–2489) | 0.92 (0.87–0.99) | 11.3 (10.5–12.2) | 57.9 (53.8–62.3) |
| Controls | 268 | 164 (153–177) | 2.08 (1.95–2.22) | 145 (138–151) | 43.5 (39.9–47.4) | 3.89 (3.72–4.06) | 2208 (2089–2333) | 0.90 (0.86–0.94) | 10.9 (10.3–11.5) | 58.5 (55.5–61.8) | |
| Nurses’ Health Study II phase 2, USA | Cases | 105 | 193 (175–213) | 2.21 (2.02–2.42) | 161 (150–173) | 40.7 (34.6–47.9) | - | 2838 (2556–3151) | 0.91 (0.85–0.98) | 9.6 (8.7–10.5) | 70.6 (65.3–76.3) |
| Controls | 203 | 186 (174–199) | 2.25 (2.11–2.40) | 163 (154–171) | 38.1 (33.9–42.9) | - | 2642 (2449–2851) | 0.91 (0.87–0.96) | 10.6 (10.0–11.3) | 62.4 (59.0–66.0) | |
| NYU WHS phase2, USA | Cases | 137 | - | - | - | - | 4.30 (3.96–4.67) | 3978 (3625–4366) | 1.01 (0.91–1.12) | 14.0 (12.4–15.8) | 48.1 (44.1–52.4) |
| Controls | 258 | - | - | - | - | 4.07 (3.83–4.33) | 3869 (3598–4161) | 0.95 (0.88–1.03) | 13.1 (11.9–14.3) | 47.8 (44.8–51.0) | |
| ORDET, Italy | Cases | 84 | 300 (274–329) | 3.66 (3.34–4.00) | - | 38.2 (32.7–44.6) | 5.26 (4.38–6.32) | 3856 (3153–4715) | 0.85 (0.75–0.97) | 9.9 (8.5–11.6) | 62.0 (56.6–68.0) |
| Controls | 336 | 282 (259–306) | 3.50 (3.23–3.79) | - | 32.4 (28.3–37.1) | 5.79 (5.38–6.23) | 3921 (3604–4265) | 0.84 (0.79–0.90) | 10.1 (9.3–10.8) | 59.8 (57.1–62.6) |
Numbers are for women with known phase of cycle and values for oestradiol (except for NYU WHS where numbers are for women with values for testosterone).
- indicates data not available.
Geometric mean hormone concentrations for Nurses’ Health Study II are obtained using the follicular phase data for oestradiol, calculated free oestradiol and oestrone and using the luteal phase data for all other hormones.
CLUE I = Washington County, MD Study “Give us a clue to cancer and heart disease”; EPIC = European Prospective Investigation into Cancer and Nutrition; NYU WHS = New York University Women’s Health Study; ORDET = Study of Hormones and Diet in the Etiology of Breast Tumors.
Sex hormones, SHBG and breast cancer risk
Figure 1 shows geometric mean concentrations of oestradiol and progesterone in cases and controls by phase of menstrual cycle at blood collection. For oestradiol, geometric mean values in cases were higher than in controls at all cycle phases except the late luteal phase. For progesterone, geometric means were lower in cases than controls in the early luteal phase, with small differences in the other phases which were not statistically significant.
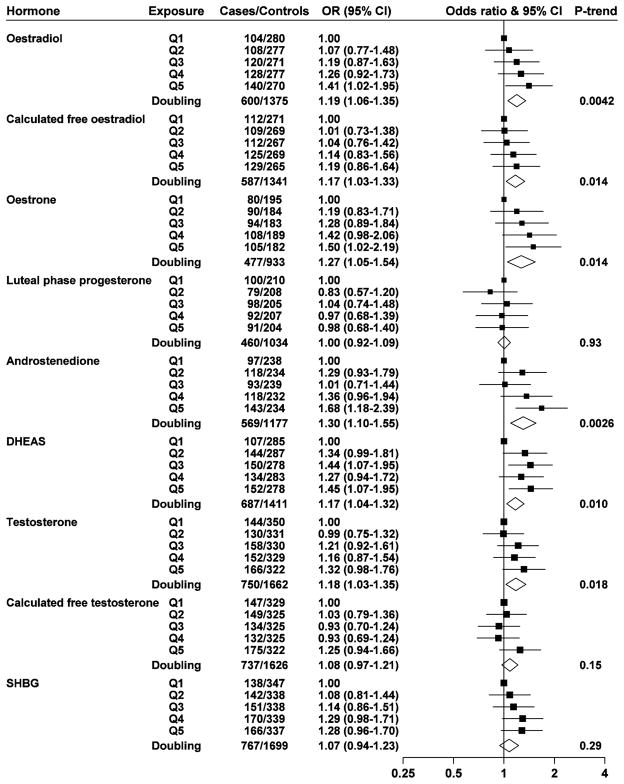
Oestradiol, calculated free oestradiol, oestrone, androstenedione, DHEAS and testosterone were positively associated with breast cancer risk, with ORs (95% CI) in the top fifth of the distribution of 1.41 (1.02–1.95), 1.19 (0.86–1.64), 1.50 (1.02–2.19), 1.68 (1.18–2.39), 1.45 (1.07–1.95), and 1.32 (0.98–1.76), respectively (Figure 2); using a doubling scale, the ORs were 1.19 (1.06–1.35) for oestradiol, 1.17 (1.03–1.33) for calculated free oestradiol, 1.27 (1.05–1.54) for oestrone, 1.30 (1.10–1.55) for androstenedione, 1.17 (1.04–1.32) for DHEAS, and 1.18 (1.03–1.35) for testosterone. Luteal phase progesterone, calculated free testosterone and SHBG were not significantly associated with risk. In a sensitivity analysis restricted to women with blood collected at ages below 45 and cases diagnosed before age 45 the results were similar (Web Figure 1). There was no significant heterogeneity between studies in the associations of these hormones with breast cancer risk (Web Figures 2 to 10). Further adjustment for age at menarche, age at first full-term pregnancy, number of full-term pregnancies, and BMI did not substantially change the ORs, except that after adjustment there was a statistically significant positive association of calculated free testosterone with risk (the OR for a doubling was 1.14, P = 0.031; Web Figure 11).
Fig. 2.
Odds ratios (ORs) for breast cancer associated with sex hormones and SHBG. The black squares indicate the ORs in fifths (study-specific fifths after adjustment for phase of cycle within each study), and the horizontal lines show the 95% confidence intervals. The area of each square is proportional to the amount of statistical information (inverse of the variance of the logarithm of the OR). The diamonds show the OR for a doubling in concentration, and the widths of the diamonds show the 95% confidence intervals. Estimates are from conditional logistic regression on case-control sets matched within each study.
Subgroup analyses
Subgroup analyses were conducted to detect heterogeneity in the associations of log hormone concentrations with breast cancer risk in subgroups according to years from blood collection to diagnosis (< 4, ≥4), stage of disease (in situ, invasive), oestrogen receptor status (positive, negative), progesterone receptor status (positive, negative), HER2 receptor status (positive, negative), phase of menstrual cycle at blood collection (except for progesterone; follicular, mid-cycle, luteal), age at menarche (<14, ≥14 years), parity (nulliparous, parous), age at first full-term pregnancy (<25, ≥25 years), mother or sister with breast cancer (no, yes), BMI (<25, ≥25 kg/m2), smoking (never or past, current), alcohol intake at recruitment (<10, ≥10 g/d), previous use of hormonal contraceptives (no, yes), and assay method for oestradiol, calculated free oestradiol, oestrone, testosterone and calculated free testosterone (extraction, non-extraction); see Web Figures 12 to 20. Among findings for nine hormones examined in relation to these factors, four out of 130 tests for heterogeneity were statistically significant: for oestradiol the OR for a doubling in concentration was 1.26 (1.10–1.44) for never or past smokers and 0.94 (0.75–1.18) for current smokers (P for heterogeneity=0.034), and 1.01 (0.82–1.24) for never users and 1.32 (1.14–1.53) for past users of hormonal contraceptives (P for heterogeneity=0.030); for oestrone the OR for a doubling in concentration was 1.74 (0.99–3.03) for progesterone receptor positive and 0.54 (0.27–1.08) for progesterone receptor negative cancers (P for heterogeneity=0.010); and for luteal phase progesterone the OR for a doubling in concentration was 1.25 (1.01–1.55) for nulliparous women and 0.99 (0.90–1.09) for parous women (P for heterogeneity=0.034).
Two sub-group analyses were of particular a priori interest. For oestrogens and androgens, the ORs were larger for oestrogen receptor positive tumours than for oestrogen receptor negative tumours, but none of these differences was statistically significant (Table 3). For oestradiol according to phase of menstrual cycle the ORs for a doubling in concentration were 1.25 (1.06–1.48) for follicular, 1.20 (0.81–1.79) for mid-cycle, and 1.13 (0.92–1.37) for luteal samples, P for heterogeneity=0.732 (Web Figure 12).
Table 3.
Odds ratios (ORs) for breast cancer associated with a doubling in concentrations of hormones and SHBG, subdivided by oestrogen receptor status
| ER positive | ER negative | ||||
|---|---|---|---|---|---|
| Hormone | Cases/controls | OR (95% CI) | Cases/controls | OR (95% CI) | P for heterogeneity |
| Oestradiol | 147/374 | 1.25 (0.95–1.65) | 71/209 | 1.09 (0.76–1.57) | 0.56 |
| Calculated free oestradiol | 147/374 | 1.22 (0.91–1.63) | 71/209 | 1.03 (0.68–1.54) | 0.50 |
| Oestrone | 107/205 | 1.26 (0.77–2.06) | 37/72 | 0.90 (0.45–1.82) | 0.45 |
| Luteal phase progesterone | 152/369 | 1.05 (0.88–1.24) | 67/184 | 1.13 (0.88–1.47) | 0.62 |
| Androstenedione | 124/237 | 1.45 (0.98–2.15) | 54/106 | 1.11 (0.58–2.14) | 0.50 |
| DHEAS | 170/327 | 1.24 (0.97–1.57) | 67/130 | 0.91 (0.62–1.34) | 0.19 |
| Testosterone | 211/495 | 1.13 (0.88–1.43) | 99/265 | 1.03 (0.76–1.39) | 0.66 |
| Calculated free testosterone | 211/495 | 1.08 (0.88–1.33) | 99/264 | 1.01 (0.78–1.30) | 0.66 |
| SHBG | 214/503 | 1.04 (0.80–1.35) | 102/271 | 1.08 (0.77–1.52) | 0.86 |
Estimates are from conditional logistic regression on case-control sets matched within each study and adjusted for phase of menstrual cycle at blood collection within study.
Associations of hormones and SHBG with BMI, parity and other factors
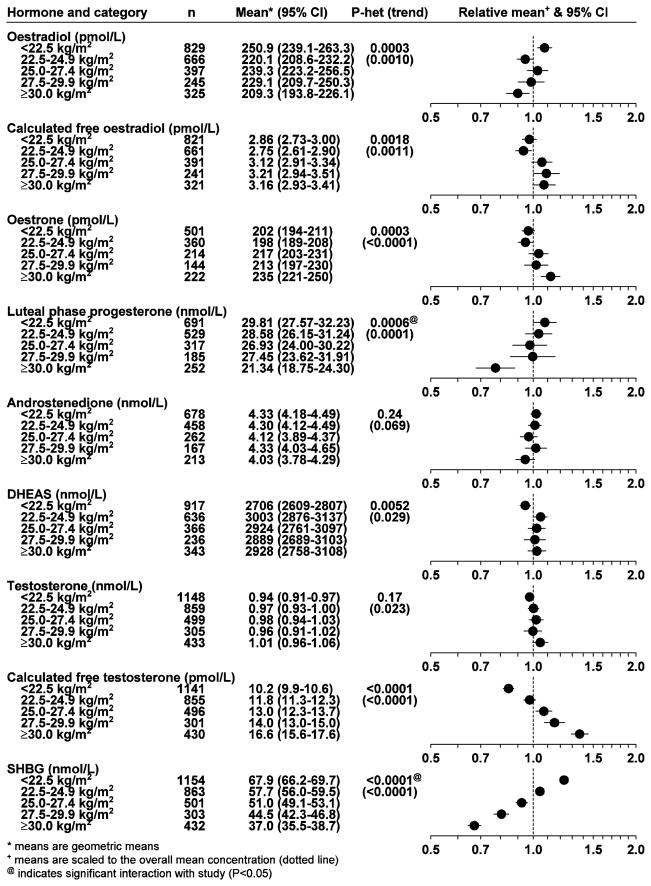
BMI was inversely associated with oestradiol, luteal phase progesterone and SHBG, with mean concentrations 17%, 28%, and 46% lower, respectively, in women with a BMI of 30 and above compared to women with a BMI of under 22.5 kg/m2; conversely, calculated free oestradiol, oestrone, DHEAS, testosterone and calculated free testosterone were positively associated with BMI, with mean concentrations 10%, 16%, 8%, 7% and 63% higher, respectively, in women with a BMI of 30 and above compared to women with a BMI of under 22.5 kg/m2 (means adjusted for age, study and cycle phase; Figure 3).
Fig. 3.
Geometric mean hormone and SHBG concentrations (with 95% confidence intervals) in controls by BMI. Adjusted for study, age at blood collection and phase of menstrual cycle.
The associations of sex hormones with age, age at menarche, parity, family history of breast cancer, smoking, alcohol intake at recruitment and previous use of hormonal contraceptives are shown in Web Figures 21 to 27. Sex hormone concentrations were lower in older than in younger women, whereas SHBG was higher in older women (Web Figure 21). Parity was inversely associated with calculated free testosterone, but was not significantly associated with concentrations of the other sex hormones or SHBG (Web Figure 23), and none of the hormones or SHBG was significantly associated with age at menarche or family history of breast cancer (Web Figures 22 and 24). Androstenedione, DHEAS, testosterone and calculated free testosterone were higher in current smokers of 15+ cigarettes per day than in never-smokers, by 21%, 12%, 12% and 13% respectively (Web Figure 25), and the same hormones were positively associated with alcohol consumption, with mean concentrations 14%, 16%, 23% and 23% higher, respectively, in women with an alcohol intake of 20 g/d and above compared to women who did not consume alcohol (Web Figure 26); further adjustment of the analyses by smoking for alcohol, and of the analyses by alcohol for smoking, had no material effect on the results (not shown). Women who had previously used hormonal contraceptives had lower concentrations of oestradiol (by 7%), oestrone (by 7%), androstenedione (by 5%) and SHBG (by 4%) (Web Figure 27).
Discussion
Sex hormones, SHBG and breast cancer risk
This worldwide collaboration has brought together and reanalysed individual participant data from seven studies with endogenous sex hormones measured in prospectively collected blood samples. All women were premenopausal and provided information on the phase of the menstrual cycle at the time of blood collection. We estimated the associations of circulating sex hormones with the risk for breast cancer diagnosis before age 50. Oestradiol, calculated free oestradiol, oestrone, androstenedione, DHEAS and testosterone were positively associated with breast cancer risk, whereas luteal phase progesterone and SHBG were not associated with risk. Calculated free testosterone was positively associated with breast cancer risk only after further adjustment for reproductive factors and BMI. These associations did not vary according to the time between blood collection and diagnosis, making reverse causality unlikely, and (with the exception of calculated free testosterone) were not materially affected by adjustment for other risk factors, suggesting that confounding is unlikely. These results therefore strongly suggest that breast cancer risk in premenopausal women increases with increasing concentrations of these sex hormones. The results are qualitatively similar to those reported in postmenopausal women, but smaller in magnitude.2–4
The analyses reported in this paper were all based on a single hormone measure for each woman. Measurements of hormone concentrations are subject to largely random error associated with assay variation, and fluctuations in serum levels within individual women. Studies of the reproducibility of sex hormones in premenopausal women for up to three years have shown intra-class correlations of ~0.6 or above for androgens and SHBG, but correlations of ~0.4 or less for oestrogens and progesterone.22,23 It is therefore likely that the observed associations between hormone concentrations and breast cancer risk are underestimates of the true associations, particularly for oestrogens, but more reproducibility data are required.
The sub-group analyses showed heterogeneity in the associations of oestradiol with risk according to smoking and previous use of hormonal contraceptives, of oestrone with risk according to progesterone receptor status, and of luteal phase progesterone with risk according to parity, but there was no significant heterogeneity according to any other combination of risk factor and hormone. All the sex hormones had larger associations with the risk of oestrogen receptor positive breast cancer than with the risk of oestrogen receptor negative disease; these differences were not statistically significant, but study power was low because of the small numbers of cases with oestrogen receptor negative disease (e.g. 71 cases for oestradiol). Since we conducted 130 sub-group analyses, some of the four analyses which were nominally statistically significant may have occurred due to chance.
For oestradiol the plot of geometric mean concentrations in cases and controls according to phase of menstrual cycle (Figure 1) suggested that concentrations in cases were higher than those in controls in the follicular phase and at mid-cycle, but not in the late luteal phase, and similarly the sub-group analyses of breast cancer risk showed larger ORs in the follicular phase and at mid-cycle than in the luteal phase, but these differences were not significant.
Associations of hormones with breast cancer risk factors in controls
All the hormones, except for androstenedione, were associated with BMI. Total oestradiol was inversely associated with BMI, whereas free oestradiol was positively associated with BMI because of the strong inverse association of SHBG with BMI. Interpretation of these observations is difficult, but if free oestradiol is a reliable index of bioavailable oestradiol then obese premenopausal women are exposed to a slightly more oestrogenic environment. Oestrone was also positively associated with BMI, perhaps because of increased peripheral aromatization of androstenedione, as in postmenopausal women.24 Progesterone was lower in obese than non-obese women, whereas DHEAS and testosterone were positively associated with BMI. Similar findings for oestrogens and progesterone have been reported among regularly menstruating women in the BioCycle Study,25 and in massively obese premenopausal women.26
Parity was not strongly associated with any of the hormones, but showed an inverse association with calculated free testosterone. Some previous studies in younger premenopausal women have suggested that early menarche and nulliparity are associated with oestrogen levels,27,28 but in the current study none of the hormones or SHBG was significantly associated with age at menarche, and none of the oestrogen measures was associated with parity.
Androstenedione, DHEAS, testosterone and free testosterone were higher in women who smoked the most cigarettes and drank the most alcohol than in non-smokers and non-drinkers, respectively. Very similar associations were seen in postmenopausal women.29 The mechanism may involve stimulation of hormone synthesis by the adrenal glands.30
Women who had previously used hormonal contraceptives had lower concentrations of oestradiol, oestrone, androstenedione, and SHBG. It is not clear whether these are causal associations, or what mechanism could be involved, though they might involve long-term effects on the liver.31
Sex hormones may mediate the effects of some risk factors on the development of breast cancer. For example, the increase in breast cancer risk caused by alcohol32 might be attributable to increased serum concentrations of sex hormones, although it could also be attributable to other effects of alcohol. BMI is inversely associated with the risk of breast cancer in premenopausal women,33 and this association might be related to the effects of obesity on hormone levels. We observed that total oestradiol was inversely related to BMI and positively associated with risk, which is compatible with the idea that the lower risk in obese women is attributable to lower oestradiol, but this interpretation is complicated by the fact that we observed that free oestradiol was positively associated with BMI, as were oestrone and the androgens DHEAS, testosterone and free testosterone. Luteal phase progesterone was also lower in obese than normal weight women, perhaps because of a higher probability of anovulatory cycles in obese women;34 our analyses do not show any association of progesterone with breast cancer risk, but the reliability of progesterone measurements is low and more data are needed before concluding that progesterone is not a determinant of breast cancer risk.
The strengths of this analysis are that the data and serum samples were collected on average several years before diagnosis, that it includes almost all the available data from published studies world-wide, and that we were able to adjust for phase of cycle and for other potential risk factors. The total sample size is moderately large for most of the hormones, but power is low for the sub-group analyses. Many statistical tests are reported, therefore some of the nominally significant results may be due to chance.
A potential weakness is that the study designs and methods for measuring hormones and other risk factors were not standardized. For example, studies variably used forward or backward dating in determining when blood was collected in the menstrual cycle, and, because of differences in progesterone measurement across study, we were unable to distinguish ovulatory versus anovulatory cycles. Further, hormone concentrations varied substantially between studies. Some of this variation in mean hormone concentrations between studies is due to differences in the timing of sample collection, for example the relatively low mean oestradiol concentrations in the follicular phase in NHS-II samples that were collected on days 3–5 of the cycle, and the relatively high mean luteal phase progesterone concentrations in NHS-II and ORDET samples that were collected in the middle of the luteal phase. Some of the variation between studies is likely to reflect differences in assay methods. The accuracy of assay methods varies, and assays which incorporate an extraction step are more accurate than “direct” non-extraction assays.35 Ideally assays would be standardized and use the most accurate methods available, but in the current analysis our aim was to make the best use of the data available. To allow for differences in absolute hormone concentrations between assay laboratories we used study-specific quintiles of hormone concentrations.21 This approach assumes that the true concentrations across the quintiles are similar in all the studies, and if this assumption is not correct then the estimates of ORs may be biased. However, because heterogeneity in risk estimates was not evident between studies or between assay methods (extraction versus non-extraction) this assumption does seem reasonable. It would be expected that the random error in laboratory estimates would lead to some underestimation of the associations observed.
Another potential weakness is that we used diagnosis of breast cancer before age 50 as a surrogate for diagnosis before menopause. The median age at menopause in western countries is typically greater than 50,36 therefore with a cut-off of <50 the majority of women would have been premenopausal at the time of breast cancer diagnosis, and the minority who were postmenopausal at diagnosis would on average have experienced menopause recently. Further, the majority of the women in these studies were of white European ethnic origin, and further data for women with other ethnic origins would be valuable.
This collaborative analysis found a positive association between sex hormones and breast cancer risk in premenopausal women. It is not known whether or not this association is causal, but there are plausible biological mechanisms which could explain such an effect, such as an increase in the mitotic rate of breast epithelial cells leading to an increased risk of mutations and the stimulation of the growth of early tumours.37 The magnitude of the observed association is modest, but the true association may be substantially larger because of measurement error in the assessment of long-term premenopausal hormone levels. More data will become available over the next few years, both from extended follow-up of some of the studies in the current collaborative analysis, and from some new large studies such as the Breakthrough Generations Study and UK Biobank.38,39 Further research is needed to provide more robust estimates of the overall associations and associations in subgroups, and to determine the environmental and genetic factors that cause differences in hormone levels among premenopausal women.
Supplementary Material
Research in context.
Systematic review
The Endogenous Hormones and Breast Cancer Collaborative Group began in 2000. Since then published literature on epidemiological studies of breast cancer has been identified using electronic searches (PubMed, 1980–2012; using combinations of the search terms “breast cancer” and “endogenous hormones”), supplemented by hand searching in review articles. Eligible studies needed to have sex hormone concentrations measured in serum or plasma collected prospectively from women who subsequently developed breast cancer and from control women who did not develop breast cancer. In addition, studies needed to have information on the stage of the menstrual cycle at blood collection. Seven eligible studies were included and principal investigators contributed information from 750 women with breast cancer. We report on the relation of circulating concentrations of eight sex hormones and sex hormone binding globulin (SHBG) with breast cancer risk, overall and by subgroups including stage of disease, receptor status of tumours, and other risk factors for breast cancer. We also describe the associations of sex hormones with risk factors for breast cancer in the control women.
Interpretation
Breast cancer risk was positively associated with circulating concentrations of oestrogens and androgens.
Acknowledgments
Funding: The central pooling, checking and analysis of data were supported by Cancer Research UK.
Funding for this collaborative reanalysis of original data was provided by Cancer Research UK. Funding for the contributing studies is described in the publications of those studies.
Members and affiliations of the Endogenous Hormones and Breast Cancer Collaborative Group
Writing committee
Prof TJ Key DPhil, PN Appleby MSc, GK Reeves PhD, RC Travis DPhil, Cancer Epidemiology Unit, Nuffield Department of Clinical Medicine, University of Oxford, Oxford, UK.
Co-authors from collaborating studies
Prof AJ Alberg PhD, Department of Epidemiology, The Johns Hopkins University Bloomberg School of Public Health, Baltimore, MD, USA; and Hollings Cancer Center, Medical University of South Carolina, Charleston, SC, USA.
A Barricarte MD, Navarre Public Health Institute, Pamplona, Spain; Consortium for Biomedical Research in Epidemiology and Public Health (CIBER Epidemiología y Salud Pública-CIBERESP), Spain.
Prof F Berrino MD, Fondazione IRCCS Istituto Nazionale Tumori, Milano, Italy.
LA Brinton PhD, National Cancer Institute, Bethesda, MD, USA.
JF Dorgan PhD, Fox Chase Cancer Center, Philadelphia, PA, USA.
L Dossus PhD, Inserm, Centre for Research in Epidemiology and Population Health (CESP), Nutrition, Hormones and Women’s Health team, Villejuif, France; Univ Paris Sud, Villejuif, France; and IGR, Villejuif, France.
Prof M Dowsett PhD, Institute of Cancer Research, London, UK.
AH Eliassen ScD, Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA, USA; and Department of Epidemiology, Harvard School of Public Health, Boston, MA, USA.
RT Fortner PhD, Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA, USA; and Department of Epidemiology, Harvard School of Public Health, Boston, MA, USA.
Prof SE Hankinson ScD, for the Nurses’ Health Study II research group, Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA, USA; Department of Epidemiology, Harvard School of Public Health, Boston, MA, USA; and Division of Biostatistics and Epidemiology, School of Public Health and Health Sciences, University of Massachusetts, Amherst, MA, USA.
KJ Helzlsouer MD, Department of Epidemiology, The Johns Hopkins University Bloomberg School of Public Health, Baltimore, MD, USA; and Prevention and Research Center, Mercy Medical Center, Baltimore, MD, USA.
J Hoffman-Bolton, Department of Epidemiology, The Johns Hopkins University Bloomberg School of Public Health, Baltimore, MD, USA; and George W Comstock Center for Public Health Research and Prevention, Washington County, MD, USA.
Prof R Kaaks PhD, Deutsches Krebsforschungszentrum, Heidelberg, Germany.
LL Kahle, Information Management Services, Rockville, MD, USA.
K Koenig PhD, Department of Environmental Medicine, New York University School of Medicine, New York, NY, USA.
V Krogh MD, Fondazione IRCCS Istituto Nazionale Tumori, Milano, Italy.
Prof P Muti MD, McMaster University, Hamilton, Canada.
Prof K Overvad PhD, Department of Clinical Epidemiology, Aarhus University Hospital, Aarhus, Denmark.
Prof PHM Peeters MD, Department of Epidemiology, Julius Center for Health Sciences and Primary Care, University Medical Center Utrecht, The Netherlands; and School of Public Health, Faculty of Medicine, Imperial College, London, UK.
Prof E Riboli MD, School of Public Health, Imperial College, London, UK.
S Rinaldi PhD, International Agency for Research on Cancer, Lyon, France.
DE Rollison PhD, Department of Interdisciplinary Oncology, H Lee Moffitt Cancer Center, Tampa, FL, USA.
S Sieri PhD, Fondazione IRCCS Istituto Nazionale Tumori, Milano, Italy.
Prof FZ Stanczyk PhD, University of Southern California Keck School of Medicine, Los Angeles, CA, USA.
Prof D Trichopoulos MD, Department of Epidemiology, Harvard School of Public Health, Boston, MA, USA; Bureau of Epidemiologic Research, Academy of Athens, Athens, Greece; and Hellenic Health Foundation, Athens, Greece.
SS Tworoger PhD, Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA, USA; Department of Epidemiology, Harvard School of Public Health, Boston, MA, USA.
Prof P Vineis MD, HuGeF Foundation, Torino, Italy; and Imperial College, London, UK.
Prof A Zeleniuch-Jacquotte MD, Department of Environmental Medicine, New York University School of Medicine, New York, NY, USA.
Footnotes
Contributors
All named members of the collaborative group are authors of this manuscript. TJK co-ordinated the collaborative group, drafted the manuscript, conducted the literature search and contributed to study design and data interpretation. PNA centralized the data, conducted the statistical analyses, and contributed to interpreting the data. GKR and RCT contributed to study design, data interpretation and writing. The authors at the secretariat (TJK, PNA, GKR, RCT) had full access to all the data and had final responsibility for the decision to submit the manuscript. All the co-authors from the collaborating studies contributed to data collection, data interpretation and writing.
Conflicts of interests
All members of the writing committee declare that they have no conflicts of interest.
References
- 1.MacMahon B, Cole P, Brown J. Etiology of human breast cancer: a review. J Natl Cancer Inst. 1973;50:21–42. doi: 10.1093/jnci/50.1.21. [DOI] [PubMed] [Google Scholar]
- 2.Endogenous Hormones and Breast Cancer Collaborative Group. Endogenous sex hormones and breast cancer in postmenopausal women: reanalysis of nine prospective studies. J Natl Cancer Inst. 2002;94:606–16. doi: 10.1093/jnci/94.8.606. [DOI] [PubMed] [Google Scholar]
- 3.Kaaks R, Rinaldi S, Key TJ, et al. Postmenopausal serum androgens, oestrogens and breast cancer risk: the European prospective investigation into cancer and nutrition. Endocr Relat Cancer. 2005;12:1071–82. doi: 10.1677/erc.1.01038. [DOI] [PubMed] [Google Scholar]
- 4.Zhang X, Tworoger SS, Eliassen AH, Hankinson SE. Postmenopausal plasma sex hormone levels and breast cancer risk over 20 years of follow-up. Breast Cancer Res Treat. 2013;137:883–92. doi: 10.1007/s10549-012-2391-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Helzlsouer KJ, Gordon GB, Alberg AJ, Bush TL, Comstock GW. Relationship of prediagnostic serum levels of dehydroepiandrosterone and dehydroepiandrosterone sulfate to the risk of developing premenopausal breast cancer. Cancer Res. 1992;52:1–4. [PubMed] [Google Scholar]
- 6.Helzlsouer KJ, Alberg AJ, Bush TL, Longcope C, Gordon GB, Comstock GW. A prospective study of endogenous hormones and breast cancer. Cancer Detect Prev. 1994;18:79–85. [PubMed] [Google Scholar]
- 7.Dorgan JF, Stanczyk FZ, Kahle LL, Brinton LA. Prospective case-control study of premenopausal serum estradiol and testosterone levels and breast cancer risk. Breast Cancer Res. 2010;12:R98. doi: 10.1186/bcr2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaaks R, Berrino F, Key T, et al. Serum sex steroids in premenopausal women and breast cancer risk within the European Prospective Investigation into Cancer and Nutrition (EPIC) J Natl Cancer Inst. 2005;97:755–65. doi: 10.1093/jnci/dji132. [DOI] [PubMed] [Google Scholar]
- 9.Thomas HV, Key TJ, Allen DS, et al. A prospective study of endogenous serum hormone concentrations and breast cancer risk in premenopausal women on the island of Guernsey. Br J Cancer. 1997;75:1075–9. doi: 10.1038/bjc.1997.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eliassen AH, Missmer SA, Tworoger SS, et al. Endogenous steroid hormone concentrations and risk of breast cancer among premenopausal women. J Natl Cancer Inst. 2006;98:1406–15. doi: 10.1093/jnci/djj376. [DOI] [PubMed] [Google Scholar]
- 11.Tworoger SS, Missmer SA, Eliassen AH, et al. The association of plasma DHEA and DHEA sulfate with breast cancer risk in predominantly premenopausal women. Cancer Epidemiol Biomarkers Prev. 2006;15:967–71. doi: 10.1158/1055-9965.EPI-05-0976. [DOI] [PubMed] [Google Scholar]
- 12.Fortner RT, Eliassen AH, Spiegelman D, Willett WC, Barbieri RL, Hankinson SE. Premenopausal endogenous steroid hormones and breast cancer risk: results from the Nurses’ Health Study II. Breast Cancer Res. 2013;15:R19. doi: 10.1186/bcr3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zeleniuch-Jacquotte A, Afanasyeva Y, Kaaks R, et al. Premenopausal serum androgens and breast cancer risk: a nested case-control study. Breast Cancer Res. 2012;14:R32. doi: 10.1186/bcr3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Micheli A, Muti P, Secreto G, et al. Endogenous sex hormones and subsequent breast cancer in premenopausal women. Int J Cancer. 2004;112:312–8. doi: 10.1002/ijc.20403. [DOI] [PubMed] [Google Scholar]
- 15.Baglietto L, Severi G, English DR, et al. Circulating steroid hormone levels and risk of breast cancer for postmenopausal women. Cancer Epidemiol Biomarkers Prev. 2010;19:492–502. doi: 10.1158/1055-9965.EPI-09-0532. [DOI] [PubMed] [Google Scholar]
- 16.Kabuto M, Akiba S, Stevens RG, Neriishi K, Land CE. A prospective study of estradiol and breast cancer in Japanese women. Cancer Epidemiol Biomarkers Prev. 2000;9:575–9. [PubMed] [Google Scholar]
- 17.Grant EJ, Neriishi K, Cologne J, et al. Associations of ionizing radiation and breast cancer-related serum hormone and growth factor levels in cancer-free female A-bomb survivors. Radiat Res. 2011;176:678–87. doi: 10.1667/rr2631.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wysowski DK, Comstock GW, Helsing KJ, Lau HL. Sex hormone levels in serum in relation to the development of breast cancer. Am J Epidemiol. 1987;125:791–9. doi: 10.1093/oxfordjournals.aje.a114596. [DOI] [PubMed] [Google Scholar]
- 19.Södergård R, Bäckström T, Shanbhag V, Carstensen H. Calculation of free and bound fractions of testosterone and estradiol-17 beta to human plasma proteins at body temperature. J Steroid Biochem. 1982;16:801–10. doi: 10.1016/0022-4731(82)90038-3. [DOI] [PubMed] [Google Scholar]
- 20.Endogenous Hormones and Breast Cancer Collaborative Group. Free estradiol and breast cancer risk in postmenopausal women: comparison of measured and calculated values. Cancer Epidemiol Biomarkers Prev. 2003;12:1457–61. [PubMed] [Google Scholar]
- 21.Key TJ, Appleby PN, Allen NE, Reeves GK. Pooling biomarker data from different studies of disease risk, with a focus on endogenous hormones. Cancer Epidemiol Biomarkers Prev. 2010;19:960–5. doi: 10.1158/1055-9965.EPI-10-0061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Muti P, Trevisan M, Micheli A, et al. Reliability of serum hormones in premenopausal and postmenopausal women over a one-year period. Cancer Epidemiol Biomarkers Prev. 1996;5:917–22. [PubMed] [Google Scholar]
- 23.Missmer SA, Spiegelman D, Bertone-Johnson ER, Barbieri RL, Pollak MN, Hankinson SE. Reproducibility of plasma steroid hormones, prolactin, and insulin-like growth factor levels among premenopausal women over a 2- to 3-year period. Cancer Epidemiol Biomarkers Prev. 2006;15:972–8. doi: 10.1158/1055-9965.EPI-05-0848. [DOI] [PubMed] [Google Scholar]
- 24.Edman CD, MacDonald PC. Effect of obesity on conversion of plasma androstenedione to estrone in ovulatory and anovulatory young women. Am J Obstet Gynecol. 1978;130:456–61. doi: 10.1016/0002-9378(78)90288-0. [DOI] [PubMed] [Google Scholar]
- 25.Yeung EH, Zhang C, Albert PS, et al. Adiposity and sex hormones across the menstrual cycle: the BioCycle Study. Int J Obes (Lond) 2013;37:237–43. doi: 10.1038/ijo.2012.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kopelman PG, Pilkington TR, White N, Jeffcoate SL. Abnormal sex steroid secretion and binding in massively obese women. Clin Endocrinol (Oxf) 1980;12:363–9. doi: 10.1111/j.1365-2265.1980.tb02721.x. [DOI] [PubMed] [Google Scholar]
- 27.Trichopoulos D, Cole P, Brown JB, Goldman MB, MacMahon B. Estrogen profiles of primiparous and nulliparous women in Athens, Greece. J Natl Cancer Inst. 1980;65:43–6. [PubMed] [Google Scholar]
- 28.Apter D, Reinilä M, Vihko R. Some endocrine characteristics of early menarche, a risk factor for breast cancer, are preserved into adulthood. Int J Cancer. 1989;44:783–7. doi: 10.1002/ijc.2910440506. [DOI] [PubMed] [Google Scholar]
- 29.Endogenous Hormones and Breast Cancer Collaborative Group. Circulating sex hormones and breast cancer risk factors in postmenopausal women: reanalysis of 13 studies. Br J Cancer. 2011;105:709–22. doi: 10.1038/bjc.2011.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mello NK. Hormones, nicotine, and cocaine: clinical studies. Horm Behav. 2010;58:57–71. doi: 10.1016/j.yhbeh.2009.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Johnson PJ. Sex hormones and the liver. Clin Sci (Lond) 1984;66:369–76. doi: 10.1042/cs0660369. [DOI] [PubMed] [Google Scholar]
- 32.Collaborative Group on Hormonal Factors in Breast Cancer. Alcohol, tobacco and breast cancer--collaborative reanalysis of individual data from 53 epidemiological studies, including 58,515 women with breast cancer and 95,067 women without the disease. Br J Cancer. 2002;87:1234–45. doi: 10.1038/sj.bjc.6600596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van den Brandt PA, Spiegelman D, Yaun SS, et al. Pooled analysis of prospective cohort studies on height, weight, and breast cancer risk. Am J Epidemiol. 2000;152:514–27. doi: 10.1093/aje/152.6.514. [DOI] [PubMed] [Google Scholar]
- 34.Grodstein F, Goldman MB, Cramer DW. Body mass index and ovulatory infertility. Epidemiology. 1994;5:247–50. doi: 10.1097/00001648-199403000-00016. [DOI] [PubMed] [Google Scholar]
- 35.Stanczyk FZ, Lee JS, Santen RJ. Standardization of steroid hormone assays: why, how, and when? Cancer Epidemiol Biomarkers Prev. 2007;16:1713–9. doi: 10.1158/1055-9965.EPI-06-0765. [DOI] [PubMed] [Google Scholar]
- 36.Stanford JL, Hartge P, Brinton LA, Hoover RN, Brookmeyer R. Factors influencing the age at natural menopause. J Chronic Dis. 1987;40:995–1002. doi: 10.1016/0021-9681(87)90113-5. [DOI] [PubMed] [Google Scholar]
- 37.Key TJ, Pike MC. The role of oestrogens and progestagens in the epidemiology and prevention of breast cancer. Eur J Cancer Clin Oncol. 1988;24:29–43. doi: 10.1016/0277-5379(88)90173-3. [DOI] [PubMed] [Google Scholar]
- 38.Swerdlow AJ, Jones ME, Schoemaker MJ, et al. The Breakthrough Generations Study: design of a long-term UK cohort study to investigate breast cancer aetiology. Br J Cancer. 2011;105:911–7. doi: 10.1038/bjc.2011.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Collins R. What makes UK Biobank special? Lancet. 2012;379:1173–4. doi: 10.1016/S0140-6736(12)60404-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.