Abstract
Background
In the mid 1980s, there was a rise in incidence rates of childhood brain tumors (CBT) in the United States that appeared to stabilize at a higher rate in the early 1990s. An updated analysis of the pattern of CBT over the past 2 decades, with commentary on whether the elevated incidence rate has continued, is past due.
Methods
We used Surveillance Epidemiology and End Results (SEER) data to examine trends in incidence of CBT from 1973 through 2009. We examined age-adjusted incidence rates (AAIRs) and secular trends for all malignant brain tumors combined (SEER classification) by histologic tumor type and anatomic site.
Results
The incidence of CBT remained stable from 1987-2009 (annual percent change (APC)=0.10; 95% confidence intervals (CI): −0.39, 0.61) with an AAIR for all CBT of 3.32 (95%CI: 3.22, 3.42). The stability of rates in these two decades contrast the change that occurred in the mid-1980s (1983-1986), when the incidence of CBT increased by 53% (APC=14.06; 95%CI: 4.05, 25.0). From 1983-1986, statistically significant rate increases were observed for pilocytic astrocytoma, PNET/medulloblastoma, and mixed glioma. Further, the rate of increase in pilocytic astrocytoma was similar to the rate of decrease for astrocytomas NOS from 1981-2009, suggesting a change from a more general to more specific classification.
Conclusion
After the increase in rates in the mid-1980s, rates of CBT over the past two decades have stabilized. Changes in incidence rates of subtypes of tumors over this time period reflect changes both in classification of CBT and in diagnostic techniques.
Keywords: childhood brain tumors, gender, histology, incidence rates, pediatric cancer, secular trends, SEER
INTRODUCTION
Brain tumors are the most frequent solid tumors in children and the most common cause of childhood cancer deaths[1]. In 2013, it is estimated that approximately 3,050 children in the United States under 15 years of age will be diagnosed with a benign or malignant primary brain tumor[2].
Ionizing radiation is one established environmental cause of childhood brain tumors (CBT), but few children are exposed to high levels of cranial ionizing radiation today. Beyond ionizing radiation, there are a number of suspected environmental causes of CBT such as exposure to pesticides, dietary nitrites, and some parental occupational exposures[1, 3, 4] but no identified environmental or lifestyle exposure appears to explain the increase in CBT rates observed in the mid-1980s. Improvements in diagnostic procedures (i.e. computed tomography (CT), introduced in the mid-1970s, and magnetic resonance imaging (MRI), introduced in the mid-1980s) as well as changes in histologic classification of CBT may have resulted in the identification of some malignant tumors that were previously undiagnosed or classified as benign[5, 6]. An analysis of Surveillance, Epidemiology, and End Results (SEER) data in the 1990s found that increasing rates were largely restricted to brain stem and low-grade cerebral tumors, which could be explained by improvements in imaging equipment[5].
In our current analysis, we used SEER data to examine trends in incidence of CBT from 1973 through 2009. We looked at incidence rates for all brain tumors combined and by histologic tumor type to better understand patterns of change over time. We additionally used incidence data from the Los Angeles County Cancer Surveillance Program (LA CSP) to examine trends in incidence rates by socioeconomic variables assembled by the registry. These details may contribute to a better understanding of the future trends in CBT and the potential risk factors contributing to the disease.
MATERIALS AND METHODS
The data for the current analysis was completed using brain cancer incidence data from the National Cancer Institute’s SEER Program, a population based cancer registry. Secular trend analyses included data from nine SEER registries (Atlanta, Connecticut, Detroit, Hawaii, Iowa, New Mexico, Utah, San Francisco/Oakland, and Seattle/Puget Sound) that covered data beginning from 1973 to 2009. Histology and anatomical site-specific incidence rates were calculated using recent data (2000-2009) based on all 18 SEER registries. Incidence rates were directly age-standardized to the U.S. 2000 standard population. Cancer incidence data by socioeconomic status (SES) was provided by the Los Angeles County Cancer Surveillance Program (LA CSP) a population-based cancer registry and participant in the SEER program[7].
All primary malignant brain tumors (using SEER classification, which includes pilocytic astrocytomas) diagnosed in children 0-14 years of age, from 1973-2009 and identified in SEER registries were included (International Classification of Diseases for Oncology (ICD-O-3) codes C71.0-C71.9). Distinctions by site of tumor were made as follows: cerebrum (C71.0-C71.4), cerebellum (C71.6), brain stem (C71.7), other brain (overlapping lesions of the brain, and not otherwise specified (NOS); C71.8, C71.9, respectively) and other (cerebral and spinal meninges, ventricles NOS, olfactory, optic, acoustic and cranial nerves and unspecified CNS; C70.0, C70.1, C70.9, C71.5, C72.0-C72.9, respectively).
Histologic categories were made based on classification schemes used by the Central Brain Tumor Registry of the United States (CBTRUS) according to ICD-O-3 morphology codes[8]: PNET/medulloblastoma/embryonal (8963, 9364, 9470-4, 9501-3, 9508), pilocytic astrocytoma (9421), astrocytoma NOS (9400), anaplastic astrocytoma/glioblastoma (9401, 9411, 9440-42), other astrocytoma (9410, 9420, 9424), oligodendroglioma (9450, 9451, 9460), mixed gliomas (9382), malignant gliomas not otherwise specified (NOS) (9380), ependymoma (9391-03), and other CBTs (8000-02, 8004, 8710, 8720, 8800-03, 8810, 8830, 8850, 8900, 8910, 9040, 9060, 9064, 9070-01, 9080-01, 9085, 9101, 9150, 9240, 9260, 9370, 9381, 9390, 9423, 9430, 9480, 9490, 9500, 9505, 9530, 9540, 9560).
Age-adjusted incidence rates (AAIRs) adjusted to the 2000 U.S. standard population and 95% confidence intervals (CIs) were calculated from the SEER data using SEER*stat 8.0.1 (www.seer.cancer.gov/seerstat). Analysis of secular trends was performed using the Joinpoint Regression Program (surveillance.cancer.gov/joinpoint/)[9]. We used the Grid Search method to fit the log-linear trend lines and the Monte Carlo permutation method to select the final model with the smallest number of joinpoints such that if one more joinpoint was added, the improvement was not statistically significant[10]. Annual percent change (APC) was calculated based on the slope of the fitted log-linear trend line for each time segment.
AAIRs for CBT by socioeconomic status (SES) were calculated using LA CSP data only. An SES classification was assigned to each cancer case in the CSP based on the case’s census tract of residence at the time of diagnosis[11]. To create the SES assignments for each census tract, the LA CSP calculated the percentage distribution of educational attainment and the median household income reported in the 1970, 1980, 1990 censuses, and linearly interpolated through these values for all other years. The distribution of the SES assignments was divided into tertiles for examination of incidence rates by categorical level of SES, for four time periods (1972-1982, 1983-1987, 1988-1999, and 2000-2006). The secular cut points were chosen to examine incidence rates based on the years where a significant change in slope was found in the data. These cut points also followed the years preceding diagnostic use of MRI (1972-1982), the initial years of MRI usage (1983-1987), and the years when diagnostic use of MRI was more commonly used in the United States (1988-1999 and 2000-2006).
RESULTS
The AAIRs per 100,000 people and 95%CIs for male and female children diagnosed with a brain tumor from 2000-2009 by histologic type are shown in Table 1. The most frequent types of CBT are pilocytic astrocytomas (26% of all cases; AAIR 0.86, 95%CI: 0.81, 0.90) and PNETs/medulloblastomas (22% of cases; AAIR 0.71, 95%CI: 0.67., 0.75). The rates of brain tumors for males and females were similar, with the exception of the PNET/medulloblastoma subgroup. Male children were 46% more likely to have a PNET/medulloblastoma than female children (relative risk (RR)=1.46; 95%CI: 1.30, 1.63).
Table 1.
Age Adjusted Incidence Rates (AAIR)a per 100,000 people for malignant primary brain tumors in children 0-14 years at diagnosis by major histologic categories, SEER, 2000-2009
| Overall | Male | Female | |||||
|---|---|---|---|---|---|---|---|
| Count | AAIRd (95%CI) | Count | AAIRd (95%CI) | Count | AAIRd (95%CI) | RRe (95%CI) | |
| Type | |||||||
| All brain tumors | 5781 | 3.27 (3.19-3.35) | 3076 | 3.40 (3.28-3.52) | 2705 | 3.13 (3.02-3.25) | 1.08 (1.03-1.14) |
| PNET/Medulloblastoma | 1266 | 0.71 (0.67-0.75) | 764 | 0.84 (0.78-0.90) | 502 | 0.58 (0.53-0.63) | 1.46 (1.30-1.63) |
| Pilocytic Astrocytoma | 1513 | 0.86 (0.81-0.90) | 748 | 0.83 (0.77-0.89) | 765 | 0.89 (0.83-0.95) | 0.93 (0.84-1.03) |
| Astrocytoma, NOS | 409 | 0.23 (0.21-0.26) | 215 | 0.24 (0.21-0.27) | 194 | 0.22 (0.19-0.26) | 1.06 (0.87-1.29) |
| Other Astrocytomab | 158 | 0.09 (0.08-0.10) | 88 | 0.10 (0.08-0.12) | 70 | 0.08 (0.06-0.10) | 1.20 (0.86-1.66) |
| Mixed Glioma | 52 | 0.03 (0.02-0.04) | 29 | 0.03 (0.02-0.05) | 23 | 0.03 (0.02-0.04) | 1.20 (0.67-2.18) |
| Glioma, NOS | 1069 | 0.61 (0.57-0.64) | 525 | 0.58 (0.53-0.63) | 544 | 0.63 (0.58-0.69) | 0.92 (0.81-1.04) |
| Anaplastic Astrocytoma | 145 | 0.08 (0.07-0.10) | 80 | 0.09 (0.07-0.11) | 65 | 0.08 (0.06-0.10) | 1.18 (0.84-1.66) |
| Glioblastoma | 205 | 0.12 (0.10-0.13) | 112 | 0.12 (0.10-0.15) | 93 | 0.11 (0.09-0.13) | 1.14 (0.86-1.52) |
| Oligodendroglioma | 110 | 0.06 (0.05-0.08) | 56 | 0.06 (0.05-0.08) | 54 | 0.06 (0.05-0.08) | 0.99 (0.67-1.47) |
| Ependymoma | 452 | 0.25 (0.23-0.28) | 241 | 0.26 (0.23-0.30) | 211 | 0.24 (0.21-0.28) | 1.09 (0.90-1.32) |
| Otherc | 402 | 0.23 (0.20-0.25) | 218 | 0.24 (0.21-0.27) | 184 | 0.21 (0.18-0.24) | 1.13 (0.93-1.39) |
| Site | |||||||
| Cerebrum | 1299 | 0.74 (0.70-0.78) | 685 | 0.76 (0.70-0.82) | 614 | 0.71 (0.66-0.77) | 1.07 (0.96-1.19) |
| Cerebellum | 1411 | 0.80 (0.76-0.84) | 804 | 0.89 (0.83-0.95) | 607 | 0.70 (0.65-0.76) | 1.26 (1.13-1.40) |
| Brain stem | 1169 | 0.66 (0.63-0.70) | 611 | 0.68 (0.62-0.73) | 558 | 0.65 (0.60-0.70) | 1.04 (0.93-1.17) |
| Other brainf | 929 | 0.52 (0.49-0.56) | 486 | 0.53 (0.49-0.58) | 443 | 0.51 (0.47-0.56) | 1.04 (0.92-1.19) |
| Otherg | 1027 | 0.58 (0.54-0.61) | 523 | 0.57 (0.53-0.63) | 504 | 0.58 (0.53-0.63) | 0.99 (0.87-1.12) |
Abbreviations: RR=Relative Risk, 95%CI=95% Confidence Interval
Age-adjusted rates per 100,000
Other Astrocytoma = ICD-O-3 9410, 9420, 9424
Other = ICD-O-3 8000-02, 8004, 8710, 8720, 8800-03, 8810, 8830, 8850, 8900, 8910, 9040, 9060, 9064, 9070-1, 9080-1, 9085, 9101, 9150, 9240, 9260, 9370, 9381, 9390, 9423, 9430, 9480, 9490, 9500, 9505, 9530, 9540, 9560
Age adjusted to the 2000 US standard population
Male to female
Other brain (overlapping) = ICD-O-3 C71.8, C71.9
Other = ICD-O-3 C70.0, C70.1, C70.9, C71.5, C72.0-C72.9
The lower half of Table 1 shows the AAIRs (per 100,000) overall and by gender for tumor diagnosis by anatomic site of the brain. For both male and female children combined, 24% of tumors were located in the cerebellum, 22% in the cerebrum and 20% were associated with the brain stem. Nearly 16% were described as overlapping in more than one region of the brain. Tumors of the cerebellum were significantly more common in male than female children (RR=1.26; 95%CI: 1.13, 1.40), which was consistent with the more common diagnosis of medulloblastoma in males.
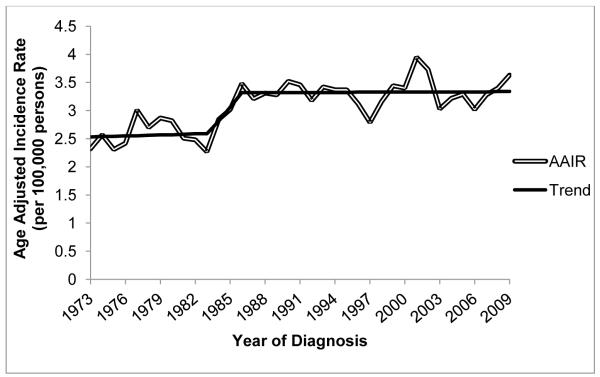
The AAIRs (represented by the double line) and secular trends based on the Joinpoint model that provided the best fit (solid black line) are shown by year for all brain tumor types combined from 1973 to 2009 (figure 1). The data show an increase in the incidence of CBT over this time period, with a significant change in slope of the linear trend line from 1983 to 1986 (p=0.03). There was a fairly constant annual rate of CBT from 1973-1982 (Annual Percent Change (APC)=1.09; 95%CI: −1.34, 3.58; AAIR=2.59; 95%CI: 2.45, 2.74), a significant increase in rates from 1983 to 1986 (APC=14.06; 95%CI: 4.05, 25.0), and a higher, but relatively constant rate of disease from 1987-2009 (APC=0.10; 95%CI: −0.39, 0.61; AAIR=3.32; 95%CI: 3.22, 3.42). We found a similar pattern across age groups for children diagnosed from birth to 14 years of age (0-4, 5-10, 11-14 years), and when analyses were completed using data from the LA CSP (data not shown).
Fig. 1.
Age-adjusted incidence rate (AAIR) per 100,000 children and best fit Joinpoint line for primary brain cancer by year of diagnosis for children 0-14 years of age, SEER 9, 1973-2009
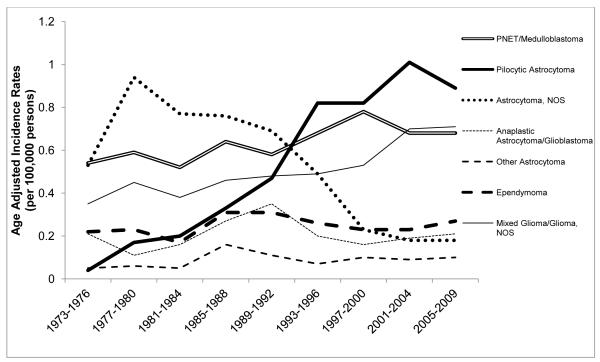
Figure 2 shows the AAIRs per 100,000 persons by major histologic types of brain tumors for children 0-14 years of age. The secular trend observed in Figure 1 for all types of CBT combined appears to be driven largely by the categories of pilocytic astrocytoma, PNET/medulloblastoma, and mixed glioma, however, there was a transient increase in all histologic tumor types except astrocytoma NOS during the mid-1980s. The AAIR of pilocytic astrocytomas increased from 0.04 per 100,000 in 1975 to 0.86 per 100,000 in 1995 (APC=11.6; 95%CI: 9.55, 13.6), but did not significantly change from 1996 through 2009 (APC=0.96; 95%CI: −1.33, 3.31). There was a significant increase in the rate of PNET/medulloblastomas (APC=0.79; 95%CI: 0.18, 1.40) and mixed gliomas and gliomas NOS (APC=2.03; 95%CI: 1.44, 2.64) over the entire time interval. A substantial decrease occurred in the diagnosis and classification of tumors as “astrocytomas NOS” from 1989 to 2009 (APC=−8.85; 95%CI: −10.8, −6.90). There was evidence of a decline beginning as early as 1977, however the change in slope from 1977 through 1988 was not statistically significant (APC= −1.86; 95%CI: −4.89, 1.27).
Fig. 2.
Age-adjusted incidence rates (AAIRs) per 100,000 persons by major histologic types of brain tumors in children 0-14 years of age at diagnosis, SEER 9, 1973-2009
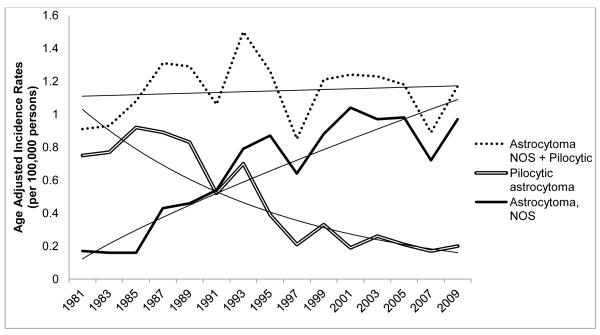
Figure 3 shows the AAIRs for astrocytomas NOS and pilocytic astrocytomas separately and combined from 1981-2009. The estimated annual percent decrease in astrocytoma, NOS over the entire interval (APC=−6.55%; 95%CI: − 7.94, −5.14) was similar to the annual percent increase in pilocytic astrocytomas (APC=4.84%; 95%CI: 3.37, 6.34). We examined these trends beginning in 1981, when rates of astrocytoma NOS and pilocytic astrocytoma began to change in opposite directions. The estimated annual percent change for the combined categories was consistent with no change in slope (APC=0.18; 95%CI: −0.55, 0.92).
Fig. 3.
Secular trends in age-adjusted incidence rates (AAIRs) for astrocytoma, NOS and pilocytic astrocytomas, SEER 9, 1981-2009
The AAIRs for all CBT in the LA CSP by socioeconomic status (SES) from 1972 to 2006 are presented in Online Resource 1. For all time periods (1972-1982, 1983-1987, 1988-1999, 2000-2006), AAIRs were lowest for children in the lowest tertile of SES compared to middle or upper tertiles. Further, the increase in the AAIRs over the 4 time periods (from 1972-1982 to 2000-2006) was greater in children of high SES (upper tertile of SES; 41% increase) or middle SES (50% increase) than low SES (31% increase).
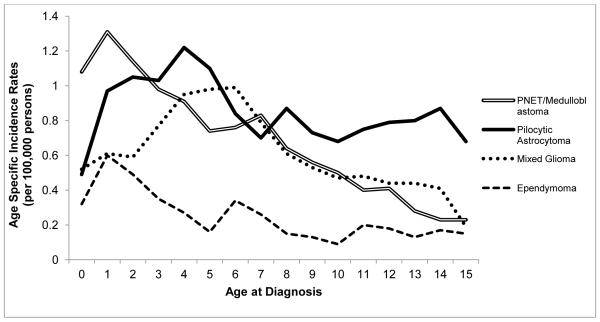
Age specific incidence rates by select histologic subtypes of CBT using SEER data from 2000-2009 are shown in figure 4. The age-specific incidence patterns and the peak-age of occurrence vary by histologic type of tumor. Pilocytic astrocytomas occur most frequently from 1 to 5 years of age. The incidence of PNET/medulloblastoma is highest at 1 year, declining until age 5, with a plateau from 5 to 7 years of age and then a decline in incidence thereafter. The incidence rate of mixed gliomas is highest at 4-6 years of age (AAIR = 0.99 per 100,000), while ependymoma incidence is highest at 1 year of age (0.60 per 100,000) with most cases occurring from birth to 4 years of age.
Fig. 4.
Age-specific incidence rate of childhood brain tumors diagnosed at 0-14 years of age by histology, SEER 18, 2000-2009
AAIRs by anatomic site within the brain are shown in Online Resource 2. Incidence rates for brain tumors of the cerebellum remained stable (APC= −0.17, 95%CI: −0.66, 0.32), while rates in the brain stem increased steadily from 1973 to 1984 (APC=7.41, 95%CI: 4.15, 10.78) but stabilized from 1985 through 2009 (APC=0.76, 95%CI: −0.12, 1.65). Rates in the cerebrum increased from 1973 through 1989 (APC=5.31, 95%CI: 2.81, 7.87) and were level, or decreasing slightly, from 1990 through 2009 (APC= −1.26, 95%CI: −2.55, 0.05).
DISCUSSION
The incidence of CBT from 1986 through 2009 has remained higher, but relatively stable, following the increase in rates observed during the mid 1980s. The increase in rates following the introduction of diagnostic imaging using CT and MRI has been described in several previous papers[5, 12-14]. In the current analyses, we found the increase in rates was present across age groups and restricted to the three major histologic subtypes (pilocytic astrocytomas, PNET/medulloblastoma, and mixed gliomas). During the same time window, there was a decrease in tumors labeled as astrocytomas, NOS.
Changes in classification may have contributed to the change in rates found for several types of brain tumors. Pilocytic astrocytoma is a newer coding designation. In the current ICD-O-3, the “Astrocytoma, NOS” category includes a designation for “Astrocytoma, cystic.” In the past, cystic appearing tumors may have been lumped into a single astrocytoma category, while currently there is more emphasis on distinguishing pilocytic astrocytomas. The classic appearance on radiographic imaging of a pilocytic astrocytoma is of a cyst with a mural nodule, however, these features may vary and the tumors can appear predominantly cystic or solid[15, 16]. In the SEER data for the 1981-2009 period, the rate of increase in pilocytic astrocytoma was similar to the rate of decrease for astrocytomas NOS, suggesting a change from a more general to more specific classification.
Changes in classification also likely influenced the increasing rate of PNET. The designation of PNET (distinct from medulloblastoma) was rarely used prior to 1986[17]. Medulloblastomas are embryonal tumors of the cerebellum with unique molecular subtypes[18-21], while PNET arise in the cerebrum or suprasellar region[22]. While medulloblastoma and PNET are now considered biologically distinct with respect to molecular genetics and treatment response[23, 24], they are typically grouped in epidemiologic studies because they appear the same histologically. In our current analysis of SEER data, the incidence rate of PNET and medulloblastomas combined increased through 1999. This pattern was described in a previous analysis of SEER data that found the incidence rate of PNET increased 23% from 1973-1977 to 1993-1998[25]. One explanation for this increasing trend may be that supratentoral embryonal tumors were previously designated based upon their anatomic location, such as cerebral neuroblastoma, but are now classified under CNS-PNET[26]. A publication using data from the Central Brain Tumor Registry of the United States (1985-2002) found no statistically significant increase in the estimated APC of medulloblastoma (1.1%; 95%CI: −0.7, 2.9), but found a significant increase (APC=1.6%; 95%CI: 0.2, 3.1) when considering both medulloblastoma and PNET combined[23]. The authors suggested that the increase of PNET and medulloblastoma combined could result from overdiagnosis of PNET due to the increased preference for this diagnostic classification[23].
Mixed gliomas or oligoastocytomas were first recognized as a tumor entity in the 1930s. The incidence rates of mixed gliomas are likely to be unstable due to the changing morphological criteria use to classify these tumors[22]. When mixed gliomas and gliomas NOS were examined separately, the increase in rates was due to changes in the mixed gliomas while the rate was relatively stable in the gliomas NOS.
Improvements in diagnostic technology are considered an important contributing factor to the higher rates of brain tumors. The use of CT and MRI imaging technologies in the US grew in the 1980s, corresponding to increasing rates of brain tumors in the mid-1980s[5, 6, 27, 28]. Diagnosis and classification of distinct histologic entities over time and by regional centers also is influenced by the availability of diagnostic technology, as well as the experience and clinical expertise of the pathologist making the diagnosis[29]. The location of a tumor within the brain is another factor that may influence diagnosis. While 90% of adult brain tumors can be found supratentorially, in children over the age of 1 year, 50% are infratentorial[31]. The most common pediatric brain tumors typically found in the posterior fossa include medulloblastoma, cerebellar astrocytoma, brain stem glioma and ependymoma, and the most common supratentorial tumors are other astrocytomas and glioneuronal tumors[15]. Both CT and MRI are fairly sensitive at detecting malignant cerebral tumors. MRI is more accurate for low-grade astrocytomas and for oligodendrogliomas, which can be missed by CT at early stages[30].
Rates of tumors in the cerebellum did not change significantly during the study period. The majority of posterior fossa pilocytic tumors are diagnosed after a child becomes symptomatic. Thus, use of MRI or CT may not result in the detection of new cerebellar pilocytic astrocytomas that previously would have gone undetected. Therefore, accurate classification may have been more influential on the increasing secular trends observed for pilocytic astrocytomas than use of improved diagnostic imaging.
Using data from Los Angeles, we found that the increase in the AAIR of brain tumors in children 14 years of age or younger at diagnosis was greater in children of high-versus low-SES. One explanation for this difference may be that children of higher income families had more access to advanced imaging technology due to their insurance coverage, which could have resulted in more definitive diagnoses necessary for inclusion in SEER registries. Working families with lower income may have had less insurance coverage and therefore more limited access to diagnostic imaging. Children of non-working families are often covered by public programs such as Medicaid[32]. Variability in rates by SES may also indicate differences in the environment or lifestyle of high versus low-income children that are responsible for disparities in risk, though an environmental risk factor to explain these patterns has not been identified.
Significant changes in requirements for cancer registry reporting by tumor behavior occurred in 2002. Prior to this time, non-malignant brain tumors were not reported to SEER, including meningiomas, pituitary adenomas, benign germ cell tumors, craniopharyngiomas and choroids plexus papillomas[33]. However, in 2002, reporting of benign tumors became mandatory by federal law. The Benign Brain Tumor Cancer Registries Amendment Act, signed into law (Public Law 107-260) in October of 2002, requires all programs participating in the National Program for Cancer Registries (NPCR) to report tumors of benign and uncertain behavior. The National Cancer Institute’s SEER and American College of Surgeons Commission on Cancer mandated reporting of benign or uncertain tumors beginning January 1, 2004. Since pilocytic astrocytomas and WHO grade I neoplasms can be considered “benign”, one could expect an impact on trends in these categories. While the Act has been critical for complete tracking of all brain tumors that could cause serious morbidity or mortality, the impact seems to be minimal on the trends of astrocytic tumors from 2002-2009.
CONCLUSION
Our investigation used SEER data to evaluate the consistency of trends in CBT in recent years, after the large increase in rates observed during the mid-1980s. This analysis indicates the overall rates, following the increase from 1983-1986, have remained elevated but relatively stable from approximately 1990 forward. The changes in rates likely reflect changes in both classification and diagnosis. Within the overall stable incidence pattern, there have been changes in the frequency of histologic subtypes. Changes in classification may explain apparent increases in subsets of tumors (e.g. pilocytic astrocytomas, PNET/medulloblastoma). The impact of classification, diagnostics, and unidentified changes in environmental factors on brain tumor trends will require ongoing evaluation as secular trends are monitored in the future.
Supplementary Material
Acknowledgements
Funding for this research was provided by the National Cancer Institute (NCI) [R01 CA116724]; the American Cancer Society [PTAPM-02-074-01]; the National Institute of Environmental Health Sciences (NIEHS) [2 T32 ES 013678-06]; the National Institute of Environmental Health Sciences Center, grant #5P30ES07048; and the Ronald Ross, MD, Cancer Research Fund at the University of Southern California Norris Comprehensive Cancer Center.
Footnotes
Conflict of interest: The authors have no conflict of interest to declare.
REFERENCES
- 1.Baldwin RT, Preston-Martin S. Epidemiology of brain tumors in childhood--a review. Toxicol Appl Pharmacol. 2004;199:118–131. doi: 10.1016/j.taap.2003.12.029. doi:10.1016/j.taap.2003.12.029. [DOI] [PubMed] [Google Scholar]
- 2.Dolecek TA, Propp JM, Stroup NE, Kruchko C. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2005-2009. Neuro Oncol. 2012;14(Suppl 5):v1–49. doi: 10.1093/neuonc/nos218. doi:10.1093/neuonc/nos218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wrensch M, Minn Y, Chew T, Bondy M, Berger MS. Epidemiology of primary brain tumors: current concepts and review of the literature. Neuro-Oncology. 2002;4:278–299. doi: 10.1093/neuonc/4.4.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Preston-Martin S. Epidemiology of primary CNS neoplasms. Neurol Clin. 1996;14:273–290. doi: 10.1016/s0733-8619(05)70256-5. [DOI] [PubMed] [Google Scholar]
- 5.Smith MA, Freidlin B, Ries LA, Simon R. Trends in reported incidence of primary malignant brain tumors in children in the United States. J Natl Cancer Inst. 1998;90:1269–1277. doi: 10.1093/jnci/90.17.1269. [DOI] [PubMed] [Google Scholar]
- 6.Steinberg EP. The status of MRI in 1986: rates of adoption in the United States and worldwide. AJR Am J Roentgenol. 1986;147:453–455. doi: 10.2214/ajr.147.3.453. [DOI] [PubMed] [Google Scholar]
- 7.Mack TM. Cancer surveillance program in Los Angeles County. Natl Cancer Inst Monogr. 1977;47:99–101. [PubMed] [Google Scholar]
- 8.Fritz AG. International classification of diseases for oncology : ICD-O. World Health Organization; Geneva: 2000. [Google Scholar]
- 9.Kim HJ, Fay MP, Feuer EJ, Midthune DN. Permutation tests for joinpoint regression with applications to cancer rates. Stat Med. 2000;19:335–351. doi: 10.1002/(sici)1097-0258(20000215)19:3<335::aid-sim336>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 10.Yu B, Barrett MJ, Kim H-J, Feuer EJ. Estimating joinpoints in continuous time scale for multiple change-point models. Computational Statistics & Data Analysis. 2007;51:2420–2427. [Google Scholar]
- 11.Liu L, Deapen D, Bernstein L. Socioeconomic status and cancers of the female breast and reproductive organs: a comparison across racial/ethnic populations in Los Angeles County, California (United States) Cancer Causes & Control. 1998;9:369–380. doi: 10.1023/a:1008811432436. [DOI] [PubMed] [Google Scholar]
- 12.Gurney JG, Davis S, Severson RK, Fang JY, Ross JA, Robison LL. Trends in cancer incidence among children in the U.S. Cancer. 1996;78:532–541. doi: 10.1002/(SICI)1097-0142(19960801)78:3<532::AID-CNCR22>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 13.Legler JM, Ries LA, Smith MA, Warren JL, Heineman EF, Kaplan RS, Linet MS. Cancer surveillance series [corrected]: brain and other central nervous system cancers: recent trends in incidence and mortality. J Natl Cancer Inst. 1999;91:1382–1390. doi: 10.1093/jnci/91.16.1382. [DOI] [PubMed] [Google Scholar]
- 14.Kohler BA, Ward E, McCarthy BJ, Schymura MJ, Ries LA, Eheman C, Jemal A, Anderson RN, Ajani UA, Edwards BK. Annual report to the nation on the status of cancer, 1975-2007, featuring tumors of the brain and other nervous system. J Natl Cancer Inst. 103:714–736. doi: 10.1093/jnci/djr077. doi:djr077 [pii] 10.1093/jnci/djr077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Poussaint TY. Magnetic resonance imaging of pediatric brain tumors: state of the art. Top Magn Reson Imaging. 2001;12:411–433. doi: 10.1097/00002142-200112000-00004. [DOI] [PubMed] [Google Scholar]
- 16.Zulch K. Histological typing of tumors of the central nervous system. World Health Organization; Geneva: 1979. [Google Scholar]
- 17.McNeil DE, Cote TR, Clegg L, Mauer A. SEER update of incidence and trends in pediatric malignancies: acute lymphoblastic leukemia. Med Pediatr Oncol. 2002;39:554–557. doi: 10.1002/mpo.10161. discussion 552-553. [DOI] [PubMed] [Google Scholar]
- 18.Kool M, Koster J, Bunt J, Hasselt NE, Lakeman A, van Sluis P, Troost D, Meeteren NS, Caron HN, Cloos J, Mrsić A, Ylstra B, Grajkowska W, Hartmann W, Pietsch T, Ellison D, Clifford SC, Versteeg R. Integrated genomics identifies five medulloblastoma subtypes with distinct genetic profiles, pathway signatures and clinicopathological features. PLoS One. 2008;3:e3088. doi: 10.1371/journal.pone.0003088. doi:10.1371/journal.pone.0003088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Northcott PA, Korshunov A, Witt H, Hielscher T, Eberhart CG, Mack S, Bouffet E, Clifford SC, Hawkins CE, French P, Rutka JT, Pfister S, Taylor MD. Medulloblastoma comprises four distinct molecular variants. J Clin Oncol. 2011;29:1408–1414. doi: 10.1200/JCO.2009.27.4324. doi:10.1200/JCO.2009.27.4324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thompson MC, Fuller C, Hogg TL, Dalton J, Finkelstein D, Lau CC, Chintagumpala M, Adesina A, Ashley DM, Kellie SJ, Taylor MD, Curran T, Gajjar A, Gilbertson RJ. Genomics identifies medulloblastoma subgroups that are enriched for specific genetic alterations. J Clin Oncol. 2006;24:1924–1931. doi: 10.1200/JCO.2005.04.4974. doi:10.1200/JCO.2005.04.4974. [DOI] [PubMed] [Google Scholar]
- 21.Parsons DW, Li M, Zhang X, Jones S, Leary RJ, Lin JC, Boca SM, Carter H, Samayoa J, Bettegowda C, Gallia GL, Jallo GI, Binder ZA, Nikolsky Y, Hartigan J, Smith DR, Gerhard DS, Fults DW, VandenBerg S, Berger MS, Marie SK, Shinjo SM, Clara C, Phillips PC, Minturn JE, Biegel JA, Judkins AR, Resnick AC, Storm PB, Curran T, He Y, Rasheed BA, Friedman HS, Keir ST, McLendon R, Northcott PA, Taylor MD, Burger PC, Riggins GJ, Karchin R, Parmigiani G, Bigner DD, Yan H, Papadopoulos N, Vogelstein B, Kinzler KW, Velculescu VE. The genetic landscape of the childhood cancer medulloblastoma. Science. 2011;331:435–439. doi: 10.1126/science.1198056. doi:10.1126/science.1198056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tumours of the Nervous System. International Agency for Research on Cancer (IARC); Lyon, France: 2000. Pathology & Genetics. [Google Scholar]
- 23.Partap S, Curran EK, Propp JM, Le GM, Sainani KL, Fisher PG. Medulloblastoma incidence has not changed over time: a CBTRUS study. J Pediatr Hematol Oncol. 2009;31:970–971. doi: 10.1097/MPH.0b013e3181bbc502. doi:10.1097/MPH.0b013e3181bbc502. [DOI] [PubMed] [Google Scholar]
- 24.Pomeroy SL, Tamayo P, Gaasenbeek M, Sturla LM, Angelo M, McLaughlin ME, Kim JY, Goumnerova LC, Black PM, Lau C, Allen JC, Zagzag D, Olson JM, Curran T, Wetmore C, Biegel JA, Poggio T, Mukherjee S, Rifkin R, Califano A, Stolovitzky G, Louis DN, Mesirov JP, Lander ES, Golub TR. Prediction of central nervous system embryonal tumour outcome based on gene expression. Nature. 2002;415:436–442. doi: 10.1038/415436a. doi:10.1038/415436a 415436a [pii] [DOI] [PubMed] [Google Scholar]
- 25.McNeil DE, Cote TR, Clegg L, Rorke LB. Incidence and trends in pediatric malignancies medulloblastoma/primitive neuroectodermal tumor: a SEER update. Surveillance Epidemiology and End Results. Med Pediatr Oncol. 2002;39:190–194. doi: 10.1002/mpo.10121. [DOI] [PubMed] [Google Scholar]
- 26.Louis DNOH, Wiestler OD, Cavenee WK, editors. WHO Classification of Tumours of the Central Nervous System. IARC; Lyon: 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Steinberg EP, Sisk JE, Locke KE. X-ray CT and magnetic resonance imagers. Diffusion patterns and policy issues. N Engl J Med. 1985;313:859–864. doi: 10.1056/NEJM198510033131405. [DOI] [PubMed] [Google Scholar]
- 28.Rapid Spending Growth and Shift to Physician Offices Indicate Need for CMS to Consider Additional Management Practices. U.S. Government Accountability Office; Washington DC: 2008. MEDICARE PART B IMAGING SERVICES; pp. 5–13. [Google Scholar]
- 29.Davis FG, Malmer BS, Aldape K, Barnholtz-Sloan JS, Bondy ML, Brannstrom T, Bruner JM, Burger PC, Collins VP, Inskip PD, Kruchko C, McCarthy BJ, McLendon RE, Sadetzki S, Tihan T, Wrensch MR, Buffler PA. Issues of diagnostic review in brain tumor studies: from the Brain Tumor Epidemiology Consortium. Cancer Epidemiol Biomarkers Prev. 2008;17:484–489. doi: 10.1158/1055-9965.EPI-07-0725. [DOI] [PubMed] [Google Scholar]
- 30.Kazner E, Wende S, Grumme T, Stochdorph FR, Claussen C, editors. Computed Tomography and Magnetic Resonance Tomography of Intracranial Tumors - A Clinical Perspective. Springer-Verlag; Berlin, Germany: 1989. [Google Scholar]
- 31.Pollack IF. Pediatric brain tumors. Semin Surg Oncol. 1999;16:73–90. doi: 10.1002/(sici)1098-2388(199903)16:2<73::aid-ssu2>3.0.co;2-0. doi:10.1002/(SICI)1098-2388(199903)16:2<73::AID-SSU2>3.0.CO;2-0 [pii] [DOI] [PubMed] [Google Scholar]
- 32.Guendelman S, Angulo V, Oman D. Access to health care for children and adolescents in working poor families: recent findings from California. Med Care. 2005;43:68–78. [PubMed] [Google Scholar]
- 33.Gurney JG, Kadan-Lottick N. Brain and other central nervous system tumors: rates, trends, and epidemiology. Curr Opin Oncol. 2001;13:160–166. doi: 10.1097/00001622-200105000-00005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.