Abstract
Purpose
To examine whether induction of autophagy is a mechanism of leukemic cell resistance to dual mTORC1/mTORC2 inhibitors in AML leukemic progenitors.
Experimental Design
Combinations of different experimental approaches were used to assess induction of autophagy, including immunoblotting to detect effects on LC3II and p62/SQTM1 expression and on ULK1 phosphorylation; immunofluoresence; and electron microscopy. Functional responses were assessed using cell viability and apoptosis assays; and clonogenic leukemic progenitor assays in methylcellulose.
Results
We provide evidence that treatment of AML cells with catalytic mTOR inhibitors results in induction of autophagy, which acts as a regulatory mechanism to promote leukemic cell survival. Such induction of autophagy by dual mTORC1/mTORC2 inhibitors partially protects primitive leukemic precursors from the inhibitory effects of such agents and limits their activities. Simultaneous blockade of the autophagic process using chloroquine or by knockdown of ULK1 results in enhanced antileukemic responses.
Conclusions
Dual targeting of mTORC2 and mTORC1 results in induction of autophagy in AML cells. Combinations of catalytic mTOR targeting agents and autophagy inhibitors may provide a unique approach to target primitive leukemic precursors in AML.
Introduction
The mammalian target of rapamycin (mTOR) pathway plays a central role in the regulation of mRNA translation of genes whose protein products promote cell proliferation and survival (1-3). There is emerging evidence that inhibition of both mTORC1 and mTORC2 complexes by catalytic targeting of mTOR may provide a powerful approach for the treatment of malignancies (1-5) and aging-related pathologies (6, 7). Beyond the classic mTOR inhibitors, the rapalogs, catalytic mTOR inhibitors have been recently developed or are in early clinical trials (8, 9). Such catalytic inhibitors of mTOR have emerged as potentially superior therapeutic options to rapalogs (rapamycin, temsirolimus, everolimus, ridaforolimus), as the clinical utility of rapalogs is limited by the inability of these agents to fully block mTOR activation in neoplastic cells. So far, two distinct complexes have been described in living mammalian cells, mTORC1 and mTORC2. mTORC1 complexes are composed of Raptor, mLST8, Pras40, Deptor and mTOR (1-3). These complexes are key and essential regulators of cellular pathways that control initiation of mRNA translation and ribosome biogenesis and exhibit important monitoring effects on cell metabolism, lipolysis, and autophagy (1-3). mTORC2 complexes are composed of mTOR, Rictor, Deptor, mLST8, Sin1 and mTOR (1-3). These complexes regulate downstream engagement of members of the AGC family of kinases, which account for prosurvival signals and control effector elements that regulate cell cycle progression and anabolism (1-3).
Acute myeloid leukemia (AML) is a heterogenous group of malignancies with diverse molecular pathogenetic lesions, characterized by an aggressive, life threatening, clinical course if left untreated (10-13). Despite extensive efforts over the years to improve survival and cure rates for this fatal disease, the treatment options remain relatively limited. As the mTOR pathway plays a central role in the survival and proliferation of malignant cells and there is evidence that it is dysregulated in AML (14-17), it provides an attractive molecular therapeutic target. Preclinical (19-21) and clinical (22, 23) evidence has suggested that the rapalogs have antileukemic properties and/or enhance the effects of chemotherapy or other antileukemic agents. Importantly, the emergence of catalytic inhibitors of mTOR which inhibit both mTORC1 and mTORC2, has led to pre-clinical efforts to assess the potential utility of these agents in AML (24-26).
A limitation in the generation of antileukemic responses by mTOR inhibitors is the activation or inhibition of regulatory feedback loops that may result in induction of cell survival mechanisms. In the present study, we provide evidence that catalytic mTOR inhibition with OSI-027 or AZD-2014 results in induction of autophagy which acts as a protective mechanism for leukemic cell survival. Concomitant treatment of primitive leukemic progenitors from AML patients with an inhibitor of autophagy potentiates the effects of dual mTORC1/2 inhibitors on leukemic precursors in vitro, while similar enhancing outcomes can be obtained in studies using RNAi to target the ULK1 kinase. Altogether, these studies provide evidence that autophagy is an escape mechanism of leukemic cells from the antileukemic properties of dual mTORC1/2 inhibitors and strongly suggest that combinations of autophagy inhibitors with catalytic mTOR inhibitors may provide a unique approach to target leukemic precursors in this disease.
Materials and Methods
Cells and Reagents
The U937, HEL, Kasumi 1, and Kasumi 3 acute leukemia cell lines were obtained from ATCC. U937 and HEL were cultured in RPMI 1640 supplemented with 10% fetal bovine serum (FBS). Kasumi 1 and Kasumi 3 were cultured in ATCC modified RPMI 1640 supplemented with 20% FBS. OSI-027 was purchased from ChemieTek and AZD-2014 was purchased from Santa Cruz Biotechnology. All antibodies were purchased from Cell Signaling Technologies except the antibody against GAPDH, which was purchased from Millipore, and p62/SQSTM1, which was purchased from Santa Cruz.
Cell lysis and Immunoblotting
For the immunoblotting experiments, cells were treated with the indicated inhibitors or DMSO (used as control for untreated cells), for the indicated times and lysed in phosphorylation lysis buffer (27-29). Unless otherwise indicated, the final concentrations of the different pharmacological agents used in these experiments were as follows: OSI-027 (5 μM), rapamycin (20 nM), chloroquine (6 μM) and AZD-2014 (0.1 or 1 μM). DMSO (diluent) was used as control treatment. Immunoblotting using an enhanced chemiluminescence (ECL) method was performed as in our previous studies (27-29).
Evaluation of Apoptosis
Apoptosis of leukemic cells after various treatments was evaluated by flow cytometric analysis for annexin V/PI staining as in our previous studies (30, 31).
Clonogenic assays in methylcellulose to assess leukemic progenitor colony formation
Peripheral blood or bone marrow samples were obtained from patients with acute myeloid leukemia (AML) after obtaining informed consent approved by the Institutional Review Board of Northwestern University. Cells were separated over Ficoll-Hypaque and cultured with the indicated concentrations of the indicated inhibitors and leukemic progenitor (CFU-L) colony formation was assessed in clonogenic assays in methylcellulose (24, 32). Experiments to assess CFU-L colony formation derived from different AML lines were performed, as indicated. The final concentrations used were OSI-027 (10 μM), AZD-2014 (0.5 μM) and chloroquine (2.5 μM), unless otherwise indicated.
RNAi targeting
Non-targeting control and ULK1 siRNA was purchased from Santa Cruz Biotechnology. siRNA-mediated knockdown of ULK1 using Amaxa™ nucleofector kits from Lonza, and assessment of the effects of such knockdown on leukemic progenitor colony formation was performed as in previous studies (33, 34).
Immunofluorescence
Immunofluorescence was performed as in our previous studies (33). Briefly, human leukemic cells were then collected and fixed with periodate/lysine/ paraformaldehyde solution before staining. Fluorescence was detected using a Zeiss LSM 510 META, confocal microscope system and images were analyzed with ImageJ.
Electron microscopy
Such studies were performed as previously described (33, 35). Briefly, fixed samples were sectioned to 70 nm thin and post-stained with 3% uranyl acetate and Reynolds lead citrate. The samples were examined under FEI Tecnai Spirit G2 TEM and images obtained on a FEI Eagle camera. Samples were processed for TEM by the Cell Imaging Facility at Northwestern University, Feinberg School of Medicine.
WST-1 assays
Cell viability/proliferation was assessed as previously described, using the WST-1 reagent (Roche) [36]. IC50 values were calculated by a sigmoidal dose-response curve fit using Prism Graphpad 6.0 for Windows.
Results
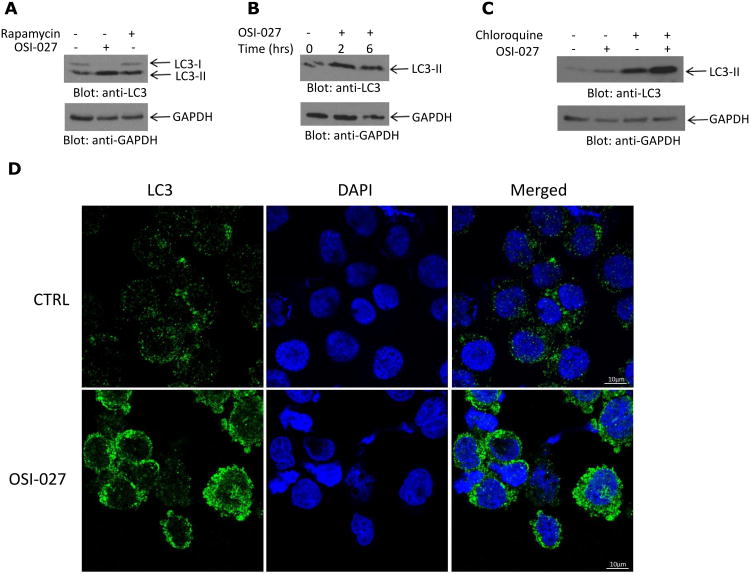
In initial studies, we sought to determine whether treatment of AML cells with mTOR inhibitors results in induction of autophagy. When U937 cells were treated with either the catalytic mTOR inhibitor OSI-027 or the selective mTORC1 inhibitor rapamycin, we found induction of autophagy as assessed in immunoblotting experiments demonstrating increased expression of LC3II (Figs. 1A and B), consistent with formation of autophagosomes. Such increase in LC3II expression was clearly more pronounced in response to treatment with OSI-027 (Fig. 1A) and was further increased by co-treatment of cells with chloroquine (Fig. 1C), an indication of autophagic flux. Induction of autophagy in response to treatment of cells with OSI-027 was further demonstrated using confocal microscopy, by monitoring leukemic cells for the presence of punctate LC3 (34) (Fig. 1D).
Figure 1.
Induction of autophagy in acute myeloid leukemia cell lines by catalytic mTOR inhibition. A. U937 cells were incubated with OSI-027 or rapamycin for 90 minutes. Total cell lysates were resolved by SDS-PAGE and immunoblotted with anti-LC3 or anti-GAPDH antibodies, as indicated. B. U937 cells were incubated for the indicated times with OSI-027. Total cell lysates were resolved by SDS-PAGE and immunoblotted with anti-LC3 or anti-GAPDH antibodies, as shown. C. U937 cells were treated with OSI-027 and/or chloroquine for 24 hours, as indicated. Total cell lysates were resolved by SDS-PAGE and immunoblotted with anti-LC3 or anti-GAPDH antibodies, as indicated. D. U937 cells were treated with OSI-027 for 24 hours and after collection were stained with anti-DAPI (blue) or anti-LC3 (green) and signals were detected by confocal microscopy. Merged panels indicate areas of overlapping images of the two fluorescing signals.
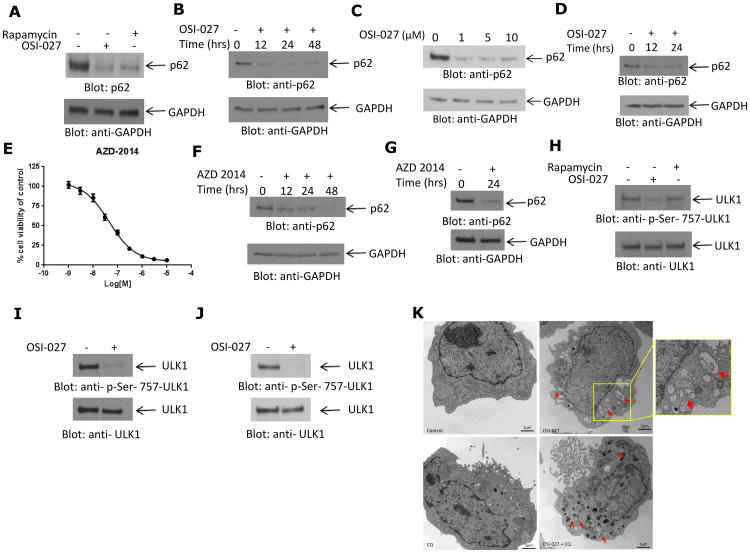
In subsequent studies, we sought to directly establish whether there is induction of autophagic flux (37) in AML cells treated with mTOR inhibitors, by assessing the expression of p62/SQSTM1, an LC3-interacting protein degraded in the autolysosomes (38). Although treatment with either OSI-027 or rapamycin resulted in a decrease in p62/SQSTM1 protein levels, the effects of OSI-027 were more pronounced (Fig. 2A-C). Suppression of p62/SQSTM1 protein levels, consistent with autophagic flux, was also seen when HEL cells were used (Fig. 2D). In other studies we used the catalytic mTOR inhibitor AZD-2014 (39), which as in the case of OSI-027 (24) inhibits proliferation of U937 cells (IC50 45 nM) (Fig. 2E). AZD-2014 also suppressed p62/SQSTM1 expression (Fig. 2F and G). Notably, catalytic mTOR inhibition resulted in suppression of phosphorylation of ULK1 on serine 757 in different AML cell lines (Figs. 2H, I and J), a site whose phosphorylation inhibits activation of ULK1 (40), suggesting a mechanism for the induction of autophagy during treatment of AML cells with dual mTORC1/2 inhibitors. Formation of autophagic structures was also documented using electron microscopy (EM) (Fig. 2K), definitely establishing induction of an autophagic state during treatment of AML cells with catalytic mTOR inhibitors. There was further increase in the number of autophagic structures when cells were treated concomitantly with OSI-027 and the autophagy inhibitor chloroquine (Fig. 2K), consistent with arrest of the autophagic process at the autophagic stage and lack of progression to autolysosome formation (38).
Figure 2.
Induction of autophagic flux by mTOR targeting in AML cells. A. U937 cells were incubated with OSI-027 or rapamycin for 24 hours. Total cell lysates were resolved by SDS-PAGE and immunoblotted with anti-p62/SQSTM1 or anti-GAPDH antibodies, as indicated. B. U937 cells treated with OSI-027 for the indicated times. Total cell lysates were resolved by SDS-PAGE and immunoblotted with anti-p62/SQSTM1 or anti-GAPDH antibodies, as indicated. C. U937 cells were treated with the indicated final concentrations of OSI-027 for 24 hours. Total cell lysates were resolved by SDS-PAGE and immunoblotted with anti-p62/SQSTM1 or anti-GAPDH antibodies, as indicated. D. HEL cells were treated with OSI-027 for the indicated times. Cell lysates were resolved by SDS-PAGE and immunoblotted with anti-p62/SQSTM1 or anti-GAPDH antibodies, as indicated. E. U937 cells were treated with AZD-2014 for 3 days and cell viability was assessed using a WST-1 assay. F-G. U937 cells were treated with AZD-2014 for the indicated times. Cell lysates were resolved by SDS-PAGE and immunoblotted with anti-p62/SQSTM1 or anti-GAPDH antibodies, as indicated. H. U937 cells were treated with OSI-027 or rapamycin for 90 minutes, as indicated. Total cell lysates were resolved by SDS-PAGE and immunoblotted with anti-phospho-Ser-757 ULK1 antibody. Equal amounts of cell lysates from the same experiment were analyzed separately by SDS-PAGE and immunoblotted with an anti-ULK1 antibody. I. HEL cells were treated with OSI-027 for 90 minutes. Total cell lysates were resolved by SDS-PAGE and immunoblotted with anti-phospho-Ser-757 ULK1 antibody. Equal amounts of cell lysates from the same experiment were analyzed separately by SDS-PAGE and immunoblotted with an anti-ULK1 antibody. J. Kasumi-1 cells were treated with OSI-027 for 90 minutes. Total cell lysates were resolved by SDS-PAGE and immunoblotted with anti-phospho-Ser-757 ULK1 antibody. Equal amounts of cell lysates from the same experiment were analyzed separately by SDS-PAGE and immunoblotted with an anti-ULK1 antibody. K. Electron microscopy was used in analysis of autophagic compartments in U937 cells treated for 24hrs with OSI-027 and/or chloroquine, as indicated. Arrows indicate autophagic compartments.
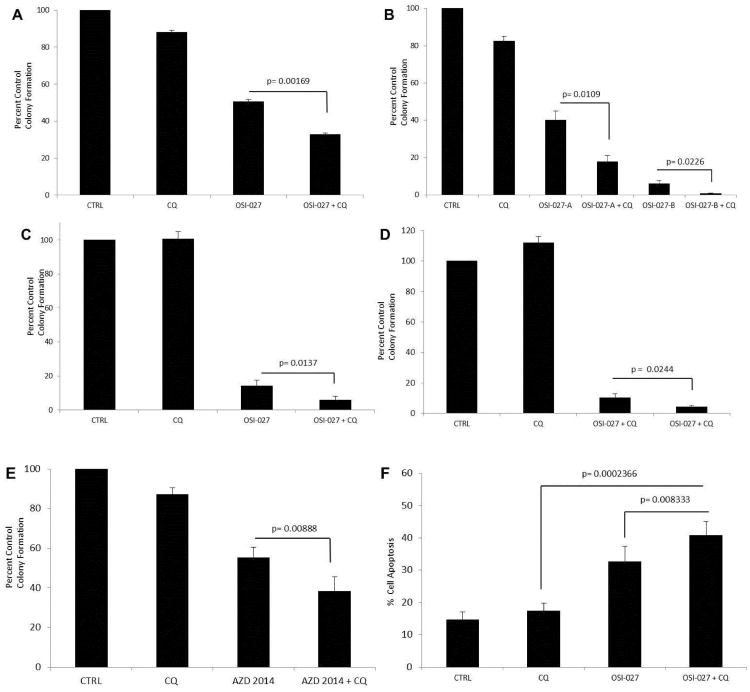
To define the functional consequences of induction of autophagy during inhibition of the mTOR pathway in AML cells, experiments were performed in which the effects of OSI-027, alone or in combination with chloroquine, on leukemic colony formation from different AML cell lines were examined. As shown in Fig. 3, concomitant treatment with chloroquine significantly enhanced the suppressive effects of OSI-027 on CFU-L colony formation from several AML lines, including the U937 (Fig. 3A), HEL (Fig. 3B), Kasumi 3 (Fig. 3C) and Kasumi 1 (Fig. 3D) leukemic cell lines. Similar suppressive effects on CFU-L colony formation were observed in AZD-2014 treated U937 cells (Fig. 3E). To determine whether the augmenting effects of chloroquine on OSI-027-dependent suppression of leukemic progenitors reflect enhanced programmed cell death, experiments were performed to examine induction of apoptosis. As shown in Fig. 3F, the addition of chloroquine promoted induction of apoptosis by OSI-027, suggesting a mechanism for the enhanced antileukemic effects.
Figure 3.
Chloroquine enhances the inhibitory effects of OSI-027 on leukemic CFU-L progenitors and promotes induction of apoptosis. A. U937 cells were plated in methylcellulose assay system with OSI-027 and/or chloroquine and CFU-L leukemic colony formation was assessed. Data are expressed as a percentage control of CFU-L for untreated cells. Means ± S.E. and the results of paired t-tests from the values of 6 independent experiments are shown. B. HEL cells were plated in methylcellulose assay system with OSI-027(A, 5 μM, B, 10 μM) and/or chloroquine and CFU-L leukemic colony formation was assessed. Data are expressed as a percentage control of CFU-L for untreated cells. Means ± S.E. and the results of paired t-tests from the values of 5 independent experiments are shown. C. Kasumi-3 cells were plated in methylcellulose assay system with OSI-027 and/or chloroquine and CFU-L leukemic colony formation was assessed. Data are expressed as a percentage control of CFU-L for untreated cells. Means ± S.E. and the results of paired t-tests from the values of 6 independent experiments are shown. D. Kasumi-1 cells were plated in methylcellulose assay system with OSI-027 and/or chloroquine and CFU-L leukemic colony formation was assessed. Data are expressed as a percentage control of CFU-L for untreated cells. Means ± S.E. and the results of paired t-tests from the values of 6 independent experiments are shown. E. U937 cells were plated in methylcellulose assay system with AZD-2014 and/or chloroquine and CFU-L leukemic colony formation was assessed. Data are expressed as a percentage control of CFU-L for untreated cells. Means ± S.E. and the results of paired t-tests from the values of 4 independent experiments are shown. F. U937 cells were treated with OSI-027 (5 μM) or chloroquine (6 μM) for 48 hours, as indicated. Apoptosis was assessed by annexin V/PI staining. Means ± SE of 7 experiments are shown and p values of paired t-tests analysis for the indicated comparisons are shown.
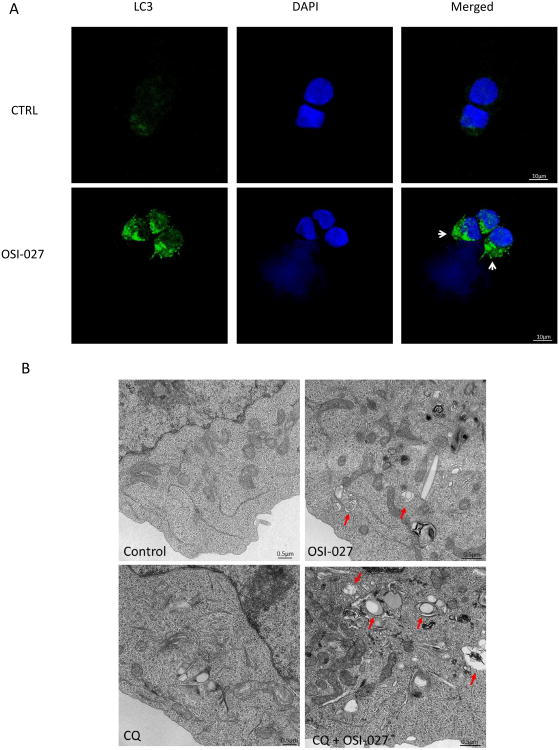
Altogether, these studies suggested that autophagy is inducible during treatment of AML cells with catalytic mTOR inhibitors and may act as a survival mechanism during targeting of the mTOR pathway. To determine whether induction of autophagy occurs in primary leukemia cells, studies were performed using primary circulating peripheral leukemic blasts from patients with AML. Induction of autophagy in response to OSI-027 treatment of the cells was observed, reflected by the increase in punctuate LC3 in primary leukemic cells (Fig. 4A). Formation of autophagic structures was also documented using electron microscopy (EM) (Fig. 4B), establishing induction of an autophagic state during treatment of AML patient-derived cells with catalytic mTOR inhibitors.
Figure 4.
Induction of autophagy by OSI-027 in primary AML leukemic blasts. A. Circulating leukemic cells from a patient with AML were treated with OSI-027 for 24 hours. After collection, cells were stained with anti-DAPI (blue) or anti-LC3 (green) and signals were detected by confocal microscopy. Merged panels indicate areas of overlapping images of the two fluorescing signals. B. Circulating leukemic cells from a patient with AML were treated for 24 hours with OSI-027 and/or chloroquine, as indicated. Electron microscopy was used for analysis of autophagic compartments. Arrows indicate autophagic compartments.
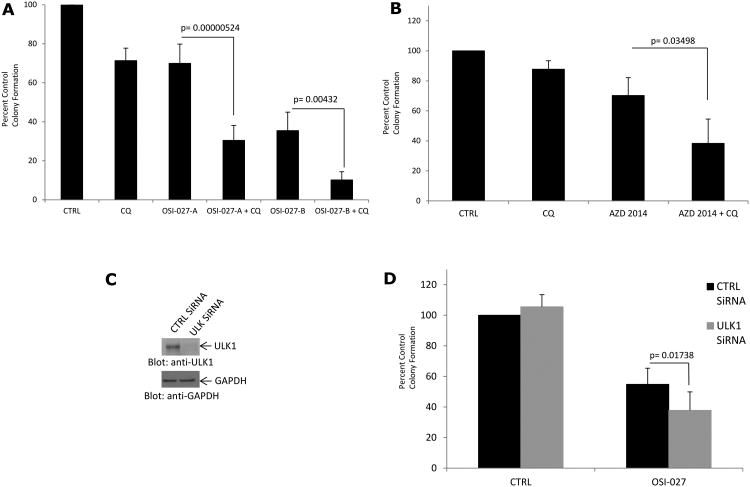
To further determine the significance of OSI-027- or AZD-2014-induced autophagy in a pathophysiologically relevant system, we performed experiments aimed to determine the role of autophagy in the inhibitory effects of OSI-027 on primitive leukemic precursors from patients with AML. As expected (24), treatment with OSI-027 (Fig. 5A) or AZD-2014 (Fig. 5B) suppressed CFU-L colony formation from primary AML cells in clonogenic assays in methylcellulose. Importantly, such effects were strongly enhanced by the combination of OSI-027 with chloroquine (Fig. 5A) or AZD-2014 with chloroquine (Fig. 5B). Notably, when ULK1 was targeted using specific siRNA (Fig. 5C)-mediated knockdown, we also found enhanced OSI-027-mediated suppression of primitive leukemic precursors from AML patients (Fig.5D). This finding underscores the significance of autophagy as a survival mechanism during treatment of primary leukemia cells with mTOR catalytic inhibitors.
Figure 5.
Inhibition of autophagy enhances the antileukemic effects of mTOR catalytic inhibitors on primitive leukemic progenitors from AML patients. A. Leukemic cells from 9 patients with AML were plated in methylcellulose culture assay system with OSI-027 (A, 1 μM, B, 5 μM) and/or chloroquine, as indicated and CFU-L colony formation was assessed. Data are expressed as a percentage control of leukemic colonies for untreated cells. Means ± S.E. and paired t-tests results of the values from 9 independent experiments are shown. B. Leukemic cells from 4 patients with AML were plated in methylcellulose culture assay system with AZD-2014 (0.1 μM) and/or chloroquine, as indicated and CFU-L colony formation was assessed. Data are expressed as a percentage control of CFU-L for untreated cells. Means ± S.E. and paired t-tests results of the values from 4 independent experiments are shown. C. U937 cells were transfected with either control siRNA or a specific siRNA against ULK1 as indicated. Total cell lysates were resolved by SDS-PAGE and immunoblotted with the indicated antibodies. D. Effects of ULK1-specific siRNA on CFU-L colony formation in the presence or absence of OSI-027 (1 μM). Data are expressed as percent control of CFU-L colony numbers for control siRNA-treated cells and represent means ± S.E. of 7 independent experiments performed with samples from different AML patients.
Discussion
mTOR is a key integrator of signals that regulate mRNA translation and metabolism and plays important roles in proliferation and survival of malignant cells (3, 4). mTORC1 and mTORC2 are attractive targets for the treatment of AML, as there is extensive evidence for dysregulation of mTOR effector pathways in AML blasts and leukemic precursors (41-43). Although selective mTORC1 inhibitors have shown some activity in AML in vitro and in vivo (19-23, 44, 45), these agents do not inhibit mTORC2 complexes, which are the complexes responsible for the activation of survival pathways downstream of AKT in malignant cells (3, 16). There is now increasing pre-clinical evidence suggesting that catalytic mTOR inhibitors may exhibit activity in AML and other myeloid malignancies (24-26, 29, 46-49). However, despite the recent emergence of catalytic mTOR inhibitors (8) and their introduction in clinical trials (50), there are potential limitations on the use of these agents as well. A key driver for the development of catalytic mTOR inhibitors was the anticipation that such compounds would be able to induce programmed cell death of neoplastic cells by inhibiting phosphorylation of AKT on serine 473 (8, 16). Indeed catalytic mTOR inhibitors have been found to induce apoptosis of some malignant cell lines and primary neoplastic cells (46, 51-53), but such effects are not always consistent. There is now evidence that in certain cases apoptosis is not seen or is minimal (46, 53), and the mechanisms of such resistance to cell death of malignant cells remain to be precisely defined. The occurrence of such resistance raises the possibility that escape mechanisms or feedback loops may be activated in malignant cells that prevent induction of programmed cell death during dual mTORC1/2 inhibition.
Autophagy is a lysosomal degradation process important for cellular homeostasis and adaptation to metabolic changes (54). Depending on the context and stimulus, autophagy can lead to divergent outcomes in the neoplastic process. It can be a caspase-independent death mechanism of malignant cells. On the contrary, it can act as an escape, and thus, protective mechanism for malignant cells during challenges by various antineoplastic agents and processes. Although the autophagic process generally impedes tumor initiation, under certain conditions it is also required for Ras-transformation (54). Autophagy has been shown to be essential for the generation of the antileukemic responses by certain agents such as arsenic trioxide and all-trans-retinoic acid (33, 34, 55), while it blocks or diminishes the antileukemic effects of other agents, such as histone deacetylase inhibitors (56). The role of autophagy in promoting or preventing cell death depending on the context, underscores the complexity of the system and the fine balance between opposing cellular regulatory responses.
In the present study, we provide evidence that during treatment of AML cells with dual mTORC1/mTORC2 inhibitors, there is induction of cellular autophagy. Our studies definitively demonstrate induction of the autophagic process in response to treatment with OSI-027 or AZD-2014 in several AML cell lines with diverse phenotypes and molecular abnormalities, indicating that it is a universal leukemic cell response to catalytic mTOR inhibitors. Consistent with our observations, another study recently provided some evidence for induction of autophagy by another catalytic mTOR inhibitor, AZD8055, although in that study, the role of autophagy in the generation of responses on primary leukemic progenitors from AML patients was not addressed (57). We also provide important information on the mechanistic process that regulates autophagy by catalytic mTOR inhibitors, as our studies demonstrate that such agents block phosphorylation of the inhibitory site serine 757 on the kinase ULK1 in AML cells, a key regulator and inducer of autophagy (3, 54). Using a combination of methodological approaches, we also demonstrate that such autophagy is inducible in primary leukemic blasts from AML patients. Importantly, our studies establish that the pharmacological or molecular inhibition of autophagy promotes the suppressive effects of OSI-027 and AZD-2014 on primitive leukemic precursors from AML patients, suggesting that the autophagic process is an important survival mechanism for leukemic AML precursors during catalytic mTOR targeting. We have previously shown that catalytic mTOR inhibitor OSI-027 which inhibits both mTORC1 and mTORC2 is a potent suppressor of leukemic progenitor colony formation (24). Such effects may reflect the ability of dual mTORC1/2 inhibitors to block mRNA translation of mitogenic genes, such as cyclin D1, in leukemic cells (24) and/or induction of apoptosis. The enhanced antileukemic effects during combined inhibition of autophagy and dual mTORC1/2 inhibition reflect induction of apoptosis and promotion of targeted cell death. It is interesting that similar targeting of autophagy appears to also promote leukemic cell apoptosis in the context of BCR-ABL leukemic transformation (46).
These findings have important implications on our overall understanding of the mechanisms of survival of leukemia cells during complete suppression of the mTOR pathway. A reasonable hypothesis is that during disruption of mTOR regulatory networks that control metabolism in leukemia initiating stem cells (LICs), ULK1-dependent induction of autophagy allows LICs to survive. This has important clinical implications and provides a rationale for the study of unique combination approaches to target early leukemic precursors. It should be also noted that catalytic mTOR inhibitors can enhance the activities of inhibitors of other cellular pathways in AML, such as inhibitors of Aurora kinase-dependent pathways (58) and chemotherapeutic agents (24); and have been shown to disrupt leukemia/stroma interactions (49). Thus, it is possible that autophagy modulators may also be useful as components of multi-agent regimens for the treatment of AML, such as combinations including dual mTORC1/2 inhibitors, chemotherapy drugs and/or other targeted therapies. Future studies should investigate the potential of such approaches, as they may provide a way to overcome resistance of primitive AML progenitors and LICs.
Statement of Translational Relevance.
Targeting the autophagic machinery may provide an approach to overcome relative resistance of acute myeloid leukemia (AML) precursors to dual mTORC1/mTORC2 inhibitors. We demonstrate that catalytic mTOR inhibitors, such OSI-027 and AZD2014, are potent inducers of autophagy in AML cells, resulting in regulatory effects on their inhibitory activities on primitive AML leukemic precursors. Concomitant inhibition of the autophagic process by pharmacological means or by molecular targeting of elements of the autophagic machinery enhances induction of antileukemic responses. Taken together, our findings suggest that combinations of mTOR targeting agents with pharmacological agents that inhibit autophagy may provide an approach to enhance antileukemic responses in AML.
Acknowledgments
This work was supported in part by National Institutes of Health grants CA121192, CA77816 and CA155566; grant I01CX000916 from the Department of Veterans Affairs; and by the HCL research foundation. EB was supported by NIH training grant T32CA070085.
Footnotes
Conflict of interest disclosure: None
References
- 1.Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell. 2012;149:274–293. doi: 10.1016/j.cell.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zoncu R, Efeyan A, Sabatini DM. mTOR: from growth signal integration to cancer, diabetes and ageing. Nat Rev Mol Cell Biol. 2011;12:21–35. doi: 10.1038/nrm3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beauchamp EM, Platanias LC. The evolution of the TOR pathway and its role in cancer. Oncogene. 2013;32:3923–32. doi: 10.1038/onc.2012.567. [DOI] [PubMed] [Google Scholar]
- 4.Dancey J. mTOR signaling and drug development in cancer. Nat Rev Clin Oncol. 2010;7:209–19. doi: 10.1038/nrclinonc.2010.21. [DOI] [PubMed] [Google Scholar]
- 5.Khokhar NZ, Altman JK, Platanias LC. Emerging roles for mammalian target of rapamycin inhibitors in the treatment of solid tumors and hematological malignancies. Curr Opin Oncol. 2011;23:578–86. doi: 10.1097/CCO.0b013e32834b892d. [DOI] [PubMed] [Google Scholar]
- 6.Johnson SC, Rabinovitch PS, Kaeberlein M. mTOR is a key modulator of ageing and age-related disease. Nature. 2013;493:338–45. doi: 10.1038/nature11861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lamming DW, Ye L, Sabatini DM, Baur JA. Rapalogs and mTOR inhibitors as anti-aging therapeutics. J Clin Invest. 2013;123:980–9. doi: 10.1172/JCI64099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Benjamin D, Colombi M, Moroni C, Hall MN. Rapamycin passes the torch: a new generation of mTOR inhibitors. Nat Rev Drug Discov. 2011;10:868–80. doi: 10.1038/nrd3531. [DOI] [PubMed] [Google Scholar]
- 9.Nelson V, Altman JK, Platanias LC. Next generation of mammalian target of rapamycin inhibitors for the treatment of cancer. Expert Opin Investig Drugs. 2013;22:715–22. doi: 10.1517/13543784.2013.787066. [DOI] [PubMed] [Google Scholar]
- 10.Döhner H, Estey EH, Amadori S, Appelbaum FR, Büchner T, Burneett AK, et al. Diagnosis and management of acute myeloid leukemia in adults: recommendations from an international expert panel, on behalf of the European LeukemiaNet. Blood. 2010;115:453–74. doi: 10.1182/blood-2009-07-235358. [DOI] [PubMed] [Google Scholar]
- 11.Walter RB, Appelbaum FR, Tallman MS, Weiss NS, Larson RA, Estey EH. Shortcomings in the clinical evaluation of new drugs: acute myeloid leukemia as paradigm. Blood. 2010;116:2420–8. doi: 10.1182/blood-2010-05-285387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Estey EH. Acute myeloid leukemia: 2013 update on risk-stratification and management. Am J Hematol. 2013;88:318–27. doi: 10.1002/ajh.23404. [DOI] [PubMed] [Google Scholar]
- 13.Altman JK, Platanias LC. Acute myeloid leukemia: potential for new therapeutic approaches targeting mRNA translation pathways. Int J Hematol Oncol. 2013;2:243–50. doi: 10.2217/ijh.13.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Min YH, Eom JI, Cheong JW, Maeng HO, Kim JY, Jeung HK, et al. Constitutive phosphorylation of Akt/PKB protein in acute myeloid leukemia: its significance as a prognostic variable. Leukemia. 2003;17:995–7. doi: 10.1038/sj.leu.2402874. [DOI] [PubMed] [Google Scholar]
- 15.Récher C, Dos Santos C, Demur C, Payrastre B. mTOR, a new therapeutic target in acute myeloid leukemia. Cell Cycle. 2005;4:1540–9. doi: 10.4161/cc.4.11.2159. [DOI] [PubMed] [Google Scholar]
- 16.Altman JK, Sassano A, Platanias LC. Targeting mTOR for the treatment of AML. New agents and new directions. Oncotarget. 2011;2:510–7. doi: 10.18632/oncotarget.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen W, Drakos E, Grammatikakis I, Schlette EJ, Li J, Leventaki V, et al. mTOR signaling is activated by FLT3 kinase and promotes survival of FLT3-mutated acute myeloid leukemia cells. Mol Cancer. 2010;9:292. doi: 10.1186/1476-4598-9-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Récher C, Beyne-Rauzy O, Demur C, Chicanne G, Dos Santos C, Mas VM, et al. Antileukemic activity of rapamycin in acute myeloid leukemia. Blood. 2005;105:2527–34. doi: 10.1182/blood-2004-06-2494. [DOI] [PubMed] [Google Scholar]
- 19.Calabro A, Tai J, Allen SL, Budman DR. In-vitro synergism of m-TOR inhibitors, statins, and classical chemotherapy: potential implications in acute leukemia. Anticancer Drugs. 2008;19:705–12. doi: 10.1097/CAD.0b013e328304ae19. [DOI] [PubMed] [Google Scholar]
- 20.Altman JK, Yoon P, Katsoulidis E, Kroczynska B, Sassano A, Redig AJ, et al. Regulatory effects of mammalian target of rapamycin-mediated signals in the generation of arsenic trioxide responses. J Biol Chem. 2008;283:1992–2001. doi: 10.1074/jbc.M705227200. [DOI] [PubMed] [Google Scholar]
- 21.Nishioka C, Ikezoe T, Yang J, Gery S, Koeffler HP, Yokoyama A. Inhibition of mammalian target of rapamycin signaling potentiates the effects of all-trans retinoic acid to induce growth arrest and differentiation of human acute myelogenous leukemia cells. Int J Cancer. 2009;125:1710–20. doi: 10.1002/ijc.24472. [DOI] [PubMed] [Google Scholar]
- 22.Perl AE, Kasner MT, Tsai DE, Vogl DT, Loren AW, Schuster SJ, et al. A phase I study of the mammalian target of rapamycin inhibitor sirolimus and MEC chemotherapyin relapsed and refractory acute myelogenous leukemia. Clin Cancer Res. 2009;15:6732–9. doi: 10.1158/1078-0432.CCR-09-0842. [DOI] [PubMed] [Google Scholar]
- 23.Amadori S, Stasi R, Martelli AM, Venditti A, Meloni G, Pane F, et al. Temsirolimus, an mTOR inhibitor, in combination with lower-dose clofarabine as salvage therapy for older patients with acute myeloid leukaemia: results of a phase II GIMEMA study (AML-1107) Br J Haematol. 2012;156:205–12. doi: 10.1111/j.1365-2141.2011.08940.x. [DOI] [PubMed] [Google Scholar]
- 24.Altman JK, Sassano A, Kaur S, Glaser H, Kroczynska B, Redig AJ, et al. Dual mTORC2/mTORC1 targeting results in potent suppressive effects on acute myeloid leukemia (AML) progenitors. Clin Cancer Res. 2011;17:4378–88. doi: 10.1158/1078-0432.CCR-10-2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Willems L, Chapuis N, Puissant A, Maciel TT, Green AS, Jacque N, et al. The dual mTORC1 and mTORC2 inhibitor AZD8055 has anti-tumor activity in acute myeloid leukemia. Leukemia. 2012;26:1195–1202. doi: 10.1038/leu.2011.339. [DOI] [PubMed] [Google Scholar]
- 26.Zeng Z, Shi YX, Tsao T, Qiu Y, Kornblau SM, Baggerly KA, et al. Targeting of mTORC1/2 by the mTOR kinase inhibitor PP242 induces apoptosis in AML cells under conditions mimicking the bone marrow microenvironment. Blood. 2012;120:2679–89. doi: 10.1182/blood-2011-11-393934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaur S, Sassano A, Dolniak B, Joshi S, Majchrzak-Kita B, Baker DP, et al. Role of the Akt pathway in mRNA translation of interferon-stimulated genes. Proc Natl Acad Sci USA. 2008;105:4808–13. doi: 10.1073/pnas.0710907105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kaur S, Sassano A, Majchrzak-Kita B, Baker DP, Su B, Fish EN, et al. Regulatory effects of mTORC2 complexes in type I IFN signaling and in the generation of IFN responses. Proc Natl Acad Sci USA. 2012;109:7723–8. doi: 10.1073/pnas.1118122109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carayol N, Katsoulidis E, Sassano A, Altman JK, Druker BJ, Platanias LC. Suppression of programmed cell death 4 (PDCD4) protein expression by BCR-ABL-regulated engagement of the mTOR/p70 S6 kinase pathway. J Biol Chem. 2008;283:8601–10. doi: 10.1074/jbc.M707934200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Giafis N, Katsoulidis E, Sassano A, Tallman MS, Higgins LS, Nebreda AR, et al. Role of the p38 mitogen-activated protein kinase pathway in the generation of arsenic trioxide-dependent cellular responses. Cancer Res. 2006;66:6763–71. doi: 10.1158/0008-5472.CAN-05-3699. [DOI] [PubMed] [Google Scholar]
- 31.Vakana E, Altman JK, Glaser H, Donato NJ, Platanias LC. Antileukemic effects of AMPK activators on BCR-ABL-expressing cells. Blood. 2011;118:6399–6402. doi: 10.1182/blood-2011-01-332783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Altman JK, Szilard A, Konicek BW, Iversen PW, Kroczynska B, Glaser H, et al. Inhibition of Mnk kinase activity by cercosporamide and suppressive effects on acute myeloid leukemia precursors. Blood. 2013;121:3675–81. doi: 10.1182/blood-2013-01-477216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goussetis DJ, Gounaris E, Wu EJ, Vakana E, Sharma B, Bogyo M, et al. Autophagic degradation of the BCR-ABL oncoprotein and generation of antileukemic responses by arsenic trioxide. Blood. 2012;120:3555–62. doi: 10.1182/blood-2012-01-402578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goussetis DJ, Altman JK, Glaser H, McNeer JL, Tallman MS, Platanias LC. Autophagy is a critical mechanism for the induction of the antileukemic effects of arsenic trioxide. J Biol Chem. 2010;285:29989–97. doi: 10.1074/jbc.M109.090530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ylä-Anttila P, Vihinen H, Jokitalo E, Eskelinen EL. Monitoring autophagy by electron microscopy in Mammalian cells. Methods Enzymol. 2009;452:143–64. doi: 10.1016/S0076-6879(08)03610-0. [DOI] [PubMed] [Google Scholar]
- 36.Tan AS, Berridge MV. Superoxide produced by activated neutrophils efficiently reduces the tetrazolium salt, WST-1 to produce a soluble formazan: a simple colorimetric assay for measuring respiratory burst activation and for screening anti-inflammatory agents. J Immunol Methods. 2000;238:59–68. doi: 10.1016/s0022-1759(00)00156-3. [DOI] [PubMed] [Google Scholar]
- 37.Mizushima N, Yoshimori T, Levine B. Methods in mammalian autophagy research. Cell. 2010;140:313–26. doi: 10.1016/j.cell.2010.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Amaravadi RK, Yu D, Lum JJ, Bui T, Christophorou MA, Evan GI, et al. Autophagy inhibition enhances therapy-induced apoptosis in a Myc-induced model of lymphoma. J Clin Invest. 2007;117:326–36. doi: 10.1172/JCI28833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pike KG, Malagu K, Hummersone MG, Menear KA, Duggan HM, Gomez S, et al. Optimization of potent and selective dual mTORC1 and mTORC2 inhibitors: the discovery of AZD8055 and AZD2014. Bioorg Med Chem Lett. 2013;23:1212–6. doi: 10.1016/j.bmcl.2013.01.019. [DOI] [PubMed] [Google Scholar]
- 40.Kim J, Kundu M, Viollet B, Guan KL. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat Cell Biol. 2013;13:132–41. doi: 10.1038/ncb2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xu Q, Simpson S, Scialla TJ, Bagg A, Carroll M. Survival of acute myeloid leukemia required PI3 kinase activation. Blood. 2003;102:972–80. doi: 10.1182/blood-2002-11-3429. [DOI] [PubMed] [Google Scholar]
- 42.Grandage VL, Gale RE, Linch DC, Khwaja A. PI3-kinase/Akt is constitutively active in primary acute myeloid leukaemia cells and regulates survival and chemoresistance via NF-κB, Mapkinase and p53 pathways. Leukemia. 2005;19:586–94. doi: 10.1038/sj.leu.2403653. [DOI] [PubMed] [Google Scholar]
- 43.Altman JK, Platanias LC. Exploiting the mammalian target of rapamycin pathway in hematologic malignancies. Curr Opin Hematol. 2008;15:88–94. doi: 10.1097/MOH.0b013e3282f3deaa. [DOI] [PubMed] [Google Scholar]
- 44.Xu Q, Thompson JE, Carroll M. mTOR regulates cell survival after etoposide treatment in primary AML cells. Blood. 2005;106:4261–8. doi: 10.1182/blood-2004-11-4468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Park S, Chapuis N, Saint Marcoux F, Recher C, Prebet T, Chevallier P, et al. A phase Ib GOELAMS study of the mTOR inhibitor RAD001 in association with chemotherapy for AML patients in first relapse. Leukemia. 2013;27:1479–86. doi: 10.1038/leu.2013.17. [DOI] [PubMed] [Google Scholar]
- 46.Carayol N, Vakana E, Sassano A, Kaur S, Goussetis DJ, Glaser H, et al. Critical roles for mTORC2- and rapamycin-insensitive mTORC1-complexes in growth and survival of BCR-ABL-expressing leukemic cells. Proc Natl Acad Sci USA. 2010;107:12469–74. doi: 10.1073/pnas.1005114107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Meng H, Jin Y, Liu H, You L, Yang C, Yang X, et al. SNS-032 inhibits mTORC1/ mTORC2 activity in acute myeloid leukemia cells and has synergistic activity with perifosine against Akt. J Hematol Oncol. 2013;6:18. doi: 10.1186/1756-8722-6-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bogani C, Bartalucci N, Martinelli S, Tozzi L, Guglielmelli P, Bosi A, et al. mTOR inhibitors alone and in combination with JAK2 inhibitors effectively inhibit cells of myeloproliferative neoplasms. PLoS One. 2013;8:e54826. doi: 10.1371/journal.pone.0054826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zeng Z, Shi YX, Tsao T, Qiu Y, Kornblau SM, Baggerly KA, et al. Targeting of mTORC1/2 by the mTOR kinase inhibitor PP242 induces apoptosis in AML cells under conditions mimicking the bone marrow microenvironment. Blood. 2012;120:2679–89. doi: 10.1182/blood-2011-11-393934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nelson V, Altman JK, Platanias LC. Next generation of mammalian target of rapamycin inhibitors for the treatment of cancer. Expert Opin Investig Drugs. 2013;22:715–22. doi: 10.1517/13543784.2013.787066. [DOI] [PubMed] [Google Scholar]
- 51.Gupta M, Hendrickson AE, Yun SS, Han JJ, Schneider PA, Koh BD, et al. Dual mTORC1/mTORC2 inhibition diminishes Akt activation and induces Puma-dependent apoptosis in lymphoid malignancies. Blood. 2012;119:476–87. doi: 10.1182/blood-2011-04-346601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Maiso P, Liu Y, Morgan B, Azab AK, Ren P, Martin MB, et al. Defining the role of TORC1/2 in multiple myeloma. Blood. 2011;118:6860–70. doi: 10.1182/blood-2011-03-342394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kampa-Schittenhelm KM, Heinrich MC, Akmut F, Rasp KH, Illing B, Döhner H, et al. Cell cycle-dependent activity of the novel dual PI3K-MTORC1/2 inhibitor NVP-BGT226 in acute leukemia. Mol Cancer. 2013;12:46. doi: 10.1186/1476-4598-12-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rubinsztein DC, Codogno P, Levine B. Autophagy modulation as a potential therapeutic target for diverse diseases. Nat Rev Drug Discov. 2012;11:709–30. doi: 10.1038/nrd3802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Isakson P, Bjørås M, Bøe SO, Simonsen A. Autophagy contributes to therapy-induced degradation of the PML/RARA oncoprotein. Blood. 2010;116:2324–31. doi: 10.1182/blood-2010-01-261040. [DOI] [PubMed] [Google Scholar]
- 56.Torgersen ML, Engedal N, Bøe SO, Hokland P, Simonsen A. Targeting autophagy potentiates the apoptotic effect of histone deacetylase inhibitors in t(8;21) AML cells. Blood. 2013;122:2467–76. doi: 10.1182/blood-2013-05-500629. [DOI] [PubMed] [Google Scholar]
- 57.Willems L, Chapuis N, Puissant A, Maciel TT, Green AS, Jacque N, et al. The dual mTORC1 and mTORC2 inhibitor AZD8055 has anti-tumor activity in acute myeloid leukemia. Leukemia. 2012;26:1195–202. doi: 10.1038/leu.2011.339. [DOI] [PubMed] [Google Scholar]
- 58.Liu LL, Long ZJ, Wang LX, Zheng FM, Fang ZG, Yan M, et al. Inhibition of mTOR pathway sensitizes acute myeloid leukemia cells to Aurora inhibitors by suppression of glycolytic metabolism. Mol Cancer Res. 2013;11:1326–36. doi: 10.1158/1541-7786.MCR-13-0172. [DOI] [PubMed] [Google Scholar]