Abstract
Introduction
It has recently been proposed that B lymphocytes are involved in sepsis pathogenesis. The goal of this study is to investigate potential abnormalities in a subset distribution and activation of circulating B lymphocytes in patients with septic shock.
Methods
This observational prospective study was conducted in a medical-surgical ICU. All patients with septic shock were eligible for inclusion. B-cell phenotypes (CD19+CD69+, CD19+CD23+, CD19+CD5+, CD19+CD80, CD19+CD86+, CD19+CD40 and CD19+CD95+) were assessed by quantitative flow cytometry upon admission to the ICU and 3, 7, 14 and 28 d later.
Results
Fifty-two patients were included. Thirty-six healthy volunteers matched for age and sex were used as controls. The patients had lymphopenia that was maintained during 28 d of follow-up. In patients with septic shock who died, the percentage of CD19+CD23+ was lower during the 7 d of follow-up than it was in survival patients. Moreover, the percentage of CD80+ and CD95+ expression on B cells was higher in patients who died than in survivors. Receiver operating characteristic curve analysis showed that a CD19+CD23+ value of 64.6% at ICU admission enabled discrimination between survivors and nonsurvivors with a sensitivity of 90.9% and a specificity of 80.0% (P = 0.0001).
Conclusions
Patients with septic shock who survive and those who don't have different patterns of abnormalities in circulating B lymphocytes. At ICU admission, a low percentage of CD23+ and a high of CD80+ and CD95+ on B cells were associated with increased mortality of patients with septic shock. Moreover, a drop in circulating B cells persisted during 28 d of ICU follow-up.
Keywords: Apoptosis, B cells, Flow cytometry, Lymphocyte, Lymphocyte activation, Phenotype, Sepsis, Septic shock
Introduction
Several mechanisms of the innate and adaptive immune responses are involved in the pathogenesis of sepsis [1,2]. The bacteria and/or bacterial components released during infection may interact with, and induce abnormal activation of, different cell types of the immune system. The involvement of monocytes and phagocytic cells in the induction of inflammatory derangement of sepsis has been clearly established [3]. Our group and other authors have described that T lymphocytes and natural killer cells show several abnormalities in patients with septic shock [4-6].
Blymphocytes are a heterogeneous cell population with different functional and phenotypical properties [7-9]. The majority of B cells are classified as conventional B2 cells, including follicular B cells, which are characterized by high CD23 and low CD21 expression, and marginal B cells that express high amounts of CD21 [10,11]. The minority B-1 B-lymphocyte population is classified into B-1a and B-1b subsets based on cell-surface CD5 expression. B-1a cells have an exclusive fetal origin and are characterized by CD5 expression (CD5+) and CD23-/low, produce natural antibodies, IL-10 and inhibition of T cells. B-1b cells can be of adult origin, do not express CD5 (and CD23-/low) and respond to particulate antigens and polysaccharide [12].
B cells play a pivotal role in both adaptive and innate immune response [13]. During the immune response against infectious agents, B lymphocytes play a relevant role by different mechanisms, including the production of antibodies and the presentation of microorganism antigens to T lymphocytes [14]. Furthermore, the interaction of several bacterial products with B cells may cause their activation and cytokine secretory function [15-17].
The role of B lymphocytes in the pathogenesis of sepsis has not been established. It has been reported that patients who have recovered from an episode of invasive pneumococcal disease show defective B-cell activation [18]. It has been demonstrated that innate response activator B (IRA-B) cells play a critical role in the response to sepsis [19,20]. It has been proposed that B cells might contribute to the immunosuppressive shift observed during sepsis [21].
In this study, we investigated the presence of abnormalities in the B-cell compartments of patients with septic shock and analyzed its relevance to the evolution of sepsis and the prognosis of these patients. In this study, we investigated the number and distribution of B cells, as well as their expression of activation/redistribution (CD69, CD23 and CD5), costimulation (CD80, CD86 and CD40) and programmed cell death regulation (CD95) antigens in 52 patients with septic shock at admission to the ICU at our institution and at days 3, 7, 14 and 28 of follow-up. Sex- and age-matched healthy donors were studied in parallel as experimental normal controls.
Materials and methods
Patient eligibility
The study was performed at the Principe de Asturias University Hospital over a period of 36 months. The study was conducted according to the guidelines of the 1975 Declaration of Helsinki. Approval was obtained from the Hospital Universitario Príncipe de Astúrias Institutional Ethics Committee. Written informed consent was obtained from each participant included in the study or from his or her relatives. The individuals enrolled were patients diagnosed with septic shock who had clinical evidence of infection, defined as the presence of a known source of infection, and who had been started on parenteral antimicrobial therapy. Septic shock was defined as sepsis-induced hypotension despite adequate fluid resuscitation along with the presence of perfusion abnormalities that could include, but were not limited to, lactic acidosis, oliguria or an acute alteration in mental status. Patients receiving inotropic or vasopressor agents for arterial hypotension were considered to be in septic shock [22]. All the patients received conventional intensive care, and included patients were treated by physicians who were not involved in this study. Patients treated with hydrocortisone for refractory hypotension were withdrawn from the study. No patient was treated with activated protein C.
The exclusion criteria were (1) subjects with immunodeficiency or who were being treated with any form of immunomodulation therapy, including low-dose corticosteroids for septic shock; (2) autoimmune or hypersensitivity diseases; (3) disseminated malignancy; and (4) participation in another research study.
Thirty-six age- and sex-matched healthy blood donors were studied in parallel with the patients. They were studied to control for the adequacy of the cytometric techniques and cellular culture procedures, as well as for characterization of the normal range of the B-lymphocyte compartment parameters analyzed.
Blood samples
Upon patients' admission to the ICU and after their informed consent had been obtained, blood samples were collected into sterilized, silicone-coated glass tubes. Blood samples were also obtained from each included patient at days 3, 7, 14 and 28 of follow-up. In patients who did not survive, the samples included in the analysis were those obtained prior to the fatal outcome. Blood samples were prepared within 1 h of sample collection for flow cytometry.
Cell separation
Peripheral blood mononuclear cells (PBMCs) were purified from blood by Ficoll-Hypaque (Lymphoprep Axis-Shield; PoC AS, Oslo, Norway) density gradient centrifugation [23]. Cells were resuspended (106 cells/ml) in RPMI 1640 medium (BioWhittaker, Basel, Switzerland) supplemented with 10% heat-inactivated fetal bovine serum (Gibco/Invitrogen, Carlsbad, CA, USA), 25 mM 2-[4-(2-hydroxyethyl)piperazin-1-yl]ethanesulfonic acid (HEPES) and 1% penicillin-streptomycin (both from BioWhittaker).
Flow cytometry analysis
B cells were phenotypically analyzed in PBMCs by four-color flow cytometry in a FACSCalibur flow cytometer using CellQuest 3.3 software (BD Biosciences, San Jose, CA, USA). PBMCs were incubated with combinations of fluorescein isothiocyanate (FITC)-, phycoerythrin (PE)- and phycoerythrin-cyanine 5 (TC, Tricolor)-labeled monoclonal antibodies (mAb). The mAb were CD3-PerCP, CD3-FITC, CD56-PE, CD95-FITC, CD69-FITC, CD80-PE, CD23-PE (BD Biosciences), CD5-FITC, CD19-TC (Caltag Laboratories, San Francisco, CA, USA) and CD86-FITC, CD40-PE (AbD Serotec, Kidlington, UK). For an adequate experimental staining control, the appropriate irrelevant anti-mouse isotype controls IgG1-FITC, PE, TC or IgG2a FITC, PE and TC (Caltag Laboratories) were used.
Assessment of absolute number of lymphocytes
The absolute numbers of B-lymphocyte subsets were calculated according to standard flow cytometry criteria for lymphocyte subset identification. First, we calculated the percentage of cells expressing CD19 in the total lymphocytes gate defined by forward and side scatter in PBMCs. The absolute number of circulatory B lymphocytes was calculated by determining the percentage of CD19+ cells in peripheral blood lymphocytes multiplied by the total number of lymphocytes per microliter measured using a Coulter LH instrument (Beckman Coulter, Fullerton, CA, USA). Next, we obtained the absolute number of CD19+ (CD23+, CD69+, CD5+, CD80+, CD86+, CD40+ or CD95+) by multiplying the total number of B lymphocytes previously calculated by the percentage of positive cells for each one of these antigens in CD19+ B cells. All absolute numbers are expressed as cells per milliliter.
Statistical analyses
All statistical tests were performed using SPSS for Windows version 15.0 software (SPSS, Chicago, IL, USA). Data are expressed as means ± SEM. Because most variables did not always fulfill the normality hypothesis, differences between groups were analyzed using the Mann-Whitney U-test for nonparametric data and analysis of variance followed by a Wilcoxon signed-rank test were used for within-group analyses. The reliability of the use of different phenotype markers concentrations or of main clinical variables to predict death due to septic shock was calculated by plotting receiver operating characteristic (ROC) curves. The level of significance was set at P < 0.05.
Results
Characteristics of patients with septic shock
During the 36-month study period, a total of 92 patients with septic shock treated at our institution were identified (Figure 1). All patients were treated with vasopressors. Patients treated with hydrocortisone for refractory hypotension were withdrawn from the study. Forty of these patients were excluded for the following reasons: 3 patients had AIDS, 18 patients were on glucocorticoid therapy, 12 patients were on chemotherapy, 5 patients had metastatic cancer, 1 patient had rheumatoid arthritis and 1 patient was excluded due to anaphylactic shock. The mean age of the healthy controls was 62.0 ± 3.4 yr vs 61.2 ± 3.2 yr in patients with septic shock (P > 0.05), and the gender distribution was similar: 24 males (66.6%) and 13 females in the healthy control group vs 36 males (69.2%) and 16 females in the group of patients with septic shock (P > 0.05).
Figure 1.
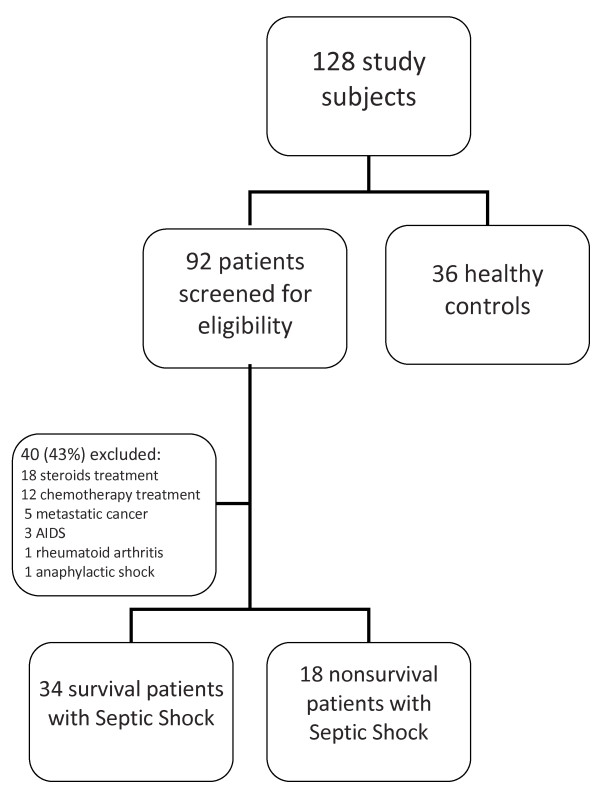
Screening and enrollment.
Table 1 provides demographic data for the 52 patients ultimately included in the study. Twenty-two patients (42.3%) had intraabdominal infections, 16 (30.7%) had pneumonia, 4 (7.4%) had bacteremia of unknown origin, 4 (7.4%) had pyelonephritis, 3 (5.7%) had soft-tissue and skin infections, 2 (3.8%) had surgical site infections and 1 (1.9%) had mediastinitis. The diagnosis of infection was made on clinical grounds in 24 patients (46.1%), and positive cultures were obtained from normally sterile sites in 28 patients (53.9%). Gram-positive cocci were isolated in 11 (21.1%) of these patients, Gram-negative organisms were isolated in 10 patients (19.2%) and polymicrobial flora were isolated in the remaining 7 patients (13.4%). The percentage of the 52 septic shock patients who had positive blood culture was 30.2%. No patient had fungal sepsis or septic shock due to viruses. Bivariate analysis failed to demonstrate a correlation between the sources of infection, the pathogens isolated and mortality. There were no differences in the lymphocyte profiles of patients who had Gram-positive bacterial sepsis versus Gram-negative bacterial sepsis.
Table 1.
Demographic data of the study patients with septic shock.
| Parameters | Survivors | Nonsurvivors | P |
| Number of patients (%) | 34 (65.4%) | 18 (34.6%) | |
| Mean age (yr) | 61.0 ± 3.2 | 64.5 ± 4.1 | 0.57 |
| Men/women (n) | 21/13 | 15/3 | 0.11 |
| Medical/surgical patients | 13/21 | 11/7 | 0.12 |
| Mean APACHE II score | 21.6 ± 1.3 | 29.2 ± 1.5 | 0.001 |
| Mean MODS score | 6.6 ± 0.5 | 9.5 ± 0.9 | 0.014 |
| Mean Δ-MODS | 2.2 ± 0.3 | 3.5 ± 0.7 | 0.169 |
| Mean SOFA score | 7.9 ± 0.6 | 10.6 ± 0.8 | 0.021 |
| Mean Δ-SOFA | 1.7 ± 0.4 | 2.9 ± 0.9 | 0.34 |
Categorical variables are expressed as number of patients, and continuous variables are expressed as means ± SEM. APACHE II, Acute Physiology and Chronic Health Evaluation II [39]; SOFA, Sepsis-Related Organ Failure Assessment [40]; MODS, Multiple Organ Dysfunction Score [41]; Δ-MODS is the difference between the maximum and initial MODS scores [42], and Δ-SOFA is the difference between the maximum and initial SOFA scores [43].
Severe lymphopenia, including B-lymphocyte population, in septic shock
Upon ICU admission, total blood lymphocyte cell count was significantly diminished in patients with septic shock compared with healthy controls (1,144 ± 67 cells/μl vs 2,095 ± 67 cells/μl, respectively; P < 0.05). This lymphopenia was maintained independently of survival outcome during 28 d of follow-up in patients with septic shock.
The absolute number of CD19+ B cells was also significantly lower in patients with septic shock than in normal donors upon ICU admission (208 ± 45 cells/μl vs 238 ± 13 cells/μl, respectively; P < 0.05). The numbers of these circulating lymphocyte populations remained significantly decreased during the 28 days of follow-up (183 ± 45 cells/μl at day 3 and 116 ± 38 cells/μl at day 7, 175 ± 54 cells/μl at day 14 and 120 ± 32 cells/μl at day 28; P < 0.05). At the times analyzed (at ICU admission and at days 3 and 7 of follow-up), because no additional patient died between days 7 and 28, there were no differences between survivors and nonsurvivors regarding the numbers of the circulating CD19+ B lymphocytes.
Redistribution of B-lymphocyte subsets in septic shock patients
Next, we investigated the subset distribution and activation of circulating CD19+ B lymphocytes by means of analysis of their expression of CD23+, CD69+ and CD5+ antigens in patients with septic shock and in healthy controls. CD23 is expressed mainly by activated regulatory B cells. CD69 is also expressed by early activated B cells. CD5+CD19+ represents the B1a subset of B cells.
We observed that the circulating numbers of the CD19+CD23+ B-lymphocyte subsets were significantly reduced in septic shock patients at ICU admission, independently of their survival outcome. However, the percentage of the CD19+CD23+ B lymphocyte subset was significantly increased in septic shock survivors compared to nonsurvivors at ICU admission and during the 7 days of follow-up. Interestingly, we observed that the significantly increased percentage of the CD19+CD23+ B-lymphocyte subset found in survivors at ICU admission normalized after 28 days of follow-up (Figure 2A). A prediction ROC curve was then used to estimate the value of the percentage of CD19+CD23+ B cells to predict death in patients with septic shock at admission. As shown in Figure 3, for the percentage of CD19+CD23+ B cells, a cut-off value of 64% showed sensitivity of 90.9% (95% confidence interval (CI) = 75.6% to 98.0%) and specificity of 80.0% (95% CI = 61.4% to 92.0%) for predicting the risk of death, with a positive predictive value of 89.4% and a negative predictive value of 82.7%. The area under the ROC curve was 0.818 ± 0.055 (95% CI = 0.701 to 0.904; P = 0.0001). Next, we selected APACHE II score for the comparative analysis of the outcome because it was the best prognostic clinical score in our series. When we analyzed the ROC curve of the Acute Physiology and Chronic Health Evaluation II (APACHE II) score for predicting death in patients with septic shock at admission, we found the area under the ROC curve to be 0.721 ± 0.83 (95% CI = 0.559 to 0.883) and a cut-off value of 23 showed sensitivity of 76.9% and specificity of 60.7% (P = 0.024).
Figure 2.
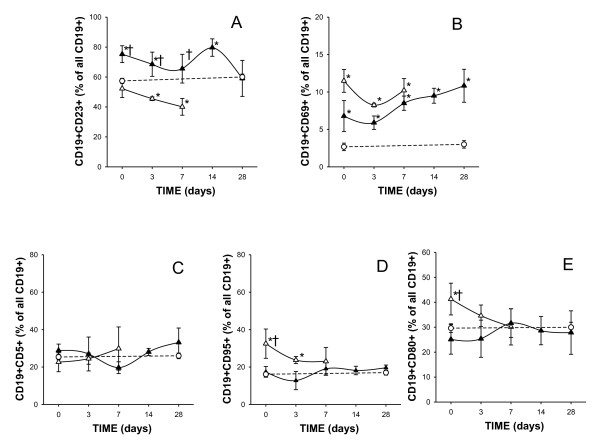
Time course of the percentages of CD19+ lymphocytes that express CD23+ (A), CD69+ (B), CD5+ (C), CD95+ (D) and CD80+ (E) antigens in patients with septic shock during their stay in the ICU. Data presented are for survivors (black triangles) and nonsurvivors (white triangles). The dotted line represents the mean value recorded in the healthy controls (n = 36). At ICU admission, study patients were 34 survivors and 18 nonsurvivors; at day 3, 34 survivors and 13 nonsurvivors; at day 7, 34 survivors and 11 nonsurvivors; at day 14 and at day 28, 34 survival patients were studied. All values are expressed as means ± SEM. *P < 0.05 for survivors or nonsurvivors vs healthy controls; †P < 0.05 for survivors vs nonsurvivors.
Figure 3.
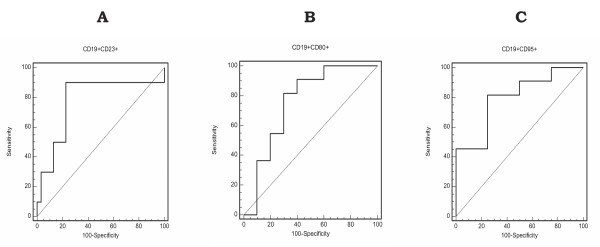
Receiver operating characteristic (ROC) curve analysis of percentages of CD19+CD23+, CD19+CD80+ and CD19+CD95+ for predicting the risk of death at ICU admission. For CD19+CD23+, a cut-off value of 64% showed sensitivity of 90.9% (95% confidence interval (CI) = 75.6% to 98.0%) and specificity of 80.0% (95% CI = 61.4% to 92.0%) for predicting the risk of death, with a positive predictive value of 89.4%, a negative predictive value of 82.7% and area under the ROC curve 0.818 ± 0.055 (95% CI = 0.701 to 0.904; P = 0.0001). For CD19+CD80+ and CD19+CD95+, respectively, cut-off values of 20% and 17% showed sensitivity of 81.8% (95% CI = 64.5% to 92.8%) and 81.9% (95% CI = 64.2% to 93.0%) and specificity of 70% (95% CI = 50.6% to 85.2%) and 75% (95% CI = 53.3% to 90.2%) for predicting the risk of death on day 28, with the area under the ROC curve 0.751 ± 0.061 (95% CI = 0.630 to 0.854) and 0.795 ± 0.058 (95% CI = 0.668 to 0.891) (P = 0.0001 for both).
Figure 4.
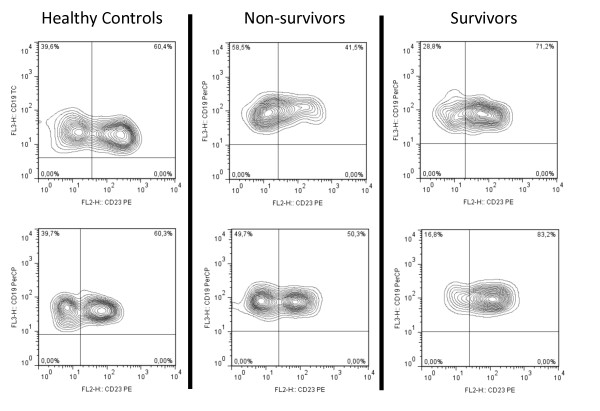
Two representative CD19+CD23+ flow cytometry samples at basal time.
In contrast to the observed diminution of the absolute number of total circulating CD19+ B lymphocytes and CD19+CD23+ B cells, we found that the CD19+CD69+ B-cell subset count was normal in both survival and nonsurvival septic shock patients at ICU admission. Furthermore, the percentage of CD19+CD69+ PBMCs was elevated in both groups of septic shock patients at ICU admission and during the 28 days of follow-up (Figure 2B).
The number of circulating CD5+CD19+ B lymphocytes was significantly reduced in patients with septic shock (at ICU admission, 46 ± 11 cells/μl; at day 3, 46 ± 21 cells/μl; at day 7, 31 ± 14 cells/μl; at day 14, 50 ± 10 cells/μl; and at day 28, 41 ± 5 cells/μl in septic shock patients and 63 ± 5 cells/μl in healthy controls). There were no differences in the percentage of circulating CD5+CD19+ cells between both groups of septic patients and healthy donors at baseline and during the follow-up (Figure 2C).
We also investigated the expression of apoptotic susceptibility CD95 antigen on the surface of B lymphocytes from patients and healthy donors. The absolute number of circulating CD19+CD95+ B cells found in septic shock patients at ICU admission was similar to that of healthy donors (Table 2). However, the percentage of CD19+CD95+ B cells was significantly higher in nonsurvivors than in survivors at ICU admission and normalized after 7 days of follow-up (Figure 2D).
Table 2.
Peripheral blood cell count of B-lymphocyte subsets and their percentage in the compartment of circulating B lymphocytes in patients with septic shock and in healthy controls at ICU admissiona
| Lymphocyte blood cell count (cells/μl) | Subset | Healthy controls | Septic shock patients |
| CD19+ | 238.5 ± 13.2 | 208.1 ± 45.4* | |
| CD19+CD23+ | 148.3 ± 11.2 | 102.5 ± 17.8* | |
| CD19+CD69+ | 5.4 ± 1.0 | 7.1 ± 2.5 | |
| CD19+CD5+ | 63.5 ± 5.2 | 44.5 ± 7.8* | |
| CD19+CD80+ | 73.6 ± 6.5 | 60.5 ± 15.8 | |
| CD19+CD86+ | 35.9 ± 3.4 | 47.6 ± 10.2 | |
| CD19+CD40+ | 223.5 ± 15.5 | 130.7 ± 21.6* | |
| CD19+CD95+ | 37.8 ± 2.7 | 30.3 ± 4.7 | |
| Percentage of CD19+ (%) | Subset | Healthy controls | Septic shock patients |
| CD19+CD23+ | 57.4 ± 1.7 | 62.7 ± 4.6 | |
| CD19+CD69+ | 2.6 ± 0.5 | 9.0 ± 2.1* | |
| CD19+CD5+ | 25.3 ± 1.5 | 24.6 ± 3.1 | |
| CD19+CD80+ | 29.6 ± 1.7 | 32.4 ± 4.4 | |
| CD19+CD86+ | 16.1 ± 1.2 | 36.7 ± 7.2* | |
| CD19+CD40+ | 94.6 ± 1.2 | 95.7 ± 1.5 | |
| CD19+CD95+ | 16.1 ± 1.1 | 25.7 ± 4.7* |
aData are expressed as mean ± SE mean. *P < 0.05 between patients with septic shock and healthy controls.
We also studied the expression of the CD80, CD86 and CD40 on B lymphocytes from patients and healthy donors. CD80, CD86 and CD40 are membrane molecules that play a critical role in the stimulation of T lymphocytes by B lymphocytes acting as antigen-presenting cells. As shown in Table 2, the absolute number of circulating CD19+ B cells expressing CD80+ or CD86+ was normal in septic shock patients at ICU admission. When we analyzed the behavior of survivors and nonsurvivors, we found that the percentage of CD19+CD80+ B cells was significantly increased in nonsurvival septic patients with respect to survivors at ICU admission and healthy controls (P < 0.05). A normalization of this percentage was observed after 3 d of follow-up (Figure 2E).
A significant reduction of circulating CD19+CD40+ B cells was found in septic shock patients at ICU admission compared to healthy donors (Table 2). There were no significant differences between the percentages of CD19+CD40+ B cells found in survivors and nonsurvivors. There were no significant differences in the number of circulating CD19+ B lymphocytes and in its cell subset distribution during the 28 d of the study (Figure 2 and data not shown).
Discussion
In this paper, we report that septic shock patients have a severe retraction of circulating B lymphocytes. This lymphopenia affects the B-cell subsets heterogeneously, with marked reduction of CD19+CD23+ and CD19+CD5+ B cells but normal numbers of CD19+CD69+ B cells. Furthermore, a different distribution of B cells subsets is found in survivor and nonsurvivor septic shock patients. The percentage of CD19+CD23+ B lymphocytes appears to be a biomarker for the prognosis of outcome of septic shock patients. These data support a role for the B-cell compartment in septic shock patients and are in agreement with those published in previous studies [24-26].
It is established that septic shock is associated with a severe exhaustion and depletion of T lymphocytes [27]. Our data support similar behavior in the B-lymphocyte compartment. We have found that the reduction of circulating B cells affects the different B-cell subsets heterogeneously in septic shock patients and that those different patterns of involvement are observed in survivor and nonsurvivors. CD23 is a low-affinity receptor for IgE located at the surface of B cells [28]. CD23 is involved in different regulatory functions, such as enhancing antigen presentation, improving cell differentiation and growth and regulating IgE synthesis [28]. Some authors have reported that CD23 is expressed on activated B cells, whereas others have suggested that peripheral blood CD23 B cells resemble classic memory cells [28]. Our data presented herein show that circulating CD19+CD23+ B lymphocytes are clearly decreased in septic shock patients because their percentage in the whole circulating B cell compartment is different in survivors and nonsurvivors. We have found that higher percentages of circulating CD19+CD23+ are associated with better clinical outcomes of patients with septic shock. Interestingly, the number of CD19+CD69+ early activated B cells in septic shock patients is similar to that found in healthy donors and is not related to the clinical prognosis of the patient.
Taking the behavior of both CD19+CD23+ and CD19+CD69+ B lymphocytes together, it can be speculated that the depletion of B cells in septic shock patients is an event that preferentially occurs after the initiation of their in vivo activation. The intensity of this event might correlate with the observed reduction of activated B cells and is associated with the clinical outcome of the patient. Moreover, we have compared this finding with the APACHE II scores, because APACHE II score was the best prognostic clinical variable that we analyzed. When we analyzed the ROC curve of APACHE II score for predicting death in patients with septic shock at admission, we found that B-lymphocyte data are significantly more accurate than the APACHE II score for predicting death.
In addition to the observed changes in the distribution of the cell subsets of the circulating B-lymphocyte compartment, we investigated the expression of the functionally critical antigens CD80 and CD86 on these B cells. In murine studies of sepsis, an important role for CD80 and CD86 antigens in the response to sepsis has been established [29,30]. Our data presented herein show a higher percentage of CD86 expression in circulating CD19+ B cells in patients with septic shock than in healthy controls. Furthermore, at ICU admission, nonsurvivors showed more elevated percentages of CD19+CD80+ B cells than found in survivors. These findings in B lymphocytes of patients with septic shock are consistent with the increased expression of CD86 and decreased expression of CD80 in dendritic cells found in human sepsis [31].
Apoptosis is the process of programmed cell death that occurs to limit damage of surrounding tissue. It is critical for the survival of many cells, including lymphocytes. Deregulated apoptotic immune cell death has been proposed to play a major role in immune dysfunction and mortality during sepsis [32,33]. Immunohistological studies of different tissues have demonstrated increased apoptosis of cells of the innate and adaptive immune system in sepsis [25,34,35]. In this work, we show that the total number of circulating B lymphocytes was low in patients with septic shock at ICU admission and during the following 28 days. We also observe that circulating B cells of septic shock patients showed increased expression of CD95 antigen. Similar findings have been described in circulating T cells [36]. In support of the concept that lymphocyte apoptosis is detrimental to host survival, a number of studies have shown an inverse correlation between lymphocyte count and survival [34,36]. In agreement with this, we found a higher percentage of CD95 expression on B cells from nonsurvivors than from survivor patients or healthy controls. This finding suggests that the increased expression of CD95 on B cells from septic shock patients might be involved in the mechanism of the observed reduction of circulating B cells in these patients.
Several limitations of our study should be noted. The peripheral blood may not represent the situation in all lymphoid compartments of the body, and these findings do suggest that not all cell populations may play equal roles in driving inflammatory and anti-inflammatory responses in septic shock [21]. All blood samples were collected upon ICU admission within 12 hours after inclusion criteria were met, but the length of time that the patients were in shock in the emergency room before admission or in the operating room is a source of inaccuracy. Recently, early alterations of the innate and adaptive immune status according to the type of underlying infection in sepsis have been reported [37]. In addition, other factors, such as host genetic polymorphisms or the characteristics of the pathogen, may also have introduced variability. The average of age of our population was 61 years. It is known that the overall number of B cells seems to moderately decline with age, but B-lymphocyte subset studies are frequently controversial [38]. This is the reason why our control group was age-matched. CD23 is the low-affinity immunoglobulin E (IgE) receptor and binds both IgE and CD21 and, through these interactions, regulates the synthesis of IgE, the antibody isotype that mediates the allergic response. The expression of CD23 on B cells is higher in persons with asthma or atopy and in patients with disorders characterized by chronic inflammation [28]. We excluded patients who were undergoing glucocorticoid therapy or were in anaphylactic shock, but patients with a personal history of chronic asthma without hypersensitivity disease were included.
Conclusions
Septic shock is associated with a severe abnormality of circulating B lymphocytes. At ICU admission, the expression of CD23+, CD95+ or CD80+ on B cells was significantly associated with increased 28-day mortality. These results highlight the potential importance of B lymphocytes in septic shock.
Key messages
• Sepsis-induced immune dysfunction may contribute to mortality to a great degree. It is increasingly clear that B-cell function beyond the production of immunoglobulins. However, little is known about how B cells affect innate immunity during bacterial sepsis.
• We found that patients with septic shock had B-cell lymphopenia that was maintained during 28 days of follow-up. The increased expression of CD95 on B cells suggests that apoptosis susceptibility is involved in the reduction of circulating B cells in these patients.
• B-cell lymphopenia affects the B-cell subsets heterogeneously, with marked reduction of CD19+CD23+ B cells (activated regulatory B cells) and CD19+CD5+ B cells (natural responder B-1a cells), but with normal numbers of CD19+CD69+ early activated B cells.
• At ICU admission, a higher percentage (64%) of CD19+CD23+, a marker of activation and regulation, appears to be a reliable biomarker of good outcome for patients with septic shock, whereas the percentages of CD80+ (a T-cell costimulation marker) and CD95+ (a marker of apoptosis susceptibility) on B cells were significantly lower in survivors than in nonsurvivors.
Abbreviations
AIDS: acquired immunodeficiency syndrome; APACHE II: Acute Physiology and Chronic Health Evaluation II; CD: cluster of differentiation; CI: confidence interval; FITC: fluorescein isothiocyanate; ICU: intensive care unit; IL-10: interleukin 10; IRA-B cells: innate response activator B cells; mAb: monoclonal antibodies; PBMC: peripheral blood mononuclear cell; PE: phycoerythrin; ROC: receiver-operating characteristic; SEM: standard error of the mean; TC: phycoerythrin-cyanine 5 tricolor.
Competing interests
The authors have no direct or otherwise commercial association that might lead to a conflict of interest.
Authors' contributions
RP, JM, AP and MAM made substantial contributions to the conception and design of the study and the analysis and interpretation of data, and they were involved in drafting and revising the manuscript. RP, JM and DDM made substantial contributions to the acquisition, analysis and interpretation of data. MRZ and AH were involved in analysis and interpretation of data and in drafting and revising the manuscript critically for important intellectual content. All authors read and approved the final manuscript.
Contributor Information
Jorge Monserrat, Email: jorge.monserrat@uah.es.
Raul de Pablo, Email: raul.depablo@uah.es.
David Diaz-Martín, Email: david.diaz@uah.es.
Manuel Rodríguez-Zapata, Email: manuel.rodriguezz@uah.es.
Antonio de la Hera, Email: antonio.delahera@uah.es.
Alfredo Prieto, Email: alfredo.prieto@uah.es.
Melchor Alvarez-Mon, Email: mademons@gmail.com.
Acknowledgements
The authors thank the valuable help of the nursing and medical staff of our ICU, the Department of Medicine and the University of Alcalá. This work was partially funded by grants from Fondo de Investigación de la Seguridad Social, Ministerio de Economia y Competitividad (MEC) (Spain), Consejeria de Educación, Comunidad de Madrid, MITIC-CM (S-2010/BMD-2502) and Instituto de Salud Carlos III, MEC (PI051871, CIBERehd).
References
- Hotchkiss RS, Karl IE. The pathophysiology and treatment of sepsis. N Engl J Med. 2003;17:138–150. doi: 10.1056/NEJMra021333. [DOI] [PubMed] [Google Scholar]
- Russell JA. Management of sepsis. N Engl J Med. 2006;17:1699–1713. doi: 10.1056/NEJMra043632. [DOI] [PubMed] [Google Scholar]
- Döcke WD, Randow F, Syrbe U, Krausch D, Asadullah K, Reinke P, Volk HD, Kox W. Monocyte deactivation in septic patients: restoration by IFN-γ treatment. Nat Med. 1997;17:678–681. doi: 10.1038/nm0697-678. [DOI] [PubMed] [Google Scholar]
- de Pablo R, Monserrat J, Torrijos C, Martín M, Prieto A, Alvarez-Mon M. The predictive role of early activation of natural killer cells in septic shock. Crit Care. 2012;17:413. doi: 10.1186/cc11204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasten KR, Tschöp J, Adediran SG, Hildeman DA, Caldwell CC. T cells are potent early mediators of the host response to sepsis. Shock. 2010;17:327–336. doi: 10.1097/SHK.0b013e3181e14c2e. [DOI] [PubMed] [Google Scholar]
- Monserrat J, de Pablo R, Reyes E, Díaz D, Barcenilla H, Zapata MR, De la Hera A, Prieto A, Alvarez-Mon M. Clinical relevance of the severe abnormalities of the T cell compartment in septic shock patients. Crit Care. 2009;17:R26. doi: 10.1186/cc7731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frasca D, Blomberg BB. Aging affects human B cell responses. J Clin Immunol. 2011;17:430–435. doi: 10.1007/s10875-010-9501-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy RR, Hayakawa K. B cell development pathways. Annu Rev Immunol. 2001;17:595–621. doi: 10.1146/annurev.immunol.19.1.595. [DOI] [PubMed] [Google Scholar]
- Sanz I, Wei C, Lee FE, Anolik J. Phenotypic and functional heterogeneity of human memory B cells. Semin Immunol. 2008;17:67–82. doi: 10.1016/j.smim.2007.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weller S, Braun MC, Tan BK, Rosenwald A, Cordier C, Conley ME, Plebani A, Kumararatne DS, Bonnet D, Tournilhac O, Tchernia G, Steiniger B, Staudt LM, Casanova JL, Reynaud CA, Weill JC. Human blood IgM "memory" B cells are circulating splenic marginal zone B cells harboring a prediversified immunoglobulin repertoire. Blood. 2004;17:3647–3654. doi: 10.1182/blood-2004-01-0346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zouali M, Richard Y. Marginal zone B-cells, a gatekeeper of innate immunity. Front Immunol. 2011;17:63. doi: 10.3389/fimmu.2011.00063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas KM, Poe JC, Steeber DA, Tedder TF. B-1a and B-1b cells exhibit distinct developmental requirements and have unique functional roles in innate and adaptive immunity to S. pneumoniae. Immunity. 2005;17:7–18. doi: 10.1016/j.immuni.2005.04.011. [DOI] [PubMed] [Google Scholar]
- Kelly-Scumpia KM, Scumpia PO, Weinstein JS, Delano MJ, Cuenca AG, Nacionales DC, Wynn JL, Lee PY, Kumagai Y, Efron PA, Akira S, Wasserfall C, Atkinson MA, Moldawer LL. B cells enhance early innate immune responses during bacterial sepsis. J Exp Med. 2011;17:1673–1682. doi: 10.1084/jem.20101715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan AT, Roghanian A, Cragg MS. B cells: masters of the immunoverse. Int J Biochem Cell Biol. 2011;17:280–285. doi: 10.1016/j.biocel.2010.12.005. [DOI] [PubMed] [Google Scholar]
- Booth J, Wilson H, Jimbo S, Mutwiri G. Modulation of B cell responses by Toll-like receptors. Cell Tissue Res. 2011;17:131–140. doi: 10.1007/s00441-010-1031-3. [DOI] [PubMed] [Google Scholar]
- Mauri C, Bosma A. Immune regulatory function of B cells. Annu Rev Immunol. 2012;17:221–241. doi: 10.1146/annurev-immunol-020711-074934. [DOI] [PubMed] [Google Scholar]
- Rawlings DJ, Schwartz MA, Jackson SW, Meyer-Bahlburg A. Integration of B cell responses through Toll-like receptors and antigen receptors. Nat Rev Immunol. 2012;17:282–294. doi: 10.1038/nri3190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darton TC, Wing JB, Lees A, Heath AW, Read RC. Adult survivors of invasive pneumococcal disease exhibit defective B cell function. Clin Infect Dis. 2011;17:1133–1136. doi: 10.1093/cid/cir126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauch PJ, Chudnovskiy A, Robbins CS, Weber GF, Etzrodt M, Hilgendorf I, Tiglao E, Figueiredo JL, Iwamoto Y, Theurl I, Gorbatov R, Waring MT, Chicoine AT, Mouded M, Pittet MJ, Nahrendorf M, Weissleder R, Swirski FK. Innate response activator B cells protect against microbial sepsis. Science. 2012;17:597–601. doi: 10.1126/science.1215173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins CS, Swirski FK. Newly discovered innate response activator B cells: crucial responders against microbial sepsis. Expert Rev Clin Immunol. 2012;17:405–407. doi: 10.1586/eci.12.32. [DOI] [PubMed] [Google Scholar]
- Shubin NJ, Monaghan SF, Ayala A. Anti-inflammatory mechanisms of sepsis. Contrib Microbiol. 2011;17:108–124. doi: 10.1159/000324024. [DOI] [PubMed] [Google Scholar]
- Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, Knaus WA, Schein RM, Sibbald WJ. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest. 1992;17:1644–1655. doi: 10.1378/chest.101.6.1644. [DOI] [PubMed] [Google Scholar]
- Böyum AJ. Isolation of mononuclear cells and granulocytes from human blood. Scand J Clin Lab Invest. 1968;17:77–89. doi: 10.3109/00365516809076979. [DOI] [PubMed] [Google Scholar]
- Venet F, Davin F, Guignant C, Larue A, Cazalis MA, Darbon R, Allombert C, Mougin B, Malcus C, Poitevin-Later F, Lepape A, Monneret G. Early assessment of leukocyte alterations at diagnosis of septic shock. Shock. 2010;17:358–363. doi: 10.1097/SHK.0b013e3181dc0977. [DOI] [PubMed] [Google Scholar]
- Hotchkiss RS, Tinsley KW, Swanson PE, Schmieg RE Jr, Hui JJ, Chang KC, Osborne DF, Freeman BD, Cobb JP, Buchman TG, Karl IE. Sepsis-induced apoptosis causes progressive profound depletion of B and CD4+ T lymphocytes in humans. J Immunol. 2001;17:6952–6963. doi: 10.4049/jimmunol.166.11.6952. [DOI] [PubMed] [Google Scholar]
- Jiménez-Ibáñez EO, Castillejos-López M, Hernández A, Gorocica P, Alvarado-Vásquez N. High mortality associated with hyperglycemia, neutrophilia, and lymphopenia in critically ill patients. Tohoku J Exp Med. 2012;17:213–220. doi: 10.1620/tjem.226.213. [DOI] [PubMed] [Google Scholar]
- Boomer JS, Shuherk-Shaffer J, Hotchkiss RS, Green JM. A prospective analysis of lymphocyte phenotype and function over the course of acute sepsis. Crit Care. 2012;17:R112. doi: 10.1186/cc11404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenwasser LJ, Meng J. Anti-CD23. Clin Rev Allergy Immunol. 2005;17:61–72. doi: 10.1385/CRIAI:29:1:061. [DOI] [PubMed] [Google Scholar]
- Nolan A, Kobayashi H, Naveed B, Kelly A, Hoshino Y, Hoshino S, Karulf MR, Rom WN, Weiden MD, Gold JA. Differential role for CD80 and CD86 in the regulation of the innate immune response in murine polymicrobial sepsis. PLoS One. 2009;17:e6600. doi: 10.1371/journal.pone.0006600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolan A, Weiden M, Kelly A, Hoshino Y, Hoshino S, Mehta N, Gold JA. CD40 and CD80/86 act synergistically to regulate inflammation and mortality in polymicrobial sepsis. Am J Respir Crit Care Med. 2008;17:301–308. doi: 10.1164/rccm.200703-515OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flohé SB, Agrawal H, Schmitz D, Gertz M, Flohé S, Schade FU. Dendritic cells during polymicrobial sepsis rapidly mature but fail to initiate a protective Th1-type immune response. J Leukoc Biol. 2006;17:473–481. doi: 10.1189/jlb.0705413. [DOI] [PubMed] [Google Scholar]
- van der Poll T, van Zoelen MA, Wiersinga WJ. Regulation of pro- and anti-inflammatory host responses. Contrib Microbiol. 2011;17:125–136. doi: 10.1159/000324026. [DOI] [PubMed] [Google Scholar]
- Hotchkiss RS, Nicholson DW. Apoptosis and caspases regulate death and inflammation in sepsis. Nat Rev Immunol. 2006;17:813–822. doi: 10.1038/nri1943. [DOI] [PubMed] [Google Scholar]
- Hotchkiss RS, Swanson PE, Freeman BD, Tinsley KW, Cobb JP, Matuschak GM, Buchman TG, Karl IE. Apoptotic cell death in patients with sepsis, shock, and multiple organ dysfunction. Crit Care Med. 1999;17:1230–1251. doi: 10.1097/00003246-199907000-00002. [DOI] [PubMed] [Google Scholar]
- Hotchkiss RS, Tinsley KW, Swanson PE, Grayson MH, Osborne DF, Wagner TH, Cobb JP, Coopersmith C, Karl IE. Depletion of dendritic cells, but not macrophages, in patients with sepsis. J Immunol. 2002;17:2493–2500. doi: 10.4049/jimmunol.168.5.2493. [DOI] [PubMed] [Google Scholar]
- Roth G, Moser B, Krenn C, Brunner M, Haisjackl M, Almer G, Gerlitz S, Wolner E, Boltz-Nitulescu G, Ankersmit HJ. Susceptibility to programmed cell death in T-lymphocytes from septic patients: a mechanism for lymphopenia and Th2 predominance. Biochem Biophys Res Commun. 2003;17:840–846. doi: 10.1016/S0006-291X(03)01482-7. [DOI] [PubMed] [Google Scholar]
- Gogos C, Kotsaki A, Pelekanou A, Giannikopoulos G, Vaki I, Maravitsa P, Adamis S, Alexiou Z, Andrianopoulos G, Antonopoulou A, Athanassia S, Baziaka F, Charalambous A, Christodoulou S, Dimopoulou I, Floros I, Giannitsioti E, Gkanas P, Ioakeimidou A, Kanellakopoulou K, Karabela N, Karagianni V, Katsarolis I, Kontopithari G, Kopterides P, Koutelidakis I, Koutoukas P, Kranidioti H, Lignos M, Louis K. et al. Early alterations of the innate and adaptive immune statuses in sepsis according to the type of underlying infection. Crit Care. 2010;17:R96. doi: 10.1186/cc9031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ongradi J, Kovesdi V. Numerical alterations of ageing B lymphocyte subsets. Acta Physiol Hung. 2011;17:99–104. doi: 10.1556/APhysiol.98.2011.2.1. [DOI] [PubMed] [Google Scholar]
- Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. 1985;17:818–829. doi: 10.1097/00003246-198510000-00009. [DOI] [PubMed] [Google Scholar]
- Vincent JL, de Mendonça A, Cantraine F, Moreno R, Takala J, Suter PM, Sprung CL, Colardyn F, Blecher S. Working Group on "Sepsis-Related Problems" of the European Society of Intensive Care Medicine. Use of the SOFA score to assess the incidence of organ dysfunction/failure in intensive care units: results of a multicenter, prospective study. Crit Care Med. 1998;17:1793–1800. doi: 10.1097/00003246-199811000-00016. [DOI] [PubMed] [Google Scholar]
- Marshall JC, Cook DJ, Christou NV, Bernard GR, Sprung CL, Sibbald WJ. Multiple organ dysfunction score: a reliable descriptor of a complex clinical outcome. Crit Care Med. 1995;17:1638–1652. doi: 10.1097/00003246-199510000-00007. [DOI] [PubMed] [Google Scholar]
- Jacobs S, Zuleika M, Mphansa T. The Multiple Organ Dysfunction Score as a descriptor of patient outcome in septic shock compared with two other scoring systems. Crit Care Med. 1999;17:741–744. doi: 10.1097/00003246-199904000-00027. [DOI] [PubMed] [Google Scholar]
- Moreno R, Vincent JL, Matos R, de Mendonça A, Cantraine F, Thijs L, Takala J, Sprung C, Antonelli M, Bruining H, Willatts S. Working Group on Sepsis-Related Problems of the ESICM. The use of maximum SOFA score to quantify organ dysfunction/failure in intensive care: results of a prospective, multicentre study. Intensive Care Med. 1999;17:686–696. doi: 10.1007/s001340050931. [DOI] [PubMed] [Google Scholar]


