Abstract
Nanotechnology provides exciting opportunities for the development of novel, clinically relevant diagnostic and therapeutic multifunctional systems. Fluorescent carbon nanoparticles (CNPs) due to their intrinsic fluorescence and high biocompatibility are among the best candidates. As innovative nanomaterials, CNPs could be utilized both as nontoxic drug delivery system and bioimaging. We foresee a great future for CNPs in cancer diagnostic and therapy.
Personalized medicine is a major goal in cancer therapy, aiming to increase drug efficacy and reduce toxicity. All the available chemotherapeutic drugs currently in use for cancer treatment have some undesired side effects. One strategy to overcome side effects and increase efficacy is delivering chemotherapeutic drugs in close proximity to the tumor. In this regard, in the last years, nanotechnology-based Drug Delivery Systems (DDSs) have been developed and tested in vitro and in vivo. Among these, gold nanoparticles-based DDSs have been investigated extensively.1 The strong Au–S interactions make it very convenient to conjugate various sulfur containing molecules and/or thiol-modified biomolecules (proteins, peptides, and nucleic acids) to the surface of Au nanoparticles. However, the major problem with AuNPs-based DDSs is the toxicity since these particles are made-up of heavy metal, which limits their applications in clinics.2 Moreover, the conjugation of drug molecules or targeting chemicals to the gold nanoparticles usually occurs through thiols, which reduce the choices to medicinal chemists for drug loading through chemical conjugation. Gold NPs are also known to quench fluorescence of fluorophores, which makes them difficult to track in vivo.3
Recently discovered, a new class of carbon nanomaterials termed fluorescent carbon nanoparticles (CNPs) could be a potential technological alternative due to their high water solubility, flexibility in surface modification with various chemicals, excellent biocompatibility, good cell permeability, and high photostability.4,5 These CNPs are made up of only carbon with inherent fluorescence properties, so their toxicity should be minimal. On the basis of their synthesis, these particles may contain different functional groups on their surface; viz., −COOH, −OH, >CO, and −NH2, which imparts them excellent water solubility and possibilities for covalent conjugation with the chemotherapeutic agent, targeting agent, and/or antibody (Figure 1).6,7 Chemotherapeutic drug in combination with targeting agent could be easily tethered to the CNPs through covalent linkage with these functional groups. Chemical synthesis of fluorescent carbon nanoparticles generally involves nonhazardous experimental procedures including either the carbonization of carbohydrates of different molecular weight or oxidation of carbon soot with nitric acid, which could be considered as green.6−8 In order to increase the fluorescence, their surface could be passivated with poly ethylene glycol or other polymers.
Figure 1.
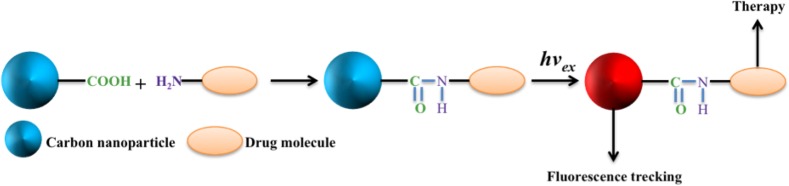
Carbon nanoparticle bearing −COOH group at their surface and the drug molecule (or antibody) containing −NH2 conjugated through amide bond. This carbon nanoparticle-based drug delivery system provides a unique possibility for tracking them inside the biological system due to the intrinsic fluorescence of carbon nanoparticle.
The toxicity studies of carbon nanoparticles show that these particles are nontoxic. In vitro studies demonstrate that under biological relevant concentration range, cell treated with carbon nanoparticles exhibited more than 80% of survival rate, clearly manifesting minimal toxicity.6,9 In vivo toxicity of carbon nanoparticles were also carried out on mice. Different amounts of carbon nanoparticles were administered to mice intravenously.10 After 4 weeks there were no sign of toxicity and adverse clinical symptoms. Hepatic indicators, blood urea nitrogen, kidney function, uric acid, and creatinine were found to be similar as the control untreated mice. Furthermore, no abnormality or necrosis were seen in the harvested organs. This study demonstrates that the particles are almost nontoxic and are biocompatible.
CNPs possess distinct optical and chemical properties that allow us to (i) have optical properties compatible with living cells, (ii) modify with suitable exogenous chemicals, and (iii) be biocompatible and nontoxic. These properties provide the opportunity to medicinal chemists for the development of a new multimodal drug delivery system, which could be used for simultaneous drug delivery and fluorescent tracking. To date there are several approaches reported in the literature for the synthesis of carbon nanoparticles; however, they possess low fluorescence mainly in the blue-green region. Thus, the development of new methodologies for the synthesis of highly fluorescent carbon nanoparticles with fine-tuned fluorescence and engineered surface functionalization is urgently required. Carbon nanoparticles having fluorescence in the red or NIR range could be the best candidate for tracking and delivery, which will avoid background noise from the endogenous fluorophores during bioimaging. We believe that the development of highly biocompatible and fluorescent drug delivery systems based on fluorescent carbon nanoparticles holds great promises for specific drug delivery with minimal side effect and toxicity in cancer patients and provide valuable tools to medicinal chemists for the synthesis of site-specific carriers of various therapeutic agents with possible application also as imaging systems. A possible future could be foreseen for CNPs in medicine and clinics providing virtually no general toxicity and long circulation times to seek out and destroy cancer cells.
Authors are thankful to Special Program Molecular Clinical Oncology, 5x1000 (No. 12214), European Research Council, Programme “Ideas”, Proposal No. 269051, and Italian Ministry of Education MIUR (FIRB prot. RBAP11ETKA) for funding.
Views expressed in this editorial are those of the author and not necessarily the views of the ACS.
The authors declare no competing financial interest.
References
- Dreaden E. C.; Alkilany A. M.; Huang X.; Murphy C. J.; El-Sayed M. A. The golden age: gold nanoparticles for biomedicine. Chem. Soc. Rev. 2012, 41, 2740–2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkilany A. M.; Murphy C. J. Toxicity and cellular uptake of gold nanoparticles: what we have learned so far?. J. Nanopart. Res. 2010, 12, 2313–2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulkeith E.; Morteani A. C.; Niedereichholz T.; Klar T. A.; Feldmann J.; Levi A. A.; van Veggel F. C. J. M.; Reinhoudt D. N.; Möller M.; Gittins D. I. Fluorescence quenching of dye molecules near gold nanoparticles: radiative and nonradiative effects. Phys. Rev. Lett. 2002, 89, 203002–1– 4. [DOI] [PubMed] [Google Scholar]
- Baker S. N.; Baker G. A. Luminescent carbon nanodots: emergent nanolights. Angew. Chem., Int. Ed. 2010, 49, 6726–6744. [DOI] [PubMed] [Google Scholar]
- Ding C.; Zhu A.; Tian Y. Functional Surface Engineering of C-Dots for fluorescent biosensing and in vivo bioimaging. Acc. Chem. Res. 2013, 10.1021/ar400023s. [DOI] [PubMed] [Google Scholar]
- Bhunia S. K.; Saha A.; Maity A. R.; Ray S. C.; Jana N. R. Carbon nanoparticle-based fluorescent bioimaging probes. Sci. Rep. 2013, 3, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X.; Cao L.; Yang S.-T.; Lu F.; Meziani M. J.; Tian L.; Sun K. W.; Bloodgood M. A.; Sun Y.-P. Bandgap-like strong fluorescence in functionalized carbon nanoparticles. Angew. Chem., Int. Ed. 2010, 49, 5310–5314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H.; Ye T.; Mao C. Fluorescent carbon nanoparticles derived from candle soot. Angew. Chem., Int. Ed. 2007, 46, 6473–6475. [DOI] [PubMed] [Google Scholar]
- Ko H. Y.; Chang Y. W.; Paramasivam G.; Jeong M. S.; Cho S.; Kim S. In vivo imaging of tumour bearing near-infrared fluorescence-emitting carbon nanodots derived from tire soot. Chem. Commun. 2013, 49, 10290–10292. [DOI] [PubMed] [Google Scholar]
- Yang S.-T.; Wang X.; Wang H.; Lu F.; Luo P. G.; Cao L.; Meziani M. J.; Liu J.-H.; Liu Y.; Chen M.; Huang Y.; Sun Y.-P. Carbon dots as nontoxic and high-performance fluorescence imaging agents. J. Phys. Chem. C 2009, 113, 18110–18114. [DOI] [PMC free article] [PubMed] [Google Scholar]


