Abstract
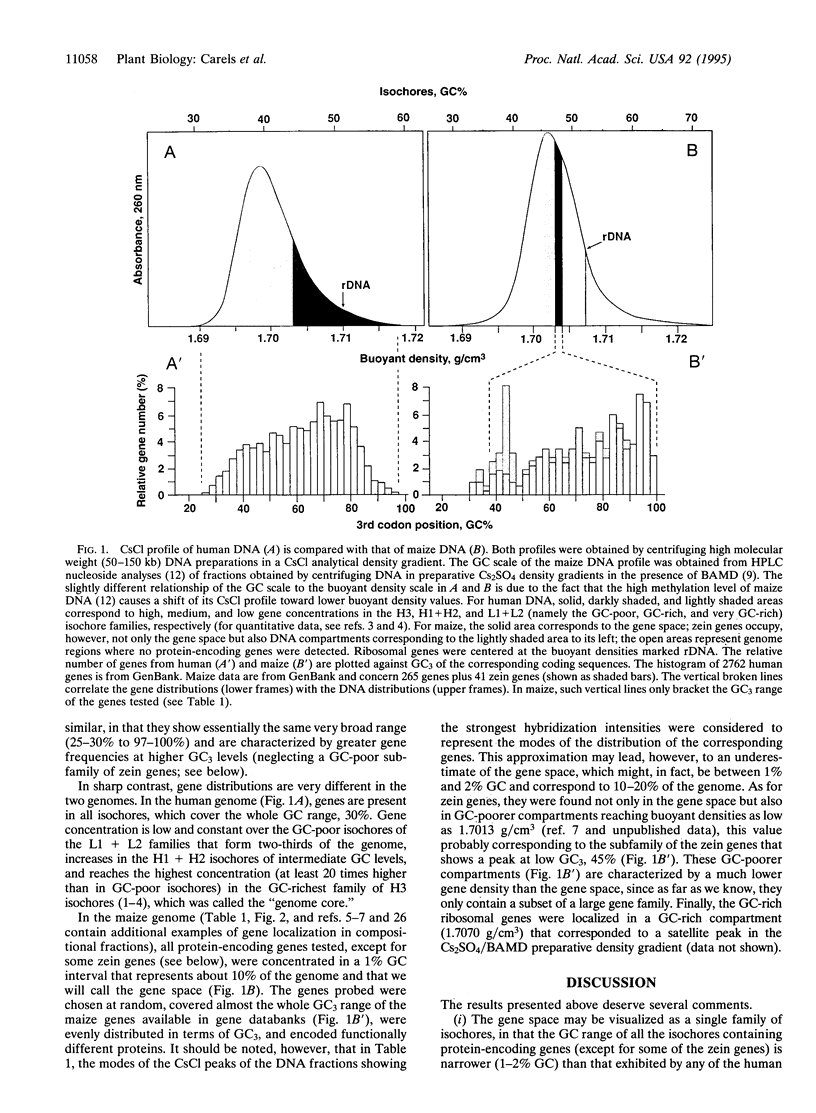
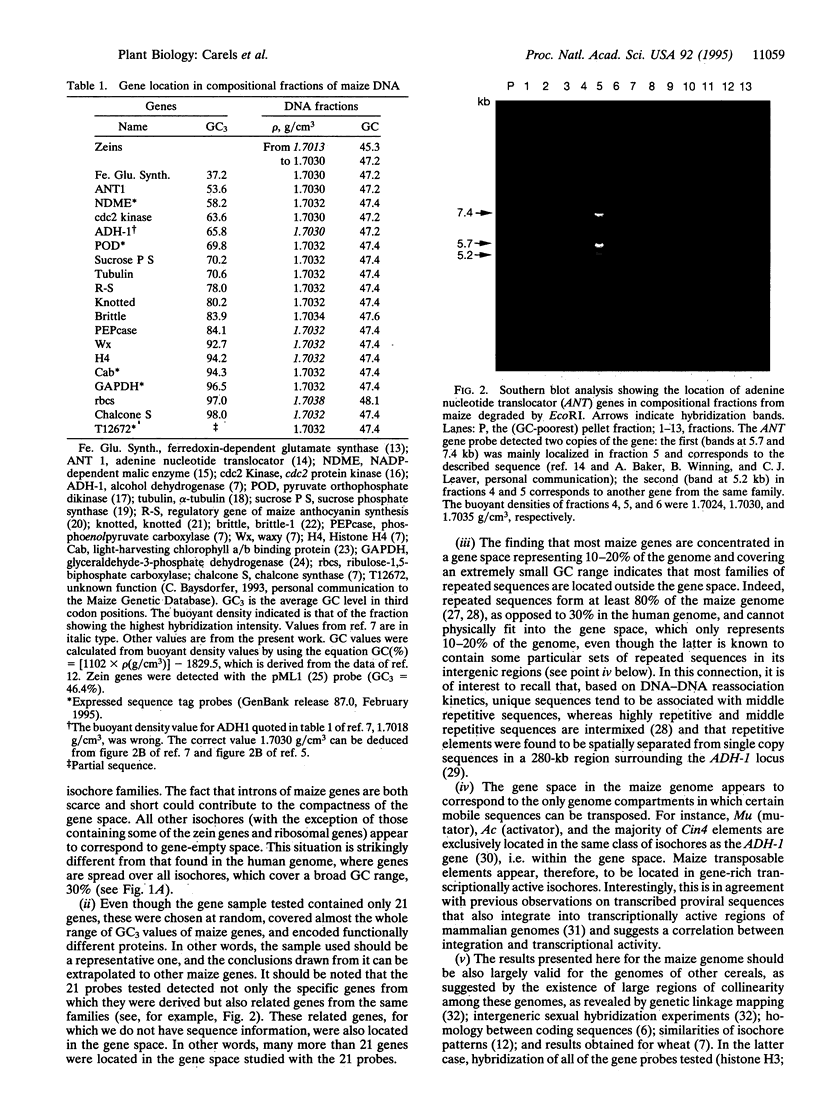
Previous investigations from our laboratory showed that the genomes of plants, like those of vertebrates, are mosaics of isochores, i.e., of very long DNA segments that are compositionally homogeneous and that can be subdivided into a small number of families characterized by different GC levels (GC is the mole fraction of guanine+cytosine). Compositional DNA fractions corresponding to different isochore families were used to investigate, by hybridization with appropriate probes, the gene distribution in vertebrate genomes. Here we report such a study on the genome of a plant, maize. The gene distribution that we found is most striking, in that almost all genes are present in isochores covering an extremely narrow (1-2%) GC range and only representing 10-20% of the genome. This gene distribution, which seems to characterize other Gramineae as well, is remarkably different from the gene distribution previously found in vertebrate genomes.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahn S., Tanksley S. D. Comparative linkage maps of the rice and maize genomes. Proc Natl Acad Sci U S A. 1993 Sep 1;90(17):7980–7984. doi: 10.1073/pnas.90.17.7980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antequera F., Bird A. P. Unmethylated CpG islands associated with genes in higher plant DNA. EMBO J. 1988 Aug;7(8):2295–2299. doi: 10.1002/j.1460-2075.1988.tb03072.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennetzen J. L., Freeling M. Grasses as a single genetic system: genome composition, collinearity and compatibility. Trends Genet. 1993 Aug;9(8):259–261. doi: 10.1016/0168-9525(93)90001-x. [DOI] [PubMed] [Google Scholar]
- Bernardi G., Olofsson B., Filipski J., Zerial M., Salinas J., Cuny G., Meunier-Rotival M., Rodier F. The mosaic genome of warm-blooded vertebrates. Science. 1985 May 24;228(4702):953–958. doi: 10.1126/science.4001930. [DOI] [PubMed] [Google Scholar]
- Bernardi G. The isochore organization of the human genome and its evolutionary history--a review. Gene. 1993 Dec 15;135(1-2):57–66. doi: 10.1016/0378-1119(93)90049-9. [DOI] [PubMed] [Google Scholar]
- Bernardi G. The isochore organization of the human genome. Annu Rev Genet. 1989;23:637–661. doi: 10.1146/annurev.ge.23.120189.003225. [DOI] [PubMed] [Google Scholar]
- Bünemann H., Dattagupta N. On the binding and specificity of 3,6-bis-(acetatomercurimethyl)-dioxane to DNAs of different base composition. Biochim Biophys Acta. 1973 Dec 21;331(3):341–348. doi: 10.1016/0005-2787(73)90020-8. [DOI] [PubMed] [Google Scholar]
- Capel J., Montero L. M., Martinez-Zapater J. M., Salinas J. Non-random distribution of transposable elements in the nuclear genome of plants. Nucleic Acids Res. 1993 May 25;21(10):2369–2373. doi: 10.1093/nar/21.10.2369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler V. L., Radicella J. P., Robbins T. P., Chen J., Turks D. Two regulatory genes of the maize anthocyanin pathway are homologous: isolation of B utilizing R genomic sequences. Plant Cell. 1989 Dec;1(12):1175–1183. doi: 10.1105/tpc.1.12.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church G. M., Gilbert W. Genomic sequencing. Proc Natl Acad Sci U S A. 1984 Apr;81(7):1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colasanti J., Tyers M., Sundaresan V. Isolation and characterization of cDNA clones encoding a functional p34cdc2 homologue from Zea mays. Proc Natl Acad Sci U S A. 1991 Apr 15;88(8):3377–3381. doi: 10.1073/pnas.88.8.3377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortadas J., Macaya G., Bernardi G. An analysis of the bovine genome by density gradient centrifugation: fractionation in Cs2SO4/3,6-bis(acetatomercurimethyl)dioxane density gradient. Eur J Biochem. 1977 Jun 1;76(1):13–19. doi: 10.1111/j.1432-1033.1977.tb11565.x. [DOI] [PubMed] [Google Scholar]
- Flavell R. B., Bennett M. D., Smith J. B., Smith D. B. Genome size and the proportion of repeated nucleotide sequence DNA in plants. Biochem Genet. 1974 Oct;12(4):257–269. doi: 10.1007/BF00485947. [DOI] [PubMed] [Google Scholar]
- Gyapay G., Morissette J., Vignal A., Dib C., Fizames C., Millasseau P., Marc S., Bernardi G., Lathrop M., Weissenbach J. The 1993-94 Généthon human genetic linkage map. Nat Genet. 1994 Jun;7(2 Spec No):246–339. doi: 10.1038/ng0694supp-246. [DOI] [PubMed] [Google Scholar]
- Matassi G., Melis R., Kuo K. C., Macaya G., Gehrke C. W., Bernardi G. Large-scale methylation patterns in the nuclear genomes of plants. Gene. 1992 Dec 15;122(2):239–245. doi: 10.1016/0378-1119(92)90211-7. [DOI] [PubMed] [Google Scholar]
- Matassi G., Melis R., Macaya G., Bernardi G. Compositional bimodality of the nuclear genome of tobacco. Nucleic Acids Res. 1991 Oct 25;19(20):5561–5567. doi: 10.1093/nar/19.20.5561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matassi G., Montero L. M., Salinas J., Bernardi G. The isochore organization and the compositional distribution of homologous coding sequences in the nuclear genome of plants. Nucleic Acids Res. 1989 Jul 11;17(13):5273–5290. doi: 10.1093/nar/17.13.5273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuoka M., Ozeki Y., Yamamoto N., Hirano H., Kano-Murakami Y., Tanaka Y. Primary structure of maize pyruvate, orthophosphate dikinase as deduced from cDNA sequence. J Biol Chem. 1988 Aug 15;263(23):11080–11083. [PubMed] [Google Scholar]
- Montero L. M., Salinas J., Matassi G., Bernardi G. Gene distribution and isochore organization in the nuclear genome of plants. Nucleic Acids Res. 1990 Apr 11;18(7):1859–1867. doi: 10.1093/nar/18.7.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montoliu L., Rigau J., Puigdomènech P. A tandem of alpha-tubulin genes preferentially expressed in radicular tissues from Zea mays. Plant Mol Biol. 1990 Jan;14(1):1–15. doi: 10.1007/BF00015650. [DOI] [PubMed] [Google Scholar]
- Moore G., Abbo S., Cheung W., Foote T., Gale M., Koebner R., Leitch A., Leitch I., Money T., Stancombe P. Key features of cereal genome organization as revealed by the use of cytosine methylation-sensitive restriction endonucleases. Genomics. 1993 Mar;15(3):472–482. doi: 10.1006/geno.1993.1097. [DOI] [PubMed] [Google Scholar]
- Pintor-Toro J. A., Langridge P., Feix G. Isolation and characterization of maize genes coding for zein proteins of the 21000 dalton size class. Nucleic Acids Res. 1982 Jul 10;10(13):3845–3860. doi: 10.1093/nar/10.13.3845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quigley F., Martin W. F., Cerff R. Intron conservation across the prokaryote-eukaryote boundary: structure of the nuclear gene for chloroplast glyceraldehyde-3-phosphate dehydrogenase from maize. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2672–2676. doi: 10.1073/pnas.85.8.2672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothermel B. A., Nelson T. Primary structure of the maize NADP-dependent malic enzyme. J Biol Chem. 1989 Nov 25;264(33):19587–19592. [PubMed] [Google Scholar]
- Saccone S., De Sario A., Della Valle G., Bernardi G. The highest gene concentrations in the human genome are in telomeric bands of metaphase chromosomes. Proc Natl Acad Sci U S A. 1992 Jun 1;89(11):4913–4917. doi: 10.1073/pnas.89.11.4913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saccone S., De Sario A., Wiegant J., Raap A. K., Della Valle G., Bernardi G. Correlations between isochores and chromosomal bands in the human genome. Proc Natl Acad Sci U S A. 1993 Dec 15;90(24):11929–11933. doi: 10.1073/pnas.90.24.11929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakakibara H., Watanabe M., Hase T., Sugiyama T. Molecular cloning and characterization of complementary DNA encoding for ferredoxin-dependent glutamate synthase in maize leaf. J Biol Chem. 1991 Feb 5;266(4):2028–2035. [PubMed] [Google Scholar]
- Salinas J., Matassi G., Montero L. M., Bernardi G. Compositional compartmentalization and compositional patterns in the nuclear genomes of plants. Nucleic Acids Res. 1988 May 25;16(10):4269–4285. doi: 10.1093/nar/16.10.4269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer P. S., Edwards K. J., Bennetzen J. L. DNA class organization on maize Adh1 yeast artificial chromosomes. Proc Natl Acad Sci U S A. 1994 Feb 1;91(3):863–867. doi: 10.1073/pnas.91.3.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan T. D., Strelow L. I., Illingworth C. A., Phillips R. L., Nelson O. E., Jr Analysis of maize brittle-1 alleles and a defective Suppressor-mutator-induced mutable allele. Plant Cell. 1991 Dec;3(12):1337–1348. doi: 10.1105/tpc.3.12.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veit B., Vollbrecht E., Mathern J., Hake S. A tandem duplication causes the Kn1-O allele of Knotted, a dominant morphological mutant of maize. Genetics. 1990 Jul;125(3):623–631. doi: 10.1093/genetics/125.3.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viret J. F., Schantz M. L., Schantz R. Nucleotide sequence of a maize cDNA coding for a light-harvesting chlorophyll a/b binding protein of photosystem II. Nucleic Acids Res. 1990 Dec 11;18(23):7179–7179. doi: 10.1093/nar/18.23.7179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winning B. M., Day C. D., Sarah C. J., Leaver C. J. Nucleotide sequence of two cDNAs encoding the adenine nucleotide translocator from Zea mays L. Plant Mol Biol. 1991 Aug;17(2):305–307. doi: 10.1007/BF00039511. [DOI] [PubMed] [Google Scholar]
- Worrell A. C., Bruneau J. M., Summerfelt K., Boersig M., Voelker T. A. Expression of a maize sucrose phosphate synthase in tomato alters leaf carbohydrate partitioning. Plant Cell. 1991 Oct;3(10):1121–1130. doi: 10.1105/tpc.3.10.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoubak S., Richardson J. H., Rynditch A., Höllsberg P., Hafler D. A., Boeri E., Lever A. M., Bernardi G. Regional specificity of HTLV-I proviral integration in the human genome. Gene. 1994 Jun 10;143(2):155–163. doi: 10.1016/0378-1119(94)90091-4. [DOI] [PubMed] [Google Scholar]




