Abstract
OBJECTIVES
Retained haemothorax and pneumothorax are the most common complications after blunt chest traumas. Lung lacerations derived from fractures of the ribs are usually found in these patients. Video-assisted thoracoscopic surgery (VATS) is usually used as a routine procedure in the treatment of retained pleural collections. The objective of this study was to find out if there is any advantage in adding the procedure for repairing lacerated lungs during VATS.
METHODS
Patients who were brought to our hospital with blunt chest trauma were enrolled into this prospective cohort study from January 2004 to December 2011. All enrolled patients had rib fractures with type III lung lacerations diagnosed by CT scans. They sustained retained pleural collections and surgical drainage was indicated. On one group, only evacuation procedure by VATS was performed. On the other group, not only evacuations but also repair of lung injuries were performed. Patients with penetrating injury or blunt injury with massive bleeding, that required emergency thoracotomy, were excluded from the study, in addition to those with cardiovascular or oesophageal injuries.
RESULTS
During the study period, 88 patients who underwent thoracoscopy were enrolled. Among them, 43 patients undergoing the simple thoracoscopic evacuation method were stratified into Group 1. The remaining 45 patients who underwent thoracoscopic evacuation combined with resection of lung lacerations were stratified into Group 2. The rates of post-traumatic infection were higher in Group 1. The durations of chest-tube drainage and ventilator usage were shorter in Group 2, as were the lengths of patient intensive care unit stay and hospital stay.
CONCLUSIONS
When compared with simple thoracoscopic evacuation methods, repair and resection of the injured lungs combined may result in better clinical outcomes in patients who sustained blunt chest injuries.
Keywords: Blunt thoracic injury, Haemothorax, Pneumothorax, Thoracotomy, Video-assisted thoracoscopic surgery
INTRODUCTION
Blunt chest injury is the most common among all chest traumas. Most of these injuries need only conservative treatments such as bed rest and pain relief. However, when accompanied with haemothorax and pneumothorax, additional management strategies may be needed [1, 2]. The majority of these pleural collections could be diagnosed by chest images during initial surveys and most of them could be managed successfully by tube thoracostomies only [3–5].
Computed tomography (CT) is a tool used in surveys of chest wall injuries and lung parenchymal injuries [3, 4, 6]. There are three types of lung parenchymal lacerations classified by Wagner et al. [3] according to their locations and trauma mechanisms (Table 1). The type III laceration is located peripherally because it is usually associated with rib fractures (Fig. 1). This lesion is an important factor in the induction of haemothorax and pneumothorax [4]. Accompanied with chest tube obstructions, retained pleural collections could occur. Further surgical intervention(s) should be considered to prevent post-traumatic complications [7, 8].
Table 1:
Wagner's classification for lung laceration diagnosed from computed tomography
| Type I | Centrally located lesion, produced from shearing between the lung parenchyma and the tracheobronchial tree |
| Type II | Tubular lesion, located at the lower lobes; the lower chest is suddenly compressed, squeezing the lower lobes against the vertebral bodies |
| Type III | Small, rounded and peripherally located, frequently associated with rib fractures and pneumothorax |
| Type IV | Shearing of the lung from traction of previously formed pleuropulmonary adhesions over the parenchyma |
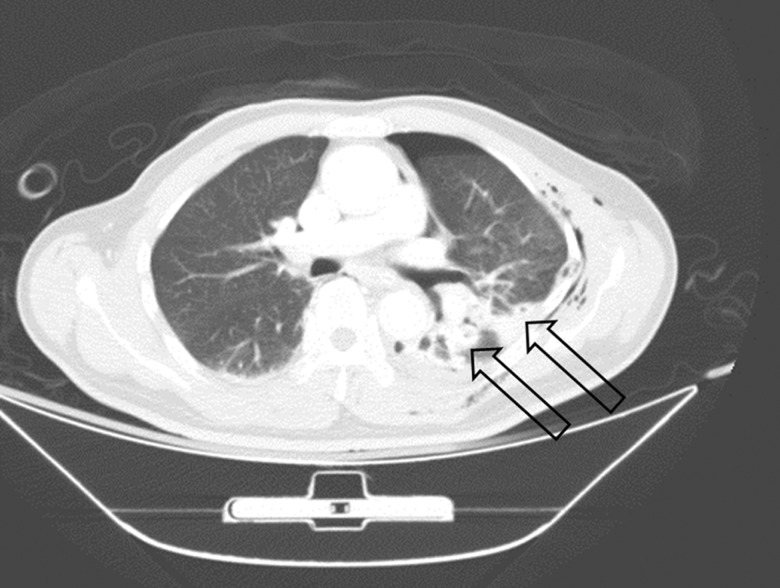
Figure 1:
A patient has left-sided multiple rib fractures with haemothorax and pneumothorax. Two lacerations of the lung parenchyma could be noted on computed tomography (arrows).
Video-assisted thoracoscopic surgery (VATS) has become a practical reality in chest surgery since 1990, due to the advances in endoscopic technology [9–11]. Thoracic surgeons use VATS in the treatment of retained haemothorax or pneumothorax; however, some thoracic surgeons have focused on adequate drainage without management of lung parenchymal injury, while others have advanced the procedure to repair lung lacerations along with adequate drainage. The objective of this study was to find out if there is any advantage in repairing or resecting traumatic lung laceration to reduce the incidence of infections, with any benefits in terms of patient clinical outcomes.
MATERIALS AND METHODS
Patients and setting
This prospective cohort study from January 2004 to December 2011 included patients with blunt chest trauma. Patients with thoracic injuries were brought to the Kaohsiung Veterans General Hospital either by the emergency medical system (after an accident) or transferred from another hospital after primary management. Once admitted, patients were taken immediately to the trauma bay, surveyed by the trauma teams and managed according to Advanced Trauma Life Support (ATLS) guidelines. Resuscitation was initiated right away if the patients were identified as having unstable vital signs. Those with more stable vital signs underwent detailed medical history evaluations with complete physical examinations. CT-scan was routinely performed for further evaluation of any lesions found on chest X-ray. After the completion of primary treatment in the trauma bay, patients were admitted to the intensive care unit (ICU) for further care. A written informed consent was obtained from family members of each patient. This study was approved by the Institution Review Board of Kaohsiung Veterans General Hospital to review the medical record.
All patients included in this study were patients having type III lung lacerations that were diagnosed by chest CT scans during the initial assessment, and post-traumatic haemothorax or pneumothorax were managed with tube thoracostomy in the trauma bay. Blood loss was recorded initially and several hours after insertion of the chest tube. During the in-hospital period, age, gender, trauma mechanisms, abbreviated injury score (AIS) according to the 2005 edition and length of hospitalization were all recorded. All post-traumatic complications were recorded, including retained thoracic collections, respiratory failure, post-traumatic pneumonia and post-traumatic empyema. This study excluded patients with penetrating injury or blunt injury with massive bleeding that required emergent or urgent thoracotomy for checking the bleeding sites. Patients who had cardiovascular or oesophageal injuries were also excluded. Although associated injuries were all recorded, those having an AIS equal to or over grade 3 were also excluded from this study.
Type III laceration of lung parenchyma according to Wagner's classification is diagnosed by chest CT [3, 6]. This lesion appears as a pneumatocele located near the lung surface, and usually has fractured ribs beside it. An air–fluid level could sometimes be seen in the lesion. In addition to these findings, subcutaneous emphysema, haemothorax and pneumothorax could also be found together. Lung contusions are also diagnosed by chest CT simultaneously, and the pulmonary contusion score would be calculated and recorded [12]. All these lung lacerations were confirmed by attending radiological physicians in our hospital. Two conditions signify massive bleeding in chest trauma. One is a haemothorax of >1500 ml initially drained from a tube thoracostomy. The other is the rate of bleeding >250 ml/h and lasting at least 4 h. For both these conditions exploratory thoracotomy is indicated to check bleeding sites, which were excluded from this study.
Post-traumatic infections were defined as positive microbial culture of sputum or pleural effusion. A retained thoracic collection was defined as persistent haemothorax or pneumothorax that could not be drained after tube thoracostomies, lasting for at least 72 h. These findings were monitored by checking of chest X-rays, obtained once daily after admission. Increased density and blunting of the costophrenic angle were estimated to be retained collections associated with obstruction of the chest tube and a repeat chest CT was indicated to validate the chest X-ray findings. Surgical interventions were considered in the event of retained pleural collections.
Operative methods
There are two thoracic surgery teams in our hospital. All thoracic surgeons receive similar training programmes and the operative equipment is the same. Patients with chest injuries admitted to our hospital were equally distributed to the two thoracic surgical teams. The first group comprised patients admitted on odd-numbered dates and the other group comprised patients admitted on even-numbered dates. The first group underwent thoracoscopy for treating pleural collections only by the ‘evacuation’ method. This method focuses on the management of pleural collections without repair of lung lesions. All patients underwent a tube thoracostomy at the ER and the incision was made along the anterior axillary line at the fifth intercostal space. A 0° angle, 10-mm thoracoscope was used. Another thoracostomy was made along the mid axillary line at the seventh intercostal space. Pleural effusion and blood clots were removed by suction tube, referred to as the evacuation procedure. This procedure focuses on adequate drainage and re-expansion of the collapsed lungs. After these processes were performed, two new chest tubes were placed; 32-Fr straight and curved chest tubes were usually used. Continuous suction of the chest tubes with −15 cmH2O was performed and they were removed when there was no air leakage or if the amount of drainage from the chest tube was <100 ml per day.
The second group underwent thoracoscopy that had small differences from the former, referred to as the ‘evacuation with suture-resection’ method. As for the first group, another thoracostomy was made at the seventh intercostal space along the mid axillary line. After this drainage, thorough inspection of the lung surface was done to look for lacerations noted at the previous chest CT, especially the lung surface attached to the site of fractured ribs. When these lesions were found under thoracoscopic vision, the previously used 10.5 mm 0° angle scope was changed to a 5 mm 0° angle thoracoscope. A 5-mm Endo-clinch grasper was applied parallel to the thoracoscope through the same site. When the lesions were checked again, both edges of the lacerated lung were grasped and repaired using an endoscopic auto-stapler (Specialist Surgical Product, Covidien Taiwan Limited) inserted through the previous thoracostomy (usually in the fifth intercostal space, anterior axillary line). As in the previous intervention for the first group, 32-Fr straight and curved chest tubes were placed.
All patients were admitted to the ICU postoperatively for close observation, with the duration of ventilator usage recorded. Patients were weaned off the ventilator when their vital signs were stable along with normal oximeter readings. The chest tubes were connected to continuous low-pressure suction and the volume of chest tube drainage was recorded daily. The chest tube was removed when the pleural effusion in the past 24 h amounted to <100 ml, without continuous air leakage. All patients enrolled were followed up at our outpatient clinics for 1 year.
Statistical analysis
The objective was to determine the superiority of repairing lung lacerations, when compared with patients with no repair. A decrease of 20% in the duration of ventilator use (mean ± standard deviation (SD): 15 ± 5) and the length of hospital stay (mean ± SD: 15 ± 5) was assumed. Per group, 44 patients were needed to achieve a power of 80% to detect a difference between groups with a one-sided level of significance of 0.025. Simple means were used for frequency and percentages for the categorical variables, while SDs were used for the continuous variables. Categorical variables were compared by the χ2 test or Fisher's exact test, and numerical variables were compared by the t-test or the Wilcoxon rank-sum test. A value of P < 0.05 was considered statistically significant. Data analyses were performed using the SPSS software (Version 16; SPSS, Inc., Chicago, IL, USA).
RESULTS
During this study period, 1958 patients with blunt chest injuries were admitted to our hospital. In total, 1352 patients complicated with haemothorax or pneumothorax were treated with tube thoracostomies. About 15% of patients (203 patients) had retained pleural collections so that surgical interventions had to be arranged. Eighty-eight patients having type III lung lacerations diagnosed by chest CT were enrolled, including 71 males and 17 females. There was a wide variation in the age distribution, ranging from 18 to 87 years, with a mean age of 53.57 (±16.10) years. The cohort included 54 patients with motorcycle accidents, 11 with walking or bicycle accidents, 12 with falls and 11 with car accidents. Of these, only 7 patients had single chest trauma, while the remainder had two or more sites of injury, including 32 head injuries, 28 abdominal injuries and 51 fractured limbs. The mean AIS of the chest was 3.25. Eighty-five patients were admitted to the ICU for close observation.
Forty-three patients who underwent thoracoscopic evacuation methods were stratified into Group 1. The remaining 45 patients who underwent evacuation plus resection and repair by thoracoscopic methods were stratified into Group 2. The characteristics and demographics are listed in Table 2. The distributions of age and gender of the two groups were comparable, along with a comparable proportion of flail chest, number of rib fractures and mean AIS of chest. However, Group 2 had slightly higher grades in injury severity scores (ISSs), but there was no statistical and clinical significance (16.40 ± 4.46 vs 18.07 ± 5.84, P = 0.134).
Table 2:
Comparison of patient characteristics and demographics between two groups with different surgical interventions
| Evacuation (43) Group 1 |
Evacuation with resection-repair (45) Group 2 |
P-value | |
|---|---|---|---|
| Age | 54.95 ± 14.87 | 52.24 ± 17.26 | 0.432 |
| Gender (male) | 34 (79.1%) | 37 (82.2%) | 0.708 |
| Number of fractured ribs | 5.93 ± 2.93 | 5.36 ± 2.52 | 0.327 |
| Flail chest | 18 (41.9%) | 18 (40.0%) | 0.859 |
| Patients with lung contusion involving more than two lobes | 40 (93.0%) | 43 (95.6%) | 0.608 |
| Pulmonary contusion score | 7.84 ± 2.79 | 8.47 ± 1.63 | 0.203 |
| Acute respiratory failure in 4 h after trauma | 19 (44.2%) | 15 (33.3%) | 0.296 |
| Time from trauma to VATS | 7.30 ± 6.54 | 6.31 ± 2.59 | 0.356 |
| ISS | 16.40 ± 4.46 | 18.07 ± 5.84 | 0.134 |
| AIS chest | 3.19 ± 0.39 | 3.31 ± 0.47 | 0.178 |
AIS: abbreviated injury score; ISS: injury severity score.
The pulmonary contusion scores were calculated and recorded according to the chest radiological images. Nearly all patients had lung contusions involving more than two lobes of the lung (93.0 vs 95.6%, P = 0.608) and pulmonary contusion scores were nearly equal between the two groups (7.84 ± 2.79 vs 8.47 ± 1.63, P = 0.203). Because most patients had multiple traumas, the overall mean time from trauma to the operation was 6.79 ± 4.92 days. The timing of operation was not statistically significant between the two groups (7.30 ± 6.54 days vs 6.31 ± 2.59 days, P = 0.356). Thirty-four patients received endotracheal tube intubation within 4 h after trauma and the percentage of immediate acute respiratory failures was nearly the same in both groups (44.2 vs 33.3%, P = 0.296).
The clinical outcomes are listed in Table 3. Post-traumatic infections occurred in 44 patients. Group 1 patients had more post-traumatic infections confirmed by higher positive microbial cultured rates in sputum and pleural effusions (65.1 vs 35.6%, P = 0.006; 46.5 vs 17.8%, P = 0.004). The mean ventilator utilization time was 10.32 (±12.26) days. In Group 1, the mean ventilator usage times were longer than Group 2 (13.61 ± 14.88 vs 7.18 ± 8.05 days, P = 0.015). The overall mean duration of chest tube usage was 13.88 (±9.70) days, with a shorter duration in Group 2 when compared with Group 1 (16.65 ± 12.56 vs 11.24 ± 4.55 days, P = 0.010). The overall mean length of stay (LOS) in the ICU was 12.26 (±12.97) days, with Group 2 having a shorter stay in the ICU when compared with Group 1 (16.60 ± 16.90 vs 8.11 ± 4.91 days, P = 0.003). The total in-hospital LOS in Group 2 was much shorter than for Group 1 (34.07 ± 24.23 days compared with 19.38 ± 11.39 days, P = 0.001). The blood loss during VATS was difficult to estimate due to surgical haemorrhage being mixed with retained effusions. Because no patient was converted to thoracotomy in this study, we thought the blood loss in the VATS procedure was small. Group 2 had slightly higher evacuated volume than the other, but there was no statistical significance (448.37 ± 84.23 vs 470.22 ± 63.73, P = 0.175).
Table 3:
Comparison of clinical outcomes between two groups with different surgical interventions
| Evacuation (43) Group 1 |
Evacuation with resection and repair (45) Group 2 |
P-value | |
|---|---|---|---|
| Duration of ventilator support (days) | 13.61 ± 14.88 | 7.18 ± 8.05 | 0.015 |
| Duration of chest tube use | 16.65 ± 12.56 | 11.24 ± 4.55 | 0.010 |
| Positive microbial cultures in sputum | 28 (65.1%) | 16 (35.6%) | 0.006 |
| Positive microbial cultures in pleural effusions | 20 (46.5%) | 8 (17.8%) | 0.004 |
| Secondary VATS | 6 (14.0%) | 2 (4.4%) | 0.121 |
| ICU LOS | 16.60 ± 16.90 | 8.11 ± 4.91 | 0.003 |
| In-hospital LOS | 34.07 ± 24.23 | 19.38 ± 11.39 | 0.001 |
| Mortality | 2 (4.7%) | 0 | 0.143 |
LOS: length of stay; ICU: intensive care unit.
Eight patients had postoperative complications with reaccumulating pleural collections. Six patients were Group 1 and the others were Group 2. All of them underwent a secondary VATS procedure for drainage. In Group 1, the percentage of this complication was higher than in Group 2, but without statistical significance (14.0 vs 4.4%, P = 0.121). There were several small surgical complications after VATS in both groups. Two in Group 1 and 3 in Group 2 were complicated with wound oozing. All of them were treated successfully with compression or resutured. Only 1 patient in Group 2 had lung parenchymal injury due to the instrument being introduced into the pleural cavity. This complication was treated with auto-stapler suturing immediately without further complications. Eight patients underwent a secondary VATS procedure. All of them underwent evacuation procedures. There was no complication after the secondary VATS procedure.
Two patients expired in this study; both were from Group 1. These 2 patients expired by severe infection after trauma. One patient had a head injury with subarachnoid haemorrhage. This head injury induced aspiration pneumonia, which worsened the lung infection. The other patient was elderly, over 80 years of age. Post-traumatic pneumonia leading to sepsis was the main reason for his death. Although no patient expired in Group 2, there were no statistical differences in mortality between the two groups.
DISCUSSION
Post-traumatic infections are dangerous complications of blunt chest injuries, which could increase both mortality and morbidity [1, 2]. Retained pleural collection is one of the important causes of induced infections. The origin of microbes is not only airways but also the epidermis carried by tube thoracostomies [7, 8, 13]. Adequate drainage accompanied with antibiotics should be performed. Since 1995, VATS has been widely applied in trauma patients as a popular method instead of secondary tube thoracostomies [9, 14]. The main target of VATS is evacuation of retained pleural collection [10, 11, 15]. Intrathoracic lesions without life-threatening conditions are usually managed with observation conservatively.
Chest CT is now a routine procedure performed for blunt chest trauma in many hospitals. It could provide clear visualizations for chest wall and lung parenchyma injuries, including lung lacerations [4, 6]. According to the classification of Wagner et al. [3], type III lung parenchymal laceration is highly associated with rib fractures. Most of these lesions are located at the surface of the lungs that could evolve into haemothorax and pneumothorax. However, this type of lung laceration could be confirmed easily during VATS, and usually there is cessation of bleeding at initial insertion of the thoracoscope. Rebleeding from these lesions may occur after contact with a suction tube or other endoscopic instruments. In addition, air leakage could also re-occur. Necrotic lung tissues or pulmonary haematomas are usually found around these lacerations.
In this study, we found that post-traumatic infection rates were lower in the surgical resection group. Once the infections decreased, the ventilator usage period and LOS in hospital could both be shortened. Although the lung lacerations could be found easily by CT or during operations, surgical repair or resections are still controversial. The endoscopic suture repair is used mainly for treating pneumothorax with continuous air leakage [5, 11]. Evacuation is still the main method during VATS; however, if lung lacerations are large enough to be seen in chest CT, there is high potential to induce retained pleural collection. In addition to this, necrotic lung tissue also provides a good cultural medium for bacterial growth. In combination with retained pleural collections, infection rates would be elevated. The results of the study found that the rates of positive microbial cultures including sputum and pleural effusions were both lower in Group 2. Thus, we consider that resection could prevent and decrease post-traumatic infections. Once the infections were prevented, lung functions could be restored more rapidly. Besides, in Group 1, the higher infection rates also increased mortality.
In this study, we also found that the duration of chest tube usage could be decreased in Group 2. Surgical resection and repair of the lacerated lung could stop oozing and micro air leakage faster and hence facilitate early removal of chest tubes. Most of them could be removed within 1 week. This is an important factor to influence the in-hospital LOS. In Group 1 patients, the un-sutured lungs could produce another chance of retained pleural collections again after the first VATS evacuation. Although the incidence rate of secondary retained pleural effusions had no statistical difference between the two groups, we thought Group 1 had a higher rate because of the ease of oozing from the injured lung.
In our study, most of these procedures could be finished in 40 min. The procedures of VATS to repair and resect are not complicated. Only a few complications during VATS occurred in Group 2. Most of them were related to the procedure itself, including 3 patients who had bleedings from incision wounds and one from introduction of instruments into the lung parenchyma. The bleeding from incision wounds resolved spontaneously or was managed with tight sutures, and punctured lung parenchyma was treated with an endoscopic auto-stapler during the surgery. Moreover, these visible type III lung lacerations are located near the surface of the lungs. Surgical resections of these lesions are safe, and might not influence residual lung functions.
There are several limitations in this study. During an 8-year period, only 88 patients were included. The case numbers were difficult to accumulate in a short time because most blunt chest traumas could be treated with non-surgical methods and only about 10% of blunt chest injuries will evolve to retained pleural collections where surgical interventions should be arranged. However, the strength of this study is that the two groups were divided near-randomly. All the demographics in the two groups were similar. Surgical timing and indications for these two groups had no differences. The only difference was that Group 2 patients had undergone surgical repair and resection of the lung lacerations. Besides, to decrease the bias between the groups, patients with severe medical diseases such as liver cirrhosis, chronic renal failure and chronic heart–lung diseases were excluded. The gender, age and trauma mechanism between the two groups were matched. In addition, due to blunt trauma usually resulting in multiple injuries with these conditions creating great variation between patients, the ISS and regional AIS between the two groups were also matched in this study. The associated injuries that were severe enough to influence the clinical outcomes such as patients needing emergency thoracotomy were also excluded. All of the above could decrease the types of patients enrolled and patient numbers. Furthermore, lung contusion is another important factor but is difficult to quantify. Destroyed chest structures could influence lung function after trauma. The numbers of rib fractures and a flail chest are important factors in worsening the lung functions. In our study, we used the pulmonary contusion score to evaluate the condition of lung parenchymal injuries although this method may also have bias because of subjective recognition based only on chest X-ray from chart reviews. However, all patients having haemothorax and pneumothorax that could influence the exact judgments for the pulmonary contusion score were reviewed by a senior surgeon with the same criteria. In addition, the timing of VATS introduced for retained pleural collections is another limitation. Although the surgical indications in our hospital are similar for all thoracic surgeons, the decision for the timing of operations is varied, and may be due to associated injuries. In this study, the mean time from trauma to surgical intervention was 7 days. The timing in Group 2 was slightly shorter than the evacuation-only group. This might be due to a higher chest AIS and pulmonary contusion score in Group 2.
In conclusion, for patients with lung lacerations noted on chest CT accompanied with residual pleural collection, intervention by VATS intervention for evacuation of pleural collection with suture repair of the injured lung could provide better clinical outcomes.
Conflict of interest: none declared.
REFERENCES
- 1.Poole GV, Jr., Myers RT. Morbidity and mortality rates in major blunt trauma to the upper chest. Ann Surg. 1981;193:70–5. doi: 10.1097/00000658-198101000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Richardson JD, Miller FB, Carrillo EH, Spain DA. Complex thoracic injuries. Surg Clin North Am. 1996;76:725–48. doi: 10.1016/s0039-6109(05)70477-1. [DOI] [PubMed] [Google Scholar]
- 3.Wagner RB, Crawford WO, Jr., Schimpf PP. Classification of parenchymal injuries of the lung. Radiology. 1988;167:77–82. doi: 10.1148/radiology.167.1.3347751. [DOI] [PubMed] [Google Scholar]
- 4.Blostein PA, Hodgman CG. Computed tomography of the chest in blunt thoracic trauma: results of a prospective study. J Trauma. 1997;43:13–8. doi: 10.1097/00005373-199707000-00006. [DOI] [PubMed] [Google Scholar]
- 5.Meredith JW, Hoth JJ. Thoracic trauma: when and how to intervene. Surg Clin North Am. 2007;87:95–118. doi: 10.1016/j.suc.2006.09.014. vii. [DOI] [PubMed] [Google Scholar]
- 6.Sangster GP, Gonzalez-Beicos A, Carbo AI, Heldmann MG, Ibrahim H, Carrascosa P, et al. Blunt traumatic injuries of the lung parenchyma, pleura, thoracic wall, and intrathoracic airways: multidetector computer tomography imaging findings. Emerg Radiol. 2007;14:297–310. doi: 10.1007/s10140-007-0651-8. [DOI] [PubMed] [Google Scholar]
- 7.Eddy AC, Luna GK, Copass M. Empyema thoracis in patients undergoing emergent closed tube thoracostomy for thoracic trauma. Am J Surg. 1989;157:494–7. doi: 10.1016/0002-9610(89)90643-0. [DOI] [PubMed] [Google Scholar]
- 8.Etoch SW, Bar-Natan MF, Miller FB, Richardson JD. Tube thoracostomy. Factors related to complications. Arch Surg. 1995;130:521–5. doi: 10.1001/archsurg.1995.01430050071012. discussion 25–6. [DOI] [PubMed] [Google Scholar]
- 9.Landreneau RJ, Keenan RJ, Hazelrigg SR, Mack MJ, Naunheim KS. Thoracoscopy for empyema and hemothorax. Chest. 1996;109:18–24. doi: 10.1378/chest.109.1.18. [DOI] [PubMed] [Google Scholar]
- 10.Manlulu AV, Lee TW, Thung KH, Wong R, Yim AP. Current indications and results of VATS in the evaluation and management of hemodynamically stable thoracic injuries. Eur J Cardiothorac Surg. 2004;25:1048–53. doi: 10.1016/j.ejcts.2004.02.017. [DOI] [PubMed] [Google Scholar]
- 11.Ahmed N, Jones D. Video-assisted thoracic surgery: state of the art in trauma care. Injury. 2004;35:479–89. doi: 10.1016/S0020-1383(03)00289-4. [DOI] [PubMed] [Google Scholar]
- 12.Tyburski JG, Collinge JD, Wilson RF, Eachempati SR. Pulmonary contusions: quantifying the lesions on chest X-ray films and the factors affecting prognosis. J Trauma. 1999;46:833–8. doi: 10.1097/00005373-199905000-00011. [DOI] [PubMed] [Google Scholar]
- 13.Helling TS, Gyles NR, III, Eisenstein CL, Soracco CA. Complications following blunt and penetrating injuries in 216 victims of chest trauma requiring tube thoracostomy. J Trauma. 1989;29:1367–70. doi: 10.1097/00005373-198910000-00013. [DOI] [PubMed] [Google Scholar]
- 14.Heniford BT, Carrillo EH, Spain DA, Sosa JL, Fulton RL, Richardson JD. The role of thoracoscopy in the management of retained thoracic collections after trauma. Ann Thorac Surg. 1997;63:940–3. doi: 10.1016/s0003-4975(97)00173-2. [DOI] [PubMed] [Google Scholar]
- 15.Carrillo EH, Richardson JD. Thoracoscopy for the acutely injured patient. Am J Surg. 2005;190:234–8. doi: 10.1016/j.amjsurg.2005.05.018. [DOI] [PubMed] [Google Scholar]