Abstract
Exacerbated inflammation in renal ischemia-reperfusion injury, the major cause of intrinsic acute renal failure, is a key trigger of kidney damage. During disease endogenous danger signals stimulate innate immune cells via Toll-like receptor (TLR)-2 and -4 and accelerate inflammatory responses. Here we show that production of soluble biglycan, a small leucine-rich proteoglycan, is induced during reperfusion and that it functions as endogenous agonist of TLR-2/4. Biglycan-mediated activation of TLR-2/4 initiates an inflammatory response in native kidneys, which is marked by the release of cytokines and chemokines and recruitment of inflammatory cells. Overexpression of soluble circulating biglycan proteoglycan before ischemic reperfusion enhanced plasma and renal levels of TNF-α, CXCL1, CCL2 and CCL5, caused influx of neutrophils, macrophages and T cells and overall worsened renal function in wild type mice. We provide robust genetic evidence for TLR-2/4 requirement insofar as biglycan biological effects were markedly dampened in mice deficient in both innate immune receptors, Tlr2−/−;Tlr4−/− mice. Thus, signaling of soluble biglycan via TLR-2/4 could represent a novel therapeutic target for the prevention and possibly treatment of patients with acute renal ischemia-reperfusion injury.
Keywords: Proteoglycan, inflammation, innate immunity, Toll-like receptor, extracellular matrix, danger signal
1. Introduction
Renal ischemia-reperfusion injury (IRI) is the major cause of intrinsic acute renal failure in native kidneys (Hassan et al., 2009). Due to a lack of suitable therapies, this disease has a high mortality rate reaching 50%. A sudden restriction of blood supply with subsequent reperfusion elicits acute renal failure through endothelial dysfunction, leukocyte-mediated inflammation and decreased microvascular blood flow (Munshi et al., 2011). A similar pathophysiology also occurs in shock, transplantation, myocardial infarction, certain infections and trauma (Arumugam et al., 2009).
Following ischemia, reperfusion is accompanied by an inflammatory process including innate and adaptive immunity that cause renal injury and long-term structural changes. Up to now the complex molecular and cellular interactions among endothelial, inflammatory and injured epithelial cells remain poorly understood (Bonventre and Yang, 2011). Toll-like receptors (TLR) have been shown to influence IRI in experimental models (Rusai et al., 2010). During acute kidney injury, TLR-activation initiates inflammatory responses marked by NF-κB-dependent cytokine (e.g. TNF-α, IL-1β) and chemokine production and attraction of immune cells (Arumugam et al., 2009; Bonventre and Yang, 2011). Recent work implies a central role for TLR-2/4 in renal IRI (Bonventre and Yang, 2011; Leemans et al., 2005; Mittag et al., 2011; Pulskens et al., 2008; Shigeoka et al., 2013; Wu et al., 2007) as both are upregulated upon kidney IRI, particularly in renal tubular epithelial cells and leukocytes (Arumugam et al., 2009; Bonventre and Yang, 2011). Further, Tlr2−/−;Tlr4−/− mice exhibit lower level of serum creatinine and less tubular damage after IRI (Krüger et al., 2009; Leemans et al., 2005; Pulskens et al., 2008; Shigeoka et al., 2013; Wu et al., 2007).
Besides recognizing pathogen associated molecular patterns, TLR-2/4 respond to endogenous ligands released during tissue stress and injury occurring e.g. during IRI (Erridge, 2010; Frey et al., 2013; Krüger et al., 2009; Newton and Dixit, 2012; Wu et al., 2007). Indeed, expression of endogenous TLR-4 ligands HMBG1, HSP70, hyaluronan and biglycan is up regulated upon renal ischemic reperfusion (Wu et al., 2007). Thus, sterile inflammation is crucial for IRI pathophysiology and hence is targeted for therapeutic interventions. Progress has been made in defining major components of this inflammatory process; yet complex molecular and cellular interactions among endothelial cells and immune cells and their modulation by endogenous danger signals like soluble biglycan remain poorly understood (Bonventre and Yang, 2011).
Biglycan is a danger associated molecular pattern of extracellular origin (Schaefer et al., 2005). Upon tissue stress or injury, biglycan is proteolytically released from the extracellular matrix to translate danger to the immune system (Schaefer, 2010). In the soluble form biglycan now triggers TLR-2/4 on macrophages and dendritic cells, thereby activating p38, ERK and NF-κB pathways and subsequently inducing proinflammatory cytokines like TNF-α (Babelova et al., 2009; Moreth et al., 2010; Schaefer et al., 2005; Zeng-Brouwers et al., 2013). Further, biglycan cross-links P2X4 and P2X7 receptors with TLR-2/4 and mediates maturation and secretion of IL-1β in macrophages (Babelova et al., 2009). It is now well accepted that biglycan correlates with organ dysfunction in sterile renal inflammation (Babelova et al., 2009; Moreth et al., 2010; Schaefer, 2011). In an experimental model of lupus nephritis, biglycan induces chemoattractants such as CCL2, CCL5 and CXCL13 in macrophages and dendritic cells. Thereby, migration of neutrophils, macrophages, T cells and B cells into the kidney is promoted (Moreth et al., 2010; Zeng-Brouwers et al., 2013). Moreover, overexpression of soluble biglycan accelerates inflammation and organ damage in a TLR-2/4-dependent manner (Zeng-Brouwers et al., 2013). In contrast, lack of biglycan reduces cytokine and chemokine production, resulting in attenuation of lupus nephritis. Biglycan also plays a crucial role in MHC I and MHC II-restricted T cell cross-priming by acting through TLR-2/4 and their adaptor proteins (Popovic et al., 2011).
To better understand the pathophysiology of IRI and its modulation by biglycan, we analyzed the impact of biglycan-triggered TLR-2/4 signaling on inflammatory responses in a murine model of IRI. We verified direct binding of soluble biglycan to TLR-2/4 under pure buffer conditions by microscale thermophoresis and in cell based assays. Moreover, we discovered that overexpression of biglycan was pro-inflammatory and required both TLR-2/4. Thus, interfering with biglycan signaling attenuate renal damage induced by IRI through amelioration of various immune responses.
2. Results
2.1 Biglycan is a ligand for TLR-2 and TLR-4
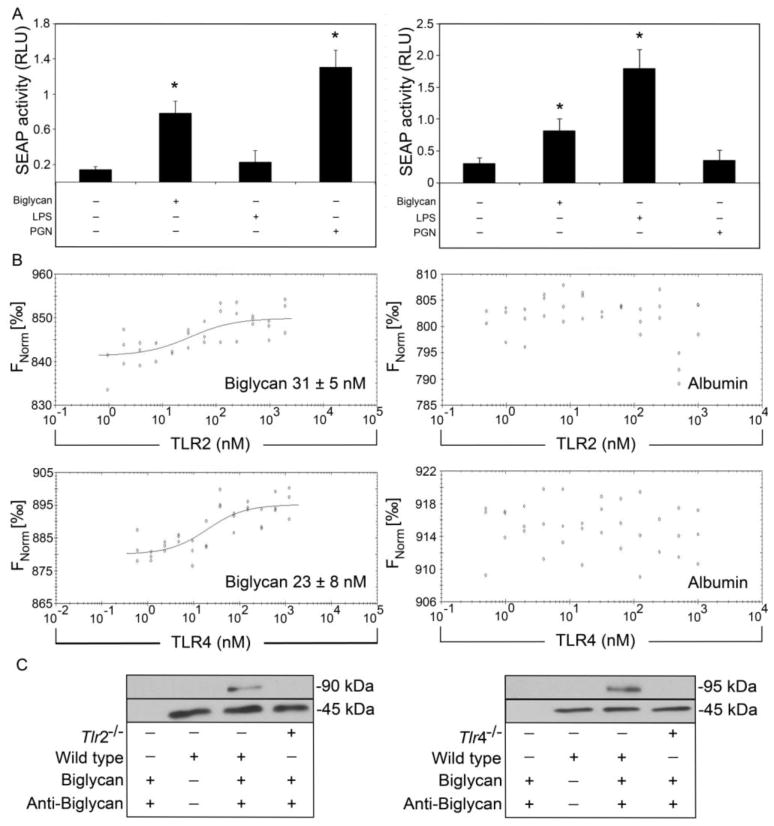
Previously we showed that soluble biglycan activates macrophages and dendritic cells via TLR-2 and TLR-4 (Babelova et al., 2009; Moreth et al., 2010; Schaefer et al., 2005). TLR stimulation directly activates NF-κB signaling. To test if biglycan binding elicited NF-κB activation we used a reporter gene assay where transcriptional activation of NF-κB was monitored by active secreted alkaline phosphatase (SEAP). Stimulation of HEK-Blue-hTLR-2 (Fig. 1A, left panel) and HEK-Blue-hTLR-4 cells (Fig. 1A, right panel) with biglycan led to increased SEAP activity indicating NF-κB activity. In control experiments NF-κB activity was only detected when HEK-Blue-hTLR-4 cells were stimulated with TLR-4 ligand lipopolysaccharide (LPS) and when HEK-Blue-hTLR-2 cells were stimulated with TLR-2 ligand peptidoglycan (PGN). However, neither LPS-stimulation of HEK-Blue-hTLR-2 cells, nor PGN-stimulation of HEK-Blue-hTLR-4 cells induced SEAP activity. Contamination of biglycan preparations was excluded as purity of biglycan was monitored as reported before (Merline et al., 2011).
Fig. 1. Biglycan directly binds Toll-like receptor-2 and -4.
(A) NF-κB activity depicted as activity of secreted alkaline phosphatase (SEAP) in HEK-Blue-hTLR2 (left) and HEK-Blue-hTLR4 (right) stimulated with biglycan (4 μg/ml), LPS (100 ng/ml) or PGN (5 μg/ml) shown as relative light units (RLU). (B) Binding of biglycan (left panel) or albumin (right panel) to NT-647-labeled recombinant human TLR-2 (upper) or TLR-4 (lower) using microscale thermophoresis. KD was calculated from three independent thermophoresis measurements. (C) Western blots show immunoprecipitation of TLR-2 (left, 90 kDa) and TLR-4 (right, 95 kDa) from wild type or Tlr2−/− or Tlr4−/− macrophages with biglycan. β-Actin served as loading control (45 kDa). Data represent means ± SD of three independent experiments. *p < 0.05.
To investigate if biglycan-induced TLR-2- or TLR-4-signaling depends on cofactors we performed microscale thermophoresis. Fluorochrome-labeled recombinant human TLR-2 and TLR-4 were incubated with increasing concentrations of biglycan until saturation of all binding sites was obtained. Biglycan bound to TLR-2 with a KD (dissociation constant) of 31 ± 5 nM and to TLR-4 with a KD of 28 ± 8 nM (Fig. 1B, left panel). To confirm specific binding of biglycan to TLR-2 and TLR-4 we used albumin as a control. As pictured, thermophoretic movements of TLR-2 and TLR-4 did not change in presence of albumin (Fig. 1B, right panels).
As human and murine biglycan are highly-conserved proteins, we tested whether human biglycan would also bind murine TLR-2/4. Therefore, human biglycan-coated beads were incubated with total lysates of macrophages from wild type, Tlr2−/−, and Tlr4−/− mice and immunoprecipitation was performed. Precipitation of murine TLR-2/4 occurred only in the presence of human biglycan, indicating that it specifically binds murine TLR-2/4 (Fig. 1C).
Together, these results demonstrate a physical interaction between soluble biglycan and TLR-2/4 in absence of any cofactors and that biglycan potently induces NF-κB transcription.
2.2 Biglycan is enhanced in plasma from mice after ischemia-reperfusion kidney injury
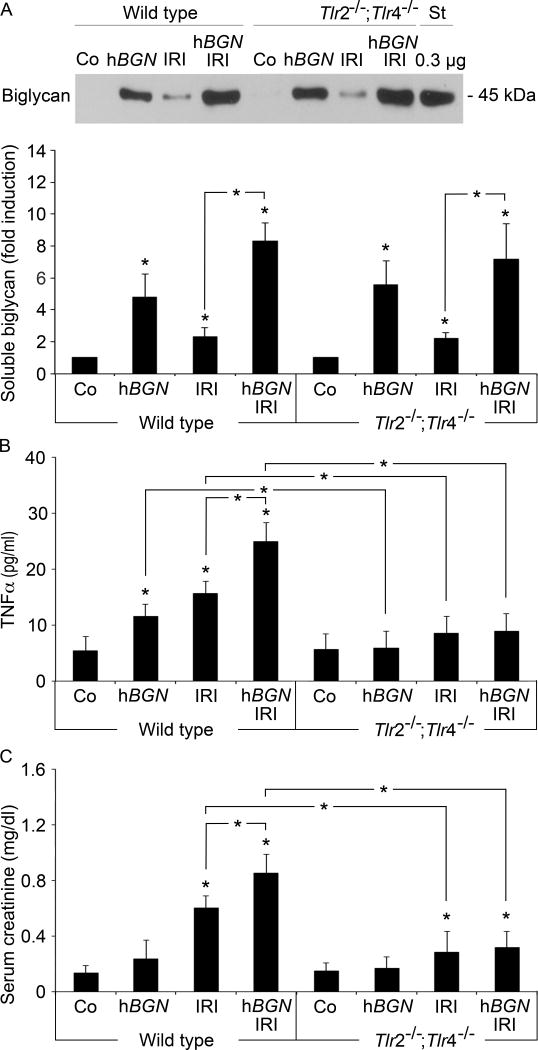
Clamping of the renal pedicle is a well-established mouse model for renal ischemia-reperfusion injury (IRI), as it mimics the pathophysiology of human acute renal failure (Hassan et al., 2009). By mediating TLR-2/4 signaling, soluble biglycan acts as endogenous proinflammatory trigger in various renal diseases (Babelova et al., 2009; Moreth et al., 2010; Schaefer, 2011; Schaefer et al., 2005). To test whether biglycan-induced TLR signaling would also play a biological role in the pathogenesis of acute renal damage, we induced renal ischemia in wild type, and Tlr2−/−;Tlr4−/− mice for 25 min followed by 30 h of reperfusion. Afterwards plasma levels of biglycan were determined. Following renal ischemia and reperfusion, circulating soluble biglycan was upregulated in wild type (4.8±1.4-fold compared to control) and Tlr2−/−;Tlr4−/− mice (5.6±1.5-fold compared to control) whereas untreated control mice did not show any detectable levels of soluble biglycan in their sera (Fig. 2A). Thus, elevated biglycan levels associate with IRI pathology and further these data demonstrate a TLR-2/4-independent biglycan production after IRI.
Fig. 2. Biglycan-mediated increases serum TNF-α and renal dysfunction in TLR-2- and TLR-4-dependent manner.
(A) Upper panel: Western blot for biglycan protein core after semi purification from plasma of wild type or Tlr2−/−;Tlr4−/− mice that underwent 30 h post-ischemia reperfusion in absence (IRI) or presence of biglycan overexpression (hBGN IRI). Sham operation (Co) or biglycan overexpression alone (hBGN) served as control. Recombinant biglycan protein core (0.3 μg) was used as standard (St). Lower panel: Quantification of serum biglycan concentrations given as fold induction over control mice. Data represent means ± SD of n = 3 experiments. (B, C) ELISA for TNF-α (B) and creatinine (C) from serum of wild type or Tlr2−/−;Tlr4−/− mice treated as in (A). Data represent means ± SD of n = 6 experiments. * p < 0.05. Asterisks indicate significance between defined groups; asterisks above bars indicate significance versus respective control.
2.3 Ischemic reperfusion-induced biglycan mediates TLR-2/4-dependent proinflammatory responses and worsens renal function
To investigate the role of biglycan-induced TLR signaling in pathophysiological processes following IRI, we overexpressed soluble human biglycan three days before ischemic reperfusion in wild type and Tlr2−/−;Tlr4−/− mice. We utilized transient liver-specific expression of human biglycan using pLIVE expression vectors. This experimental strategy leads to deposition of human biglycan in kidneys and other organs between one and 14 days post injection of hBGN pLIVE (Zeng-Brouwers et al., 2013). Four days after injection of hBGN pLIVE, plasma of wild type and Tlr2−/−;Tlr4−/− mice showed comparable levels of soluble biglycan that were further enhanced when mice additionally underwent IRI (Fig. 2A).
Next, as serum levels of proinflammatory cytokine TNF-α are elevated after ischemia (Patschan et al., 2012), we monitored the severity of inflammation by analyzing TNF-α in peripheral blood 30 h after reperfusion. Interestingly, in wild type mice without ischemic reperfusion, overexpression of soluble biglycan alone induced strong TNF-α production (Fig. 2B). Comparable amounts of TNF-α were also detectable in wild type mice that underwent IRI, whereas in sham-operated littermates no TNF-α was detected (Fig. 2B). In wild type mice, IRI and biglycan overexpression synergistically increased TNF-α in peripheral blood (Fig. 2B). However, in the absence of TLR-2/4, IRI, biglycan overexpression or their combination failed to induce TNF-α production (Fig. 2B). These findings clearly show that biglycan-mediated TNF-α production depends on both TLR-2 and TLR-4 signaling.
To investigate if soluble biglycan alone mediates renal damage, we assessed renal function by quantifying creatinine levels in the sera. Interestingly, overexpression of soluble biglycan alone did not worsen renal function in wild type mice (Fig. 2C). However, ischemic reperfusion significantly increased creatinine in wild type mice and it was further enhanced when soluble biglycan was over expressed in addition (Fig. 2C). IRI in Tlr2−/−;Tlr4−/− mice elicited lower levels of creatinine in sera compared to wild type mice. Also, as biglycan signaling required TLR-2/4, biglycan overexpression did not worsen renal function in these mice (Fig. 2C). Thus, these data suggest that biglycan acts as a TLR-2/4-dependent proinflammatory mediator of renal damage in the course of ischemic injury.
2.4 Biglycan-induced exacerbation of renal histopathology is abolished in Tlr2−/−;Tlr4−/− IRI mice
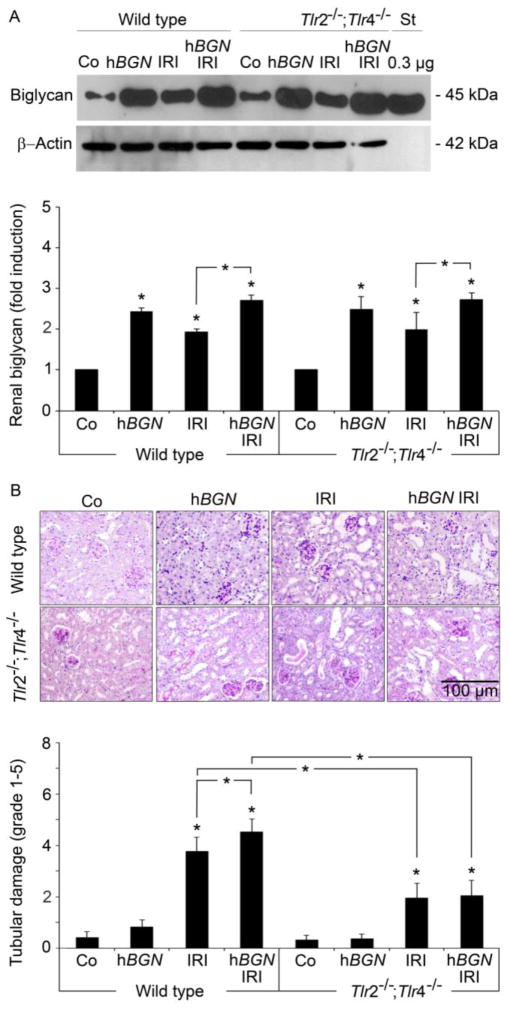
It is well established that the levels of soluble biglycan are elevated systemically in response to injury, and thus biglycan functions as endogenous mediator of inflammation and autoinflammation (Iozzo et al., 2011; Merline et al., 2012; Moreth et al., 2010; Schaefer and Iozzo, 2012). To study the influence of local biglycan levels in inflamed kidneys after IRI, we determined the accumulation of renal biglycan and tubular damage. Overexpression of soluble biglycan drastically enhanced accumulation of biglycan in the kidney (Fig. 3A). Interestingly, elevated biglycan levels were associated with significantly aggravated IRI-induced renal damage in wild type but not in Tlr2−/−;Tlr4−/− mice. Specifically, there was widespread tubular dilatation, loss of brush border, cast formation and tubular necrosis (Fig. 3B and C). Already kidney IRI alone induced comparably strong biglycan accumulation in the kidney of wild type (1.9±0.07-fold compared to sham operated controls) and Tlr2−/−/;Tlr4−/− mice (2.1±0.56-fold). However, despite comparable biglycan levels, Tlr2−/−;Tlr4−/− mice undergoing IRI showed ameliorated histopathology and reduced tubular damage compared to wild type littermates (Fig. 3B). Overexpression of soluble biglycan alone was insufficient to induce tubular damage and also sham operated control mice showed no signs of renal damage (Fig. 3B). Thus, biglycan exacerbates tubular damage in a TLR-2/4-dependent fashion after ischemic reperfusion and therefore plays a crucial role in pathogenesis of acute renal injury.
Fig. 3. Biglycan-induced exacerbation of renal histopathology in IRI mice is abolished in absence of TLR-2 and TLR-4.
(A) Western blots (upper panel) and quantification (lower panel) for biglycan following isolation of total proteoglycan from wild type or Tlr2−/−;Tlr4−/− kidney homogenates from sham operated (Co) or biglycan overexpressing mice (hBGN) or mice that underwent 30 h post-ischemia reperfusion injury in absence (IRI) or presence of biglycan overexpression (hBGN IRI). Biglycan levels were normalized to β-Actin. Data represent the means ± SD of three independent experiments. (B) Upper panel: PAS-stained kidney sections from mice treated as in (A). Bar indicates 100 μm. Lower panel: Quantification of tubular damage. Data are given as mean ± SD. Asterisks indicate significance between defined groups and asterisks above bars indicate significance versus respective control; *p < 0.05.
2.5 Biglycan-mediated TLR-2/4 signaling induces proinflammatory cytokines and chemokines during ischemia-reperfusion injury
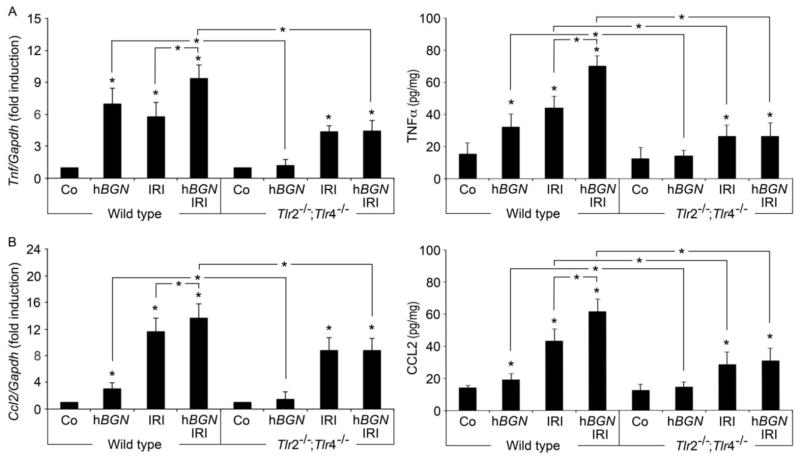
Increasing experimental evidence suggests TLR-engagement by endogenous ligands as a trigger of inflammation in response to ischemia (Wu et al., 2007). A critical factor of inflammation during renal IRI is cytokine and chemokine production (Furuichi et al., 2002). To determine effects of biglycan-mediated signaling after IRI, we examined renal expression of TNF-α and selected chemokines (CXCL1, CCL2, CCL5). Overexpression of soluble biglycan led to robust induction of TNF-α mRNA and protein in the kidney of wild type mice that was absent in Tlr2−/−/;Tlr4−/− mice (Fig. 4A). In both mouse strains, renal TNF-α was also induced after ischemic injury; however only in wild type mice TNF-α production was further enhanced when soluble biglycan was concurrently overexpressed (Fig. 4A). Sham-operated control animals showed no detectable levels of TNF-α mRNA or protein in the kidneys.
Fig. 4. Biglycan-induced TLR-2- and TLR-4-signaling mediates renal TNF-α and CCL2 production during IRI.
Determination of mRNA (left panel, normalized to Gapdh) or protein levels of renal TNF-α (A) and CCL2 (B) in total kidney homogenates of wild type or Tlr2−/−;Tlr4−/− mice. Mice underwent 30 h post-ischemia reperfusion injury in absence (IRI) or presence of overexpressed biglycan (hBGN IRI). Sham operated mice (Co) and mice that overexpressed biglycan (hBGN) served as controls. Data are given as mean ± SD of six independent experiments. Asterisks indicate significance between defined groups, asterisks above bars indicate significance versus respective control. *p < 0.05.
Next, we discovered that chemokines CXCL1 (neutrophil attraction), CCL2 (macrophage) and CCL5 (T cell attraction) were also induced by soluble biglycan. This induction was TLR-2/4-dependent, as these chemokines were not detectable in Tlr2−/−;Tlr4−/− mice (Fig. 4B and Table 1). Chemokines were also upregulated in wild type and Tlr2−/−/;Tlr4−/− mice that underwent IRI but only in wild type mice, production was further increased after concurrent overexpression of soluble biglycan.
Table 1.
Renal CXCL1 and CCL5 expression in wild type and Tlr2−/−;Tlr4−/− mice following overexpression of biglycan in the cause of IRI
| Wild type | Tlr2−/−;Tlr4−/− | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Co | hBGN | IRI | hBGN IRI | Co | hBGN | IRI | hBGN IRI | ||
| CXCL1 | Protein | 16±6 | 34±8A | 93±19A,B | 180±40A | 19±3 | 20±2E | 48±19A,C | 51±13A,D |
| mRNA | 1±0 | 3.9±2.7A | 15±3 A,B | 19±5A | 1±0 | 1.2±0.83E | 8.9±4.5A,C | 8.4±5.4A,D | |
| CCL5 | Protein | 15±4 | 59±12A | 70±14 A,B | 104±12A | 12±8 | 15±6E | 53±9 A,C | 54±14A,D |
| mRNA | 1±0 | 10±3A | 4.8±2.6A,B | 12±6A | 1±0 | 2.1±1.1E | 3.1±2.5A,C | 3.6±1.6A,D | |
Co: sham operated controls. hBGN: mice transiently transfected for 4 days with human biglycan inserted into the pLIVE vector. IRI: mice that underwent 30 h postischemic reperfusion injury. hBGN IRI: mice transiently transfected for 4 days with human biglycan inserted into the pLIVE vector and additional 30 h postischemic reperfusion injury performed on day 3. Levels of CXCL1 and CCL5 are given as pg/ml for protein and as fold induction (normalized to Gapdh) for mRNA. Values are given as mean ± SD; n=6 mice in each group.
p < 0.05 compared to age-matched wild type control,
p < 0.05 compared to age-matched wild type hBGN IRI,
p < 0.05 compared to age-matched wild type IRI,
p < 0.05 compared to age-matched wild type hBGN IRI,
p < 0.05 compared to age-matched wild type hBGN.
Collectively, our data underline the requirement of TLR-2/4 in mediating the function of soluble biglycan as endogenous danger signal in the course of ischemic injury. Further, the biglycan- TLR-2/4 axis strongly impacts induction, maintenance and acceleration of renal damage following ischemic reperfusion by inducing proinflammatory cytokines and chemokines.
2.6 Biglycan-mediated infiltration of immune cells into kidney after ischemic reperfusion requires TLR-2/4 signaling
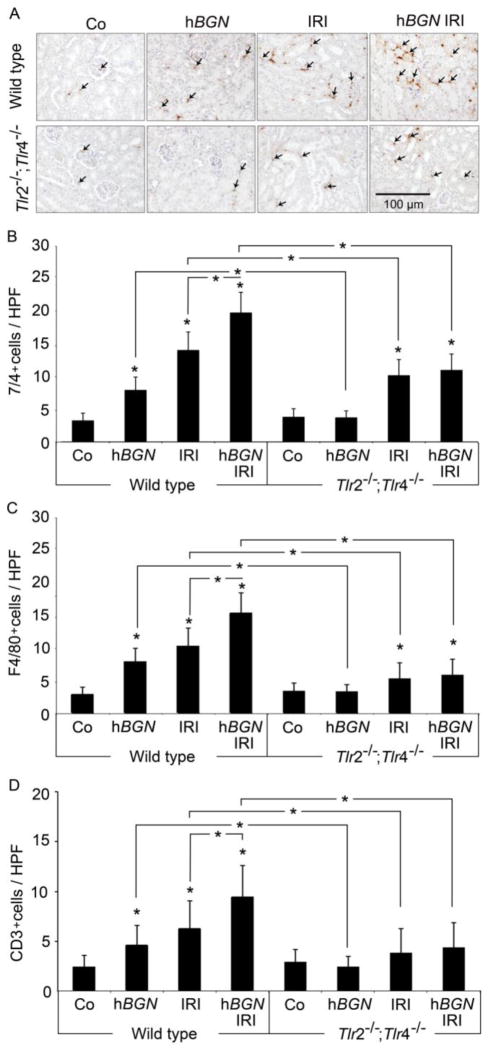
Innate and adaptive immune cells are important contributors to ischemic kidney injury (Bonventre and Yang, 2011). To analyze if biglycan-induced chemokines promote infiltration of immune cells, we performed immunohistochemistry of kidneys 30 h following ischemic reperfusion and determined frequencies of 7/4+ neutrophils, F4/80+ macrophages, and CD3+ T cells.
Renal IRI induced a strong infiltration of all tested cell populations into the kidney of wild type mice that was amplified after in vivo overexpression of soluble biglycan (Fig. 5). Monitored immune cell infiltration was indeed depended on biglycan, as renal infiltration in kidneys of Tlr2−/−;Tlr4−/− mice was strongly reduced albeit not abolished completely (Fig. 5B–D). In line, overexpression of soluble biglycan in wild type mice but not in Tlr2−/−;Tlr4−/− mice induced infiltration of neutrophils (Fig. 5A and B), macrophages (Fig. 5C) and T cells (Fig. 5D). Thus, these data show that IRI-mediated synthesis and release of biglycan induces TLR-2/4 signaling that promotes infiltration of immune cells into the inflamed kidney by secretion of chemokines.
Fig. 5. Biglycan-induced inflammatory cell influx during IRI is diminished in absence of TLR-2 and TLR-4.
(A) Representative immunohistochemical staining of 7/4+ neutrophils (brown, some depicted by arrows) and (B–D) quantifications of (B) 7/4+ neutrophils, (C) F4/80+ macrophages, and (D) CD3+ T cells in kidneys of wild type or Tlr2−/−;Tlr4−/− mice. Infiltrating cells in renal sections from mice that underwent 30 h post-ischemia reperfusion injury in absence (IRI) or presence of overexpressed biglycan (hBGN IRI), sham operated controls (Co) and mice overexpressing biglycan (hBGN) were determined, and quantified as described in Methods section (HPF, x200). Bar indicates 100 μm. Data are given as mean ± SD. Asterisks indicate significance between defined groups and asterisks above bars indicate significance versus respective control, *p < 0.05.
3. Discussion
In this study we report increasing levels of soluble biglycan in the course of ischemia-reperfusion injury (IRI). Utilizing microscale thermophoresis we demonstrate that soluble biglycan is an endogenous ligand for TLR-2 and TLR-4. Biglycan-binding to these TLR is required for induction of proinflammatory cytokines like TNF-α and chemokines (CXCL1, CCL5 and CCL2) as induction was prevented in TLR-2- and TLR-4-deficient mice. These TLR-2- and TLR-4-mediated processes promote infiltration of immune cell populations into the inflamed kidney and lead to renal tubular damage.
In the course of ischemia-reperfusion, tissue is damaged and endogenous danger signals are released. In particular, ligands for TLR-2 and TLR-4 like heat-shock proteins, HMGB1, hyaluronan, fibronectin, heparan sulfate and biglycan (Wu et al., 2010) are produced and subsequently mediate sterile inflammation. Here, we show elevated levels of biglycan in peripheral blood and kidneys after renal IRI. Recent data show that biglycan is also increased in lupus nephritis and sepsis, where it can be detected in plasma and inflamed organs (Moreth et al., 2010; Schaefer et al., 2005). As it has been shown that cytokines like IL-1β, IL-6, and TGF-β induce biglycan production in immune and renal cells, high biglycan concentrations in the course of IRI are most likely due to de novo synthesis during inflammation (Brandan and Gutierrez, 2013; Merline et al., 2012; Schaefer et al., 2005). Besides de novo synthesis, soluble biglycan is generated by proteolytic release from the extracellular matrix. Proteolytic enzymes mediating this cleavage are released from infiltrating or resident cells. Thereby, production of biglycan and its action as danger mediator enables a rapid response to tissue damage (Iozzo and Karamanos, 2010; Nikitovic et al., 2012; Schaefer, 2011).
Thirty hours of reperfusion following renal ischemia, animals show marked deterioration of renal function associated with significantly increased creatinine levels. Renal dysfunction correlates with severe tubular damage visualized by histology, and both renal function and damage in wild type mice are accelerated when soluble biglycan is overexpressed before ischemia. However, deficiency for TLR-2 and TLR-4 ameliorates biglycan-mediated acceleration of renal damage.
During the first 24 hours of reperfusion, cytokines and chemokines continuously increase and neutrophils and macrophages infiltrate the kidney (Rabb et al., 2000; Swaminathan and Griffin, 2008; Wu et al., 2010). Afterwards, further macrophages, dendritic cells and T cells are recruited (Li et al., 2008; Park et al., 2002; Wu et al., 2010) that differentiate into effector populations. Subsequently, tubular cells become necrotic or apoptotic and renal function worsens (Bonventre and Yang, 2011; Stroo et al., 2010; Swaminathan and Griffin, 2008). In this report, we show renal infiltration of neutrophils, macrophages and T cells which further increases after biglycan overexpression. Interestingly, this biglycan-accelerated influx was abolished in mice lacking TLR-2/4, thereby suggesting that biglycan might act as an endogenous mediator of sterile inflammation. Recruitment of immune cells coincides with increased chemoattractants for neutrophils (CXCL1), macrophages (CCL2) and T cells (CCL5). Again, increase in chemokines is enhanced when soluble biglycan is de novo overexpressed. However, we are fully aware of potential limitations in overinterpreting data generated with Tlr2−/−;Tlr4−/− mice insofar as other endogenous ligands might be concurrently induced upon IRI (e.g. HSP70, hyaloronan and HMBG1) (Leemans et al., 2005; Shigeoka et al., 2013; Wu et al., 2010) and this might contribute to protection. Indeed, neutralization of HMGB1 in C57BL/6J mice is protective in renal IRI (Wu et al., 2010).
Our recent work reported that biglycan stimulation of macrophages leads to TLR-2/4 -dependent induction of TNF-α and MIP-2 by activation of p38, ERK and NF-κB (Iozzo and Schaefer, 2010; Schaefer et al., 2005). However, direct interaction of biglycan with TLR-2 or TLR-4 has never been shown. Utilizing microscale thermophoresis we demonstrate here that highly-purified biglycan binds TLR-2/4 by itself without the need of any cofactor. The observed dissociation constants are remarkably small but consistent with data for other endogenous TLR ligands (Allam et al., 2012; Merline et al., 2011; Parroche et al., 2007). We found that biglycan binds TLR-4 with an affinity higher than TLR-2. These findings are in agreement with reports showing strong reduction of biglycan-induced TNF-α, MIP-2 and IL-1β in Tlr4−/− macrophages but only slight reduction in Tlr2−/−/− macrophages (Babelova et al., 2009; Moreth et al., 2010; Schaefer et al., 2005). Further, weak signals for TLR-2 in mass spectrometry after immunoprecipitation with biglycan could be explained by lower affinities of biglycan (Schaefer et al., 2005). Interestingly, the structurally closely related small-leucine rich proteoglycan decorin, which controls inflammation and tumor growth, shows similar binding affinities towards TLR-2 and TLR-4 (Merline et al., 2011; Rutnam et al., 2013).
Absence of TLR-2 and TLR-4 signaling, however, does not completely prevent renal injury, suggesting that biglycan-signaling pathways are independent of TLR (Rusai et al., 2010). Recently, involvement of biglycan in oxidative stress has been reported (Babelova et al., 2009; Moreth et al., 2010), further biglycan can bind complement protein C1q (Groeneveld et al., 2005). It is well accepted that besides others, formation of reactive oxygen species or complement activation promote local tissue damage during renal ischemia-reperfusion (Arumugam et al., 2009). Moreover, we showed that biglycan is able to orchestrate Tlr2−/−;Tlr4−/− with P2X7/4 receptors, thereby autonomously activating the Nlrp3 inflammasome with subsequent secretion of mature IL-1β (Babelova et al., 2009). As biglycan is induced upon renal IRI, it is reasonable that inflammasome activation would worsen renal injury. Interestingly, mice deficient in either Nlrp3 or ASC have reduced levels of urea and creatinine in plasma and show decreased renal infiltration of neutrophils (Iyer et al., 2009).
Our present findings suggest interference of interaction of biglycan with Tlr2−/−;Tlr4−/− as a new therapeutic approach for treatment of acute renal injury and progression of kidney failure. Prevention of biglycan binding to Tlr2−/−;Tlr4−/− could possibly ameliorate production of proinflammatory cytokines and chemokines ad subsequent infiltration of inflammatory cells. Thereby kidney tissue would be protected from progressive tubular damage and recovery from renal IRI would be improved. Renoprotective potential has already been demonstrated as pathogen-induced inflammation like sepsis or autoinflammatory responses like lupus nephritis are reduced in absence of biglycan (Moreth et al., 2010). Circumvention of biglycan signaling could be accomplished by blocking the biglycan-TLR binding site with antibodies. Such an approach may also improve the outcome of systemic inflammatory responses to IRI, as biglycan-deficiency improves survival of septic shock (Schaefer et al., 2005). Whether biglycan-induced Tlr2−/−;Tlr4−/−-signaling contributes to IRI of other organs such as heart, lung or brain, needs to be further investigated.
In conclusion, our study demonstrates that biglycan acts as an endogenous danger mediator in a Tlr2−/−;Tlr4−/−-dependent manner in renal IRI. The physical interaction between biglycan and Tlr2−/−;Tlr4−/− occurs directly without any cofactors and with higher affinities towards TLR-4 than TLR-2. During reperfusion, soluble biglycan exacerbates immune responses by evoking proinflammatory cytokines and chemokines, and by promoting lymphocytic infiltration of the renal parenchyma. Further characterization of biglycan-triggered Tlr2−/−;Tlr4−/− signaling will add to our understanding of renal IRI and will help to develop new therapeutic strategies for patients with acute kidney injury.
4. Experimental procedures
4.1 Animal experiments
C57BL/6J (wild type) mice were purchased from Jackson Laboratories (Bar Harbor, ME). Tlr2−/−;Tlr4−/− mice were generously provided by Dr. M. Freudenberg (Max Planck Institute of Immunology, Freiburg, Germany). All animal work was conducted in accordance with the German Animal Protection Law and was approved by the Ethics Review Committee for laboratory animals of the District Government of Darmstadt, Germany.
4.1.1 In vivo transfection
To generate mice overexpressing soluble biglycan, human biglycan cDNA (hBGN) was inserted into the BamHI/SacII site of the pLIVE™ (Liver In Vivo Expression) vector containing the mouse albumin promoter (Mirus Bio, USA). 10-week-old male wild type and Tlr2−/−;Tlr4−/− mice received a single intravenous injection of 40 μg hBGN pLIVE vector in 300 μl 5% glucose and 7.2 μl ExGenInVivo solution (Thermo Fisher Scientific, Germany). Therefore, mice were anesthetized under 2% isofluoran (Abbott, Germany) and 1 L/min oxygen. For controls, vehicle or empty pLIVE vector were used and mice were divided into three experimental groups (injected with solvent, pLIVE or hBGN-pLIVE), each consisting of a minimum of six animals. Mice were sacrificed for collection of blood and organs at day 4 post injection.
4.1.2 Induction of renal ischemia reperfusion injury
For induction of kidney ischemia-reperfusion injury, mice were anesthetized with Ketamin/Xylazin (100 and 10 mg, respectively) per kg body weight; Ketavet from Pfizer, Germany and Rompun from Bayer, Germany). A midline incision was made, right renal pedicle was tied off with suture, cut distal to suture and kidney was removed. Next, renal artery of the left kidney was clamped for 25 min with atraumatic microaneurysm clamps. During ischemia body temperature was maintained by placing mice on a 37°C heating pad and to maintain fluid balance, kidneys were wet with saline every few minutes. After clamp removal kidneys were inspected for 1 min for restoration of blood flow, returning to their original color then incision was closed. Sham-operated mice (control, n=6 per group) received identical surgical procedures except that neither nephrectomy nor application of the microaneurysm clamps was performed. Mice were sacrificed 30 h after reperfusion. Serum was taken for ELISA and proteoglycan isolation. Kidney tissues were divided up to be snap frozen (for subsequent RNA extraction, proteoglycan isolation and ELISA), and fixed in 4% paraformaldehyde (for histology and immunohistochemistry).
4.2 Determination of renal functional and histopathology
Serum creatinine was determined by colorimetric Microplate Assay (Oxford Biomedical Research, Biotrend, Germany). Serial sections (4 μm) of paraffin-embedded kidneys from mice were stained with PAS (Schaefer et al., 2001). The severity of renal lesions after IRI was graded from 0 to 5 by determining percentage of necrotic tubules, loss of brush border, cast formation and tubule dilation as follows: 0, none; 1, < 10%; 2, 11%–25%; 3, 26%–45%; 4, 46%–75%; and 5, > 76% (Oh et al., 2008). At least 10 high-power fields (magnification, x200) per section for each sample were examined. For immunohistochemistry primary antibodies included: rat anti-mouse F4/80 (Serotec), rat anti-mouse 7/4 (Abcam) and rat-anti mouse CD3 (Santa Cruz Biotechnology). Incubation with the primary antibody was performed overnight at 4 °C. Counterstaining was performed with Meyer’s Heamatoxylin (Sigma Aldrich, Munich, Germany). Specificity controls included omitting or replacement of primary antiserum with normal rat IgG or goat serum. The number of neutrophils, macrophages and T cells was estimated per field (hpf 400x, with a minimum of 10 fields counted) (Soft Imaging System, Olympus, Hamburg, Germany). Histological examinations were performed by two observers blinded to the conditions.
4.3 Isolation and semi quantification of biglycan from tissue and plasma
Biglycan in homogenates from whole kidneys and plasma samples (n=3 per group) was extracted and semi purified as described previously (Moreth et al., 2010; Schaefer et al., 2001). The proteoglycan was then digested with Chondroitinase ABC (Seikagaku Corporation, Japan) to remove glycosaminoglycan chains and subjected to polyacrylamide gel electrophoresis followed by Western blotting with subsequent quantification as described previously (Moreth et al., 2010; Schaefer et al., 2001). Goat anti-human biglycan (Acris Antibodies, Germany) antibody was used as primary antibody. Signal detection was performed using the ECL Western blotting detection system (GE Healthcare, Germany) and optical density of bands was obtained using Scion Image software (Scion Corporation). For validation graded known amounts of intact biglycan (containing protein core and glycosaminoglycan chains) were used as positive controls (Moreth et al., 2010). All samples were purified and digested with Chondroitinase ABC in parallel and under same conditions. Renal biglycan content was calculated per mg protein of kidney homogenate used for proteoglycan isolation. Approximately 150 mg kidney and 200 μl plasma was used for biglycan isolation. The results of three samples per group were averaged.
4.4 Real-time quantitative polymerase chain reaction
Total RNA was extracted from kidneys using the TRIreagent (Sigma Aldrich, Germany) as described (Schaefer et al., 2001). QuantitativeTaqMan PCRs for murine Tnf (Mm_00443260_g1), Cxcl1 (Mm_04207460_m1), Ccl2 (Mm_00441242_m1), Ccl5 (Mm_01302427_m1), Gapdh (Mm_03302249_g1) (Life Technologies) were performed using TaqMan ROX Low Master Mix (Thermo Scientific, Germany). Amplification was detected by an increased fluorescent signal of 5-[(N-(30-diphenylphosphinyl-40-methoxycarbonyl) phenyl-carbonyl) aminoacetamido] fluorescein (FAM) using AbiPrism 7500 sequence detection system (Applied Biosystems). Relative changes in gene expression compared to control and normalized to Gapdh were quantified by the 2−ΔΔCt method.
4.5 ELISA and Western blotting
Mouse plasma and tissue homogenates were used to determine TNF-α, CXCL1, CCL2 and CCL5 (R&D Systems, Germany) protein levels by mouse specific ELISA. Tissue was homogenized and sonicated in lysis buffer containing 137 mM NaCl, 20 mM Tris/HCl, pH 8.0, 5 mM EDTA, 10% glycerol, 1% Triton X-100, centrifuged at 4°C at 13000 rpm for 15 min and supernatants were snap-frozen and stored at −80°C. Cytokine and chemokine levels were normalized to protein content of tissue homogenates. Assays were performed in duplicate; experiments were carried out at least three times. Western blots were performed and quantified as described (Schaefer et al., 2001) using an antibody against the C-terminus of human biglycan (Acris Antibodies).
4.6 SEAP NF-κB activity assays
105 HEK-Blue-hTLR4 and HEK-Blue-hTLR2 cells containing the SEAP reporter gene (InvivoGen, France) for monitoring NF-κB activation were used. To validate specificity additional stimulation with lipopolysaccharide from Salmonella minnesota (100 ng/ml, InvivoGen) and peptidoglycan from Staphylococcus aureus (5 μg/ml, Sigma Aldrich) were performed. Cells were stimulated with biglycan (4 μg/ml) for 4 h and activation of TLR-2 and TLR-4 signaling was analyzed by measuring secreted SEAP according manufacturer’s instruction (InvivoGen).
4.7 Immunoprecipitation assays
Immunoprecipitation of biglycan with TLR-2 and TLR-4 from murine peritoneal macrophage cell lysates (Schaefer et al., 2005) was performed using protein A-agarose affinity chromatography matrix (Roche) immobilized with biglycan and anti-biglycan antibody complexes by means of an irreversible cross-linker (BS3, Thermo Fisher Scientific) following manufacturer’s instruction. Briefly, pre-cleared lysates were added to biglycan-coated beads and incubated overnight at 4°C on a rotor. Immunoprecipitated proteins were eluted and analyzed by SDS-PAGE and Western blotting using anti-TLR-4 (IMG-577, Imgenex, Biomol), anti-TLR-2 (IMG-410A, Imgenex, Biomol) and donkey anti-rabbit-HRP (GE Healthcare, Germany) antibodies. For controls, protein A-agarose was incubated (i) without cross-linker, (ii) with antibody and peritoneal macrophages deficient for either TLR-2 or TLR-4 and without biglycan and (iii) with antibody and biglycan in presence or absence of cell lysates.
4.8 Microscale thermophoresis
Protein-protein interactions of biglycan with recombinant human TLR-2 and human TLR-4 were determined by changes in thermophoretic movement (NanoTemper Technologies, Germany) (Wienken et al., 2010). TLR-2 and TLR-4 were labeled with fluorophore NT-647 (Monolith NT Protein Labeling Kit, NanoTemper Technologies). A 12-fold titration series of biglycan (5.5 μM to 1.95 nM) diluted 1:1 with PBS + 0.05% Tween 20 was performed. Concentrations of NT-647-labeled TLR-2 or TLR-4 were kept constant (350 nM). Binding partners were incubated with biglycan for 30 min in the dark, reaction was then aspirated into glass capillaries, sealed with wax and thermophoretic movement of labeled proteins was monitored with a laser on for 30 s and off for 5 s at a laser voltage of 40%. To demonstrate that changed thermophoresis was actually due to biglycan-interaction we used albumin (Thermo Fisher Scientific) as negative control. Fluorescence was measured before laser heating (FInitial) and after 30 s of laser-on time (FHot). Normalized fluorescence FNorm= Fhot/FInitial reflects concentration ratio of labeled molecules. FNorm was plotted directly and multiplied by a factor of 10, yielding a relative change in fluorescence per mill. KD was calculated from three independent thermophoresis measurements with NanoTemper Software (NanoTemper Technologies).
4.9 Cell lines and cell culture
Human embryonic kidney (HEK) 293 cells (InvivoGen) were cultured in MEM (InvitroGen) with 1 g/l glucose (PAA), 10% fetal calf serum and penicillin/streptomycin (PAA) and transfected with the following constructs in pcDNA3.1 vector: human biglycan, human TLR4 (Glu24 to Lys631) and human TLR2 (Met1 to Leu590) all containing a C-terminal 6xHis tag. For transfections, 3 μl FuGENE 6 reagent (Roche) per 1 μg DNA were employed following manufacturer’s instruction. Stable transfected cells were selected with 750 μg/ml G418 (Carl Roth, Germany). Cell culture supernatants were collected twice per week and frozen until use. His-tagged proteins were isolated from cell culture supernatants by binding to nickel-nitrilotriacetic acid (NiNTA) agarose (Qiagen, Germany) following manufacturer’s instruction for isolation of His-tagged proteins under native conditions. Protein concentrations were determined using BCA assay (Thermo Fisher Scientific), purity of preparations was controlled by gel electrophoresis. Expression of human biglycan in HEK293 cells and purification of the native proteoglycan, containing two chondroitin/dermatan sulfate chains, has been described previously (Babelova et al., 2009).
4.10 Statistics
All data are expressed as means ±SD. Student’s t test was used to evaluate significance of differences between groups. Differences were considered significant at p values <0.05. Note that p values are not corrected for multiple testing.
Highlights.
Renal ischemia-reperfusion injury (IRI) elevates biglycan in plasma and kidney.
Soluble biglycan directly interacts with TLR-2 and TLR-4.
Overexpression of soluble biglycan worsens pathophysiology of renal IRI.
Disabled biglycan-triggered TLR-2/TLR-4 signaling in renal IRI is renoprotective.
Acknowledgments
This work was supported by the by the German Research Council (SFB 815, project A5, SFB 1039, project B2, Excellence Cluster ECCPS to L.S., and GRK1172 to M.V.N. and L.S), LOEWE program Ub-Net (L.S.) and by National Institutes of Health grants RO1 CA39481, RO1 CA47282 and RO1 CA164462 (R.V.I.).
Abbreviations
- CCL
chemokine (C-C motif) ligand
- CCR2
C-C chemokine receptor type 2
- CXCL
neutrophil chemoattractant chemokine C-X-C motif ligand
- IL-1β
interleukin-1β
- IRI
ischemia-reperfusion injury
- LPS
lipopolysaccharide
- NF-κB
nuclear factor kappa-light-chain enhancer of activated B cells
- NLRP3
NLR family, pyrin domain-containing 3
- PGN
peptidoglycan
- SEAP
secreted alkaline phosphatase
- TLR
Toll-like receptor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allam R, Scherbaum CR, Darisipudi MN, Mulay SR, Hägele H, Lichtnekert J, Hagemann JH, Rupanagudi KV, Ryu M, Schwarzenberger C, Hohenstein B, Hugo C, Uhl B, Reichel Ca, Krombach F, Monestier M, Liapis H, Moreth K, Schaefer L, Anders HJ. Histones from dying renal cells aggravate kidney injury via TLR2 and TLR4. J Am Soc Nephrol. 2012;23:1375–88. doi: 10.1681/ASN.2011111077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arumugam TV, Okun E, Tang SC, Thundyil J, Taylor SM, Woodruff TM. Toll-like receptors in ischemia-reperfusion injury. Shock. 2009;32:4–16. doi: 10.1097/SHK.0b013e318193e333. [DOI] [PubMed] [Google Scholar]
- Babelova A, Moreth K, Tsalastra-Greul W, Zeng-Brouwers J, Eickelberg O, Young MF, Bruckner P, Pfeilschifter J, Schaefer RM, Gröne HJ, Schaefer L. Biglycan, a danger signal that activates the NLRP3 inflammasome via toll-like and P2X receptors. J Biol Chem. 2009;284:24035–48. doi: 10.1074/jbc.M109.014266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonventre JV, Yang L. Science in medicine Cellular pathophysiology of ischemic acute kidney injury. J Clin Invest. 2011;121:4210–4221. doi: 10.1172/JCI45161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandan E, Gutierrez J. Role of skeletal muscle proteoglycans during myogenesis. Matrix Biol. 2013;32:289–97. doi: 10.1016/j.matbio.2013.03.007. [DOI] [PubMed] [Google Scholar]
- Erridge C. Endogenous ligands of TLR2 and TLR4: agonists or assistants? J Leukoc Biol. 2010;87:989–99. doi: 10.1189/jlb.1209775. [DOI] [PubMed] [Google Scholar]
- Frey H, Schroeder N, Manon-jensen T, Iozzo RV, Schaefer L. Biological interplay between proteoglycans and their innate immune receptors in inflammation. FEBS J. 2013;280:2165–2179. doi: 10.1111/febs.12145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuichi K, Wada T, Yokoyama H, Kobayashi K. Role of Cytokines and Chemokines in Renal Ischemia-Reperfusion Injury. Drugs News Perspect. 2002;15:477–482. doi: 10.1358/dnp.2002.15.8.840067. [DOI] [PubMed] [Google Scholar]
- Groeneveld TWL, Oroszlán M, Owens RT, Faber-krol MC, Bakker AC, Arlaud GJ, Mcquillan DJ, Kishore U, Daha MR, Roos A, Oroszla M, Arlaud J. Interactions of the Extracellular Matrix Proteoglycans Decorin and Biglycan with C1q and Collectins. J Immunol. 2005;175:4715–4723. doi: 10.4049/jimmunol.175.7.4715. [DOI] [PubMed] [Google Scholar]
- Hassan IR, Gronert K, Alerts E. Acute changes in dietry w-3 and w-6 polyunsaturated fatty acids have a pronounced impact on survival following ischemia renal injury and formation of renoprotective docosahexaenoic acid-derived protectin d1. J Immunol. 2009;182:3223–3232. doi: 10.4049/jimmunol.0802064. [DOI] [PubMed] [Google Scholar]
- Iozzo RV, Goldini S, Berendsen A, Young MF. Small leucine-rich proteoglycans. In: Mecham RP, editor. The Extracellular Matrix: An Overview. Springer; 2011. pp. 197–231. [Google Scholar]
- Iozzo RV, Karamanos N. Proteoglycans in health and disease: emerging concepts and future directions. FEBS J. 2010;277:3863. doi: 10.1111/j.1742-4658.2010.07796.x. [DOI] [PubMed] [Google Scholar]
- Iozzo RV, Schaefer L. Proteoglycans in health and disease: novel regulatory signaling mechanisms evoked by the small leucine-rich proteoglycans. FEBS J. 2010;277:3864–75. doi: 10.1111/j.1742-4658.2010.07797.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyer SS, Pulskens WP, Sadler JJ, Butter LM, Teske GJ, Ulland TK, Eisenbarth SC, Florquin S, Flavell Ra, Leemans JC, Sutterwala FS. Necrotic cells trigger a sterile inflammatory response through the Nlrp3 inflammasome. Proc Natl Acad Sci U S A. 2009;106:20388–93. doi: 10.1073/pnas.0908698106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krüger B, Krick S, Dhillon N, Lerner SM, Ames S, Bromberg JS, Lin M, Walsh L, Vella J, Fischereder M, Krämer BK, Colvin RB, Heeger PS, Murphy BT, Schröppel B. Donor Toll-like receptor 4 contributes to ischemia and reperfusion injury following human kidney transplantation. Proc Natl Acad Sci U S A. 2009;106:3390–5. doi: 10.1073/pnas.0810169106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leemans JC, Stokman G, Claessen N, Rouschop KM, Teske GJD, Kirschning CJ, Akira S, van der Poll T, Weening JJ, Florquin S. Renal-associated TLR2 mediates ischemia/reperfusion injury in the kidney. J Clin Invest. 2005;115:2894–903. doi: 10.1172/JCI22832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Huang L, Sung SSJ, Vergis AL, Rosin DL, Rose CE, Lobo PI, Okusa MD. The chemokine receptors CCR2 and CX3CR1 mediate monocyte/macrophage trafficking in kidney ischemia-reperfusion injury. Kidney Int. 2008;74:1526–37. doi: 10.1038/ki.2008.500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merline R, Moreth K, Beckmann J, Nastase MV, Zeng-Brouwers J, Tralhão JG, Lemarchand P, Pfeilschifter J, Schaefer RM, Iozzo RV, Schaefer L. Signaling by the matrix proteoglycan decorin controls inflammation and cancer through PDCD4 and MicroRNA-21. Sci Signal. 2011;4:ra75. doi: 10.1126/scisignal.2001868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merline R, Nastase M-V, Iozzo RV, Schaefer L. Small Leucine-rich proteoglycans: multifunctional signaling effectors. In: Karamanos N, editor. Extracellular Matrix: Pathobiology and Signaling. Walter de Gruytier GmbH & Co; Berlin: 2012. pp. 185–196. [Google Scholar]
- Mittag D, Proietto AI, Loudovaris T, Mannering SI, Vremec D, Shortman K, Wu L, Harrison LC. Human dendritic cell subsets from spleen and blood are similar in phenotype and function but modified by donor health status. J Immunol. 2011;186:6207–17. doi: 10.4049/jimmunol.1002632. [DOI] [PubMed] [Google Scholar]
- Moreth K, Brodbeck R, Babelova A, Gretz N, Spieker T, Zeng-brouwers J, Pfeilschifter J, Young MF, Schaefer RM, Schaefer L. The proteoglycan biglycan regulates expression of the B cell chemoattractant CXCL13 and aggravates murine lupus nephritis. J Clin Invest. 2010;120:4251–4272. doi: 10.1172/JCI42213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munshi R, Hsu C, Himmelfarb J. Advances in understanding ischemic acute kidney injury. BMC Med. 2011;9:11. doi: 10.1186/1741-7015-9-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton K, Dixit VM. Signaling in innate immunity and inflammation. Cold Spring Harb Perspect Biol. 2012;4 doi: 10.1101/cshperspect.a006049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikitovic D, Aggelidakis J, Young MF, Iozzo RV, Karamanos NK, Tzanakakis GN. The biology of small leucine-rich proteoglycans in bone pathophysiology. J Biol Chem. 2012;287:33926–33. doi: 10.1074/jbc.R112.379602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh D, Dursun B, He Z, Lu L, Hoke TS, Ljubanovic D, Faubel S, Edelstein CL. Fractalkine receptor (CX3CR1) inhibition is protective against ischemic acute renal failure in mice. Am J Ren Physiol. 2008;294:264–271. doi: 10.1152/ajprenal.00204.2007. [DOI] [PubMed] [Google Scholar]
- Park P, Haas M, Cunningham PN, Bao L, Alexander JJ, Quigg RJ. Injury in renal ischemia-reperfusion is independent from immunoglobulins and T lymphocytes. Am J Physiol Renal Physiol. 2002;282:F352–7. doi: 10.1152/ajprenal.00160.2001. [DOI] [PubMed] [Google Scholar]
- Parroche P, Lauw FN, Goutagny N, Latz E, Monks BG, Visintin A, Halmen Ka, Lamphier M, Olivier M, Bartholomeu DC, Gazzinelli RT, Golenbock DT. Malaria hemozoin is immunologically inert but radically enhances innate responses by presenting malaria DNA to Toll-like receptor 9. Proc Natl Acad Sci U S A. 2007;104:1919–24. doi: 10.1073/pnas.0608745104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popovic ZV, Wang S, Papatriantafyllou M, Kaya Z, Porubsky S, Meisner M, Burgdorf S, Young MF. The Proteoglycan Biglycan Enhances Antigen-Specific T Cell Activation Potentially via MyD88 and TRIF Pathways and Triggers Autoimmune Perimyocarditis. J Immunol. 2011;187:6217–6226. doi: 10.4049/jimmunol.1003478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulskens WP, Teske GJ, Butter LM, Roelofs JJ, van der Poll T, Florquin S, Leemans JC. Toll-like receptor-4 coordinates the innate immune response of the kidney to renal ischemia/reperfusion injury. PLoS One. 2008;3:e3596. doi: 10.1371/journal.pone.0003596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabb H, Daniels F, O’Donnell M, Haq M, Saba SR, Keane W, Tang WW. Pathophysiological role of T lymphocytes in renal ischemia-reperfusion injury in mice. Am J Physiol Renal Physiol. 2000;279:F525–31. doi: 10.1152/ajprenal.2000.279.3.F525. [DOI] [PubMed] [Google Scholar]
- Rusai K, Sollinger D, Baumann M, Wagner B, Strobl M, Schmaderer C, Roos M, Kirschning C, Heemann U, Lutz J. Toll-like receptors 2 and 4 in renal ischemia/reperfusion injury. Pediatr Nephrol. 2010;25:853–60. doi: 10.1007/s00467-009-1422-4. [DOI] [PubMed] [Google Scholar]
- Rutnam ZJ, Wight TN, Yang BB. miRNAs regulate expression and function of extracellular matrix molecules. Matrix Biol. 2013;32:74–85. doi: 10.1016/j.matbio.2012.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer L. Extracellular matrix molecules: endogenous danger signals as new drug targets in kidney diseases. Curr Opin Pharmacol. 2010;10:185–90. doi: 10.1016/j.coph.2009.11.007. [DOI] [PubMed] [Google Scholar]
- Schaefer L. Small Leucine-Rich Proteoglycans in Kidney Disease. J Am Soc Nephrol. 2011;22:1200–1207. doi: 10.1681/ASN.2010050570. [DOI] [PubMed] [Google Scholar]
- Schaefer L, Babelova A, Kiss E, Hausser H, Baliova M, Krzyzankova M, Marsche G, Young MF, Mihalik D, Götte M, Malle E, Schaefer RM, Gröne H. The matrix component biglycan is proinflammatory and signals through Toll-like receptors 4 and 2 in macrophages. J Clin Invest. 2005;115:26–28. doi: 10.1172/JCI23755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer L, Iozzo RV. Small leucine-rich proteoglycans, at the crossroad of cancer growth and inflammation. Curr Opin Genet Dev. 2012;22:56–7. doi: 10.1016/j.gde.2011.12.002. [DOI] [PubMed] [Google Scholar]
- Schaefer L, Raslik I, Gröne H, Schönherr K, Ugorcakova J, Budny S, Schaefer RM, Kresse H. Small proteoglycans in human diabetic nephropathy: Discrepancy between glomerular expression and protein accumulation of decorin, biglycan, lumican, and fibromodulin. FASEB J. 2001;15:559–561. doi: 10.1096/fj.00-0493fje. [DOI] [PubMed] [Google Scholar]
- Shigeoka AA, Holscher TD, King AJ, Hall FW, Kiosses WB, Tobias PS, Mckay DB, Mackman N. TLR2 Is Constitutively Expressed within the Kidney and Participates in Ischemic Renal Injury through Both MyD88-Dependent and -Independent Pathways. J Immunol. 2013;178:6252–6258. doi: 10.4049/jimmunol.178.10.6252. [DOI] [PubMed] [Google Scholar]
- Stroo I, Stokman G, Teske GJD, Raven A, Butter LM, Florquin S, Leemans JC. Chemokine expression in renal ischemia/reperfusion injury is most profound during the reparative phase. Int Immunol. 2010;22:433–442. doi: 10.1093/intimm/dxq025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swaminathan S, Griffin MD. First responders: understanding monocyte-lineage traffic in the acutely injured kidney. Kidney Int. 2008;74:1509–11. doi: 10.1038/ki.2008.555. [DOI] [PubMed] [Google Scholar]
- Wienken CJ, Baaske P, Rothbauer U, Braun D, Duhr S. Protein-binding assays in biological liquids using microscale thermophoresis. Nat Commun. 2010;1:100. doi: 10.1038/ncomms1093. [DOI] [PubMed] [Google Scholar]
- Wu H, Chen G, Wyburn KR, Yin J, Bertolino P, Eris JM, Alexander SI, Sharland AF, Chadban SJ. TLR4 activation mediates kidney ischemia/reperfusion injury. J Clin Invest. 2007;117:2847–2859. doi: 10.1172/JCI31008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H, Ma J, Wang P, Corpuz TM, Panchapakesan U, Wyburn KR, Chadban SJ. HMGB1 contributes to kidney ischemia reperfusion injury. J Am Soc Nephrol. 2010;21:1878–90. doi: 10.1681/ASN.2009101048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng-Brouwers J, Beckmann J, Nastase M-V, Iozzo RV, Schaefer L. De novo expression of circulating biglycan evokes an innate inflammatory tissue response via MyD88/TRIF pathways. Matrix Biol. 2013 doi: 10.1016/j.matbio.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]