Abstract
Purpose
We assessed patient perceptions of regular intermittent self-dilation in men with urethral stricture.
Materials and Methods
We constructed and distributed a visual analog questionnaire to evaluate intermittent self-dilation via catheterization by men referred for urethral stricture management at a total of 4 institutions. Items assessed included patient duration, frequency, difficulty and pain associated with intermittent self-dilation as well as interference of intermittent self-dilation with daily activity. The primary outcome was patient perceived quality of life. Multivariate analysis was performed to assess factors that affected this outcome.
Results
Included in the study were 85 patients with a median age of 68 years, a median of 3.0 years on intermittent self-dilation and a median frequency of 1 dilation per day. On a 1 to 10 scale the median intermittent self-dilation difficulty was 5.0 ± 2.7, the median pain score was 3.0 ± 2.7 and median interference with daily life was 2.0 ± 1.3. Overall quality of life in patients with stricture was poor (median score 7.0 ± 2.6 with poor quality of life defined as 7 or greater). On univariate analysis younger age (p <0.01), interference (p = 0.03), pain (p <0.01) and difficulty performing intermittent self-dilation (p = 0.03) correlated with poor quality of life in a statistically significant manner. On multivariate analysis only difficulty catheterizing (p <0.01) and younger age (p = 0.05) were statistically significant predictors. Patients with stricture involving the posterior urethra had a statistically significant increase in difficulty and decrease in quality of life (each p = 0.04).
Conclusions
Most patients with urethral stricture who are on intermittent selfdilation rate difficulty and pain as moderate, and inconvenience as low but report poor quality of life.
Keywords: urethral stricture, urinary bladder neck obstruction, contracture, catheterization, questionnaires
Voiding dysfunction was commonly managed by indwelling urethral or suprapubic catheters until 1972, when Lapides et al reported that ISC is a safe, effective option.1 ISC is generally considered a simple, painless procedure that obviates complex surgical reconstruction and decreases the complications of indwelling catheters, such as urethral erosion, bladder stones, recurrent infection and cancer.2,5 ISC has proved to be well tolerated by patients with chronic neurogenic bladder in whomsimple pharmacological or surgical solutions are not readily available.6,7 ISC has since been popularized as a conservative means of managing neurogenic bladders, benign prostatic obstruction and bladder neck contracture.8,9
As ISC has grown increasingly accepted as an option for lower urinary tract management, extrapolating these results to patients with urethral stricture was inevitable. More recent studies championed ISC for urethral stricture as an alternative to office or surgical dilation10 after internal urethrotomy.11 Unlike in patients with a neurogenic etiology, the purpose of ISC in patients with stricture is primarily to maintain patency and secondarily to evacuate urine. It is important to distinguish ISD of urethral strictures from ISC, as outlined.
To our knowledge the effect of ISD on QOL in men with obstructive urethral pathology has not been studied. Patients with stricture differ from those with neurogenic bladder since they tend to be fully sensate and fully ambulatory, and have an otherwise normal lower urinary tract that is focally disrupted by a discrete lesion amenable to various straightforward reconstructive techniques. Our impression is that many men on chronic ISD for urethral obstruction are suffering. We hypothesized that this questionnaire could be used to stratify patient groups and their perceptions of chronic ISD as a treatment. We present the findings of our prospective, multi-institutional, patient reported study of ISD in men with urethral stricture disease and bladder neck contracture.
METHODS
Population
We prospectively analyzed patients with urethral stricture from a total of 4 tertiary referral centers, including University of Texas Southwestern Medical Center, University of California-San Francisco, Wake Forest Baptist Medical Center and Alfred Health. All patients were referred for management of urethral obstruction and had been on an ISD regimen for urethral stricture or bladder neck contracture between May 2011 and August 2012. Patients with stricture with no history of ISD and those with concomitant neurogenic voiding dysfunction were excluded from analysis.
Patient age, and information on stricture site and length were recorded. Stricture location was defined as panurethral, posterior, bulbar or pendulous/fossa navicularis. Panurethral indicated strictures that involved each portion of the anterior urethra. Bladder neck contracture was included with posterior urethral stricture.
Questionnaire
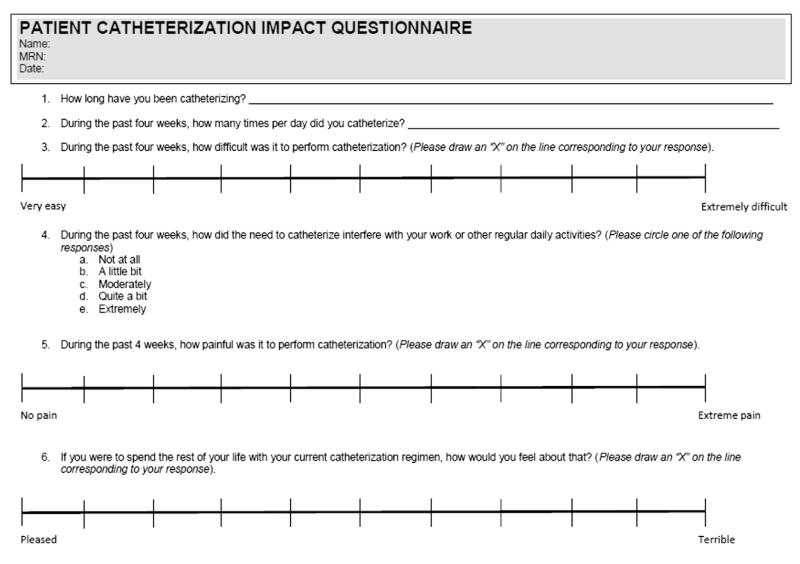
Since to our knowledge a validated questionnaire to assess QOL in patients on ISD was not available, we constructed a visual analog questionnaire based on a QOL questionnaire previously used to assess the impact of ISD in a population of predominantly patients with neurogenic bladder (fig. 1).11 Modifications were made to adaptthis questionnaire toward urethral stricture, including a question on the duration that the patient had catheterized. The QOL question was changed to reflect willingness to continue ISD.
Figure 1.
Visual analog scale
Visual analog scales were validated to provide an accurate assessment of pain.12 Tick marks on the visual analog scale implicitly corresponded to numbers from 0dnone to 10dsevere. Responses of 1 to 3 were considered mild, easy or good, 4 to 6 were categorized as moderate and 7 or greater were interpreted as severe or poor. Items assessed included the patient history, frequency, difficulty and pain associated with ISD. Impact on QOL was assessed by the level of patient interest in continuing ISD for the duration of his life (question 6). The questionnaire was distributed to patients with stricture and bladder neck contracture who regularly performed ISD. The primary outcome was patient perceived QOL.
Statistical Analysis
Data were tabulated in Excel® and analyzed using SPSS®, version 18. To establish variables that correlated with QOL we calculated the Kendall t-b for nonparametric variables. Multivariate analysis was done for factors that correlated with QOL on univariate analysis. Patient demographics and complications were compared using the independent sample t-test for numerical variables and the chi-square test for categorical variables. Statistical significance was considered at p ≤0.05 and reported p values are 2-sided.
RESULTS
Of the approximately 400 patients with stricture from a total of 4 centers who were evaluated during the study period we surveyed 85 with a median age of 68 years (range 15.5 to 94.0) who were treated with ISD. The 85 men were from the 4 participating institutions, including 30 from University of Texas Southwestern Medical Center, 6 from University of California-San Francisco, 13 from Wake Forest Baptist Medical Center and 36 from Alfred Health. Table 1 shows the overall survey responses. Median time on ISD was 3.0 years (range 1 to 20) and the median number of catheterizations per day was 1.0 (range 1 to 10). Of the 85 patients 18 (21%) catheterized at a regular interval of less than once per day. This group included patients who catheterized 1 to 3 times per week.
Table 1. Demographics and questionnaire results of 85 patients.
| Median duration (range) | 3.0 (0.04–20) | |
| Mean ± SD No. catheterizations/day | 1.0 ± 2.02 | |
| Difficulty: | ||
| Mean ± SD | 5.0 ± 2.67 | |
| No. easy (%) | 33 | (38.8) |
| No. moderate (%) | 30 | (35.3) |
| No. severe (%) | 22 | (25.9) |
| Interference: | ||
| Mean ± SD | 2.0 ± 1.3 | |
| No. minimal (%) | 53 | (63.2) |
| No. moderate (%) | 17 | (20.2) |
| No. severe (%) | 14 | (16.6) |
| Pain: | ||
| Mean ± SD | 3.0 ± 2.67 | |
| No. minimal (%) | 44 | (51.8) |
| No. moderate (%) | 27 | (31.7) |
| No. severe (%) | 14 | (16.5) |
| QOL: | ||
| Mean ± SD | 7.0 ± 2.62 | |
| No. good (%) | 11 | (12.9) |
| No. moderate (%) | 27 | (31.8) |
| No. poor (%) | 4 | (55.3) |
Median ISD difficulty with catheterization was moderate (5.0 ± 2.7/10) with 22 patients (26%) reporting severe difficulty. The median pain score was 3.0 ± 2.7, which was divided approximately evenly between 41 patients (48%) who considered pain moderate to severe and 44 (52%) who categorized pain as none to minimal. Interference with daily activity was low with 53 men (63%) reporting it as minimal compared with 14 (17%) who reported it as severe. Despite the relative ease of catheterization and minimal interference with daily life 47 of 85 patients (55%) rated overall QOL as poor with a median score of 7.0 ± 2.6. Only 11 patients (13%) considered QOL as good.
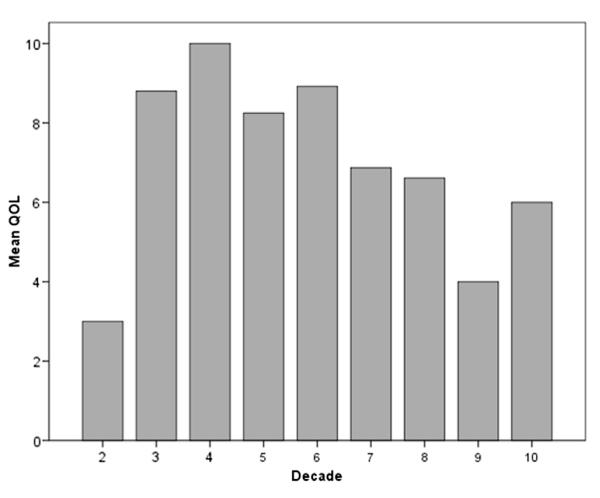
On initial analysis at the univariate level younger age, interference, difficulty catheterizing and pain significantly correlated with poor QOL (each p <0.01). However, on multivariate analysis only difficulty catheterizing (p <0.01) and younger age (p = 0.05) remained statistically significant. Table 2 summarizes these results. Figure 2 shows the inverse relationship when age by decade was plotted against the mean QOL of patients in each age range.
Table 2. QOL predictors.
| Univariate |
Multivariate |
|||
|---|---|---|---|---|
| Coefficient | p Value | B | p Value | |
| Age | −0.28 | <0.00 | −0.29 | <0.00 |
| Duration | −0.03 | 0.74 | – | – |
| No. catheterizations/day | 0.09 | 0.27 | – | – |
| Interference | 0.19 | 0.03 | 0.14 | 0.17 |
| Difficulty | 0.31 | <0.00 | 0.32 | 0.05 |
| Pain | 0.26 | <0.00 | 0.08 | 0.62 |
Figure 2.
Association of younger age with poor QOL score
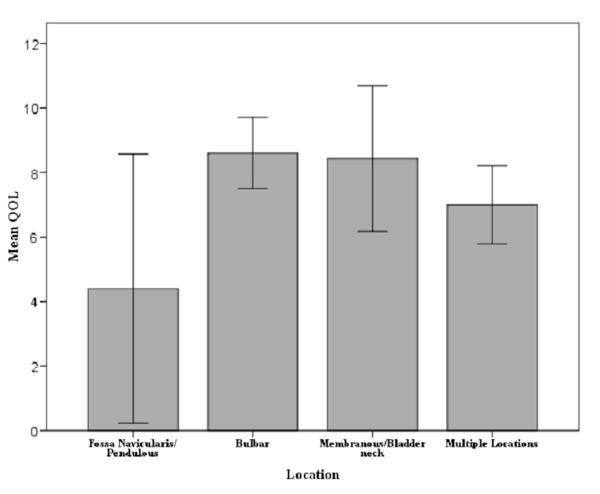
In the subset of patients for whom information on stricture location (37) and length (31) was available location was classified into 4 categories, including panurethral, posterior, bulbar and pendulous/fossa navicularis (table 3). Strictures most commonly involved the bulbar urethra (67% of cases), followed by more proximal sites (41%). The pendulous urethra and fossa navicularis were involved in only 22% of cases. Proximal strictures showed a statistically significant increase in difficulty and decrease in QOL (each p = 0.04, fig. 3). Stricture length did not correlate with pain, difficult catheterization,interference or QOL. Although there was a trend toward patients with posterior stricture to experience more pain compared with bulbar or pendulous and fossa navicularis strictures, this difference did not attain statistical significance (average score 6.9 ± 2.6 vs 3.3 ± 2.7 and 3.4 ± 2.8, respectively, p = 0.06).
Table 3. Stricture population.
| Site | No. Pts (%) | Mean ± SD Age | Mean ± SD Pain | Mean ± SD Difficulty | Mean ± SD Interference | Mean ± SD QOL |
|---|---|---|---|---|---|---|
| Fossa navicularis/pendulous | 5 (13.5) | 63.3 ± 7.3 | 3.4 ± 2.79 | 5 ± 3.67 | 2.6 ± 1.82 | 4.4 ± 3.36 |
| Bulbar | 15 (40.6) | 59.9 ± 3.5 | 3.33 ± 2.66 | 4.3 ± 2.61 | 2.4 ± 1.5 | 8.6 ± 1.99 |
| Posterior | 7 (18.9) | 68.9 ± 2.8 | 6.86 ± 2.61 | 7.43 ± 2.22 | 2.86 ± 1.46 | 8.43 ± 2.44 |
| Panurethral/multiple: | 10 (27.0) | 67.2 ± 5.2 | 5.0 ± 3.16 | 5.6 ± 2.59 | 2.7 ± 1.34 | 7.0 ± 1.7 |
| Bulbar | 9 (100) | |||||
| Membranous | 6 (66.7) | |||||
| Pendulous | 3 (33.3) | |||||
| Bladder neck | 1 (11.1) | |||||
| p Value | – | 0.06 | 0.06 | 0.04 | 0.67 | 0.04 |
Figure 3.
Association of stricture site with QOL score in patients with ISD.
DISCUSSION
Indications for implementing ISC primarily center around neurogenic conditions resulting in 1) poor detrusor contractility or 2) bladder outlet obstruction, which prevents efficient bladder emptying. ISC has gained broad acceptance as a means to promote greater independence of patients with spinal cord injury while decreasing the number of catheter related complications.13 The urological literature demonstrates the superiority of ISC over indwelling catheters and many patients with neurogenic bladder prefer ISC.2 Previous studies in patients with neurogenic bladder described improved QOL associated with ISC6 but these benefits seemed to depend on proper patient selection, technique, support and education.14 Patient noncompliance is associated with complications such as urinary tract infection, epididymitis and urethral trauma along with more severe complications, including bladder perforation or bladder necrosis.15 Girotti et al identified female gender, neurogenic voiding function and age less than 40 years as factors predicting strong adherence to ISC, while about half of evaluated male patients and patients with nonneurogenic bladder were noncompliant.16
While ISD has been recommended to maintain patency in various stricture populations,10,17 few studies have evaluated the QOL of patients on an ISD regimen.7,16,18 To our knowledge none has specifically addressed the role of ISD in a non-neurogenic male population with obstructing urethral lesions. Our patient population is unique in that all men had normal sensation and an otherwise anatomically normal urinary tract. In addition, the resistance caused by spongiofibrosis in our cases probably made catheterization more challenging and increased the risk of urethral bleeding, false passage and/or other trauma.
Our univariate and multivariate analyses strongly suggest that younger men who perform ISD had greater QOL impairment. The reason for the underlying discontent in young men was not captured by the survey and may be related to poor psychosocial acceptance of the regimen or sexuality concerns. A proximal urethral stricture location was strongly associated with greater difficulty and decreased QOL, probably due to the greater angulation and penetration associated with deep bulbar urethral catheter insertion. Stricture length, catheterization duration and number of catheterizations daily had no impact on QOL.
Our study population is also distinct from neurogenic cases because definitive surgical options exist for urethral stricture disease and bladder neck contracture. The success rate of urethroplasty is 75% to 95% at 6 years.19 The success rate of transurethral incision of bladder neck contracture is similar.20 ISD in these cases may not only deny effective reconstructive options but also make subsequent reconstruction more difficult.21
There are several limitations to this study. Referral of these patients to reconstructive centers may have resulted in selection bias for those dissatisfied with management by ISD. Although the questionnaire was not validatedfor thisuse, we believe that it is sufficient as an initial attempt to assess patient perceptions in this poorly studied treatment modality. Our impression is that QOL was greatly enhanced after urethroplasty but patients were not administered the same QOL questionnaire post-operatively for comparison. Finally, the influence of catheter type or size was not assessed. It is possible that smaller or more lubricious catheters may be better tolerated.
CONCLUSIONS
ISD remains a reasonable palliative option for patients with stricture who cannot undergo or do not elect urethral reconstructive surgery. However, in young men with obstructing proximal urethral lesions ISD appears to adversely affect QOL. Further study is warranted to explore the poor acceptance of ISD in young men and the impact of urethroplasty on QOL in these patients. However, results suggest that the strategy of implementing chronic ISD in young patients with stricture amenable to reconstruction is inappropriate.
Abbreviations and Acronyms
- ISC
intermittent self-catheterization
- ISD
intermittent self-dilation
- QOL
quality of life
REFERENCES
- 1.Lapides J, Diokno AC, Silber SJ, et al. Clean, intermittent self-catheterization in the treatment of urinary tract disease. J Urol. 1972;107:458. doi: 10.1016/s0022-5347(17)61055-3. [DOI] [PubMed] [Google Scholar]
- 2.Hakvoort RA, Thijs SD, Bouwmeester FW, et al. Comparing clean intermittent catheterisation and transurethral indwelling catheterisation for incomplete voiding after vaginal prolapse surgery: a multicentre randomised trial. BJOG. 2011;118:1055. doi: 10.1111/j.1471-0528.2011.02935.x. [DOI] [PubMed] [Google Scholar]
- 3.Kuhn W, Rist M, Zaech GA. Intermittent urethral self-catheterisation: long term results (bacteriological evolution, continence, acceptance, complications) Paraplegia. 1991;29:222. doi: 10.1038/sc.1991.33. [DOI] [PubMed] [Google Scholar]
- 4.Rovner ES, Goudelocke CM, Gilchrist A, et al. Transvaginal bladder neck closure with posterior urethral flap for devastated urethra. Urology. 2011;78:208. doi: 10.1016/j.urology.2010.11.054. [DOI] [PubMed] [Google Scholar]
- 5.Wyndaele JJ, Brauner A, Geerlings SE, et al. Clean intermittent catheterization and urinary tract infection: review and guide for future research. BJU Int. 2012;110:E910. doi: 10.1111/j.1464-410X.2012.11549.x. [DOI] [PubMed] [Google Scholar]
- 6.Kessler TM, Ryu G, Burkhard FC. Clean intermittent self-catheterization: a burden for the patient? Neurourol Urodyn. 2009;28:18. doi: 10.1002/nau.20610. [DOI] [PubMed] [Google Scholar]
- 7.Kessler TM, Khan S, Panicker J, et al. Clean intermittent self-catheterization after botulinum neurotoxin type A injections: short-term effect on quality of life. Obstet Gynecol. 2009;113:1046. doi: 10.1097/AOG.0b013e3181a1f5ea. [DOI] [PubMed] [Google Scholar]
- 8.Brannan W. Management of urethral strictures. J Urol. 1985;133:442. doi: 10.1016/s0022-5347(17)49013-6. [DOI] [PubMed] [Google Scholar]
- 9.Ghalayini IF, Al-Ghazo MA, Pickard RS. A prospective randomized trial comparing transurethral prostatic resection and clean intermittent self-catheterization in men with chronic urinary retention. BJU Int. 2005;96:93. doi: 10.1111/j.1464-410X.2005.05574.x. [DOI] [PubMed] [Google Scholar]
- 10.Newman LH, Stone NN, Chircus JH, et al. Recurrent urethral stricture disease managed by clean intermittent self-catheterization. J Urol. 1990;144:1142. doi: 10.1016/s0022-5347(17)39676-3. [DOI] [PubMed] [Google Scholar]
- 11.Bodker A, Ostri P, Rye-Andersen J, et al. Treatment of recurrent urethral stricture by internal urethrotomy and intermittent self-catheterization: a controlled study of a new therapy. J Urol. 1992;148:308. doi: 10.1016/s0022-5347(17)36580-1. [DOI] [PubMed] [Google Scholar]
- 12.Price DD, McGrath PA, Rafii A, et al. The validation of visual analogue scales as ratio scale measures for chronic and experimental pain. Pain. 1983;17:45. doi: 10.1016/0304-3959(83)90126-4. [DOI] [PubMed] [Google Scholar]
- 13.Bermingham SL, Hodgkinson S, Wright S, et al. Intermittent self catheterisation with hydrophilic, gel reservoir, and non-coated catheters: a systematic review and cost effectiveness analysis. BMJ. 2013;346:e8639. doi: 10.1136/bmj.e8639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oh SJ, Ku JH, Lim SH, et al. Effect of a ‘centralized intensive education system’ for clean intermittent self-catheterization in patients with voiding dysfunction who start catheterization for the first time. Int J Urol. 2006;13:905. doi: 10.1111/j.1442-2042.2006.01438.x. [DOI] [PubMed] [Google Scholar]
- 15.Igawa Y, Wyndaele JJ, Nishizawa O. Catheterization: possible complications and their prevention and treatment. Int J Urol. 2008;15:481. doi: 10.1111/j.1442-2042.2008.02075.x. [DOI] [PubMed] [Google Scholar]
- 16.Girotti ME, MacCornick S, Perisse H, et al. Determining the variables associated to clean intermittent self-catheterization adherence rate: one-year follow-up study. Int Braz J Urol. 2011;37:766. doi: 10.1590/s1677-55382011000600013. [DOI] [PubMed] [Google Scholar]
- 17.Gnanaraj J, Devasia A, Gnanaraj L, et al. Intermittent self catheterization versus regular outpatient dilatation in urethral stricture: a comparison. Aust N Z J Surg. 1999;69:41. doi: 10.1046/j.1440-1622.1999.01490.x. [DOI] [PubMed] [Google Scholar]
- 18.van Achterberg T, Holleman G, Cobussen-Boekhorst H, et al. Adherence to clean intermittent self-catheterization procedures: determinants explored. J Clin Nurs. 2008;17:394. doi: 10.1111/j.1365-2702.2006.01893.x. [DOI] [PubMed] [Google Scholar]
- 19.Zimmerman WB, Santucci RA. A simplified and unified approach to anterior urethroplasty. Nat Rev Urol. 2010;7:386. doi: 10.1038/nrurol.2010.79. [DOI] [PubMed] [Google Scholar]
- 20.Yurkanin JP, Dalkin BL, Cui H. Evaluation of cold knife urethrotomy for the treatment of anastomotic stricture after radical retropubic prostatectomy. J Urol. 2001;165:1545. [PubMed] [Google Scholar]
- 21.Terlecki RP, Steele MC, Valadez C, et al. Urethral rest: role and rationale in preparation for anterior urethroplasty. Urology. 2011;77:1477. doi: 10.1016/j.urology.2011.01.042. [DOI] [PubMed] [Google Scholar]