Abstract
Cancer is one of the major causes of mortality worldwide and advanced techniques for therapy are urgently needed. The development of novel nanomaterials and nanocarriers has allowed a major drive to improve drug delivery in cancer. The major aim of most nanocarrier applications has been to protect the drug from rapid degradation after systemic delivery and allowing it to reach tumor site at therapeutic concentrations, meanwhile avoiding drug delivery to normal sites as much as possible to reduce adverse effects. These nanocarriers are formulated to deliver drugs either by passive targeting, taking advantage of leaky tumor vasculature or by active targeting using ligands that increase tumoral uptake potentially resulting in enhanced antitumor efficacy, thus achieving a net improvement in therapeutic index. The rational design of nanoparticles plays a critical role since structural and physical characteristics, such as size, charge, shape, and surface characteristics determine the biodistribution, pharmacokinetics, internalization and safety of the drugs. In this review, we focus on several novel and improved strategies in nanocarrier design for cancer therapy.
Keywords: nanoparticles, nanomedicine, drug delivery, cancer therapy
Introduction
Cancer is one of the leading causes of morbidity and mortality worldwide and it is expected to be the major cause of death in the coming decades (Bray et al., 2012). Despite the advances and extensive research on novel approaches, current treatments are still limited to surgery, radiotherapy, chemotherapy, immunotherapy. Treatment failure is related to either drug resistance, pharmacological or toxicity issues in most instances. In contrary, utilization of nanocarriers leads to increased therapeutic index and tumor tissue concentrations of the drugs and can enhance the efficacy of currently used regimens by providing superior pharmacokinetic features, extended blood circulation time, cellular uptake, volume of distribution, and half-life are major factors for an improved therapeutic window and subsequent clinical success. Advances in nanotechnology are also expected to provide foundation for development of novel therapeutics and wide applications of diagnostic methods in cancer.
Key factors in selecting biomaterials are biocompatibility, biodegradability, safety and ease of assembly in the structures with the desired characteristics. Taken together, biomaterials and nanotechnology offer a unique opportunity to improve survival in cancer patients. In this review, we will focus on strategies of nanoparticle design and highlight the latest developments in cancer nanomedicine.
Nanoparticles
The history of nanoparticles starts in 1950s with a polymer-drug conjugate that was designed by Jatzkewitz (Jatzkewitz, 1954), followed by Bangham who discovered the liposomes in mid-1960s (Bangham and Horne, 1964), (Bangham et al., 1965). In 1972, Scheffel and colleagues first reported albumin based nanoparticles (Scheffel et al., 1972), which formed the basis of albumin-bound paclitaxel (Abraxane). Abraxane was approved in 2005 by US Food and Drug Administration (FDA) for the treatment of breast cancer (Gradishar et al., 2005) and recently approved for the treatment of lung cancer (Casaluce et al., 2012). Abelcet, amphotericin B lipid complex, was approved by FDA (Chonn and Cullis, 1995) for the treatment of invasive fungal infections and it is widely used to treat systemic fungal disease, which is a source of major morbidity in cancer patients (Herbrecht, 1996).
In the 1980s, Maeda and colleagues observed the enhanced accumulation of nanoparticles in the tumor site due to the altered structure of tumor vasculature (Matsumura and Maeda, 1986). Blood vessels in tumors are different compared to normal blood vessels due to abnormal and leaky architecture. Impaired regulation in blood vessels leads to ‘enhanced permeability and retention (EPR) effect’ (Maeda et al., 2006). The reduced lymphatic derange, increased size of fenestrations and gaps between endothelial cells, varies from 200 to 1200 nm, in contrast to normal endothelium with pores with 10 to 50 nm contributes to EPR effect. This effect has become a hallmark of the solid tumor vasculature leading to increased nanoparticle accumulation in the tumor site due to ‘passive targeting’. Hereby drug carriers exhibit enhanced therapeutic efficacy in tumors, in addition to reduced side effects and toxicity.
Despite the advantages of passive targeting approaches, several limitations exist that still needs to be eliminated in the future. Certain tumors are difficult to deliver due to lack of EPR effect, hence the permeability in blood vessels may not be identical throughout the same tumor (Yuan et al., 1995). To overcome these limitations, nanoparticles are designed to bind to specific targets (active targeting) through the ligands that recognize particular receptors in target cells.
Active Targeting
Various receptors on the tumor cell surface have been studied as potential sites to achieve selective delivery. Nanoparticle surface can be modified by a variety of conjugation chemistries to attach specific receptor ligands (Torchilin, 2005). Nanoparticles recognize and bind to their targets with subsequent uptake through receptor mediated endocytosis. Once internalized, the drug or payload is released in the cytoplasm or nucleus. Such receptor ligands may be peptides, vitamins, antibodies, carbohydrates and other chemical structures. For instance, the overexpression of transferrin and folate in certain tumors have been exploited to deliver nanoparticles conjugated with these receptor’s ligands (Yang et al., 2010), (Fernandes et al., 2008). Another example is the αvβ3 integrin, which is overexpressed in a wide range of tumors and angiogenic tumor-associated endothelium, and is largely absent in normal tissues. Han and colleagues have recently reported that the administration of chitosan nanoparticles conjugated with cyclic Arg-Gly-Asp (RGD) led to increased tumor delivery and enhanced anti-tumor activity in ovarian cancer models (Han et al., 2010) (Fig. 1). A variety of targeting agents such as monoclonal antibodies (mAbs) and nucleic acids (aptamers) are also used to enhance tumoral uptake of nanoparticles. Using mAbs for targeting in cancer therapy was first described by Milstein in 1981 (Warenius et al., 1981). Since then, antibody-based targeting has made a significant progression as a feasible strategy in cancer therapy. Clinically approved and widely used mAbs include rituximab (Rituxan) for the treatment of non-Hodgkin’s lymphoma (James and Dubs, 1997), trastuzumab (Herceptin) for breast cancer treatment (Albanell and Baselga, 1999), bevacizumab as an angiogenesis inhibitor in colorectal cancer (Ferrara, 2005). Since 1997, 12 mAb-based therapy have been approved and a large number of antibody-based strategy is in progress for preclinical or clinical trials (Scott et al., 2012). Conjugation of an antibody directly to a therapeutic agent has been also explored. Mylotarg was the first approved formulation with this regard in clinic. Calicheamicin is a chemotherapeutic agent and it was conjugated with the CD33 antibody (Peer et al., 2007). Zevalin and Bexxar are radio-immunoconjugates formulated by using CD20 antibody and approved for the treatment of non-Hodgkin’s lymphoma (Grillo-López, 2002), (Blagosklonny, 2004).
FIGURE 1. Targeted therapy with RGD-Chitosan Nanoparticles.
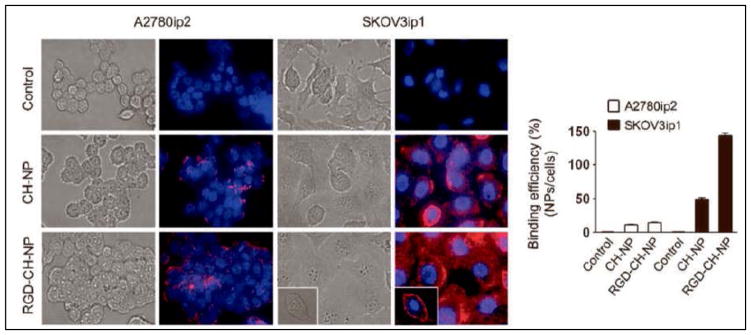
Binding efficiency of Alexa555 siRNA (red fluorescence) incorporated chitosan nanoparticles (CH-NP)- conjugated with RGD peptide (RGD-CH-NP) in SKOV3ip1 or A2780ip2 ovarian cancer cells (blue for nuclei) by fluorescence microscopy (Han et al., 2010).
HAN, H. D., MANGALA, L. S., LEE, J. W., SHAHZAD, M. M., KIM, H. S., SHEN, D., NAM, E. J., MORA, E. M., STONE, R. L. & LU, C. 2010. Targeted gene silencing using RGD-labeled chitosan nanoparticles. Clinical Cancer Research, 16, 3910–3922.
Recently, nucleic acid aptamers have gained immediate attention after the in vitro selection of functional nucleic acids (termed SELEX) that was discovered in 1990 (Ellington and Szostak, 1990, Tuerk and Gold, 1990). Aptamers are single stranded oligonucleotides that can modulate molecular targets with high specificity and affinity through their three-dimensional structures. Aptamers exhibit significant advantages such as the technical possibility in selection and chemical modification, specificity to target any given molecule, its prosperous bio-activity in vivo, the low production costs, the simplicity in synthesis and storage for the marketing (Scaggiante et al., 2013) There are currently several aptamers that are in clinical trials (Scott et al., 2012). For instance, Pegaptanib was approved by FDA and used as a VEGF-specific aptamer that binds to VEGF and blocks the interaction with its receptor (VEGFR) thereby inhibiting its activity (Gragoudas et al., 2004). Moreover, aptamers seem alluring to modify the surface of nanoparticles for the design of targeted drug delivery systems.
Drug Delivery Systems
Liposomes
Liposomes are self-assembling nanoparticles formed by dispersion of phospholipids with hydrophilic heads and hydrophobic anionic/cationic long chain tails, creating closed membrane structures (Fig. 2). Hydrophilic agents such as drugs and siRNA or hydrophobic drugs can be incorporated into the inner compartments and, into the hydrophobic membranes respectively. Currently, several liposomal anticancer drugs are used successfully as carriers in the clinic or studied in advanced stages of clinical trials. For instance, doxorubicin loaded liposomes were modified with polyethylene glycol (PEG) that alters the plasma pharmacokinetics and tissue distribution of doxorubicin and this PEGylated liposomal doxorubicin (Doxil) carriers, were approved by FDA for the treatment of Kaposi’s sarcoma (Patel, 1996). Along with Doxil, approved liposomal formulations include non-pegylated liposomal doxorubicin (Myocet by Elan), liposomal daunorubicin (DaunoXome by Gilead), liposomal amphotericin B (abelcet), liposomal cytarabine (DepoCyte by SkyePharma/Enzon/Mundipharma) and liposomal cisplatin (Lipoplatin by Regulon) (Huwyler et al., 2008). On the other hand, antisense oligonucleotides are also attractive to be used in liposomal formulations for cancer therapy (Tari et al., 1995). Antisense oligonucleotides can selectively inhibit disease-causing genes and thereby inhibiting the production of disease associated-proteins. For instance, liposomal formulation of bcl-2 oligos was demonstrated to inhibit bcl-2 protein production thereby leading to a growth inhibition in follicular lymphoma cell lines (Tormo et al., 1998). Furthermore, liposomal bcl-2 antisense oligos were studied to evaluate the in vivo behavior in rodents. The liposomes were widely distributed and no significant toxicity was observed over 6-week treatment of intravenously administered liposomal Bcl-2 oligos (Gutiérrez-Puente et al., 1999). Another example is raf antisense oligonucleotide that inhibits c-raf that leads to enhanced sensitivity to radiation and chemotherapy. LErafAON is the liposomal formulation of raf oligonucleotide that showed success for advanced solid tumors in its Phase I study (McGinnis et al., 2012).
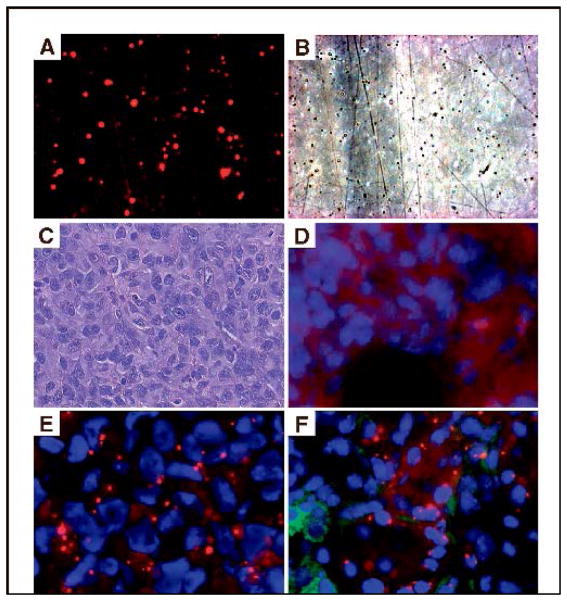
FIGURE 2. Accumulation of siRNA with DOPC nanoliposomes in vivo.
Fluorescent (A) and phase (B) view of liposomes after siRNA incorporation.
C. Hematoxylin &Eosin stain of HeyA8 ovarian tumor.
D. Autofluorescence in tumor 48 hours after intravenous administration of nonfluorescent control siRNA.
E. Tumor accumulation of Alexa 555 siRNA (red fluoresce) incorporated in DOPC.
F. Alexa 555 siRNA is seen in both tumor cells and surrounding macrophages (green) (Landen et al., 2005).
LANDEN, C. N., CHAVEZ-REYES, A., BUCANA, C., SCHMANDT, R., DEAVERS, M. T., LOPEZ-BERESTEIN, G. & SOOD, A. K. 2005. Therapeutic EphA2 gene targeting in vivo using neutral liposomal small interfering RNA delivery. Cancer research, 65, 6910–6918.
Polymeric nanoparticles
Polymer based delivery systems show great promise for biomedical applications due to their high biocompatibility and flexibility in which their structures can be modified to engineer multifunctional nanoparticles with desired shape, size, internal and external morphology as well as surface modifications. During the preparation stage of nanoparticles, polymers can be utilized through isolation from their natural sources such as chitosan that is produced from chitin or they can be synthesized in the desired structure such as poly-lactic-co-glycolic acid (PLGA). PLGA, arginine, chitosan, human serum albumin, alginate, and hyaluronic acid have been widely used in preclinical studies for drug delivery. Polymer based nanoparticles shows great promise in preclinical studies. For example, chitosan nanoparticles are one of the most popular polymeric delivery system that is widely used in particular gene delivery. Chitosan nanoparticles serve as an attractive candidate for small interfering RNA (siRNA) delivery due its positive charge. Electrostatic interactions between negatively charged siRNA and positively charged chitosan create a safe carrier for siRNA in the blood circulation. Kim and coworkers, analyzed the therapeutic effects of src and fgr inhibition using siRNA incorporated chitosan nanoparticles in orthotopic models of ovarian cancer. Dual silencing of src and fgr with chitosan nanoparticles in vivo, led to a significant reduction in tumor growth (Kim et al., 2011).
For clinical studies, albumin bound paclitaxel (abraxane) is the first polymeric formulation that is approved by FDA for the treatment of metastatic breast cancer (Gradishar et al., 2005) and it is recently approved for the treatment of lung cancer. Abraxane exploited the ability of albumin to bind to 60-kDa glycoprotein (gp60) receptor (albondin)(Miele et al., 2009). After this receptor-ligand interaction, albumin-gp60 complex triggers caveolin-1 mediated uptake of protein bound plasma molecules. On the other hand, albumin also binds to osteonectin (secreted protein acid rich in cysteine [SPARC]) due to a sequence homology with gp60. SPARC is highly expressed in particular neoplasms (breast, prostate, and lung cancer) and contributes to intratumor accumulation of all albumin-bound drugs (Hawkins et al., 2008). In addition, Livatag (Doxorubicin Transdug) is a poly (isohexyl cyanoacrylate) nanoparticle formulation loaded with doxorubicin and approved for the treatment of multidrug-resistant protein-overexpressing hepatocellular carcinoma (Sultana et al., 2013).
Polymeric micelles
Polymeric micelles are formed from self-assembly of amphiphilic-block copolymers ranging between 10–100 nm in size. They are composed of a hydrophobic core and a hydrophilic corona. Micelles can improve the bioavailability of hydrophobic drugs, confer protection and inactivation of the drugs under the effect of biological surroundings (Torchilin, 2001). Polymeric micelle formulations are used for both passive and active targeting in anticancer therapy. For example, Genexol-PM is currently under investigation as a paclitaxel loaded polymeric micelle formulation for the treatment of breast, lung, and pancreatic cancer. Pluronic and NK911 are doxorubicin loaded micelle formulations that are also currently studied in Phase I (Sultana et al., 2013). NC-6004 is carboplatin loaded formulation that is also studied in early clinical trials for the treatment of solid tumors (Wilson et al., 2008). Furthermore, there are polymeric micelle formulations that are designed for active targeting and modified with different ligands such as folate (binds to folate receptor) and mAb C225 (binds to EGF receptor). In a nude mice xenograft model, doxorubicin loaded PLGA-b-PEG polymeric micelle formulation has been shown to increase tumoral uptake and significant tumor regression (Yoo and Park, 2004).
Dendrimers
Dendrimers are hyperbranched nanoparticles composed of a core, branching units and functionalized terminal groups. The major advantage of dendrimers is that multiple anticancer agents can be incorporated in the central core or conjugated to functional end groups (Lee et al., 2005). In addition, depolymerization of dendrimers can be controlled to modify release profiles of the payload (Wong et al., 2012). For example, polyamidoamine (PAMAM) dendrimers can be tailored to enhance their biocompatibility and release properties through PEGylation, acetylation, and modified with anionic, neutral ligand molecules (Cai et al., 2013). As an example, doxorubicin was conjugated to PEGylated PAMAM dendrimers by acid-sensitive linkages in order to trigger the release of doxorubicin in acidic conditions (Zhu et al., 2010). Evaluations of pH-dependent payload release, cytotoxicity, cellular uptake and intracellular localization were performed using SKOV-3 ovarian cancer cell line. In addition, dendrimers with highest PEGylation degree showed the maximum- accumulation in SKOV3 tumor xenografts in mice. On the other hand, polylysine dendrimers conjugated with a ligand for α5β1 also known as fibronectin receptor was designed for tumor targeting. Activated α5β1 is highly expressed in breast cancer cells compared to non-transformed cells and it plays a vital role in invasion and metastasis pathways in cancer. PHSCN peptide is a ligand that interacts with a specific region of the α5 subunit of integrin thereby blocking its activity. Polylysine dendrimers can be modified with this ligand for tumor targeting and the treatment with this carrier led to a significant reduction in the number of invasive human breast cancer cells (Yao et al., 2011). Furthermore, when tumor bearing mice were treated with polylysine dendrimers modified with integrin ligand, lung colony formation was obviously inhibited. In conclusion, despite the fact that dendrimers are extensively used for the design and development of therapeutics, further research is needed to improve its immunogenicity to assure the safety of long-term administration in clinic.
Characteristics of nanoparticles
Physical and chemical characteristics of nanoparticles including size, charge, shape, and surface properties individually play major roles for in vivo biodistribution and cellular internalization of these drug carriers. In this section, we will focus on the major parameters that determine the lifetime and delivery of the nanoparticles.
Size
Particle size is one of the crucial primary factors in determining the circulation time of the nanoparticles. After systemic administration, nanoparticles accumulate in spleen due to mechanical filtration and removed by reticulo-endothelial system (RES). For example, as the main constituent of RES, Kupffer cells play a major role for the removal of the particles accumulated in the liver (Moghimi et al., 2001). Currently, 100–200 nm is accepted as optimal size for drug delivery systems since nanocarriers take the advantage of EPR effect in tumors and avoid filtration in the spleen whereas they are large enough to avoid the uptake in the liver (Petros and DeSimone, 2010). Particles with a smaller diameter than 5nm are rapidly cleared from blood circulation through renal clearance or extravasation (Wong et al., 2008), (Alexis et al., 2008), (Choi et al., 2007). However, particles with a size up to 15 μm; accumulate in liver, spleen and bone marrow (Petros and DeSimone, 2010).
In addition, particle size has a significant impact on cellular internalization through phagocytosis, macropinocytosis, caveolar-mediated endocytosis, clathrin-mediated endocytosis. As mentioned above, size range has high influence on biodistribution and cellular internalization. In addition, recent studies show that the geometry of the particles is as important as size range in terms of cellular internalization and distribution (Geng et al., 2007), (Decuzzi et al., 2010). In addition, Gratton and coworkers studied the correlation between shape and size on the internalization frequency in HeLa cells and interestingly, the particles with different shapes but similar volumes were internalized at extremely assorted rates (Gratton et al., 2008). In a distinct study, Godin and coworkers demonstrated that the accumulation of discoidal particles in breast tumors were five times higher than spherical particles despite their similar diameters (Godin et al., 2012). As a result, accumulating evidence shows that although size is a major parameter in the design of nanocarriers for decades, the shape as well, has a high impact along with the size.
Shape
Degradation properties of nanoparticles and subsequent payload release have been shown to be dependent on particle shape (Bawa et al., 1985). The importance of surface area and diameter were also demonstrated to be critical for cellular uptake of the nanoparticles (Panyam et al., 2003), (Dunne et al., 2000). Hemi-spherical particles were generated as sustained release devices in order to achieve zero-order. Spherical particles, however, can provide different degradation profiles as their shapes are susceptible upon degradation (Champion et al., 2007). Additionally, deformability of spherical nanoparticles is also playing a key role to avoid spleen filtration since spleen exhibit asymmetric filtering units (Moghimi et al., 2001). Therefore, nanoparticles which are especially larger than 200 nm should be either deformable enough to bypass the filtration in spleen or flexible as erythrocytes that can avoid filtration even with 10 μm diameter.
In an elegant study, Decuzzi and co-workers studied the effect of size and shape of nanoparticles on biodistribution and tumor accumulation after intravenous injection. Spherical silica particles were generated in different sizes ranging from 700 nm to 3μm also in different shapes such as quasi-hemispherical, discoidal, and cylindrical silicon based particles. After a single, intravenous particle injection to tumor bearing mice, tumors and the major organs including liver, spleen, heart, lungs, kidneys, and brain were analyzed for silicon content and histological evaluation. This study elucidated the importance of shape properties of nanoparticles in addition to size distribution, indicating that geometry of the nanoparticles contributes to opsonization, in vivo biodistribution, the strength of adhesion and internalization rate in the cells (Decuzzi et al., 2009).
Surface characteristics
Surface properties play a key role on the period of nanoparticles in blood circulation subsequent systemic administration. After administration, nanoparticles may be associated with proteins which are known as ‘opsonins’, such as immunoglobulins and complement proteins that contribute to recognition of nanoparticles by macrophages. Therefore, opsonization is the key factor that determines the fate of nanoparticles to an extent in blood circulation. Modifying the surface of nanoparticles can be used as a strategy to enhance or reduce their circulation time in blood and tissues. For instance, negatively charged nanoparticles result in rapid RES clearance from circulation (Zahr et al., 2006). Cationic surfaces may induce cell membrane permeability and enhance cellular uptake (Chen et al., 2009) however, cationic nanoparticles prepared from polycationic polymers such as polyethyleneimine and diethylaminoethyl-dextran can induce disruption in the cell, through formation of holes, membrane thinning and membrane erosion in lipid bilayers (Leroueil et al., 2008). On the other hand, the use of neutrally charged particles as well as particles coated with polyethylene glycol (PEG) lead to a major reduction of particle uptake by the RES (Torchilin and Trubetskoy, 1995, Otsuka et al., 2003).
The surface modification of PEGylated liposomes with rat serum albumin (RAS), compared with non-modified PEGylated liposomes, showed prolonged blood circulation in rats. To further analyze, total serum protein amounts were determined quantitatively in the absence and presence of RAS coating. As a result, RAS-modified liposomes significantly reduced the total amount of serum proteins that can induce opsonization in serum (Furumoto et al., 2007). In addition, doxorubicin-loaded and albumin-modified liposomes demonstrated enhanced pharmacokinetics and tissue distribution of doxorubicin (Yokoe et al., 2008). Tumor accumulation and therapeutic index of albumin-modified PEGylated liposomal doxorubicin was significantly higher than non-modified PEGylated liposomal doxorubicin indicating that surface modification of nanoparticles with albumin, enhances their safety and effectiveness.
In addition, nanoparticle surface can be modified with ligands that recognize and bind to specific receptors. Also, monoclonal antibodies can be conjugated onto nanoparticle surface to provide specificity. For instance, nanoparticles modified with HER2 specific antibody, delivers the drug, particularly HER2 expressing cells (Kirpotin et al., 2006). Torchilin’s group has also designed different approaches for active targeted delivery to the tumor with liposomes and micellar delivery systems. They have developed monoclonal antibody 2C5-modified doxorubicin loaded liposomes to enhance the therapeutic activity of the payload in brain tumor xenografts (Gupta and Torchilin, 2007) These studies demonstrate that surface characteristics are fundamentally important for nanoparticles to avoid their rapid clearance from the blood circulation before reaching the tumor site, and to provide active targeting through surface modifications with antibodies or ligands.
Release characteristics
The release properties of nanoparticles determine the efficiency of the treatment at target sites. Conventional drugs used in clinic have a narrow therapeutic window due to rapid increase and decrease of plasma drug levels after systemic administration, resulting in bordering doses with subsequent side effects. However, drug delivery systems aims at delivering the desired concentration of the drug within the therapeutic range at target site, culminating minimized side effects and discomfort in patients. Constant plasma drug levels over a long period of time can be attained through zero-order release kinetics that can be achieved by using osmotic pressure, mechanical pumping, and electrokinetic transportation (Sakamoto et al., 2010). Besides, biocompatible polymeric nanoparticles are also used to prolong the period of drug release due to their long biodegration time in a range from days to months. Particularly, molecular weight is a major parameter in biodegradation rate of polymers. For instance, poly lactide-co-glycolide (PLGA) and poly lactic acid (PLA) were both used in order to study the sustained release of docetaxel after intravenous administration (Musumeci et al., 2006). Release rate of the drug has been shown to highly associate with molecular weight of the polymers. Furthermore, polymer with high molecular weight led to slower degradation of the material, compared to the polymer with low molecular weight, resulting in sustained release of the payload.
Multistage delivery system is an additional alternative approach providing sustained release of the payload where mesoporous silicon particles (MSP) offer unique opportunities for drug delivery (Tanaka et al., 2010). MSPs size, charge, shape, porosity are among the characteristics that can be tailored for particular applications and objectives of its use. We have used MSPs loaded with nanoliposomes carrying small interfering RNA (siRNA) that leads to target mRNA degradation. In this study, degradation of silicon particles allowed for the long term release of siRNA to the target site (Shen et al., 2013, Tanaka et al., 2010). (Fig. 3)
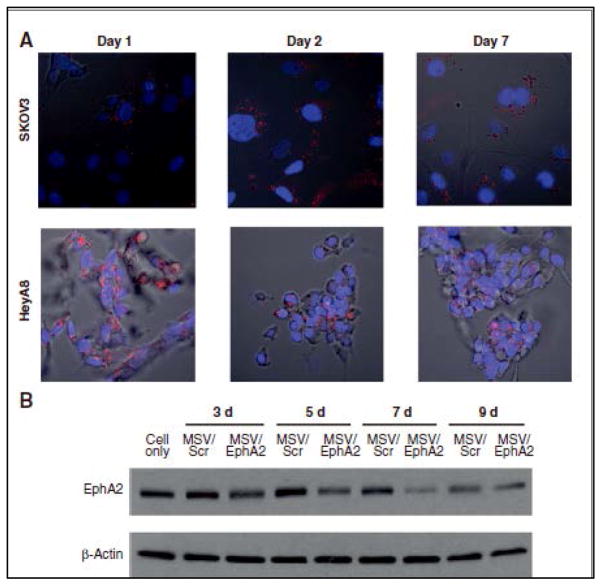
FIGURE 3. Sustained release of liposomal EphA2 siRNA in tumor cells.
A. Alexa555-labeled siRNA oligos (red fluorescence) were packaged in DOPC nanoliposomes and loaded into Multistage Vector (MSV). Human ovarian tumor cells SKOV3ip2 and HeyA8 (nuclei in blue) were incubated with MSV/Alexa555 siRNA and release of Alexas555 siRNA from MSV was monitored by confocal microscopy over the next 7 days.
B. Western blot analysis of EphA2 expression in SKOV3 cells incubated with MSV/EphA2 siRNA indicating inhibition in protein expression more than 7 days (Shen et al., 2013).
SHEN, H., RODRIGUEZ-AGUAYO, C., XU, R., GONZALEZ-VILLASANA, V., MAI, J., HUANG, Y., ZHANG, G., GUO, X., BAI, L. & QIN, G. 2013. Enhancing chemotherapy response with sustained EphA2 silencing using multistage vector delivery. Clinical Cancer Research.
Another strategy to control the release of the payload can be using the environment of target site as a driving mechanism. Environment responsive nanocarriers offer a unique strategy, in particular, when the stimulus is specific to the disease pathology (Ganta et al., 2008). The approach seems promising since the stimuli trigger the payload to diffuse out of the particles through a controlled drug release. The biological stimuli include pH, temperature, and redox microenvironment (Shenoy et al., 2005), (Kommareddy and Amiji, 2005). Recently, Chen and colleagues have designed dual responsive-doxorubicin loaded polymeric micelles that release the payload in response to temperature and pH (Chen et al., 2012). In this study, drug release was analyzed at different pH conditions such as physiological condition (pH 7.4), endosomal (pH 6.6 and 6.0), lysosomal (pH 5.4), and different temperature conditions. Doxorubicin release rate was associated with increased temperature and decreased pH. Furthermore, they have demonstrated enhanced antitumor activity in tumor bearing mice that were generated by subcutaneously injected HeLa cells. On the other hand, external stimuli can be used to trigger the release such as magnetic field, mild temperature increase or ultrasound (MacEwan et al., 2010). For instance, ultrasound triggers the degradation of polymers, slightly increases the temperature and cell membrane permeability, ultimately resulting in the release of the drug at target site (Mitragotri, 2005). Cisplatin release upon low frequency ultrasound has been demonstrated by Schroeder and colleagues (Schroeder et al., 2009). In this study, cisplatin-loaded liposomes were intraperitoneally administered into tumor bearing mice and the release of cisplatin was triggered by ultrasound at tumor site. Despite the tremendous progress in the design and development of nanoparticles, further preclinical studies are still required to conduct clinical trials for cancer therapy.
Conclusion and future perspectives
Advances in nanomedicine offer new opportunities to improve the anticancer armamentarium. Targeted and nontargeted nanoparticles are currently in preclinical and clinical phases indicating the impact of delivery systems on the field. Further studies in nanomedicine will improve therapeutic window of drugs with immensely reduced side effects leading to improved patient outcomes.
Acknowledgments
This work was supported in part by CPRIT (RP120406, RP120214), NIH (CA093459, U54CA151668, U54CA096300, UH2TR000943, R44GM084552, R21CA167505, R01CA151372), DOD (W81XWH-09-1-0385, OC073399, W81XWH-10-1-0158, BC085265), the Marcus Foundation, the Blanton-Davis Ovarian Cancer Research Program, and the Betty Anne Asche Murray Distinguished Professorship.
Footnotes
Declaration of interest
The authors report no conflict of interest. The authors are responsible for the content and writing of the paper.
References
- ALBANELL J, BASELGA J. Trastuzumab, a humanized anti-HER2 monoclonal antibody, for the treatment of breast cancer. Drugs Today (Barc) 1999;35:931–946. [PubMed] [Google Scholar]
- ALEXIS F, PRIDGEN E, MOLNAR LK, FAROKHZAD OC. Factors affecting the clearance and biodistribution of polymeric nanoparticles. Molecular pharmaceutics. 2008;5:505–515. doi: 10.1021/mp800051m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BANGHAM A, HORNE R. Negative staining of phospholipids and their structural modification by surface-active agents as observed in the electron microscope. Journal of molecular biology. 1964;8:660-IN10. doi: 10.1016/s0022-2836(64)80115-7. [DOI] [PubMed] [Google Scholar]
- BANGHAM A, STANDISH M, WATKINS J. Diffusion of univalent ions across the lamellae of swollen phospholipids. Journal of molecular biology. 1965;13:238-IN27. doi: 10.1016/s0022-2836(65)80093-6. [DOI] [PubMed] [Google Scholar]
- BAWA R, SIEGEL AR, MARASCA B, KAREL M, LANGER R. An explanation for the controlled release of macromolecules from polymers. Journal of Controlled Release. 1985;1:259–267. [Google Scholar]
- BLAGOSKLONNY MV. Analysis of FDA approved anticancer drugs reveals the future of cancer therapy. Cell Cycle. 2004;3:1033–1040. [PubMed] [Google Scholar]
- BRAY F, JEMAL A, GREY N, FERLAY J, FORMAN D. Global cancer transitions according to the Human Development Index (2008–2030): a population-based study. The Lancet Oncology. 2012 doi: 10.1016/S1470-2045(12)70211-5. [DOI] [PubMed] [Google Scholar]
- CAI X, HU J, XIAO J, CHENG Y. Dendrimer and cancer: a patent review (2006–present) Expert opinion on therapeutic patents. 2013:1–15. doi: 10.1517/13543776.2013.761207. [DOI] [PubMed] [Google Scholar]
- CASALUCE F, SGAMBATO A, ROSSI A, MULSHINE JL. The US FDA has approved Abraxane® for the treatment of non-small-cell lung cancer Aurora: a new light for targeted therapy in small-cell lung cancer. Lung Cancer. 2012;1:251–254. [Google Scholar]
- CHAMPION JA, KATARE YK, MITRAGOTRI S. Particle shape: a new design parameter for micro-and nanoscale drug delivery carriers. Journal of Controlled Release. 2007;121:3–9. doi: 10.1016/j.jconrel.2007.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHEN J, HESSLER JA, PUTCHAKAYALA K, PANAMA BK, KHAN DP, HONG S, MULLEN DG, DIMAGGIO SC, SOM A, TEW GN. Cationic nanoparticles induce nanoscale disruption in living cell plasma membranes. The Journal of Physical Chemistry B. 2009;113:11179–11185. doi: 10.1021/jp9033936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHEN Y-C, LIAO L-C, LU P-L, LO C-L, TSAI H-C, HUANG C-Y, WEI K-C, YEN T-C, HSIUE G-H. The accumulation of dual pH and temperature responsive micelles in tumors. Biomaterials. 2012 doi: 10.1016/j.biomaterials.2012.02.059. [DOI] [PubMed] [Google Scholar]
- CHOI HS, LIU W, MISRA P, TANAKA E, ZIMMER JP, IPE BI, BAWENDI MG, FRANGIONI JV. Renal clearance of quantum dots. Nature biotechnology. 2007;25:1165–1170. doi: 10.1038/nbt1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHONN A, CULLIS PR. Recent advances in liposomal drug-delivery systems. Current opinion in Biotechnology. 1995;6:698–708. doi: 10.1016/0958-1669(95)80115-4. [DOI] [PubMed] [Google Scholar]
- DECUZZI P, GODIN B, TANAKA T, LEE SY, CHIAPPINI C, LIU X, FERRARI M. Size and shape effects in the biodistribution of intravascularly injected particles. Journal of Controlled Release. 2010;141:320–327. doi: 10.1016/j.jconrel.2009.10.014. [DOI] [PubMed] [Google Scholar]
- DECUZZI P, PASQUALINI R, ARAP W, FERRARI M. Intravascular delivery of particulate systems: does geometry really matter? Pharmaceutical research. 2009;26:235–243. doi: 10.1007/s11095-008-9697-x. [DOI] [PubMed] [Google Scholar]
- DUNNE M, CORRIGAN O, RAMTOOLA Z. Influence of particle size and dissolution conditions on the degradation properties of polylactide-< i> co</i>-glycolide particles. Biomaterials. 2000;21:1659–1668. doi: 10.1016/s0142-9612(00)00040-5. [DOI] [PubMed] [Google Scholar]
- ELLINGTON AD, SZOSTAK JW. In vitro selection of RNA molecules that bind specific ligands. Nature. 1990;346:818–822. doi: 10.1038/346818a0. [DOI] [PubMed] [Google Scholar]
- FERNANDES JC, WANG H, JREYSSATY C, BENDERDOUR M, LAVIGNE P, QIU X, WINNIK FM, ZHANG, DAI K, SHI Q. Bone-protective Effects of Nonviral Gene Therapy With Folate–Chitosan DNA Nanoparticle Containing Interleukin-1 Receptor Antagonist Gene in Rats With Adjuvant-induced Arthritis. Molecular Therapy. 2008;16:1243–1251. doi: 10.1038/mt.2008.99. [DOI] [PubMed] [Google Scholar]
- FERRARA N. VEGF as a therapeutic target in cancer. Oncology. 2005;69:11–16. doi: 10.1159/000088479. [DOI] [PubMed] [Google Scholar]
- FURUMOTO K, YOKOE JI, OGAWARA KI, AMANO S, TAKAGUCHI M, HIGAKI K, KAI T, KIMURA T. Effect of coupling of albumin onto surface of PEG liposome on its in vivo disposition. International journal of pharmaceutics. 2007;329:110–116. doi: 10.1016/j.ijpharm.2006.08.026. [DOI] [PubMed] [Google Scholar]
- GANTA S, DEVALAPALLY H, SHAHIWALA A, AMIJI M. A review of stimuli-responsive nanocarriers for drug and gene delivery. Journal of Controlled Release. 2008;126:187–204. doi: 10.1016/j.jconrel.2007.12.017. [DOI] [PubMed] [Google Scholar]
- GENG Y, DALHAIMER P, CAI S, TSAI R, TEWARI M, MINKO T, DISCHER DE. Shape effects of filaments versus spherical particles in flow and drug delivery. Nature Nanotechnology. 2007;2:249–255. doi: 10.1038/nnano.2007.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GODIN B, CHIAPPINI C, SRINIVASAN S, ALEXANDER JF, YOKOI K, FERRARI M, DECUZZI P, LIU X. Drug Delivery: Discoidal Porous Silicon Particles: Fabrication and Biodistribution in Breast Cancer Bearing Mice (Adv. Funct. Mater. 20/2012) Advanced Functional Materials. 2012;22:4186–4186. doi: 10.1002/adfm.201200869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRADISHAR WJ, TJULANDIN S, DAVIDSON N, SHAW H, DESAI N, BHAR P, HAWKINS M, O’SHAUGHNESSY J. Phase III trial of nanoparticle albumin-bound paclitaxel compared with polyethylated castor oil–based paclitaxel in women with breast cancer. Journal of clinical oncology. 2005;23:7794–7803. doi: 10.1200/JCO.2005.04.937. [DOI] [PubMed] [Google Scholar]
- GRAGOUDAS ES, ADAMIS AP, CUNNINGHAM ET, JR, FEINSOD M, GUYER DR. Pegaptanib for neovascular age-related macular degeneration. New England Journal of Medicine. 2004;351:2805–2816. doi: 10.1056/NEJMoa042760. [DOI] [PubMed] [Google Scholar]
- GRATTON SE, ROPP PA, POHLHAUS PD, LUFT JC, MADDEN VJ, NAPIER ME, DESIMONE JM. The effect of particle design on cellular internalization pathways. Proceedings of the National Academy of Sciences. 2008;105:11613–11618. doi: 10.1073/pnas.0801763105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRILLO-LÓPEZ AJ. Zevalin: the first radioimmunotherapy approved for the treatment of lymphoma. Expert review of anticancer therapy. 2002;2:485–493. doi: 10.1586/14737140.2.5.485. [DOI] [PubMed] [Google Scholar]
- GUPTA B, TORCHILIN VP. Monoclonal antibody 2C5-modified doxorubicin-loaded liposomes with significantly enhanced therapeutic activity against intracranial human brain U-87 MG tumor xenografts in nude mice. Cancer Immunology, Immunotherapy. 2007;56:1215–1223. doi: 10.1007/s00262-006-0273-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GUTIÉRREZ-PUENTE Y, TARI AM, STEPHENS C, ROSENBLUM M, GUERRA RT, LOPEZ-BERESTEIN G. Safety, pharmacokinetics, and tissue distribution of liposomal P-ethoxy antisense oligonucleotides targeted to Bcl-2. Journal of Pharmacology and Experimental Therapeutics. 1999;291:865–869. [PubMed] [Google Scholar]
- HAN HD, MANGALA LS, LEE JW, SHAHZAD MM, KIM HS, SHEN D, NAM EJ, MORA EM, STONE RL, LU C. Targeted gene silencing using RGD-labeled chitosan nanoparticles. Clinical Cancer Research. 2010;16:3910–3922. doi: 10.1158/1078-0432.CCR-10-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAWKINS MJ, SOON-SHIONG P, DESAI N. Protein nanoparticles as drug carriers in clinical medicine. Advanced Drug Delivery Reviews. 2008;60:876–885. doi: 10.1016/j.addr.2007.08.044. [DOI] [PubMed] [Google Scholar]
- HERBRECHT R. The changing epidemiology of fungal infections: are the lipid-based forms of amphotericin B an advance? European Journal of Haematology. 1996;56:12–17. doi: 10.1111/j.1600-0609.1996.tb01347.x. [DOI] [PubMed] [Google Scholar]
- HUWYLER J, DREWE J, KRÄHENBÜHL S. Tumor targeting using liposomal antineoplastic drugs. International journal of nanomedicine. 2008;3:21. [PMC free article] [PubMed] [Google Scholar]
- JAMES J, DUBS G. FDA approves new kind of lymphoma treatment. Food and Drug Administration. AIDS treatment news. 1997:2. [PubMed] [Google Scholar]
- JATZKEWITZ H. Incorporation of physiologically-active substances into a colloidal blood plasma substitute. I. Incorporation of mescaline peptide into polyvinylpyrrolidone. Hoppe-Seyler’s Zeitschrift für physiologische Chemie. 1954;297:149. [PubMed] [Google Scholar]
- KIM HS, HAN HD, ARMAIZ-PENA GN, STONE RL, NAM EJ, LEE JW, SHAHZAD MM, NICK AM, LEE SJ, ROH JW. Functional roles of Src and Fgr in ovarian carcinoma. Clinical Cancer Research. 2011;17:1713–1721. doi: 10.1158/1078-0432.CCR-10-2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIRPOTIN DB, DRUMMOND DC, SHAO Y, SHALABY MR, HONG K, NIELSEN UB, MARKS JD, BENZ CC, PARK JW. Antibody targeting of long-circulating lipidic nanoparticles does not increase tumor localization but does increase internalization in animal models. Cancer research. 2006;66:6732–6740. doi: 10.1158/0008-5472.CAN-05-4199. [DOI] [PubMed] [Google Scholar]
- KOMMAREDDY S, AMIJI M. Preparation and evaluation of thiol-modified gelatin nanoparticles for intracellular DNA delivery in response to glutathione. Bioconjugate chemistry. 2005;16:1423–1432. doi: 10.1021/bc050146t. [DOI] [PubMed] [Google Scholar]
- LEE CC, MACKAY JA, FRÉCHET JM, SZOKA FC. Designing dendrimers for biological applications. Nature biotechnology. 2005;23:1517–1526. doi: 10.1038/nbt1171. [DOI] [PubMed] [Google Scholar]
- LEROUEIL PR, BERRY SA, DUTHIE K, HAN G, ROTELLO VM, MCNERNY DQ, BAKER JR, ORR BG, BANASZAK HOLL MM. Wide varieties of cationic nanoparticles induce defects in supported lipid bilayers. Nano letters. 2008;8:420–424. doi: 10.1021/nl0722929. [DOI] [PubMed] [Google Scholar]
- MACEWAN SR, CALLAHAN DJ, CHILKOTI A. Stimulus-responsive macromolecules and nanoparticles for cancer drug delivery. Nanomedicine. 2010;5:793–806. doi: 10.2217/nnm.10.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAEDA H, GREISH K, FANG J. Polymer Therapeutics II. Springer; 2006. The EPR effect and polymeric drugs: a paradigm shift for cancer chemotherapy in the 21st century. [Google Scholar]
- MATSUMURA Y, MAEDA H. A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer research. 1986;46:6387–6392. [PubMed] [Google Scholar]
- MCGINNIS AC, CHEN B, BARTLETT MG. Chromatographic methods for the determination of therapeutic oligonucleotides. Journal of Chromatography B. 2012;883:76–94. doi: 10.1016/j.jchromb.2011.09.007. [DOI] [PubMed] [Google Scholar]
- MIELE E, SPINELLI GP, MIELE E, TOMAO F, TOMAO S. Albumin-bound formulation of paclitaxel (Abraxane® ABI-007) in the treatment of breast cancer. International journal of nanomedicine. 2009;4:99. doi: 10.2147/ijn.s3061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MITRAGOTRI S. Healing sound: the use of ultrasound in drug delivery and other therapeutic applications. Nature Reviews Drug Discovery. 2005;4:255–260. doi: 10.1038/nrd1662. [DOI] [PubMed] [Google Scholar]
- MOGHIMI SM, HUNTER AC, MURRAY JC. Long-circulating and target-specific nanoparticles: theory to practice. Pharmacological reviews. 2001;53:283–318. [PubMed] [Google Scholar]
- MUSUMECI T, VENTURA CA, GIANNONE I, RUOZI B, MONTENEGRO L, PIGNATELLO R, PUGLISI G. PLA/PLGA nanoparticles for sustained release of docetaxel. International journal of pharmaceutics. 2006;325:172–179. doi: 10.1016/j.ijpharm.2006.06.023. [DOI] [PubMed] [Google Scholar]
- OTSUKA H, NAGASAKI Y, KATAOKA K. PEGylated nanoparticles for biological and pharmaceutical applications. Advanced Drug Delivery Reviews. 2003;55:403–419. doi: 10.1016/s0169-409x(02)00226-0. [DOI] [PubMed] [Google Scholar]
- PANYAM J, DALI MM, SAHOO SK, MA W, CHAKRAVARTHI SS, AMIDON GL, LEVY RJ, LABHASETWAR V. Polymer degradation and in vitro release of a model protein from poly (d, l-lactide-< i> co</i>-glycolide) nano-and microparticles. Journal of Controlled Release. 2003;92:173–187. doi: 10.1016/s0168-3659(03)00328-6. [DOI] [PubMed] [Google Scholar]
- PATEL J. Liposomal doxorubicin: Doxil®. Journal of Oncology Pharmacy Practice. 1996;2:201–210. [Google Scholar]
- PEER D, KARP JM, HONG S, FAROKHZAD OC, MARGALIT R, LANGER R. Nanocarriers as an emerging platform for cancer therapy. Nature nanotechnology. 2007;2:751–760. doi: 10.1038/nnano.2007.387. [DOI] [PubMed] [Google Scholar]
- PETROS RA, DESIMONE JM. Strategies in the design of nanoparticles for therapeutic applications. Nature Reviews Drug Discovery. 2010;9:615–627. doi: 10.1038/nrd2591. [DOI] [PubMed] [Google Scholar]
- SAKAMOTO JH, VAN DE VEN AL, GODIN B, BLANCO E, SERDA RE, GRATTONI A, ZIEMYS A, BOUAMRANI A, HU T, RANGANATHAN SI. Enabling individualized therapy through nanotechnology. Pharmacological Research. 2010;62:57–89. doi: 10.1016/j.phrs.2009.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCAGGIANTE B, DAPAS B, FARRA R, GRASSI M, POZZATO G, GIANSANTE C, FIOTTI N, TAMAI E, TONON F, GRASSI G. Aptamers as Targeting Delivery Devices or Anti-cancer Drugs for Fighting Tumors. Current drug metabolism. 2013 doi: 10.2174/13892002113149990010. [DOI] [PubMed] [Google Scholar]
- SCHEFFEL U, WAGNER HN, RHODES BA, NATARAJA TK. Albumin Microspheres for Study of Reticuloendothelial System. Journal of Nuclear Medicine. 1972;13:498. [PubMed] [Google Scholar]
- SCHROEDER A, HONEN R, TURJEMAN K, GABIZON A, KOST J, BARENHOLZ Y. Ultrasound triggered release of cisplatin from liposomes in murine tumors. Journal of Controlled Release. 2009;137:63–68. doi: 10.1016/j.jconrel.2009.03.007. [DOI] [PubMed] [Google Scholar]
- SCOTT AM, WOLCHOK JD, OLD LJ. Antibody therapy of cancer. Nature Reviews Cancer. 2012;12:278–287. doi: 10.1038/nrc3236. [DOI] [PubMed] [Google Scholar]
- SHEN H, RODRIGUEZ-AGUAYO C, XU R, GONZALEZ-VILLASANA V, MAI J, HUANG Y, ZHANG G, GUO X, BAI L, QIN G. Enhancing chemotherapy response with sustained EphA2 silencing using multistage vector delivery. Clinical Cancer Research. 2013 doi: 10.1158/1078-0432.CCR-12-2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHENOY D, LITTLE S, LANGER R, AMIJI M. Poly (ethylene oxide)-modified poly (β-amino ester) nanoparticles as a pH-sensitive system for tumor-targeted delivery of hydrophobic drugs: part 2. In vivo distribution and tumor localization studies. Pharmaceutical research. 2005;22:2107–2114. doi: 10.1007/s11095-005-8343-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SULTANA S, KHAN MR, KUMAR M, KUMAR S, ALI M. Nanoparticles-mediated drug delivery approaches for cancer targeting: a review. Journal of drug targeting. 2013;21:107–125. doi: 10.3109/1061186X.2012.712130. [DOI] [PubMed] [Google Scholar]
- TANAKA T, MANGALA LS, VIVAS-MEJIA PE, NIEVES-ALICEA R, MANN AP, MORA E, HAN HD, SHAHZAD MM, LIU X, BHAVANE R. Sustained small interfering RNA delivery by mesoporous silicon particles. Cancer research. 2010;70:3687–3696. doi: 10.1158/0008-5472.CAN-09-3931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TARI AM, LOPEZ-BERESTEIN G, DEISSEROTH AB. Liposomal antisense methyl phosphonate oligonucleotides and methods for their preparation and use. Google Patents 1995 [Google Scholar]
- TORCHILIN VP. Structure and design of polymeric surfactant-based drug delivery systems. Journal of controlled release: official journal of the Controlled Release Society. 2001;73:137. doi: 10.1016/s0168-3659(01)00299-1. [DOI] [PubMed] [Google Scholar]
- TORCHILIN VP. Recent advances with liposomes as pharmaceutical carriers. Nature Reviews Drug Discovery. 2005;4:145–160. doi: 10.1038/nrd1632. [DOI] [PubMed] [Google Scholar]
- TORCHILIN VP, TRUBETSKOY VS. Which polymers can make nanoparticulate drug carriers long-circulating? Advanced Drug Delivery Reviews. 1995;16:141–155. [Google Scholar]
- TORMO M, TARI AM, MCDONNELL TJ, CABANILLAS F, GARCIA-CONDE J, LOPEZ-BERESTEIN G. Apoptotic induction in transformed follicular lymphoma cells by Bcl-2 downregulation. Leukemia & lymphoma. 1998;30:367–380. doi: 10.3109/10428199809057548. [DOI] [PubMed] [Google Scholar]
- TUERK C, GOLD L. Systematic evolution of ligands by exponential enrichment: RNA ligands to bacteriophage T4 DNA polymerase. Science. 1990;249:505–510. doi: 10.1126/science.2200121. [DOI] [PubMed] [Google Scholar]
- WARENIUS H, GALFRE G, BLEEHEN N, MILSTEIN C. Attempted targeting of a monoclonal antibody in a human tumour xenograft system. European Journal of Cancer and Clinical Oncology. 1981;17:1009–1015. doi: 10.1016/s0277-5379(81)80006-5. [DOI] [PubMed] [Google Scholar]
- WILSON R, PLUMMER R, ADAM J, EATOCK M, BODDY A, GRIFFIN M, MILLER R, MATSUMURA Y, SHIMIZU T, CALVERT H. Phase I and pharmacokinetic study of NC-6004, a new platinum entity of cisplatin-conjugated polymer forming micelles. J Clin Oncol. 2008;26:2573. [Google Scholar]
- WONG AD, DEWIT MA, GILLIES ER. Amplified release through the stimulus triggered degradation of self-immolative oligomers, dendrimers, and linear polymers. Advanced Drug Delivery Reviews. 2012;64:1031–1045. doi: 10.1016/j.addr.2011.09.012. [DOI] [PubMed] [Google Scholar]
- WONG J, BRUGGER A, KHARE A, CHAUBAL M, PAPADOPOULOS P, RABINOW B, KIPP J, NING J. Suspensions for intravenous (IV) injection: a review of development, preclinical and clinical aspects. Advanced drug delivery reviews. 2008;60:939–954. doi: 10.1016/j.addr.2007.11.008. [DOI] [PubMed] [Google Scholar]
- YANG SJ, LIN FH, TSAI KC, WEI MF, TSAI HM, WONG JM, SHIEH MJ. Folic acid-conjugated chitosan nanoparticles enhanced protoporphyrin IX accumulation in colorectal cancer cells. Bioconjugate chemistry. 2010;21:679–689. doi: 10.1021/bc9004798. [DOI] [PubMed] [Google Scholar]
- YAO H, VEINE DM, FAY KS, STASZEWSKI ED, ZENG ZZ, LIVANT DL. The PHSCN dendrimer as a more potent inhibitor of human breast cancer cell invasion, extravasation, and lung colony formation. Breast cancer research and treatment. 2011;125:363–375. doi: 10.1007/s10549-010-0826-y. [DOI] [PubMed] [Google Scholar]
- YOKOE JI, SAKURAGI S, YAMAMOTO K, TERAGAKI T, OGAWARA KI, HIGAKI K, KATAYAMA N, KAI T, SATO M, KIMURA T. Albumin-conjugated PEG liposome enhances tumor distribution of liposomal doxorubicin in rats. International journal of pharmaceutics. 2008;353:28–34. doi: 10.1016/j.ijpharm.2007.11.008. [DOI] [PubMed] [Google Scholar]
- YOO HS, PARK TG. Folate receptor targeted biodegradable polymeric doxorubicin micelles. Journal of Controlled Release. 2004;96:273–283. doi: 10.1016/j.jconrel.2004.02.003. [DOI] [PubMed] [Google Scholar]
- YUAN F, DELLIAN M, FUKUMURA D, LEUNIG M, BERK DA, TORCHILIN VP, JAIN RK. Vascular permeability in a human tumor xenograft: molecular size dependence and cutoff size. Cancer research. 1995;55:3752–3756. [PubMed] [Google Scholar]
- ZAHR AS, DAVIS CA, PISHKO MV. Macrophage uptake of core-shell nanoparticles surface modified with poly (ethylene glycol) Langmuir. 2006;22:8178–8185. doi: 10.1021/la060951b. [DOI] [PubMed] [Google Scholar]
- ZHU S, HONG M, ZHANG L, TANG G, JIANG Y, PEI Y. PEGylated PAMAM dendrimer-doxorubicin conjugates: in vitro evaluation and in vivo tumor accumulation. Pharmaceutical research. 2010;27:161–174. doi: 10.1007/s11095-009-9992-1. [DOI] [PubMed] [Google Scholar]