Abstract
Bacterial lipopolysaccharide (LPS) is an established animal model to study the innate immune response to Gram-negative bacteria mimicking symptoms of infection including reduction of food intake. LPS decreases acyl ghrelin associated with decreased concentrations of circulating ghrelin-O-acyltransferase (GOAT) likely contributing to the anorexigenic effect. We also recently described the prominent expression of the novel anorexigenic hormone, nucleobindin2 (NUCB2)/nesfatin-1 in gastric X/A-like cells co-localized with ghrelin in different pools of vesicles. To investigate whether LPS would affect gastric and circulating NUCB2/nesfatin-1 concentration, ad libitum fed rats were equipped with an intravenous (iv) catheter. LPS was injected intraperitoneally (ip, 100 μg/kg) and blood was withdrawn before and at 2, 5, 7 and 24 h post injection and processed for NUCB2/nesfatin-1 radioimmunoassay. Gastric corpus was collected to measure NUCB2 mRNA expression by RT-qPCR and NUCB2/nesfatin-1 protein concentration by Western blot. Injection of LPS increased plasma NUCB2/nesfatin-1 concentrations by 43%, 78% and 62% compared to vehicle at 2 h, 5 h and 7 h post injection respectively (p < 0.05) and returned to baseline at 24 h. The plasma NUCB2/nesfatin-1 increase at 2 h was associated with increased corpus NUCB2 mRNA expression (p < 0.01), whereas NUCB2 mRNA was not detectable in white blood cells. Likewise, gastric NUCB2 protein concentration was increased by 62% after LPS compared to vehicle (p < 0.01). These data show that gastric NUCB2 production and release are increased in response to LPS. These changes are opposite to those of ghrelin in response to LPS supporting a differential gastric regulation of NUCB2/nesfatin-1 and ghrelin expression derived from the same cell by immune challenge.
Keywords: Endotoxin, Ghrelin, Nucleobindin2, Stomach, X/A-like cell
1. Introduction
Lipopolysaccharide (LPS) is a component of Gram-negative bacteria cell walls and as such a well established model to induce features of acute systemic infections including increased body temperature and reduced appetite [19,20]. We reported previously that LPS markedly reduces fasting plasma levels of total ghrelin in rats [4,39] and extended these observations by showing that plasma acyl ghrelin is more rapidly suppressed compared to desacyl ghrelin resulting in a reduced acyl/desacyl ghrelin ratio at 2 h post LPS injection [31]. This was associated with a decrease of plasma ghrelin-O-acyltransferase (GOAT), the recently identified ghrelin acylating enzyme [44], whereas gastric GOAT protein concentration was increased [31].
Ghrelin is mainly produced in the stomach [1] in gastric X/A-like cells which were long thought to be restricted to the production of ghrelin [33]. However, we recently identified the expression of another product in these cells, nucleobindin2 (NUCB2) [36] which may be cleaved in the hypothalamus into nesfatin-1, nesfatin-2 and nesfatin-3 [24]. NUCB2/nesfatin-1 was described in rat brain food intake regulatory nuclei and implicated in the regulation of food intake [24]. Several studies reported a reduction of food intake following central injection of nesfatin-1 in mice and rats [2,21,24,32,45] and one study after peripheral injection in mice [29]. These effects are in opposition to those of ghrelin which is well established to stimulate food intake after central [41] as well as peripheral injection in rodents [41] and humans [40]. Recently, several studies showed an effect of nesfatin-1 on glucose homeostasis with nesfatin-1 release from rat and mouse pancreatic islets following glucose administration [9,15] and stimulation of insulin release following incubation with nesfatin-1 [15,22] likely to contribute to the observed anti-hyperglycemic effect of nesfatin-1 [37]. On the other hand, ghrelin injected peripherally inhibited the glucose-stimulated secretion of ghrelin [28], an inhibitory effect that has also been described in humans after intravenous injection of ghrelin resulting in increased blood glucose levels [7]. In line with these findings, an inhibition of GOAT by a novel GOAT antagonist, GOCoA-Tat increases glucose-stimulated insulin secretion resulting in lower glucose levels under conditions of a glucose tolerance test in mice [3] pointing to an anti-hyperglycemic effect of GOAT inhibition and therefore decreased acyl ghrelin signaling.
Since ghrelin and nesfatin-1 are co-expressed in gastric X/A-like cells in different pools of vesicles and based on the oppositional effects, a differential regulation of these two hormones is postulated. In the present study we investigated the regulation of NUCB2/nesfatin-1 in the circulation by radioimmunoassay following intraperitoneal (ip) injection of LPS. To characterize the source of circulating NUCB2/nesfatin-1, gastric and white blood cell NUCB2 mRNA expression was assessed by real-time quantitative RT-PCR. In addition, to monitor changes in the tissue, gastric corpus NUCB2/nesfatin-1 protein expression was determined using semi-quantitative Western blot analysis.
2. Methods
2.1. Animals
Adult male Sprague-Dawley rats (Harlan, San Diego, CA) weighing 280–320 g were housed 4/cage under controlled illumination (06:00–18:00 h) and temperature (21–23 °C). Animals had ad libitum access to standard rodent chow (Prolab RMH 2500; LabDiet, PMI Nutrition, Brentwood, MO) and tap water. All animal studies were approved by the subcommittee on animal studies, Long Beach VAMC research health care group (151) with the protocol number 0909-909. Experiments were started between 09:00 and 10:00 h.
2.2. Surgery
Intravenous (iv) catheterization was performed as described before [39]. Rats were anesthetized with a mixture of ketamine (75 mg/kg ip; Fort Dodge Laboratories, Fort Dodge, IA) and xylazine (5 mg/kg ip; Mobay, Shawnee, KS). The right external jugular vein was catheterized using a sterile PE-50 tube filled with sterile saline, the catheter exteriorized between the scapulae via subcutaneous tunneling, secured to the skin, filled with heparin solution (Roche Diagnostics Corp., Indianapolis, IN, 200 units/ml) to maintain patency (200 units/ml) and closed using a wire obturator. Rats were single housed after surgery and allowed to recover for 3–4 days during which they were accustomed to the experimental procedures including handling for intraperitoneal (ip) injection and light hand-restraint for blood withdrawal. Body weight was monitored before surgery and during the recovery period to assure complete recovery and reinstatement of an anabolic state.
2.3. Blood sampling and processing
Single housed, ad libitum fed rats (n = 5/group) were injected ip with vehicle (pyrogen-free saline, 300 μl) or LPS (Escherichia coli, serotype 055:B5; Sigma, St. Louis, MO, 100 μg/kg body weight in 300 μl saline). Repeated blood withdrawals (0.8 ml) were performed from the jugular vein catheter of conscious lightly hand-restrained rats directly before and at 2, 5, 7 and 24 h post injection. During that time rats had ad libitum access to food and water. Blood was collected in tubes containing EDTA (7.5%, 10 μl/0.5 ml blood; Sigma Chemical Co., St. Louis, MO) and aprotinin (0.6 U trypsin inhibitor/0.5 ml blood; ICN Pharmaceuticals, Costa Mesa, CA) and centrifuged at 3000 × g for 15 min at 4 °C. Plasma was collected and stored at −80 °C until further processing. Plasma nesfatin-1 was measured by radioimmunoassay (rat nesfatin-1, detection range 125–16,000 pg/ml; Phoenix Pharmaceuticals, Burlingame, CA). Samples were analyzed in two radioimmunoassay batches and the inter- and intra-assay variability was 10 and 4%.
2.4. Gel electrophoresis and Western blot analysis of NUCB2/nesfatin-1 in gastric corpus
Ad libitum fed rats (n = 5/group) were injected ip (300 μl) with LPS (100 μg/kg body weight in saline) or vehicle (saline) and euthanized by decapitation at 2 h after injection. The supernatants were collected and stored at −80 °C. The stomachs were quickly removed and opened, rinsed, the corpus mucosa scraped off and homogenized (Dounce, Wheaton, Millville, NY) on ice in ice-cold phosphate-buffered saline (PBS) containing one tablet of protease inhibitor cocktail (Roche Applied Science, Indianapolis, IN) and phenylmethylsulphonyl fluoride (PMSF, 1 mM). Crude protein fractions were prepared as described before [35]. Briefly, homogenates were centrifuged in the Sorvall centrifuge at 12,000 × g for 20 min at 4 °C to remove cell debris and nuclei. Protein concentrations for stomach mucosa and plasma were immediately determined using a BCA protein assay according to the manufacturer's protocol (Pierce Biotechnology, Rockford, IL). Gel samples were prepared by mixing protein samples with gel sample buffer [4% sodium dodecyl sulfate (SDS), 0.05% bromphenol blue (w/v), 20% glycerol, 1% mercaptoethanol (v/v) in 0.1 Tris buffer, pH 6.8]. The samples were boiled for 1 min before gel electrophoresis and equal amounts of protein (20 μg/lane) were loaded on a 4–12% SDS-polyacrylamide gel (SDS-PAGE, NuPage; Invitrogen, Carlsbad, CA) and run in 2-(N-morpholino)ethanesulfonic acid buffer. After SDS-PAGE, proteins were transferred by electrophoresis to nitrocellulose membranes (BioPlot-NC; Costar, Cambridge, MA) for 1 h at 4 °C. Membranes were washed in distilled water and stained in Ponceau-S in 3% trichloroacetic acid solution, and an image was taken. Membranes were washed twice with Tween-Tris-buffered saline (TBS; 10 mM Tris, 150 mM NaCl, and 0.05% Tween, v/v) and incubated in Tween-TBS containing 5% (w/v) nonfat milk (Carnation, Nestlé, Glendale, CA). After 60 min, the membranes were incubated in antinesfatin-1 polyclonal antibody (Catalog No. H-003-22, Phoenix Pharmaceuticals) diluted 1:1000 in Tween-TBS. This antibody was raised against the full length of rat nesfatin-1 (amino acids 1–82) and has been established for the use in Western blots detecting full length NUCB2 (47 kDa) as well as exogenous nesfatin-1 (10 kDa) [36]. After 1 h, membranes were washed five times with Tween-TBS and incubated with the secondary antibody solution (anti-rabbit IgG conjugated to alkaline phosphatase; Promega, Madison, WI) diluted 1:2000 in Tween-TBS. After 1 h, membranes were washed three times before color development in alkaline phosphatase buffer [100 mM Tris, 100 mM NaCl, and 5 mM MgCl2 (pH 9.5)] containing 0.3% nitroblue tetrazolium solution (v/v) and 0.15% 5-bromo-4-chloro-3-indolyl-l-phosphate solution (v/v) according to the manufacturer's instructions for 5–10 min. The same Western blot was stained again for β-actin (MW 45 kDa) using a polyclonal antibody (1:1000, Ab #4967, Cell Signaling Technology Inc., Danvers, MA) following the protocol described above. The Western blot (four squares of 352 pixels each/lane) was analyzed using Scion Image 4.0.3 (Scion Corp., Frederick, MD). Gastric NUCB2 protein expression was normalized to the housekeeping protein, ß-actin.
2.5. RT-qPCR for NUCB2 mRNA expression in gastric corpus mucosa and white blood cells
Total RNA from the gastric corpus mucosa and white blood cells was isolated at 2 h post LPS or vehicle injection in the same groups of rats described in Section 2.4. White blood cells were obtained from the buffy coat of trunk blood samples centrifuged for 15 min at 3000 × g and 4 °C. RNA was denatured at 65 °C for 5 min and used to synthesize first-strand cDNA by reverse transcription with the ThermoScriptTM RT-PCR system (Invitrogen, CA). RT-qPCR for NUCB2 mRNA expression was performed using DNA Engine Opticon1 2 Detection System interfaced to the Opticon MONITORTM Analysis Software version 2.01 (MJ Research Inc., Waltham, MA) in a 20 μl reaction volume. The optimized reaction contained 10 μl of SYBR1 Premix Ex TaqTM (Perfect Real Time, Takara Mirus Bio Inc., Madison, WI), 1 μl each of oligonucleotide primers (10 mM), 1 μl of the cDNA synthesis reaction, and 7 μl of H2O. Primers for NUCB2 (GenBank accession no. NM 021663, sense CCA TCC AAG CAC GGT ACT GTT TTC, antisense CCA GTG TCT TGA AGG GCA TCC), glyceraldehyde 3-phosphate dehydrogenase (GAPDH, NM 017008, sense GGG TGA TGC TGG TGC TGA GTA TGT, antisense CAG TGG ATG CAG GGA TGA TGT TCT) and β-actin (mRNA accession no. not available, sense CAT CAC TAT CGG CAA TGA GC, antisense GAC AGC ACT GTG TTG GCA TA) were used. Each amplification was followed by a melting curve resulting in only one peak for each amplicon indicative of amplification of only one product. This was confirmed by agarose gel electrophoresis of the RT-PCR products. The cycle of threshold C(T) was determined as the fluorescent signal (binding of SYBR green to double-stranded cDNA) of 1 SD over background. All reactions were carried out in duplicate, and three separate amplifications for each primer pair were performed. Standard curves were constructed with four serial dilution points of control cDNA (combined cDNA from all samples, 100 ng–100 pg). Data presented were derived from starting quantity (SQ) values of each sample normalized to the housekeeping genes, GAPDH and β-actin. The relative expression ratio of the target gene compared to the reference genes was calculated using the Pfaffl equation [26].
2.6. Statistical analysis
Data are expressed as mean ± SEM and were analyzed by ANOVA followed by all pair-wise multiple comparison procedures (Tukey post hoc test). p < 0.05 was considered significant.
3. Results
3.1. LPS increases plasma NUCB2/nesfatin-1 levels
In ad libitum fed rats plasma NUCB2/nesfatin-1 concentrations did not differ before ip injection of LPS or vehicle respectively (223.3 ± 12.4 pg/ml vs. 211.6 ± 17.8 pg/ml, p > 0.05; Fig. 1). Ip injection of vehicle did not significantly change the NUCB2/nesfatin-1 plasma levels at any time point (p > 0.05; Fig. 1). LPS (100 μg/kg body weight, ip) significantly increased plasma NUCB2/nesfatin-1 by 43% compared to vehicle at 2 h (274.8 ± 40.5 pg/ml vs. 192.9 ± 19.5 pg/ml, p < 0.05), by 78% at 5 h (379.8 ± 29.2 pg/ml vs. 213.7 ± 24.3 pg/ml, p < 0.001) and by 62% at 7 h (380.0 ± 58.3 pg/ml vs. 234.4 ± 25.2 pg/ml, p < 0.05), whereas at 24 h no significant differences were detected between the two groups (311.6 ± 83.9 pg/ml vs. 249.9 ± 29.5 pg/ml, p > 0.05; Fig. 1).
Fig. 1.
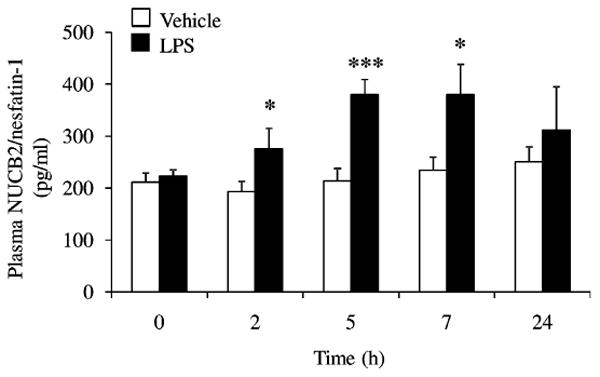
LPS increases plasma concentrations of NUCB2/nesfatin-1 in conscious rats. LPS (100 μg/kg body weight) or vehicle was injected ip during the light phase in ad libitum fed rats chronically implanted with a venous catheter. Blood was withdrawn before and 2, 5, 7 and 24 h post injection and NUCB2/nesfatin-1 plasma levels were assessed by radioimmunoassay. Each bar represents the mean ± SEM of 5–7 rats/group. *p < 0.05 and ***p < 0.001 vs. vehicle.
3.2. LPS increases gastric corpus NUCB2 mRNA levels
Since we observed a significant increase of plasma NUCB2/nesfatin-1 concentrations at 2 h post LPS injection, we investigated whether it was associated with alterations in gastric NUCB2 mRNA expression at that time. LPS injected ip at 100 μg/kg body weight significantly increased gastric corpus NUCB2 mRNA concentration by 109% compared to vehicle (NUCB2 mRNA expression normalized to housekeeping genes, GAPDH and β-actin: 1.22 ± 0.11 vs. 0.58 ± 0.17, p < 0.01; Fig. 2A). To corroborate the gastric source of NUCB2 and exclude a direct production in the blood, we also assessed NUCB2 mRNA expression in white blood cells (red blood cells do not have nuclei and do not produce mRNA). NUCB2 mRNA was undetectable in white blood cells in both groups (Fig. 2B).
Fig. 2.
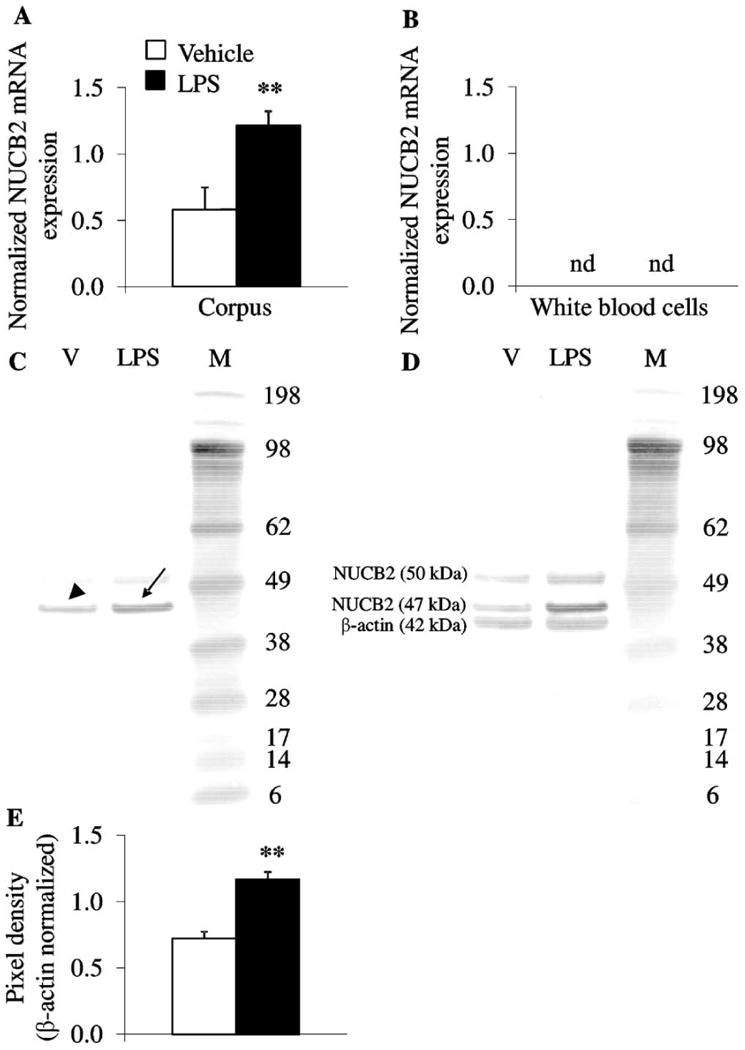
LPS increases gastric corpus NUCB2 mRNA and protein concentration, whereas NUCB2 mRNA is not detectable in white blood cells. Ad libitum fed rats were injected ip with LPS (100 μg/kg body weight) or vehicle (pyrogen-free saline) and trunk blood and stomach were collected at 2 h post injection. (A and B) Gastric corpus and white blood cell mRNA were isolated and assessed for NUCB2, GAPDH and β-actin. After injection of LPS, NUCB2 mRNA expression (normalized for housekeeping genes, GAPDH and β-actin) was increased in the gastric corpus compared to vehicle (A) but was not detectable in white blood cells (B). (C–E) Equal amounts of gastric corpus protein were loaded and NUCB2/nesfatin-1 concentrations assessed using Western blot followed by semi-quantitative analysis. Lane 1 contains corpus after vehicle injection, lane 2 corpus proteins after LPS and lane 3 the molecular weight standards (C). The Western blot shows full length NUCB2 with (∼50 kDa) or without signal sequence (∼ 47 kDa) but not mature nesfatin-1 (∼10 kDa). Injection of LPS increased NUCB2 (arrow) compared to vehicle (arrowhead, C). Re-staining of the Western blot with ß-actin (42 kDa) demonstrated equal gastric corpus mucosal protein concentration (D). Quantification of NUCB2 stomach protein expression is shown in (E). Each bar represents the mean ± SEM of 5 rats/group. **p < 0.01 vs. vehicle.
3.3. LPS increases gastric corpus NUCB2 protein concentration
At the time point of increased gastric NUCB2 mRNA and circulating NUCB2/nesfatin-1 protein concentrations, we also investigated gastric NUCB2/nesfatin-1 protein concentrations. In the gastric corpus mucosa, full length NUCB2 was visible by Western blot analysis, whereas nesfatin-1 1-82 (∼10 kDa) was not detectable (Fig. 2C) as described before [36]. Injection of LPS (100 μg/kg body weight, ip) significantly increased gastric corpus NUCB2 protein concentration at 2 h post injection by 62% compared to vehicle (pixel density normalized to β-actin: 1.17 ± 0.06 vs. 0.72 ± 0.05, p < 0.01; Fig. 2C and E).
4. Discussion
In the present study we show that LPS injected intraperitoneally at a low dose (100 μg/kg) increases circulating NUCB2/nesfatin-1 in freely fed rats. This increase was observed at 2 h post injection with a peak increase at 5 h which plateaued at 7 h and levels were normalized at 24 h. Under a similar regimen of LPS administration, we previously found a similar time course of changes in acyl and desacyl ghrelin levels, however, in the opposite direction since plasma levels of these hormones were decreased [31]. These data indicate a differential regulation of ghrelin and NUCB2/nesfatin-1 under conditions of acute inflammation. The concept of differential regulation is also supported by previous experimental and clinical studies showing (1) the co-localization of ghrelin and NUCB2/nesfatin-1 in the same cell in different pools of vesicles allowing differential release in rats [36], (2) differential regulation of ghrelin and NUCB2/nesfatin-1 under conditions of fasting with increased production and release of ghrelin [17,38] and decreased gastric expression and circulating levels of NUCB2/nesfatin-1 in rats [32,36] and (3) the decreased plasma levels of NUCB2/nesfatin-1 and increased circulating concentrations of acyl ghrelin in human subjects with anorexia nervosa which is reflected by a negative correlation of circulating NUCB2/nesfatin-1 and acyl ghrelin [23]. Therefore, previous and present evidence indicates that the circulating levels of ghrelin and NUCB2/nesfatin-1 are altered in an opposite manner under various physiological (food intake/food restriction) and pathophysiological (reduced body weight, acute inflammation) conditions.
The source of NUCB2/nesfatin-1 accounting for the increased circulating levels in response to LPS is likely to be the stomach. We found that at 2 h post LPS injection, when plasma levels of NUCB2/nesfatin-1 were elevated by 43%, gastric corpus NUCB2 mRNA and protein expression was increased by 109% and 62% respectively. These data indicate that LPS induced a rapid up-regulation of NUCB2 synthesis and transcription in the X/A-like cells where NUCB2 has been identified [36]. The lack of NUCB2 mRNA in white blood cells argues against the blood as direct source of NUCB2/nesfatin-1. However, at this point it cannot be ruled out that also other production sites such as pancreas [9,16,36], adipose tissue [27] or pituitary [36] also contribute to the observed increase in circulating NUCB2/nesfatin-1. A previous study showed an activation of nesfatin-1 immunoreactive neurons in various brain nuclei encompassing the paraventricular nucleus, supraoptic nucleus, arcuate nucleus and the nucleus of the solitary tract following intraperitoneal injection of a higher dose of LPS (0.25 mg/kg) [5]. These data suggest a role for central nesfatin-1 in the response to an immunological stressor. However, the release of central nesfatin-1 into the circulation has not been shown so far and several studies suggested local actions based on the restriction of the immunostaining to the axon and absence in dendrites [6,8,11,14]. In summary, these data point toward a peripheral source of nesfatin-1/NUCB2 measured in the present study.
It is still to be established, whether and if so, where NUCB2 is cleaved into nesfatin-1 and other fragments. In the present study, only the full length NUBC2 was detected by Western blot. The antibody used was raised against mature nesfatin-1 amino acids 1–82 and shown before to detect synthetic nesfatin-1 peptide as well as full length NUCB2 which also contains the epitope [36]. So far, only one study described the occurrence of mature nesfatin-1 peptide in the cerebrospinal fluid of rats, whereas in brain nuclei only full length NUCB2 was detected [24]. In all subsequent reports, only full length NUCB2 was detected by Western blot [8,27,36] or studies performing immunostaining did not distinguish between NUCB2 protein and nesfatin-1 peptide [5,6,8,10,13,14,16,18,25,32,34,42,43]. Since also full length NUCB2 exerts an anorexigenic effect it is also possible that this protein is the active circulating form.
Whereas the role for central NUCB2 starts to be better understood (for review see [30]), the function of peripheral NUCB2/nesfatin-1 remains to be established. Consistent studies showed biological activity of peripherally injected nesfatin-1 on glucose homeostasis in ex vivo and in vivo studies in rats and mice [9,15,22,37] while an effect on food intake has been observed in one study [29] and not in another [12].
In summary, these data show that NUCB2 production and release are increased under conditions of inflammation in response to LPS. The stomach is likely to represent the source of NUCB2/nesfatin-1 as gastric mRNA and protein content was increased at the same time indicating stimulated production, whereas NUCB2 mRNA was not detectable in circulating white blood cells. These changes are in opposition to those observed for ghrelin and support the assumption of a differential regulation of these two peptides derived from the same cell.
Acknowledgments
This work was supported by Department of Veterans Affairs Merit Awards (N.W.G.L. and Y.T.), VA Research Career Scientist Award (Y.T.), NIH DK-33061 (Y.T.) and Center grant DK-41301 (Animal Core, Y.T.).
References
- 1.Ariyasu H, Takaya K, Tagami T, Ogawa Y, Hosoda K, Akamizu T, et al. Stomach is a major source of circulating ghrelin, and feeding state determines plasma ghrelin-like immunoreactivity levels in humans. J Clin Endocrinol Metab. 2001;86:4753–8. doi: 10.1210/jcem.86.10.7885. [DOI] [PubMed] [Google Scholar]
- 2.Atsuchi K, Asakawa A, Ushikai M, Ataka K, Tsai M, Koyama K, et al. Centrally administered nesfatin-1 inhibits feeding behaviour and gastroduodenal motility in mice. Neuroreport. 2010;21:1008–11. doi: 10.1097/WNR.0b013e32833f7b96. [DOI] [PubMed] [Google Scholar]
- 3.Barnett BP, Hwang Y, Taylor MS, Kirchner H, Pfluger PT, Bernard V, et al. Glucose and weight control in mice with a designed ghrelin O-acyltransferase inhibitor. Science. 2010;330:1689–92. doi: 10.1126/science.1196154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Basa NR, Wang L, Arteaga JR, Heber D, Livingston EH, Taché Y. Bacterial lipopolysaccharide shifts fasted plasma ghrelin to postprandial levels in rats. Neurosci Lett. 2003;343:25–8. doi: 10.1016/s0304-3940(03)00312-4. [DOI] [PubMed] [Google Scholar]
- 5.Bonnet MS, Pecchi E, Trouslard J, Jean A, Dallaporta M, Troadec JD. Central nesfatin-1 expressing neurons are sensitive to peripheral inflammatory stimulus. J Neuroinflammation. 2009;6:27. doi: 10.1186/1742-2094-6-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brailoiu GC, Dun SL, Brailoiu E, Inan S, Yang J, Chang JK, et al. Nesfatin-1: distribution and interaction with a G protein-coupled receptor in the rat brain. Endocrinology. 2007;148:5088–94. doi: 10.1210/en.2007-0701. [DOI] [PubMed] [Google Scholar]
- 7.Broglio F, Benso A, Gottero C, Prodam F, Gauna C, Filtri L, et al. Non-acylated ghrelin does not possess the pituitaric and pancreatic endocrine activity of acylated ghrelin in humans. J Endocrinol Invest. 2003;26:192–6. doi: 10.1007/BF03345156. [DOI] [PubMed] [Google Scholar]
- 8.Foo K, Brismar H, Broberger C. Distribution and neuropeptide coexistence of nucleobindin-2 mRNA/nesfatin-like immunoreactivity in the rat CNS. Neuroscience. 2008;156:563–79. doi: 10.1016/j.neuroscience.2008.07.054. [DOI] [PubMed] [Google Scholar]
- 9.Foo KS, Brauner H, Ostenson CG, Broberger C. Nucleobindin-2/nesfatin in the endocrine pancreas: distribution and relationship to glycaemic state. J Endocrinol. 2010;204:255–63. doi: 10.1677/JOE-09-0254. [DOI] [PubMed] [Google Scholar]
- 10.Fort P, Salvert D, Hanriot L, Jego S, Shimizu H, Hashimoto K, et al. The satiety molecule nesfatin-1 is co-expressed with melanin concentrating hormone in tuberal hypothalamic neurons of the rat. Neuroscience. 2008;155:174–81. doi: 10.1016/j.neuroscience.2008.05.035. [DOI] [PubMed] [Google Scholar]
- 11.Goebel-Stengel M, Wang L, Stengel A, Taché Y. Localization of nesfatin-1 neurons in the mouse brain and functional implication. Brain Res. 2011;1396:20–34. doi: 10.1016/j.brainres.2011.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goebel M, Stengel A, Wang L, Taché Y. Central nesfatin-1 reduces the nocturnal food intake in mice by reducing meal size and increasing inter-meal intervals. Peptides. 2011;32:36–43. doi: 10.1016/j.peptides.2010.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goebel M, Stengel A, Wang L, Taché Y. Restraint stress activates nesfatin-1-immunoreactive brain nuclei in rats. Brain Res. 2009;1300:114–24. doi: 10.1016/j.brainres.2009.08.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goebel M, Stengel A, Wang L, Lambrecht NWG, Taché Y. Nesfatin-1 immunoreactivity in rat brain and spinal cord autonomic nuclei. Neurosci Lett. 2009;452:241–6. doi: 10.1016/j.neulet.2009.01.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gonzalez R, Reingold BK, Gao X, Gaidhu MP, Tsushima RG, Unniappan S. Nesfatin-1 exerts a direct, glucose-dependent insulinotropic action on mouse islet beta- and MIN6 cells. J Endocrinol. 2011;208:R9–16. doi: 10.1530/JOE-10-0492. [DOI] [PubMed] [Google Scholar]
- 16.Gonzalez R, Tiwari A, Unniappan S. Pancreatic beta cells colocalize insulin and pronesfatin immunoreactivity in rodents. Biochem Biophys Res Commun. 2009;381:643–8. doi: 10.1016/j.bbrc.2009.02.104. [DOI] [PubMed] [Google Scholar]
- 17.Kim MS, Yoon CY, Park KH, Shin CS, Park KS, Kim SY, et al. Changes in ghrelin and ghrelin receptor expression according to feeding status. Neuroreport. 2003;14:1317–20. doi: 10.1097/01.wnr.0000078703.79393.d2. [DOI] [PubMed] [Google Scholar]
- 18.Kohno D, Nakata M, Maejima Y, Shimizu H, Sedbazar U, Yoshida N, et al. Nesfatin-1 neurons in paraventricular and supraoptic nuclei of the rat hypothalamus coexpress oxytocin and vasopressin and are activated by refeeding. Endocrinology. 2008;149:1295–301. doi: 10.1210/en.2007-1276. [DOI] [PubMed] [Google Scholar]
- 19.Langhans W. Anorexia of infection: current prospects. Nutrition. 2000;16:996–1005. doi: 10.1016/s0899-9007(00)00421-4. [DOI] [PubMed] [Google Scholar]
- 20.Langhans W. Bacterial products and the control of ingestive behavior: clinical implications. Nutrition. 1996;12:303–15. doi: 10.1016/s0899-9007(96)80052-9. [DOI] [PubMed] [Google Scholar]
- 21.Maejima Y, Sedbazar U, Suyama S, Kohno D, Onaka T, Takano E, et al. Nesfatin-1-regulated oxytocinergic signaling in the paraventricular nucleus causes anorexia through a leptin-independent melanocortin pathway. Cell Metab. 2009;10:355–65. doi: 10.1016/j.cmet.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 22.Nakata M, Manaka K, Yamamoto S, Mori M, Yada T. Nesfatin-1 enhances glucose-induced insulin secretion by promoting Ca(2+) influx through L-type channels in mouse islet beta-cells. Endocr J. 2011;58:305–13. doi: 10.1507/endocrj.k11e-056. [DOI] [PubMed] [Google Scholar]
- 23.Ogiso K, Asakawa A, Amitani H, Nakahara T, Ushikai M, Haruta I, et al. Plasma nesfatin-1 concentrations in restricting-type anorexia nervosa. Peptides. 2010 doi: 10.1016/j.peptides.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 24.Oh IS, Shimizu H, Satoh T, Okada S, Adachi S, Inoue K, et al. Identification of nesfatin-1 as a satiety molecule in the hypothalamus. Nature. 2006;443:709–12. doi: 10.1038/nature05162. [DOI] [PubMed] [Google Scholar]
- 25.Okere B, Xu L, Roubos EW, Sonetti D, Kozicz T. Restraint stress alters the secretory activity of neurons co-expressing urocortin-1, cocaine- and amphetamine-regulated transcript peptide and nesfatin-1 in the mouse Edinger-Westphal nucleus. Brain Res. 2010;1317C:92–9. doi: 10.1016/j.brainres.2009.12.053. [DOI] [PubMed] [Google Scholar]
- 26.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ramanjaneya M, Chen J, Brown JE, Tripathi G, Hallschmid M, Patel S, et al. Identification of nesfatin-1 in human and murine adipose tissue: a novel depot-specific adipokine with increased levels in obesity. Endocrinology. 2010;151:3169–80. doi: 10.1210/en.2009-1358. [DOI] [PubMed] [Google Scholar]
- 28.Reimer MK, Pacini G, Ahren B. Dose-dependent inhibition by ghrelin of insulin secretion in the mouse. Endocrinology. 2003;144:916–21. doi: 10.1210/en.2002-220819. [DOI] [PubMed] [Google Scholar]
- 29.Shimizu H, Oh IS, Hashimoto K, Nakata M, Yamamoto S, Yoshida N, et al. Peripheral Administration of Nesfatin-1 Reduces Food Intake in Mice: the leptin-independent mechanism. Endocrinology. 2009;150:662–71. doi: 10.1210/en.2008-0598. [DOI] [PubMed] [Google Scholar]
- 30.Stengel A, Goebel M, Taché Y. Nesfatin-1: a novel inhibitory regulator of food intake and body weight. Obes Rev. 2011;12:261–71. doi: 10.1111/j.1467-789X.2010.00770.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stengel A, Goebel M, Wang L, Reeve JR, Jr, Taché Y, Lambrecht NW. Lipopolysaccharide differentially decreases plasma acyl and desacyl ghrelin levels in rats: potential role of the circulating ghrelin-acylating enzyme GOAT. Peptides. 2010;31:1689–96. doi: 10.1016/j.peptides.2010.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stengel A, Goebel M, Wang L, Rivier J, Kobelt P, Mönnikes H, et al. Central nesfatin-1 reduces dark-phase food intake and gastric emptying in rats: differential role of corticotropin-releasing factor2 receptor. Endocrinology. 2009;150:4911–9. doi: 10.1210/en.2009-0578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stengel A, Goebel M, Wang L, Taché Y. Ghrelin, des-acyl ghrelin and nesfatin-1 in gastric X/A-like cells: role as regulators of food intake and body weight. Peptides. 2010;31:357–69. doi: 10.1016/j.peptides.2009.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stengel A, Goebel M, Wang L, Taché Y. Abdominal surgery activates nesfatin-1 immunoreactive brain nuclei in rats. Peptides. 2010;31:263–70. doi: 10.1016/j.peptides.2009.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stengel A, Goebel M, Wang L, Taché Y, Sachs G, Lambrecht NW. Differential distribution of ghrelin-O-acyltransferase (GOAT) immunoreactive cells in the mouse and rat gastric oxyntic mucosa. Biochem Biophys Res Commun. 2010;392:67–71. doi: 10.1016/j.bbrc.2009.12.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stengel A, Goebel M, Yakubov I, Wang L, Witcher D, Coskun T, et al. Identification and characterization of nesfatin-1 immunoreactivity in endocrine cell types of the rat gastric oxyntic mucosa. Endocrinology. 2009;150:232–8. doi: 10.1210/en.2008-0747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Su Y, Zhang J, Tang Y, Bi F, Liu JN. The novel function of nesfatin-1: antihyperglycemia. Biochem Biophys Res Commun. 2010;391:1039–42. doi: 10.1016/j.bbrc.2009.12.014. [DOI] [PubMed] [Google Scholar]
- 38.Toshinai K, Mondal MS, Nakazato M, Date Y, Murakami N, Kojima M, et al. Upregulation of Ghrelin expression in the stomach upon fasting, insulininduced hypoglycemia, and leptin administration. Biochem Biophys Res Commun. 2001;281:1220–5. doi: 10.1006/bbrc.2001.4518. [DOI] [PubMed] [Google Scholar]
- 39.Wang L, Basa NR, Shaikh A, Luckey A, Heber D, St-Pierre DH, et al. LPS inhibits fasted plasma ghrelin levels in rats: role of IL-1 and PGs and functional implications. Am J Physiol Gastrointest Liver Physiol. 2006;291:G611–20. doi: 10.1152/ajpgi.00533.2005. [DOI] [PubMed] [Google Scholar]
- 40.Wren AM, Seal LJ, Cohen MA, Brynes AE, Frost GS, Murphy KG, et al. Ghrelin enhances appetite and increases food intake in humans. J Clin Endocrinol Metab. 2001;86:5992. doi: 10.1210/jcem.86.12.8111. [DOI] [PubMed] [Google Scholar]
- 41.Wren AM, Small CJ, Abbott CR, Dhillo WS, Seal LJ, Cohen MA, et al. Ghrelin causes hyperphagia and obesity in rats. Diabetes. 2001;50:2540–7. doi: 10.2337/diabetes.50.11.2540. [DOI] [PubMed] [Google Scholar]
- 42.Xu L, Bloem B, Gaszner B, Roubos EW, Kozicz T. Sex-specific effects of fasting on urocortin 1, cocaine- and amphetamine-regulated transcript peptide and nesfatin-1 expression in the rat Edinger-Westphal nucleus. Neuroscience. 2009;162:1141–9. doi: 10.1016/j.neuroscience.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 43.Xu L, Bloem B, Gaszner B, Roubos EW, Kozicz T. Stress-related changes in the activity of cocaine- and amphetamine-regulated transcript and nesfatin neurons in the midbrain non-preganglionic Edinger-Westphal nucleus in the rat. Neuroscience. 2010;170:478–88. doi: 10.1016/j.neuroscience.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 44.Yang J, Brown MS, Liang G, Grishin NV, Goldstein JL. Identification of the acyltransferase that octanoylates ghrelin, an appetite-stimulating peptide hormone. Cell. 2008;132:387–96. doi: 10.1016/j.cell.2008.01.017. [DOI] [PubMed] [Google Scholar]
- 45.Yosten GL, Samson WK. The anorexigenic and hypertensive effects of nesfatin-1 are reversed by pretreatment with an oxytocin receptor antagonist. Am J Physiol Regul Integr Comp Physiol. 2010;298:R1642–7. doi: 10.1152/ajpregu.00804.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]


