Abstract
Background
To understand the dynamic process of cessation fatigue (i.e., the tiredness of trying to quit smoking) with respect to its average trend, effect on relapse, time-varying relations with craving and negative affect, and differences among genders and treatment groups.
Method
Randomized placebo-controlled clinical trial. Participants received either placebo, monotherapy (bupropion SR, nicotine patch, nicotine lozenge), or combined pharmacotherapy (bupropion SR + nicotine lozenge, nicotine patch + nicotine lozenge). Data were collected from 1504 daily smokers who were motivated to quit smoking. The participants completed baseline assessments and ecological momentary assessments for 2 weeks post-quit.
Results
Cessation fatigue reduced the likelihood of 6-month post-quit abstinence (OR = 0.97, 95% CI (0.95, 0.99)), and was positively associated with craving and negative affect. After controlling for these two factors, average cessation fatigue increased over time. Compared to men, women experienced greater fatigue (t = -10.69, p < 0.0001) and a stronger relation between fatigue and craving (t = -8.80, p < 0.0001). The relationship between fatigue and negative affect was significantly stronger in men (t = 5.73, p < 0.0001). Cessation fatigue was significantly reduced by combined pharmacotherapy (t = -13.4, p < 0.0001), as well as monotherapy (t = -6.2, p < 0.0001).
Conclusions
Cessation fatigue was closely related to craving, negative affect, and cessation outcomes. Women reported greater cessation fatigue than men. Current treatments appeared to reduce fatigue and weaken its relations with craving and negative affect.
Keywords: cessation fatigue, smoking cessation, time-varying effect model, ecological momentary assessments
1. INTRODUCTION
Many smokers trying to quit ultimately relapse within a few weeks. Even with various medications and behavioral interventions, less than 30% of smokers achieve long-term abstinence (Fiore et al., 2008). Effective treatments have been shown to work by reducing cravings or negative affect, but these mechanisms only account for a small proportion of treatments’ effects on relapse (Bolt et al., 2006; Lerman et al., 2002; McCarthy et al., 2008; Piper et al., 2008; Piasecki, 2006). Thus, it is important for researchers to identify other potential factors that not only convey relapse risk but also can be modified by effective treatment.
One possible relapse risk factor that has been posited but rarely studied is cessation fatigue. Cessation fatigue, defined as tiredness of trying to quit smoking, may be an important construct in understanding the relapse process (Piasecki et al., 2002). At the beginning of a quit attempt, individuals may be enthusiastic about quitting; however, motivation may diminish over time in the presence of stressors, strong urges to smoke, and the extra effort required to cope with negative affect by means other than smoking. Cessation fatigue, manifested by decreases in self-efficacy and exhaustion of self-control resources, mounts accordingly. This is consistent with the strength model of self-regulation (Muraven et al., 1998; Hagger et al., 2010; Vohs and Heatherton, 2000; Inzlicht and Schmeichel, 2012) which holds that individuals have a limited capacity for self-regulation (i.e., a limited amount of strength or energy) and that exertion of self-control diminishes capacity for subsequent self-control efforts.
Simmons and colleagues (2010) developed a measure to assess motivation to maintain abstinence, which taps related constructs. To the best of our knowledge, however, there are little empirical studies directly measuring cessation fatigue. Because fatigue is posited to be something that develops over time, it is important to understand the dynamic process of fatigue. Further, it is important to understand how withdrawal symptoms – specifically, craving and negative affect, which motivate smoking and require self-control resources to prevent cigarette use, are related to fatigue during the course of a quit attempt. Understanding these dynamics is critical to understanding the relapse process and developing new interventions to address specific relapse risk factors. For instance, it could be that smokers can cope with craving for only a few days, but then their self-control resources are exhausted and their fatigue reaches a level that prevents them from inhibiting their desire to smoke. If this were true, then interventions could be developed to help increase self-control capacity, similar to training for a marathon (Muraven et al., 1998).
Previous research has shown that relapse rates differ by treatment (e.g., combined pharmacotherapy; Fiore et al., 2008; Smith et al., 2009; Stead et al., 2008). If cessation treatments work by suppressing craving and negative affect (Bolt et al., 2012), thereby reducing fatigue, then we would expect that participants who received the most effective treatments would show less overall cessation fatigue. Further, the relation between craving/negative affect and fatigue should be attenuated among smokers receiving treatment relative to these relations in a placebo treatment group.
Gender also influences relapse risk. Research has shown that women are less likely to be successful quitters in the long term and are particularly responsive to specific medications (Piper et al., 2010; Shiffman et al., 2005; Smith et al., 2003). However, the underlying mechanisms of women’s increased relapse risk and treatment response are unclear. Therefore, it is important to understand whether there are gender differences in fatigue that might account for the gender differences in relapse.
In the current study, we explore the dynamic process of cessation fatigue using ecological momentary assessment (EMA) data (Shiffman et al., 2008; Shiffman, 2009). We first examine its general dynamics during a quit attempt and its relation to cessation, hypothesizing that fatigue will increase over time and be positively associated with relapse (H1); second, we analyze its time-varying relations to other relapse risk factors—craving and negative affect, testing the hypothesis that craving and negative affect will be positively associated with fatigue, with the strength of association increasing during the quit attempt (H2). This research will also examine the effects of treatment and gender on the dynamic process of cessation fatigue, addressing the hypotheses that participants who received active pharmacotherapy will report less overall fatigue relative to those who received placebo (H3); and women will report more fatigue relative to men and combined pharmacotherapy will be especially helpful in attenuating fatigue among women (H4).
We addressed these questions using multilevel modeling (MLM; Raudenbush and Bryk, 2002; Schwartz and Stone, 1998; Walls and Schafer, 2006) and a relatively new analytical approach, the time-varying effect model (TVEM; Hastie and Tibshirani, 1993). MLM is a well-established parametric approach that is used to model longitudinal data. TVEM, by contrast, is a nonparametric modeling technique that may provide new insight in the same data context. In previous work (Shiyko et al., 2012; Selya et al.,), TVEM was employed to examine the dynamic associations between smoking urges and negative affect during a quit attempt. This study extends the application of TVEM to more complex models to incorporate interactions between time-varying effects of key predictors, and to the important but less studied outcome of cessation fatigue.
2. METHODS
2.1. Participants
We used data from a randomized, placebo-controlled clinical trial (N=1504) of five active smoking-cessation pharmacotherapies, in which daily smokers who were highly motivated to quit were recruited (Piper et al., 2009). The study was registered in http://clinicaltrials.gov/ with the identification number NCT00332644.
In our analysis, we removed the subjects with zero observations for the outcome or the key covariates (n=373) and those who failed to establish initial abstinence (i.e., quit for at least 24 hours in the first 7 days after the target quit date, n=127). We used only the observations before full relapse (i.e., 7 consecutive days of smoking). Ultimately, data from 1004 subjects were analyzed; 102 received placebo, 522 received monotherapy (nicotine patch, nicotine lozenge or bupropion) and 380 received combined pharmacotherapy (nicotine patch + nicotine lozenge or nicotine bupropion + nicotine lozenge). On average, participants provided 27.1 observations (SD=11.8), contributing to 27,173 EMA occasions in all. The resulting sample was 59% female and 87% White with the average age of 45.5 years (SD=10.8), reporting a baseline mean of 21.1 cigarettes per day (SD=8.8) and a mean of 26.9 years smoked (SD=11.2). No baseline characteristic differences were found across the three treatment groups due to randomization of the experiment.
2.2. Measures
Prior to quitting, participants answered questions about gender, ethnicity, age, marital status, education level, employment and smoking history features. Tobacco dependence was assessed with one item from the Fagerström Test for Nicotine Dependence (FTND; Heatherton et al., 1991): “How soon after you wake up do you smoke?” This item has strong predictive validity (Baker et al., 2007).
Participants also provided intensive longitudinal data; they responded to four EMA prompts per day (morning, night, and 2 random times) for two weeks post-quit. These EMAs assessed the number of cigarettes smoked since last prompt and how participants felt within the last 15 minutes in terms of withdrawal symptoms (e.g., negative affect (Watson et al., 1988), craving) and cessation fatigue. The withdrawal symptoms were assessed using 11 items from the Wisconsin Smoking Withdrawal Scale (WSWS; Welsch et al., 1999), such as feeling tense or anxious, feeling sad or depressed, being bothered by the desire to smoke a cigarette, and having difficulty thinking clearly, but with an eleven-point response scale to increase response variability (see McCarthy et al., 2008). Negative affect is a combination of six items from the Positive and Negative Affect Scale (PANAS; Watson et al., 1998): tense or anxious, impatient, bothered by negative moods, irritable or easily angered, sad or depressed, and hopeless or discouraged. Cessation fatigue was also measured by an eleven-point response scale to the single item recording the feeling that “I am tired of trying to quit smoking”, with scale 0 as disagree most and scale 10 as agree most.
2.3. Statistical Analysis
For Hypothesis 1, we used both the parametric approach, MLM, and the nonparametric approach, TVEM, to delineate the temporal trajectory of cessation fatigue. A generalized MLM was used to examine the relation between fatigue and cessation outcome. Both MLM and TVEM can be used to depict time-varying trends and relations. MLM typically exerts functional forms (i.e., linear, quadratic) on the outcome over time and does not allow for effects of covariates, even time-varying ones, to change with time. In contrast, TVEM is able to capture temporal changes over time because the only restriction it imposes is that change over time in the coefficient curves (intercept and effects of covariates) is smooth (Hoover et al., 1998; Li et al., 2006).
For Hypothesis 2, we explored the dynamic association between fatigue, craving, and negative affect using TVEMs, where the model coefficients were estimated non-parametrically and model selection procedures were used to determine model complexity (Shiyko et al., 2012; Tan et al., 2012). We first fit a TVEM with cessation fatigue as the outcome and craving and negative affect as the predictors (see Model 1). We also controlled for baseline dependence (i.e., FTND) and episodes of smoking at each time (LAPSE; 0 = no lapse, 1 = lapse).
| Model 1 |
In Model 1, FATIGij, CRAVij and NAij are intensively measured longitudinal variables for subject i measured at time tij. All continuous predictors were standardized. Thus, the intercept β0(tij) represents the mean value of FATIGij at time tij for a typical person with the average level on all continuous predictors and no lapse. Similarly, the slopes β1(tij) and β2(tij) represent the strength and direction of the relation between craving and fatigue, and between negative affect and fatigue, respectively, at time tij after adjusting for other covariates in the model. The fact that β0, β1 and β2 are time-specific makes the model fundamentally distinct from MLM. Interpretation of the intercept and the slope coefficients requires plotting the estimated values against time, along with the corresponding confidence intervals to determine whether the lines differ from 0 or from other curves. The random errors εij are assumed to be continuous with mean zero.
For Hypotheses 3 and 4, we included gender (1 = male; 0 = female) and its interaction with craving and negative affect to test for gender differences in the coefficient functions for β0, β1 and β2 (Model 2). We fit this model separately for each of the three treatment groups (i.e., placebo, monotherapy and combined pharmacotherapy), allowing for an assessment of treatment effects on all coefficients and coefficient functions.
| Model 2 |
2.4. Software
All MLMs were fit in SAS. The SAS macro %TVEM_normal was used to fit all TVEMs, and is available for free download at http://methodology.psu.edu (Yang et al., 2012). Please refer to the supplementary material for analytic steps and syntax for estimating a TVEM1.
3. RESULTS
3.1. Cessation Fatigue: Average Trends and Role in Relapse
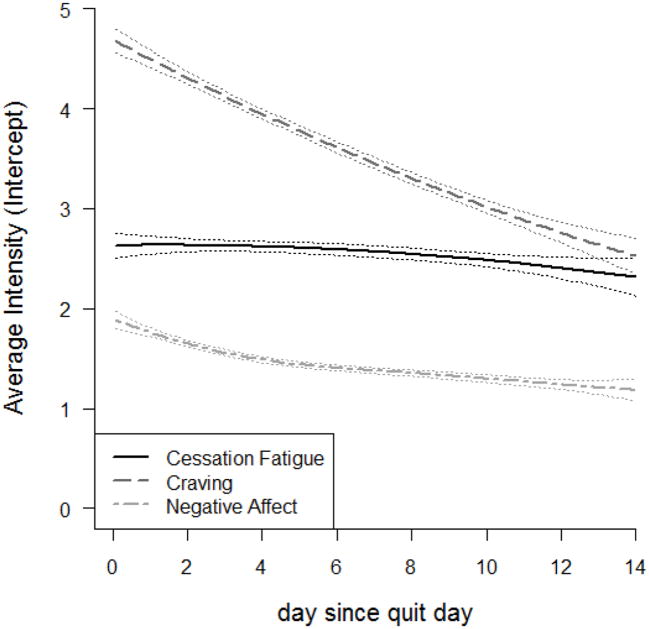
Figure. 1 displays nonparametric curves of cessation fatigue, craving, and negative affect over the first 2 weeks post-quit. All the three curves show decreasing trends, but with different slopes. One may notice by visual inspection that the overall decline in cessation fatigue is small relative to that in craving and negative affect. This difference is confirmed by the results of corresponding MLMs, which show that craving has the steepest linear decreasing trend (estimated slope = -0.16), followed by negative affect (estimated slope = -0.09) and cessation fatigue (estimated slope = -0.02).
Figure. 1.
The overall dynamics of the outcome, cessation fatigue, and the two predictors, craving and negative affect
To determine whether cessation fatigue is associated with relapse risk, we fit a generalized MLM with the 6-month post-quit abstinence status as the outcome and fatigue as the predictor. When fatigue was treated as a continuous predictor with its original scale, it was significantly negatively associated with the probability of 6-month post-quit abstinence (OR = 0.97, 95% CI (0.95, 0.99)), and thus significantly positively associated with relapse.
Together these results partially support H1: fatigue is significantly associated with relapse and participants with higher levels of fatigue are less likely to achieve long-term abstinence compared with those with lower levels of fatigue. However, these analyses showed that fatigue actually decreased over time, although to a lesser degree than other relapse risk factors.
3.2. Time-Varying Effect of Craving and Negative Affect on Cessation Fatigue
To examine how fatigue is associated with craving and negative affect at different times in the two weeks post-quit, we estimated Model 1 (Table 1 and Figure. 2). The dynamic association between craving and cessation fatigue is depicted as a linear function of time in the middle panel of Figure. 2. The confidence bands are well above zero, and the estimated effect of craving on cessation fatigue increased steadily as a function of time since quit date, indicating that the association between the two measures strengthened over time (i.e., increases in craving a week after the target quit date produce greater increases in cessation fatigue than the same increase would have produced one day after the target quit date). The coefficient function for negative affect is shown in the right panel of Figure. 2. The curve lies above zero, increases dramatically from day 0 to day 4, and decreases mildly thereafter. This pattern indicates that a participant reporting higher levels of negative affect was likely to experience greater cessation fatigue during the quit attempt, and this association strengthened considerably within the first few days after quitting.
Table 1.
Time-constant coefficient estimates of Model 1 (time-varying effect model of regressing cessation fatigue on craving and negative affect, adjusting for lapse and baseline dependence)
| Covariate | Estimate (SD) | p-value |
|---|---|---|
| Intercept | 2.53 (0.04) | <.0001 |
| Intercept*time | 0.02 (0.01) | 0.0007 |
| Craving | 0.24 (0.04) | <.0001 |
| Craving*time | 0.05 (0.01) | <.0001 |
| Lapse | 0.28 (0.08) | 0.0005 |
| Baseline dependence | 0.08 (0.02) | <.0001 |
Intercept, craving and their interactions with time are shown in the constant effects table because these terms were chosen to be of parametric forms in model selection procedure of TVEM. The corresponding plots are the left and middle panels of Fig. 2. The coefficient of NA is not included in this table because it was decided by the model selection procedure of TVEM to be a nonparametric curve with two knots. The inference for NA can be drawn from the right panel of Fig. 2.
Figure. 2.
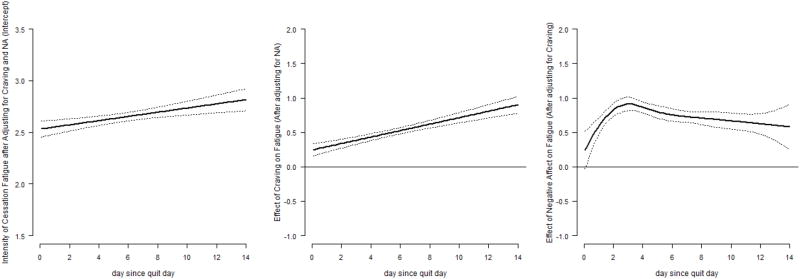
Time-varying coefficient functions of Model 1 (left panel: time-varying intercept, the average intensity of cessation fatigue after accounting for craving and negative affect; middle panel: time-varying effect of craving; right panel: time-varying effect of negative affect)
After adjusting for craving and negative affect, the trend of cessation fatigue increased over time (Figure. 2, left panel), in contrast to the downward trajectory in Figure. 1. With these findings, we conclude that the decrease in cessation fatigue in Figure. 1 was likely due to the decreases in craving and negative affect, which were positively associated with fatigue. Thus, over time, as craving and negative affect decrease, fatigue decreases as well.
It should be noted that because craving and negative affect are correlated (Piper et al., 2011), β1(tij) represents the relation between fatigue and craving only after adjusting for negative affect, and the same is true for β2(tij). For a more precise test of the hypotheses of the raw relation between fatigue, craving and negative affect, we fit two other univariate TVEMs, where either craving or negative affect is the only predictor of cessation fatigue, controlling only for nicotine dependence and lapse. The resulting plots of the coefficients for craving and negative affect (not shown) were nearly identical to those in Figure. 2.
3.3. Treatment and Gender Differences in the Dynamics of Cessation Fatigue
The MLM results indicate that the active treatment groups had significantly lower average levels of cessation fatigue than the placebo group (monotherapy vs. placebo: t=-6.2; combined pharmacotherapy vs. placebo: t=-13.4; p<0.0001), consistent with our hypothesis that active pharmacotherapy reduces fatigue. Table 2 presents average fatigue and other key variables for the different treatment groups. The combined pharmacotherapy group had even lower cessation fatigue than the monotherapy group (t=-12.4, p<0.0001). Women experienced significantly greater fatigue than men (mean for women = 2.86, mean for men = 2.30, t=-10.69, p<0.0001), consistent with hypothesis H4.
Table 2.
Descriptive statistics of study variables for the three treatment groups and the two genders
| Treatment comparison | Gender comparison | ||||
|---|---|---|---|---|---|
|
|
|||||
| Placebo Mean (SD) |
Monotherapy Mean (SD) |
Combined therapy Mean (SD) |
Male Mean (SD) |
Female Mean (SD) |
|
| Proportion of female | 55.88% | 59.00% | 59.74% | --- | --- |
| Baseline dependence | 1.86 (0.93) | 1.93 (0.87) | 2.02 (0.82) | 1.91 (0.84) | 1.86 (0.87) |
|
| |||||
| Pre-quit Craving | 3.42 (3.24) | 3.46 (3.12) | 3.48 (3.10) | 3.23* (2.67) | 3.62 (3.22) |
| Pre-quit Negative affect | 1.31 (1.35) | 1.28 (1.31) | 1.31 (1.36) | 1.21* (1.22) | 1.34 (1.40) |
| Pre-quit Smoke | 4.44 (5.19) | 4.59 (5.09) | 4.92 (5.15) | 5.24* (6.07) | 4.34 (4.63) |
|
| |||||
| Post-quit Craving | 4.12 (3.54) | 3.87* (3.41) | 3.55*† (3.37) | 3.51* (3.26) | 3.95 (3.50) |
| Post-quit Negative affect | 1.69 (1.67) | 1.52* (1.54) | 1.41*† (1.44) | 1.40* (1.42) | 1.55 (1.57) |
| Post-quit Smoke | 0.33 (1.56) | 0.16* (0.89) | 0.13*† (0.74) | 0.20 (1.06) | 0.15 (0.83) |
| Cessation fatigue | 3.29 (3.72) | 2.78* (3.51) | 2.28*† (3.21) | 2.30* (3.29) | 2.86 (3.51) |
Items in the first two rows are baseline measurements measured once at the beginning of the study; items from row 3 to row 5 are based on the pre-quit EMA measurements of craving, negative affect, and smoke. Smoke was measured by the number of cigarette smoked since last report, corresponding to the variable “Relapse” in the model; the last four rows represent the post-quit measurements assessed using EMA data. The response cessation fatigue is a post-quit variable;
in the first three columns, p < .05 compared to placebo;
p < .05 compared to monotherapy;
in the last two columns, p < .05 compared to females.
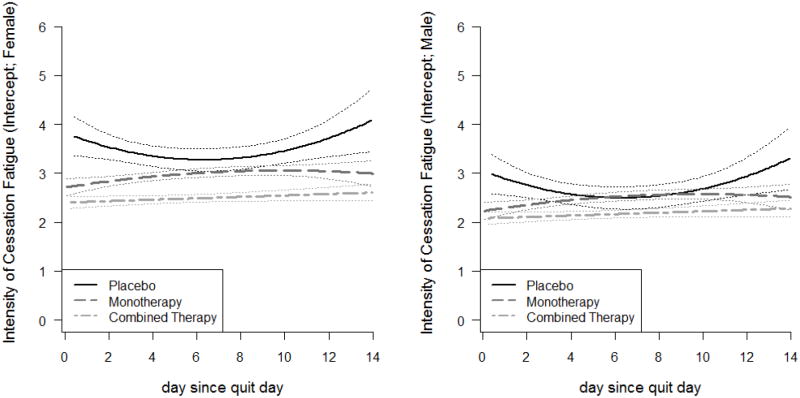
We further explored treatment and gender differences by fitting Model 2 (Table 3). Figure. 3 represents graphical summaries of the intercept functions (i.e., plots of each group mean cessation fatigue over time) for the three treatment groups by gender. For both genders, the placebo group had the highest average level of fatigue with quadratic trends, followed by the monotherapy group with similar quadratic trends but in different directions. Individuals in the combined therapy group had the lowest overall level of fatigue and experienced a slight increase during the two-week period. Comparing the two panels of the figure, we observed that all three intercept functions for men were lower than those for women in the corresponding treatment groups, indicating men had lower fatigue than women. For women (Figure. 3 left panel), the confidence bands for the three curves overlap only slightly. By comparison, the distinctions among the three treatment groups for men were less dramatic (Figure. 3 right panel), with complete separation only between the placebo and combined therapy groups. These results suggest that active treatments reduce cessation fatigue, relative to placebo, and are more effective for women than men in ameliorating cessation fatigue.
Table 3.
Time-constant coefficient estimates of Model 2 (time-varying effect model of regressing cessation fatigue on craving, negative affect, gender, the interaction between gender and craving, and the interaction between gender and negative affect, adjusting for lapse and baseline dependence, for three treatment groups respectively)
| Group | Covariate | Estimate (SD) | p-value |
|---|---|---|---|
| Placebo | Male | -0.78 (0.14) | <.0001 |
| Male*Crav | -0.69 (0.15) | <.0001 | |
| Male*NA | 0.54 (0.18) | 0.0022 | |
| Lapse | 0.24 (0.24) | 0.3234 | |
| Baseline dependence | -0.60 (0.10) | <.0001 | |
|
| |||
| Monotherapy | Male | -0.48 (0.06) | <.0001 |
| Male*Crav | -0.50 (0.07) | <.0001 | |
| Male*NA | 0.40 (0.07) | <.0001 | |
| Lapse | 0.61 (0.11) | <.0001 | |
| Baseline dependence | 0.24 (0.03) | <.0001 | |
|
| |||
| Combined therapy | Male | -0.32 (0.06) | <.0001 |
| Male*Crav | -0.24 (0.07) | 0.0006 | |
| Male*NA | 0.04 (0.09) | 0.6570 | |
| Lapse | -0.32 (0.13) | 0.0154 | |
| Baseline dependence | 0.14 (0.03) | <.0001 | |
This table contains only the coefficient estimates of the model parameters that are assumed to be time-constant, namely, β3, β4, β5, β6, and β7. The inference of the time-varying effects that are of primary interest (i.e., the intercepts, the effects of craving and the effects of negative affect) can be drawn from Fig. 3, Fig. 4 and Fig. 5.
Figure. 3.
Intercept functions for Model 2 (time-varying mean fatigue during first two weeks of quit attempt) by treatment group and gender (left panel for females and right panel for males)
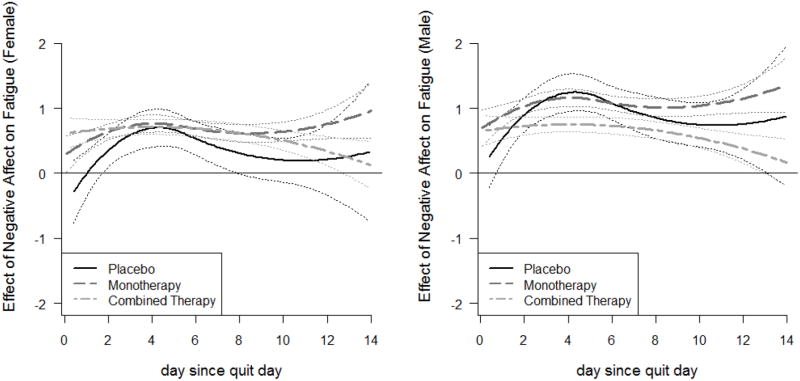
The coefficient functions for the effect of craving on cessation fatigue are presented in Figure. 4. The lines for placebo group are flat, whereas those for the active treatment groups are linearly increasing. For both genders, most of the lines fall significantly above zero, indicating a significant positive association between craving and fatigue that remained stable for the placebo group and strengthened for the active treatment groups. Thus, treatments effectively weakened the associations at the beginning of the post-quit period (the first week) but became less effective over time. At all times, and for all treatment groups, the association between craving and cessation fatigue was stronger for females than for males. In other words, women tended to have greater association between craving and fatigue than men. Moreover, since the lines for the active treatment groups are separated from that of the placebo group more consistently for women than for men, indicating a statistically significant difference at these points in time, we conclude that the active pharmacotherapies are more effective in weakening the association between craving and fatigue for women than for men, at least in the first week post-quit.
Figure. 4.
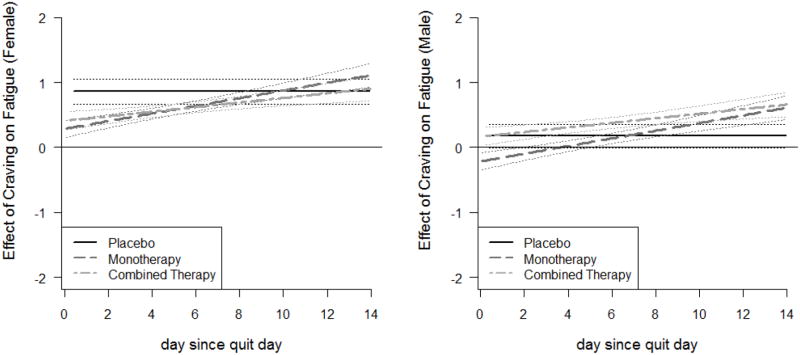
Time-varying effect of craving on cessation fatigue for Model 2, by treatment group and gender (left panel for females and right panel for males)
The coefficient functions for the effect of negative affect on cessation fatigue for the three treatment groups by gender are presented in Figure. 5. For both females (left panel) and males (right panel), more negative affect corresponded to significantly greater cessation fatigue for all treatment groups after approximately Day 2 post-quit. The two active treatment curves separate completely among males, and the combined pharmacotherapy group completely separates from the placebo group around Day 4, when negative affect has its strongest association with fatigue (Figure. 2, right panel). This suggests that combined pharmacotherapy was more effective than monotherapy at reducing the association between negative affect and fatigue, and that this advantage was more significant for men than women. On average, men had a stronger association between negative affect and cessation fatigue than women.
Figure. 5.
Time-varying effect of negative affect on cessation fatigue for Model 2, by treatment group and gender (left panel for females and right panel for males)
4. DISCUSSION
This exploratory research describes the first comprehensive analysis of the construct of cessation fatigue. We stated four hypotheses based on previous theoretical and empirical work. We hypothesized that cessation fatigue would increase over time; results were partially consistent with this hypothesis. Fatigue actually decreased over time; however, after we controlled for craving and negative affect, fatigue did increase over time. This suggests that craving and negative affect strongly influence the time course of cessation fatigue. As predicted, we found that cessation fatigue is significantly related to cessation success such that those who report low levels of fatigue are more likely to be abstinent by 6-months post-quit confirming H1. Further, we found that fatigue is ameliorated by nicotine replacement therapy, consistent with H3. In addition, smokers who received combined pharmacotherapy reported significantly less fatigue than those who received monotherapy. These findings suggest that fatigue is an important construct, related to cessation success and influenced by cessation treatments.
We also sought to understand the relations between cessation fatigue, craving, and negative affect, which were hypothesized to strengthen over time. Using TVEMs, we were able to model the time-varying relations between fatigue, craving, and negative affect. As hypothesized, craving and negative affect were both positively associated with fatigue: smokers who reported greater craving or negative affect also reported more cessation fatigue. Importantly, the associations between craving, negative affect and fatigue increased over time, supporting H2, that these smoking-related cues exert an increasing toll on smokers. However, it should be noted that the relation between negative affect and fatigue increased sharply for the first few days post-quit and then remained positive, but exhibited a slight decline through the end of the two weeks post-quit.
These findings are consistent with the strength model of self-regulation (Muraven et al., 1998; Haggeret al., 2010; Voh and Heatherton, 2000; Inzlicht and Schmeichel, 2012). The continuing need to activate self-control resources to prevent the dominant response of smoking in the face of key motivational prods exhausts these resources so that, over time, coping with a craving that would have been easy in the first days of a cessation attempt becomes much more difficult. This task, then, is related to significantly greater self-control depletion and, therefore, greater risk of relapse. Future research is needed to demonstrate the relation between cessation fatigue and self-control resources and to examine this proposed relapse mechanisms more carefully.
It is important to note that the strength model of self-regulation is distinct from a skill-based model (Muraven et al., 1998), which suggests that once someone has the necessary coping skills, s/he can execute them at any time without regard to any previous regulatory activity. Smoking treatment has focused on helping smokers develop skills to cope with smoking cues and urges (Fiore et al., 2008), but little focus has addressed this issue of capacity. The current research suggests that smokers may initially have the necessary capacity to cope with cravings or negative affect, but as they continue to succeed in doing so, they deplete their coping/self-control reserves and may ultimately reach a point where they have exhausted their capacity to resist the urge to smoke. Future research on fatigue could examine whether there is a time when need for self-control exceeds capacity and lapsing becomes inevitable. Identifying this point could help in developing treatments that prevent smokers from reaching this point of exhaustion.
Understanding gender differences in cessation success is a key public health concern. The TVEM results showed that women had greater cessation fatigue and stronger association between craving and cessation fatigue than did men, but active pharmacotherapy was more effective in reducing this association for women than for men. On the other hand, we found that men had a stronger association between negative affect and fatigue than did women, active pharmacotherapy was more effective in weakening this association for men than for women. These findings suggest that active treatment may ameliorate cessation fatigue through different mechanisms for men and women, but that reduction in cessation fatigue may be a common pathway of relapse prevention. It is important to note that these findings occurred in the context of women generally reporting higher levels of cessation fatigue as well as pre-quit and post-quit craving and negative affect. However, if these findings were only related to women reporting elevated levels of craving and fatigue, we would not see gender differences in the correlations between these variables over time (Figures 4 and 5), merely the different effectiveness of treatments between two genders (Figures 3, 4 and 5).
These findings have several limitations. This study was conducted with smokers who were motivated to quit and participate in a long-term clinical trial. Therefore, these findings might not generalize to all smokers. Moreover, by MLM results, the subjects removed from the dataset due to providing insufficient data or not being able to quit for at least 24 hours had significantly higher baseline dependence, pre-quit craving and pre-quit negative affect than those who were in the analysis. This may introduce bias to our results. In addition, most conclusions of this study are based on the novel methodology TVEM. Since this methodology was designed to depict changes in relations between variables over time, rather than to conduct specific hypothesis testing, this limits the conclusions that can be drawn from this approach. However, future advances in the development of this approach will likely lead to a broader set of hypothesis tests that can be accurately conducted. Finally, while we did not use data from participants following a return to regular smoking and we did control for smoking since the last EMA prompt, we do not have a good understanding of the impact of smoking/lapsing on cessation fatigue. Future research is needed to examine the effects of initial lapse and subsequent lapses on the development of fatigue.
Despite the limitations, this study contributes to our understanding of smoking cessation fatigue in several ways. Using EMA data and a new analytic technique, TVEM, our results supported a strength model of self-control, showing that fatigue increased over time, after controlling for craving and negative affect, and that the association between craving and negative affect and fatigue also increased over time. In sum, it appears that cessation fatigue is a key component of the relapse process that is related to both craving and negative affect, influences smoking cessation outcome, manifests differently by gender, and appears to be ameliorated to some degree by pharmacotherapies.
Supplementary Material
Acknowledgments
The authors thank Amanda Applegate for feedback on an earlier draft of this manuscript.
Role of Funding Source: This project was supported by Award Numbers P50-DA010075-15, P50 DA019706, P50-DA0197 and T32-DA017629 from the National Institute on Drug Abuse (NIDA), P50-CA84724 and R01 CA168676 from the National Cancer Institute (NCI), and M01-RR03186 from The General Clinical Research Centers Program of the National Center for Research Resources.
Footnotes
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:…
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:…
Contributors: Author Xiaoyu Liu conducted statistical analysis and wrote the main body of the manuscript. Author Runze Li provided methodology support for the data analysis. Author Megan Piper designed the study, provided the data and wrote discussion section of the manuscript. Authors Stephanie Lanza and Sara Vasilenko contributed in interpreting the results and revising the manuscript. All authors contributed to and have approved the final manuscript.
Conflict of Interest: No conflict declared.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baker TB, Piper ME, McCarthy DE, Bolt DM, Smith SS, Kim SY, Colby S, Conti D, Giovino GA, Hatsukami D, Hyland A, Krishnan-Sarin S, Niaura R, Perkins KA, Toll BA. Time to first cigarette in the morning as an index of ability to quit smoking: Implications for nicotine dependence. Nicotine Tob Res. 2007;9:S555–S570. doi: 10.1080/14622200701673480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolt DM, Piper ME, Theobald WE, Baker TB. Why two smoking cessation agents work better than one: role of craving suppression. J Consult Clin Psych. 2012;80:54–65. doi: 10.1037/a0026366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiore MC, Jaen CR, Baker TB, Bailey WC, Benowitz N, Curry SJ, et al. Treating tobacco use and dependence: 2008 update. Rockville, MD: U.S. Department of Health and Human Services, U.S. Public Health Service; 2008. [Google Scholar]
- Hagger MS, Wood C, Stiff C, Chatzisarantis NL. Ego depletion and the strength model of self-control: a meta-analysis. Psychol Bull. 2010;136:495–525. doi: 10.1037/a0019486. [DOI] [PubMed] [Google Scholar]
- Hastie T, Tibshirani R. Varying-coefficient models. J Roy Stat Soc B Methodol. 1993;55:757–779. [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The Fagerström Test for Nicotine Dependence: a revision of the Fagerström Tolerance Questionnaire. Br J Addict. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Hoover DR, Rice JA, Wu CO, Yang LP. Nonparametric smoothing estimates of time-varying coefficient models with longitudinal data. Biometrika. 1998;85:809–822. [Google Scholar]
- Inzlicht M, Schmeichel BJ. What is ego depletion? Toward a mechanistic revision of the resource model of self-control. Perspect Psychol Sci. 2012;7:450–463. doi: 10.1177/1745691612454134. [DOI] [PubMed] [Google Scholar]
- Lerman C, Roth D, Kaufmann V, Audrain J, Hawk L, Liu A, Niaura R, Epstein L. Mediating mechanisms for the impact of bupropion in smoking cessation treatment. Drug Alcohol Depend. 2002;67:219–223. doi: 10.1016/s0376-8716(02)00067-4. [DOI] [PubMed] [Google Scholar]
- Li R, Root T, Shiffman S. A local linear estimation procedure for functional multilevel modeling. In: Walls T, Schafer J, editors. Models for Intensive Longitudinal Data. Oxford University Press; New York, NY: 2006. pp. 63–83. [Google Scholar]
- McCarthy DE, Piasecki TM, Lawrence DL, Jorenby DE, Shiffman S, Baker TB. Psychological mediators of bupropion sustained-release treatment for smoking cessation. Addiction. 2008;103:1521–1533. doi: 10.1111/j.1360-0443.2008.02275.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muraven M, Tice DM, Baumeister RF. Self-control as limited resource: regulatory depletion patterns. J Pers Soc Psychol. 1998;74:774–789. doi: 10.1037//0022-3514.74.3.774. [DOI] [PubMed] [Google Scholar]
- Piasecki TM. Relapse to smoking. Clin Psychol Rev. 2006;26:196–215. doi: 10.1016/j.cpr.2005.11.007. [DOI] [PubMed] [Google Scholar]
- Piasecki TM, Fiore MC, McCarthy DE, Baker TB. Have we lost our way? The need for dynamic formulations of smoking relapse proneness. Addiction. 2002;97:1093–108. doi: 10.1046/j.1360-0443.2002.00216.x. [DOI] [PubMed] [Google Scholar]
- Piper ME, Cook JW, Schlam TR, Jorenby DE, Smith SS, Bolt DM, Loh WY. Gender, race and education differences in abstinence rates among participants in two randomized smoking cessation trials. Nicotine Tob Res. 2010;12:647–57. doi: 10.1093/ntr/ntq067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper ME, Federman EB, McCarthy DE, Bolt DM, Smith SS, Fiore MC, Baker TB. Using mediational models to explore the nature of tobacco motivation and tobacco treatment effects. J Abnorm Psych. 2008;117:94–105. doi: 10.1037/0021-843X.117.1.94. [DOI] [PubMed] [Google Scholar]
- Piper ME, Schlam TR, Cook JW, Sheffer MA, Smith SS, Loh WY, Bolt DM, Kim SY, Kaye JT, Hefner KR, Baker TB. Tobacco withdrawal components and their relations with cessation success. Psychopharmacology. 2011;216:569–578. doi: 10.1007/s00213-011-2250-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper ME, Smith SS, Schlam TR, Fiore MC, Jorenby DE, Fraser D, Baker TB. A randomized placebo-controlled clinical trial of five smoking cessation pharmacotherapies. Arch Gen Psychiatry. 2009;66:1253–1262. doi: 10.1001/archgenpsychiatry.2009.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raudenbush SW, Bryk AS. Hierarchical Linear Models: Applications and Data Analysis Methods. Sage; Thousand Oaks: 2002. [Google Scholar]
- Schwartz JE, Stone AA. Strategies for analyzing ecological momentary assessment data. Health Psychol. 1998;17:6–16. doi: 10.1037//0278-6133.17.1.6. [DOI] [PubMed] [Google Scholar]
- Selya AS, Dierker LC, Rose JS, Hedecker D, Li R, Tan X, Mermelstein RJ. Time-varying effects of smoking quantity and nicotine dependence on adolescent smoking regularity. Drug Alcohol Depend. 2012;128:230–237. doi: 10.1016/j.drugalcdep.2012.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffman S. Ecological momentary assessment (EMA) in studies of substance use. Psychol Assess. 2009;21:486–497. doi: 10.1037/a0017074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffman S, Sweeney CT, Dresler CM. Nicotine patch and lozenge are effective for women. Nicotine Tob Res. 2005;7:119–127. doi: 10.1080/14622200412331328439. [DOI] [PubMed] [Google Scholar]
- Shiyko MP, Lanza ST, Tan X, Li R, Shiffman S. Using the time-varying effects model (TVEM) to examine dynamic associations between negative affect and self-confidence on smoking urges: differences between successful quitters and relapsers. Prev Sci. 2012;13:288–299. doi: 10.1007/s11121-011-0264-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons VN, Heckman BW, Ditre JW, Brandon TH. A measure of smoking abstinence-related motivational engagement: development and initial validation. Nicotine Tob Res. 2010;12:432–437. doi: 10.1093/ntr/ntq020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffman S, Stong AA, Huffold MR. Ecological momentary assessment. Annu Rev Clin Psychol. 2008;4:1–32. doi: 10.1146/annurev.clinpsy.3.022806.091415. [DOI] [PubMed] [Google Scholar]
- Smith SS, Jorenby DE, Leischow SJ, Nides MA, Rennard SI, Johnston JA, Jamerson B, Fiore MC, Baker TB. Targeting smokers at increased risk for relapse: Treating women and those with a history of depression. Nicotine Tob Res. 2003;5:99–109. doi: 10.1080/1462220021000060437. [DOI] [PubMed] [Google Scholar]
- Smith SS, McCarthy DE, Japuntich SJ, Christiansen B, Piper ME, Jorenby DE, Fraser DL, Fiore MC, Baker TB, Jackson TC. Comparative effectiveness of 5 smoking cessation pharmacotherapies in primary care clinics. Arch Intern Med. 2009;169:2148–55. doi: 10.1001/archinternmed.2009.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stead LF, Perera R, Bullen C, Mant D, Lancaster T. Nicotine replacement therapy for smoking cessation. Cochrane Database Syst Rev. 2008;1 doi: 10.1002/14651858.CD000146.pub3. CD000146. [DOI] [PubMed] [Google Scholar]
- Tan X, Shiyko M, Li R, Li Y, Dierker L. Intensive longitudinal data and model with varying effects. Psychol Methods. 2012;17:61–77. doi: 10.1037/a0025814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vohs KD, Heatherton TF. Self-regulatory failure: a resource-depletion approach. Psychol Sci. 2000;11:249–254. doi: 10.1111/1467-9280.00250. [DOI] [PubMed] [Google Scholar]
- Walls TA, Schafer JL. Modeling for Intensive Longitudinal Data. Oxford University Press; New York, NY: 2006. [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. J Pers Soc Psychol. 1998;54:1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Welsch SK, Smith SS, Wetter DW, Jorenby DE, Fiore MC, Baker TB. Development and validation of the Wisconsin Smoking Withdrawal Scale. Exp Clin Psychopharm. 1999;7:354–361. doi: 10.1037//1064-1297.7.4.354. [DOI] [PubMed] [Google Scholar]
- Yang J, Tan X, Li R, Wagner A. TVEM (time-varying effect model) SAS Macro Suite Users’ Guide (Version 2.0.0) The Methodology Center, Penn State; 2012. [Dec 12002C 2012]. Retrieved from http://methodology.psu.edu, archived at http://www.webcitation.org/69xyvCfKw. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.