Abstract
Chronic cadmium exposure may cause disease through induction of systemic oxidative stress and inflammation. Factors that mitigate cadmium toxicity and could serve as interventions in exposed populations have not been well characterized. We used data from the 2003–2010 National Health and Nutrition Examination Survey to quantify diet’s role in modifying associations between cadmium exposure and oxidative stress and inflammation. We created a composite antioxidant and anti-inflammatory diet score (ADS) by ranking participants by quintile of intake across a panel of 19 nutrients. We identified associations and effect modification between ADS, urinary cadmium, and markers of oxidative stress and inflammation by multiple linear regression. An interquartile range increase in urinary cadmium was associated with a 47.5%, 8.8%, and 3.7% increase in C-reactive protein (CRP), gamma glutamyl transferase (GGT), and alkaline phosphatase (ALP), respectively. An interquartile range increase in ADS was associated with an 7.4%, 3.3%, 5.2%, and 2.5% decrease in CRP, GGT, ALP, and total white blood cell count respectively, and a 3.0% increase in serum bilirubin. ADS significantly attenuated the association between cadmium exposure, CRP and ALP. Dietary interventions may provide a route to reduce the impact of cadmium toxicity on the population level.
Keywords: Cadmium, Inflammation, Oxidative stress, Diet, Antioxidant, Toxicant–nutrient interaction
1. Introduction
Cadmium is a ubiquitous environmental contaminant that represents a significant health hazard to humans. Occupational studies examining the effects of chronic cadmium exposure first identified the compound as a potent nephrotoxin (Johri et al., 2010). Studies of populations with chronic low dose cadmium exposure have reported a range of adverse health outcomes, including chronic kidney disease (Navas-Acien et al., 2009), hypertension (Tellez-Plaza et al., 2008), type 2 diabetes mellitus (Schwartz et al., 2003), and cancer (Julin et al., 2012). Systemic inflammation and induction of oxidative stress (OS) are hypothesized as mechanisms by which cadmium exposure increases disease risk. Cadmium exposure has been positively associated with concentrations of gamma glutamyl transferase (GGT), a known marker of OS in population studies (Lee et al., 2006). Similarly, cadmium exposure was positively associated with the systemic inflammation markers alkaline phosphatase (ALP) and C-reactive protein (CRP) (Lin et al., 2009; Staessen and Lauwerys, 1993). The relationship between cadmium exposure and additional markers of OS, including serum concentrations of the antioxidant bilirubin, or systemic inflammation, such as total white blood cell count (WBC), have not been explored in population studies. Given the widespread nature of cadmium exposure, little research has been done to identify modifiable factors that mitigate cadmium toxicity and serve as an intervention in cadmium exposed populations.
Dietary intake has long been known to play a role in the physiological response to inflammation and OS (Singh et al., 2005). Micronutrients such as vitamin C, vitamin E, selenium, carotenoids, copper, zinc and iron mitigate OS through elimination of superoxides, peroxides hydroxyl radicals and singlet oxygen molecules from the body (Gropper et al., 2005; Machlin and Bendich, 1987). Anti-inflammatory nutrients, such as n-3 polyunsaturated fatty acids, inhibit the production of pro-inflammatory cytokines and eicosanoids by antagonizing arachadonic acid metabolism (Calder, 2006). Despite similar mechanisms of action, dietary micronutrients and macronutrients are often modeled as individual units, and composite antioxidant and anti-inflammatory scores previously described (Cavicchia et al., 2009) are not typically utilized in population based studies. Additionally, the extent to which antioxidants and anti-inflammatory compounds can moderate the effects of chronic toxicant exposure remains poorly characterized. Dietary interventions remain a low-risk intervention to prevent adverse health effects in exposed populations, exemplified by the success of calcium supplementation in reducing blood lead concentrations (Hernandez-Avila et al., 2003).
To extend previous research describing associations between cadmium exposure and systemic inflammation and OS, we used data from the 2003–2010 National Health and Nutrition Examination Survey (NHANES) to characterize associations between urinary cadmium concentrations and two markers of OS: GGT and bilirubin, as well as three markers of systemic inflammation: CRP, WBC, and ALP. Additionally, we developed a novel composite estimate of dietary antioxidant and anti-inflammatory capacity based on intake of 19 individual nutrients quantified by two 24-hour dietary recalls. We examined associations between this “Antioxidant and Anti-inflammatory Dietary Score” (ADS) and markers of OS and inflammation. Finally, we quantified effect modification of the relationship between cadmium exposure, OS, and inflammation by dietary intake, to determine a role for diet in the prevention of cadmium related toxicity.
2. Materials and methods
Data was obtained from NHANES, an ongoing survey of the civilian, non-institutionalized U.S. population designed to monitor the health status of adults and children (National Center for Health Statistics (NCHS), 2003–2010). NHANES is administered by the National Center for Health Statistics, a division of the Centers for Disease Control and Prevention (CDC).
2.1. Laboratory methods
All biomarker concentration measurements were performed by the CDC via methods previously described (CDC 2003–2010). Serum ALP was quantified via UniCel DxC800 Synchron (Beckman Coulter, Brea, CA, USA) using kinetic rate method employing a 2-amino-2-methyl-1-propanol buffer to measure enzyme activity. Serum total bilirubin was quantified via DxC800 with a timed-endpoint Diazo method. CRP was quantified in whole blood by latex-enhanced nephelometry at the University of Washington at Seattle. Serum GGT was quantified via DxC800 via enzymatic rate method. Total WBC was estimated using a MAXM hematology flow cytometer (Beckman Coulter, Brea, CA, USA).
Urinary cadmium concentrations were quantified by inductively coupled plasma-mass spectrometry, as previously described (Mulligan et al., 1990). Urinary creatinine was measured via an enzymatic method on the Roche/Hitachi Modular P Chemistry Analyzer. Serum cotinine was quantified via isotope dilution-high performance liquid chromatography/atmospheric pressure chemical ionization tandem mass spectrometry. Values below the limit of detection (LOD) for all biomarkers were estimated by the LOD divided by the square root of two.
2.2. Dietary methods
Two 24-hour dietary recall (24-HR) interviews were collected from participants; the first was administered during the mobile examination center exam, the second by telephone approximately 3–10 days later. Participants who did not complete both 24-HR interviews, were deemed unreliable reporters by NHANES or reported energy intake outside the arbitrarily set range of 500–4000 kcal (kcals)/day for women and 800–4500 kcals/day for men were excluded for this analysis. Reported energy intakes outside these ranges are rarely accurate and considered to be biologically implausible (Willett, 1998). Nutrient intakes were calculated based on the mean reported consumption from the two 24-HRs.
We developed an antioxidant and anti-inflammatory dietary score (ADS) a priori to any cadmium analyses to investigate modification of the association between cadmium exposure and markers of systemic inflammation or OS by diet. We ranked participants based on intake of 19 individual nutrients consumed from food known to be involved in anti-inflammatory, pro-inflammatory, or antioxidant processes in the body and that have been included in a previously validated dietary inflammatory score (Gropper et al., 2005; Cavicchia et al., 2009). Nutrient intake was calculated by NHANES based on foods reportedly consumed in the 24-HRs using the USDA Food and Nutrient Database for Dietary Studies. (http://www.cdc.gov/nchs/nhanes/nhanes2007-2008/DR1TOT_E.htm). For each nutrient, individuals were scored based on quintile of intake, with nutrient specific scores ranging from 0 to 4, from lowest (score 0) to highest (score 4) quintile of intake. Dietary micronutrients considered to have anti-inflammatory or antioxidant activity were vitamin A, vitamin C, vitamin E, α-carotene, β-carotene, β-cryptoxanthin, lycopene, lutein and zeaxanthin, selenium, vitamin B6, magnesium, zinc, copper, iron, monounsaturated fat, polyunsaturated fat, and fiber. The pro-inflammatory nutrients saturated fat and cholesterol were inversely ranked so that the upper quintile (score 4) represented participants with the lowest intake. Total ADS was created by summing individual nutrient scores, giving a theoretical range from 0 to 76. A higher ADS represents a greater intake of antioxidant and anti-inflammatory nutrients.
2.3. Statistical analysis
We included a total of 6344 individuals from NHANES 2003–2010 with information available on all OS or inflammatory biomarkers, urinary cadmium, dietary intake, and covariates of interest in our analyses.
Covariates identified as potential confounders or effect modifiers of associations among cadmium exposure, dietary intake, and systemic inflammation included age, sex, race/ethnicity, body mass index (BMI), and smoking status (estimated continuously via serum cotinine concentrations). All covariates of interest were significantly associated with at least one infiammatory or OS biomarker (P<0.05) in multivariate models; hence, all were included in the fully adjusted models.
Associations between urinary cadmium concentrations or ADS and markers of inflammation or OS were quantified via generalized linear regression analysis adjusting for covariates listed above. We adjusted urinary cadmium models for urinary creatinine concentrations as previously described (Barr et al., 2005). Urinary cadmium, ALP, bilirubin, CRP, GGT, WBC, serum cotinine, and urinary creatinine were all positively skewed and were natural log (ln) transformed for analysis. Estimates are presented as percent change in outcome per interquartile range (IQR) increase in predictor. We additionally tested for potential confounding of the relationship between urinary cadmium and markers of OS and inflammation by smoking in a subset analysis by categorizing individuals into never, former, and current smokers (n=4842). Individuals who reported smoking less than 100 cigarettes during their lifetime were categorized as never smokers, while individuals who reported smoking greater than 100 cigarettes but were not currently smoking cigarettes were categorized as former smokers, while individuals who reported smoking cigarettes every day or some day were categorized as current smokers. We also quantified the associations between cadmium and markers of inflammation and OS only in non-smokers (n=2590). We estimated the potential confounding effects of alcohol consumption, estimating total alcohol intake as a function of average drinks consumed per day (n=3973).
To test for effect modification of the relationship between cadmium exposure and inflammation or OS biomarker by dietary intake, we calculated the interaction between urinary cadmium (continuous) and ADS subset into tertiles (categorical). We estimated the association between cadmium exposure and inflammatory or OS biomarkers in models that stratified based on tertile of ADS. In all analyses, we set the cutoff for two-sided statistical significance at α=0.05 for both an association between exposure and outcome and interaction between urinary cadmium and anti-inflammatory intake.
Statistical analyses were conducted in R 2.15.2 (R Development Core Team, 2011) using the Survey package (Lumley, 2004) and SAS 9.3 (SAS Institute, Inc.). All analyses were adjusted for appropriate strata, cluster, and sample specific weights to account for the complex sampling design of NHANES.
3. Results
Women comprised 51.8% of the study population, which had a mean age of 43.3 years (Table 1). The geometric mean of urinary cadmium concentration was 0.20 μg/L, with a maximum value of 14.9 μg/L. The geometric mean concentrations of ALP, bilirubin, CRP, GGT, and WBC were 76.4 (69.4 U/L), 13.2 (12.3 mg/dL), 0.36 (0.15 mg/dL), 26.3 (20.1 U/L), and 7.2 (6.9 ×103 cells/μL) respectively (Table 1). Mean energy-adjusted nutrient intake by quintile is presented in (Table 2). There was a large range in intake of vitamin C, α-carotene, β-carotene, lycopene, and lutein plus zeaxanthin, with over an order of magnitude difference identified between the lowest and highest quintiles of intake for each nutrient. Across the study population, the ADS ranged from 8 to 71, with a median score of 39.
Table 1.
Survey weighted epidemiological characteristics of the study population (n=6344).
| n (%) | Geo. mean, Geo. std. error (Min, Max) | |
|---|---|---|
| Sex | ||
| Male | 3056 (48.2%) | |
| Female | 3288 (51.8%) | |
| Race/ethnicity | ||
| Mexican American | 1448 (22.3%) | |
| Other Hispanic | 426 (6.7%) | |
| Non-Hispanic White | 2906 (45.8%) | |
| Non-Hispanic Black | 1325 (20.9%) | |
| Other | 239 (3.8%) | |
| Age (years) | 38.7, 0.010 (12–85) | |
| Body mass index (kg/m2) | 27.2, 0.004 (14.9–76.1) | |
| Serum cotinine (ng/mL) | 0.28, 0.080 (0.01, 1438) | |
| Urinary cadmium (μg/L) | 0.20, 0.019 (0.03, 14.9) | |
| Alkaline phosphatase (U/L) | 69.4, 0.007 (21, 667) | |
| Bilirubin (mg/dL) | 12.3, 0.010 (1.7, 224) | |
| C-reactive protein (mg/dL) | 0.15, 0.024 (0.01, 18.01) | |
| Gamma glutamyl transferase (U/L) | 20.1, 0.014 (4, 1479) | |
| Total white blood cell count (103 cells/μL) | 6.9, 0.005 (1.5, 83.2) | |
Table 2.
Quntile-specific mean energy-adjusted nutrient intakes.
| Nutrient | Quintile 1 | Quintile 2 | Quintile 3 | Quintile 4 | Quintile 5 |
|---|---|---|---|---|---|
| Anti-inflammatory nutrients | |||||
| Vitamin A, RAE (μg) | 219 | 384 | 524 | 696 | 1163 |
| Vitamin C (mg) | 20 | 46.5 | 76.5 | 115 | 206 |
| Vitamin E (α-tocopherol) (mg) | 3.4 | 4.7 | 5.6 | 6.9 | 11.1 |
| α-Carotene (μg) | 8.6 | 28.4 | 71.4 | 293 | 1354 |
| β-Carotene (μg) | 210 | 440 | 848 | 1816 | 5481 |
| β-Cryptoxanthin (μg) | 5.6 | 20.6 | 52.4 | 121 | 407 |
| Lycopene (μg) | 406 | 1599 | 3283 | 6214 | 15,957 |
| Lutein+zeaxanthin (μg) | 237 | 448 | 664 | 1022 | 3539 |
| Selenium (μg) | 64 | 82.1 | 94.5 | 109 | 141 |
| Vitamin B6 (mg) | 1 | 1.4 | 1.7 | 2 | 3 |
| Magnesium (mg) | 169 | 211 | 243 | 281 | 366 |
| Zinc (mg) | 6.8 | 8.7 | 10.2 | 12 | 17 |
| Copper (mg) | 0.7 | 0.9 | 1.1 | 1.2 | 1.8 |
| Iron (mg) | 9.2 | 11.6 | 13.5 | 16.1 | 23.4 |
| Monounsaturated fat (g) | 17.6 | 22.4 | 25.4 | 28.5 | 34.6 |
| Polyunsaturated Fat (g) | 8.7 | 11.8 | 14.1 | 16.7 | 22.6 |
| Fiber (g) | 7.8 | 11 | 13.6 | 16.8 | 24.4 |
| Pro-inflammatory nutrients | |||||
| Saturated fat (mg) | 15.3 | 20.4 | 23.5 | 26.8 | 32.9 |
| Cholesterol (mg) | 111 | 167 | 216 | 290 | 487 |
After adjusting for relevant covariates and urinary creatinine, urinary cadmium was associated with an increase in ALP, CRP, and GGT, and a decrease in WBC (Table 3). The most striking positive association was observed for the inflammatory marker CRP, where an IQR increase in urinary cadmium (0.31 μg/L) was associated with a 47.5% increase in blood CRP concentration. Additionally, an IQR increase in urinary cadmium was associated with an 8.8% and a 3.7% increase in serum concentrations of GGT and ALP, respectively. An increase in urinary cadmium was found to be inversely associated with total WBC. No significant association was observed between urinary cadmium and the antioxidant bilirubin after adjusting for covariates, although an inverse association was observed in models only adjusting for urinary creatinine. Adjusting for smoking by categorizing smokers into never/former/current smokers or alcohol consumption did not change the regression results when compared to adjusting only for serum cotinine (Supplementary Table 1). When examining the effects of urinary cadmium in only non-smokers, the results for AP, GGT, and CRP were found to be attenuated, while the results for bilirubin and WBC were enhanced (Supplementary Table 1).
Table 3.
Percent change in biomarker of systemic inflammation or oxidative stress per IQR increase in urinary cadmium.
| Biomarker | Creatinine adjusted | 95% CI | Fully adjusteda | 95% CI |
|---|---|---|---|---|
| Alkaline phosphatase | −6.6*** | −8.5, −4.7 | 3.7 ** | 1.3, 6.3 |
| Bilirubin | −3.1* | −5.6, −0.5 | 0.2 | −3.5, 4 |
| C-reactive protein | 93.3*** | 81.8, 105.6 | 47.5*** | 39.3, 56.2 |
| Gamma glutamyl transferase | 20.7*** | 20.3, 21.1 | 8.8** | 4.6, 13.2 |
| Total white blood cell count | −1.1 | −2.6, 0.4 | −3.2 ** | −5.2, −1.1 |
P<0.05.
P<0.01.
P<0.0001.
Adjusted for age, sex, ethnicity, body mass index, urinary creatinine, and serum cotinine.
In adjusted models ADS score was found to be positively associated with bilirubin, representing an increase in antioxidant availability, and inversely associated with ALP, CRP, GGT, and WBC (Table 4). The strongest association with ADS was observed for the inflammatory marker CRP, where an IQR increase in ADS (18 points) was associated with a 7.4% decrease in CRP. An IQR increase of ADS was also associated with a 2.7% increase in serum concentration of bilirubin, a 5.2% decrease in ALP, a 3.3% decrease in GGT, and a 2.5% decrease in WBC. ADS was not associated with urinary cadmium after adjusting for relevant covariates (P=0.69).
Table 4.
Percent change in biomarker of systemic inflammation or oxidative stress per IQR increase in anti-inflammatory diet score.
| Biomarker | Unadjusted | 95% CI | Fully adjusteda | 95% CI |
|---|---|---|---|---|
| Alkaline phosphatase | −7.6*** | (−9.2, −6.1) | −5.2*** | −7.1, −3.3 |
| Bilirubin | 3.5** | −6.3, −0.1 | 2.7** | 0.8, 4.6 |
| C-reactive protein | −2.2 | −9.2, 5.3 | −7.4* | −13.2, −1.1 |
| Gamma glutamyl transferase | −3.3* | −6.3, −0.1 | −3.3* | −6.4, −0.1 |
| Total white blood cell count | −4.7*** | −5.9, −3.4 | −2.5** | −3.9, −1 |
P<0.05.
P<0.01.
P<0.0001.
Adjusted for age, sex, ethnicity, body mass index, and serum cotinine.
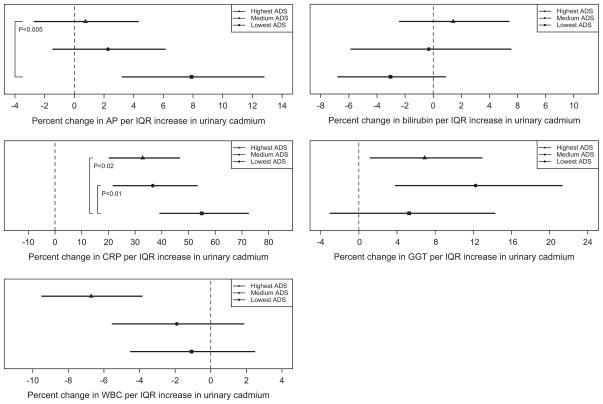
We conducted stratified analyses by tertile of ADS to quantify potential interactions between dietary intake and urinary cadmium on systemic inflammation and OS. No effect modification of the relationship between urinary cadmium and the markers of OS, bilirubin and GGT, by ADS was observed (Fig. 1). However, individuals in the lowest tertile of ADS were found to have a stronger positive association between urinary cadmium concentration and markers of systemic inflammation CRP and ALP, compared to individuals in the highest tertile of ADS.
Fig. 1.
Graphical depiction of percent change in inflammation or oxidative stress marker per IQR increase in urinary cadmium concentration by tertile of anti-inflammatory dietary intake.
4. Discussion
4.1. Cadmium and oxidative stress
In this study, we confirm and extend previous reports of associations between chronic cadmium exposure and biomarkers of OS and inflammation. The ability of cadmium to induce OS has been established in animal and in vitro studies, and substantiated in some epidemiologic studies (Liu et al., 2009; Shaikh et al., 1999). In acute cases, exposure can directly induce reactive oxygen species (ROS) production. In instances of chronic exposure, although the mechanisms are less clear, free radical release can occur as well (Patra et al., 2011). Increased OS resulting from cadmium exposure may be an important contributor to adverse health outcomes, particularly carcinogenesis (Liu et al., 2009).
Although there are a variety of biomarkers for assessing systemic OS levels, GGT may be particularly informative. GGT is involved in the regeneration of glutathione (GSH), an antioxidant crucial in the metabolism of xenobiotic substances, and consequently elevated serum GGT levels may be representative of acute increases in GSH activity (Lee and Jacobs, 2009). Alternatively, or possibly in combination, GGT may serve as a useful marker because it is directly involved in the production of ROS (Emdin et al., 2005). Under this scenario, increases in serum GGT levels may be indicative of chronic OS; because prolonged stressor exposure may deplete GSH, and consequently the body’s ability to neutralize free radicals, higher ROS and GGT levels may be observed. Therefore, positive associations observed between cadmium and GGT in our analysis may suggest either current and/or long-term systemic OS in study subjects.
The positive relationships observed here between urinary cadmium concentrations and GGT corroborate other human studies that suggest a link between long-term cadmium exposure and increases in systemic OS using GGT and other biomarkers (Lee et al., 2006). An early analysis of NHANES III data (N=10,098) observed a similar association between urinary cadmium and GGT, which persisted after adding urinary levels of antioxidant vitamins into the models (Lee et al., 2006). To our knowledge this is the only study to date examining the cadmium–OS relationship while adjusting for dietary intake. This highlights the value in our study of examining not only a more comprehensive measure of anti-oxidant and anti-inflammatory dietary intake, but also its potential for effect modification.
Bilirubin, a potent antioxidant involved in the scavenging of reactive oxygen species, may serve as a sensitive marker of OS response to xenobiotic exposure. Bilirubin concentrations have been correlated with other biomarkers of OS, such as malondialdehyde, and in epidemiological studies, bilirubin concentrations have been associated with other chemicals known to be capable of releasing ROS (Ferguson et al., 2012; Shekeeb Shahab et al., 2008). However, bilirubin has never been utilized as a biomarker of OS in relationship to cadmium. Our analyses did not identify an association between chronic cadmium exposure and serum bilirubin after adjusting for relevant covariates in the whole study population, but did measure a positive association between serum cadmium and bilirubin in non-smokers. This suggests that the route of exposure to cadmium, whether inhalational or dietary, may have differential effects on antioxidant capacity.
4.2. Cadmium and inflammation
Chronic cadmium exposure may cause an increase in systemic levels of inflammation, either as a downstream effect of cadmium-induced OS or through other mechanisms (Dong et al., 1998; Knoflach et al., 2011). This relationship has been explored to some extent in humans, particularly because cadmium associated inflammation may be an important mechanism in cadmium-related cardiovascular disease (Navas-Acien et al., 2004). Some studies have utilized the same biomarkers as our analyses. Lin et al. (2009) identified a positive association between urinary cadmium and CRP as well as fibrinogen, another marker of inflammation, in NHANES III. Cheung et al. (2009), using continuous NHANES data from 1999 to 2004, observed a positive association with ALP, although exposure measures in this study were assessed with serum cadmium levels which may be more indicative of short-term exposure. Our results confirm the associations between cadmium exposure and CRP and ALP, and also uniquely identify that relationships are strongest among individuals whose diets are lower in anti-inflammatory and antioxidant nutrients. Additionally, we observed a significant inverse association between cadmium exposure and WBC, a biomarker that has not been previously used in this setting. In other studies this measure has been associated with various other environmental contaminants, including phthalate diesters, bisphenol-A, tobacco smoke, and criteria air pollutants (Ferguson et al., 2012; Liao et al., 2005; Liu et al., 2011; Venn and Britton, 2007; Yang et al., 2009). However, while we observed an inverse association between chronic cadmium exposure and WBC, previous in vivo work has identified acute cadmium exposure as positively associated with WBC in Sprague–Dawley rats (Morgan et al., 1984). More research in human populations is necessary to understand if the associated identified in the current study represents a biologically relevant inhibition of white blood cell function or formation.
4.3. Cadmium, oxidative stress and inflammation, and chronic disease
Systemic inflammation and OS are becoming recognized as important components in the etiology of a variety of chronic diseases, including type 2 diabetes mellitus, cancer, and cardiovascular disease (Coussens and Werb, 2002; Wellen and Hotamisligil, 2005; Wu et al., 2004). As such, systemic and organ-specific inflammation and OS are hypothesized to be major mechanisms by which chronic cadmium exposure influences the initiation and progression of these diseases (Gobe and Crane, 2010; Joseph, 2009).
Cadmium exposure has been associated with development of cancer at a number of sites including the kidney (Il’yasova and Schwartz, 2005), lung (Nawrot et al., 2006), and breast (McElroy et al., 2006). Markers of inflammation and OS have also been shown to be predictive of cancer development and response to treatment. A prospective study of serum GGT concentrations and risk of cancer development in 79,279 healthy men found a dose dependent increase in overall cancer risk, and site-specific increases in risk of respiratory and digestive cancers (Strasak et al., 2008). Additionally, ALP concentrations have been reported as a useful marker in identifying individuals at high risk for bone metastases of prostate cancer (Wymenga et al., 2001), showing that inflammation and OS play an important role throughout cancer initiation and progression.
Chronic cadmium exposure has been associated with impaired glucose tolerance and the development of type 2 diabetes mellitus in both population based (Afridi et al., 2008) and in vivo studies (Merali and Singhal, 1980). OS and inflammatory markers have also been shown to be strong predictors of the development of diabetes. Serum GGT was associated with incidence of diabetes in a cohort of 4088 healthy men employed in a steel manufacturing company (Lee et al., 2003), and also in a cohort of 3260 non-diabetic Japanese men (Nakanishi et al., 2004). Elevated CRP predicted diabetes in a prospective study of 27,628 women as part of the Women’s Health Study (Pradhan et al., 2001), and in 5245 middle aged men enrolled in the West of Scotland Coronary Prevention Study (Freeman et al., 2002). Serum bilirubin was significantly decreased in prevalent diabetics in a study of 12,400 middle aged and elderly men and women from Japan (Ohnaka et al., 2010), suggesting that OS and inflammation play a role both in the development and progression of diabetes.
4.4. Dietary intake, inflammation, and oxidative stress
Numerous studies have provided evidence suggesting dietary intake modulates inflammation and OS. However, the majority of these studies have only examined associations with individual food groups (e.g. fruits and vegetables) and nutrients. As the many nutrients present in food likely interact and work synergistically to produce a stronger effect than any individually, examining the combined influence of nutrients on inflammation and OS may be more informative (Hu et al., 2000). Additionally, the generation of a single score that represents an estimate of overall anti-inflammatory and antioxidant capacity of diet simplifies the analysis and interpretation of study results. In this study, we present a novel method to calculate an anti-inflammatory diet composite score from 24-hour dietary recall data, confirming its efficacy by identifying inverse associations with markers of systemic inflammation ALP, CRP, and WBC, an inverse association with a marker of OS, GGT, and a positive association the antioxidant bilirubin.
Cavicchia et al. previously developed a scored Inflammatory Index designed to assess the inflammatory potential of individuals’ diets (Cavicchia et al., 2009). Although the researchers demonstrated the ability of the index to predict serum high sensitivity CRP, it is not easily reproducible for use in other studies, as the use of this index involves extensive weighting of variables by study design and robustness of the literature, and thus is sensitive to constantly evolving research. Furthermore, many of the nutrients included in this index are not available in the NHANES datasets. The ADS we developed was simple to calculate using NHANES data and is easily applicable in other studies, including studies using food frequency questionnaire data, and can be expanded or abbreviated based on the hypothesis of interest and breadth of data available. Furthermore, we demonstrate strong associations with not only inflammatory markers, but also markers of OS.
We identified effect modification of the relationship between cadmium, OS, and inflammation by diet, with a negative interaction observed for both ALP and CRP. Interestingly, a similar pattern of interaction was observed for the three markers of inflammation in the study, CRP, ALP, and WBC (although not statistically significant), while no significant interaction was observed for the biomarkers of OS, bilirubin and GGT. These results suggest that the ADS is particularly effective at estimating the anti-inflammatory and antioxidant potential of the diet. Dietary interventions designed to increase consumption of nutrients with anti-inflammatory and antioxidant properties may be effective in mitigating the inflammatory effects of cadmium in highly exposed populations and could be an effective mechanism to prevent cadmium-related toxicity.
4.5. Limitations
As with all NHANES analyses, our study was limited by the cross-sectional nature of the data. Hence, we are unable to identify cause–effect relationships from these results. However, the strong associations observed are useful for hypothesis generation and planning for future longitudinal studies. Other limitations in using this dataset include: use of 24-hour dietary recalls, which are self-reported and may be biased, and cadmium exposure assessment was based on spot urine samples. Overall, we would expect these limitations inherent to the NHANES dataset to bias effect estimates toward the null, and as we observed strongly significant associations despite this, our results are likely robust.
4.6. Strengths
The results presented here corroborate and extend previous findings describing the relationship between chronic cadmium exposure and OS and inflammation, describing novel associations between cadmium exposure and the OS and antioxidant capacity biomarker bilirubin as well as a lack of association between cadmium exposure and total white blood cell count. A major strength of this study lies in the large, well-characterized sample population, with available measures of exposure, diet, and bio-markers of interest. We formulated a novel dietary score to quantify overall anti-inflammatory and antioxidant potential of the diet. We also report for the first time significant effect modification by dietary intake on the role of cadmium on systemic inflammation and OS, potentially providing initial evidence in support of anti-inflammatory dietary interventions for cadmium exposed individuals.
5. Conclusions
The role of cadmium exposure in the etiology of chronic diseases like cancer, diabetes, and hypertension is well-characterized and likely mediated, at least in part, through OS and inflammatory intermediates. As dietary intake was found to significantly attenuate the relationship between cadmium, OS, and inflammation, dietary interventions may reduce the impact of cadmium toxicity on the population level.
Supplementary Material
Acknowledgments
Funding sources: Support for this study was provided by a grant from the University of Michigan School of Public Health Global Public Health Initiative. Support for JAC was provided by Institutional Training Grants from the National Institute of Environmental Health Sciences (NIEHS) (T32 ES007062) and the National Human Genome Research Institute (NHGRI) (T32 HG00040). A Rackham Predoctoral Fellowship from the University of Michigan provided support for AEA and JAC. Support for KKF was provided by NIEHS Grants R01 ES021465 and R01 ES018872.
Abbreviations
- NHANES
National Health and Nutrition Examination Survey
- ADS
antioxidant and anti-inflammatory diet score
- CRP
C-reactive protein
- GGT
gamma glutamyl transferase
- ALP
alkaline phosphatase
- OS
oxidative stress
- WBC
white blood cell count
- CDC
Centers for Disease Control and Prevention
- 24-HR
24-hour dietary recall
- kcal
kilocalories
- BMI
body mass index
- IQR
interquartile range
- ROS
reactive oxygen species
Appendix A. Supplementary data
Supplementary data associated with this article can be found in the online version at http://dx.doi.org/10.1016/j.envres.2014.02.003.
References
- Afridi HI, et al. Evaluation of status of toxic metals in biological samples of diabetes mellitus patients. Diabetes Res Clin Pract. 2008;80:280–288. doi: 10.1016/j.diabres.2007.12.021. [DOI] [PubMed] [Google Scholar]
- Barr DB, et al. Urinary creatinine concentrations in the U.S. population: implications for urinary biologic monitoring measurements. Environ Health Perspect. 2005;113:192–200. doi: 10.1289/ehp.7337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calder PC. n-3 Polyunsaturated fatty acids, inflammation, and inflammatory diseases. Am J Clin Nutr. 2006;83:S1505–1519S. doi: 10.1093/ajcn/83.6.1505S. [DOI] [PubMed] [Google Scholar]
- Cavicchia PP, et al. A new dietary inflammatory index predicts interval changes in serum high-sensitivity C-reactive protein. J Nutr. 2009;139:2365–2372. doi: 10.3945/jn.109.114025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung BM, et al. Elevated serum alkaline phosphatase and peripheral arterial disease in the United States National Health and Nutrition Examination Survey 1999–2004. Int J Cardiol. 2009;135:156–161. doi: 10.1016/j.ijcard.2008.03.039. [DOI] [PubMed] [Google Scholar]
- Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong W, et al. Toxic metals stimulate inflammatory cytokines in hepatocytes through oxidative stress mechanisms. Toxicol Appl Pharmacol. 1998;151:359–366. doi: 10.1006/taap.1998.8481. [DOI] [PubMed] [Google Scholar]
- Emdin M, et al. Gamma-glutamyltransferase, atherosclerosis, and cardiovascular disease: triggering oxidative stress within the plaque. Circulation. 2005;112:2078–2080. doi: 10.1161/CIRCULATIONAHA.105.571919. [DOI] [PubMed] [Google Scholar]
- Ferguson KK, et al. Exploration of oxidative stress and inflammatory markers in relation to urinary phthalate metabolites: NHANES 1999–2006. Environ Sci Technol. 2012;46:477–485. doi: 10.1021/es202340b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman DJ, et al. C-reactive protein is an independent predictor of risk for the development of diabetes in the west of Scotland Coronary Prevention Study. Diabetes. 2002;51:1596–1600. doi: 10.2337/diabetes.51.5.1596. [DOI] [PubMed] [Google Scholar]
- Gobe G, Crane D. Mitochondria, reactive oxygen species and cadmium toxicity in the kidney. Toxicol Lett. 2010;198:49–55. doi: 10.1016/j.toxlet.2010.04.013. [DOI] [PubMed] [Google Scholar]
- Gropper SS, et al. Advanced Nutrition and Human Metabolism. Thomson Wadsworth; Belmont, CA: 2005. [Google Scholar]
- Hernandez-Avila M, et al. Dietary calcium supplements to lower blood lead levels in lactating women: a randomized placebo-controlled trial. Epidemiology. 2003;14:206–212. doi: 10.1097/01.EDE.0000038520.66094.34. [DOI] [PubMed] [Google Scholar]
- Hu FB, et al. Prospective study of major dietary patterns and risk of coronary heart disease in men. Am J Clin Nutr. 2000;72:912–921. doi: 10.1093/ajcn/72.4.912. [DOI] [PubMed] [Google Scholar]
- Il’yasova D, Schwartz GG. Cadmium and renal cancer. Toxicol Appl Pharmacol. 2005;207:179–186. doi: 10.1016/j.taap.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Johri N, et al. Heavy metal poisoning: the effects of cadmium on the kidney. Biometals. 2010;23:783–792. doi: 10.1007/s10534-010-9328-y. [DOI] [PubMed] [Google Scholar]
- Joseph P. Mechanisms of cadmium carcinogenesis. Toxicol Appl Pharmacol. 2009;238:272–279. doi: 10.1016/j.taap.2009.01.011. [DOI] [PubMed] [Google Scholar]
- Julin B, et al. Dietary cadmium exposure and risk of postmenopausal breast cancer: a population-based prospective cohort study. Cancer Res. 2012;72:1459–1466. doi: 10.1158/0008-5472.CAN-11-0735. [DOI] [PubMed] [Google Scholar]
- Knoflach M, et al. Non-toxic cadmium concentrations induce vascular inflammation and promote atherosclerosis. Circ J. 2011;75:2491–2495. doi: 10.1253/circj.cj-11-0196. [DOI] [PubMed] [Google Scholar]
- Lee D, et al. Graded associations of blood lead and urinary cadmium concentrations with oxidative-stress-related markers in the US population: results from the third National Health and Nutrition Examination Survey. Environ Health Perspect. 2006;114:350. doi: 10.1289/ehp.8518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DH, et al. Gamma-glutamyltransferase and diabetes—a 4 year follow-up study. Diabetologia. 2003;46:359–364. doi: 10.1007/s00125-003-1036-5. [DOI] [PubMed] [Google Scholar]
- Lee DH, Jacobs DR. Is serum gamma-glutamyltransferase a marker of exposure to various environmental pollutants? Free Radic Res. 2009;43:533–537. doi: 10.1080/10715760902893324. [DOI] [PubMed] [Google Scholar]
- Liao D, et al. Association of criteria pollutants with plasma hemostatic/ inflammatory markers: a population-based study. J Expo Anal Environ Epidemiol. 2005;15:319–328. doi: 10.1038/sj.jea.7500408. [DOI] [PubMed] [Google Scholar]
- Lin YS, et al. Cadmium exposure is associated with elevated blood C-reactive protein and fibrinogen in the US population: the third National Health and Nutrition Examination Survey (NHANES III, 1988–1994) Ann Epidemiol. 2009;19:592–596. doi: 10.1016/j.annepidem.2009.02.005. [DOI] [PubMed] [Google Scholar]
- Liu J, et al. Relationship between biomarkers of cigarette smoke exposure and biomarkers of inflammation, oxidative stress, and platelet activation in adult cigarette smokers. Cancer Epidemiol Biomark Prev. 2011;20:1760–1769. doi: 10.1158/1055-9965.EPI-10-0987. [DOI] [PubMed] [Google Scholar]
- Liu J, et al. Role of oxidative stress in cadmium toxicity and carcinogenesis. Toxicol Appl Pharmacol. 2009;238:209–214. doi: 10.1016/j.taap.2009.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumley T. Analysis of complex survey samples. J Statist Softw. 2004;9:1–19. [Google Scholar]
- Machlin LJ, Bendich A. Free radical tissue damage: protective role of antioxidant nutrients. FASEB J. 1987;1:441–445. [PubMed] [Google Scholar]
- McElroy JA, et al. Cadmium exposure and breast cancer risk. J Natl Cancer Inst. 2006;98:869–873. doi: 10.1093/jnci/djj233. [DOI] [PubMed] [Google Scholar]
- Merali Z, Singhal RL. Diabetogenic effects of chronic oral cadmium adminstration to neonatal rats. Br J Pharmacol. 1980;69:151–157. doi: 10.1111/j.1476-5381.1980.tb10895.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan RM, et al. Interaction of cadmium chloride and gamma irradiation on blood parameters of the young adult rat. Environ Res. 1984;35:362–372. doi: 10.1016/0013-9351(84)90143-9. [DOI] [PubMed] [Google Scholar]
- Mulligan KJ, et al. Feasibility of the direct analysis of urine by inductively coupled argon plasma mass spectrometry for biological monitoring of exposure to metals. J Anal At Spectrom. 1990;5:301–306. [Google Scholar]
- Nakanishi N, et al. Serum -glutamyltransferase and risk of metabolic syndrome and type 2 diabetes in middle-aged Japanese men. Diabetes Care. 2004;27:1427–1432. doi: 10.2337/diacare.27.6.1427. [DOI] [PubMed] [Google Scholar]
- National Center for Health Statistics (NCHS) National Health and Nutrition Examination Survey Data. Centers for Disease Control and Prevention, U.S. Department of Health and Human Services; 2003–2010. [Google Scholar]
- Navas-Acien A, et al. Lead, cadmium, smoking, and increased risk of peripheral arterial disease. Circulation. 2004;109:3196–3201. doi: 10.1161/01.CIR.0000130848.18636.B2. [DOI] [PubMed] [Google Scholar]
- Navas-Acien A, et al. Blood cadmium and lead and chronic kidney disease in US adults: a joint analysis. Am J Epidemiol. 2009;170:1156–1164. doi: 10.1093/aje/kwp248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawrot T, et al. Environmental exposure to cadmium and risk of cancer: a prospective population-based study. Lancet Oncol. 2006;7:119–126. doi: 10.1016/S1470-2045(06)70545-9. [DOI] [PubMed] [Google Scholar]
- Ohnaka K, et al. Inverse associations of serum bilirubin with high sensitivity C-reactive protein, glycated hemoglobin, and prevalence of type 2 diabetes in middle-aged and elderly Japanese men and women. Diabetes Res Clin Pract. 2010;88:103–110. doi: 10.1016/j.diabres.2009.12.022. [DOI] [PubMed] [Google Scholar]
- Patra RC, et al. Oxidative stress in lead and cadmium toxicity and its amelioration. Vet Med Int. 2011;2011:457327. doi: 10.4061/2011/457327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pradhan AD, et al. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. J Am Med Assoc. 2001;286:327–334. doi: 10.1001/jama.286.3.327. [DOI] [PubMed] [Google Scholar]
- R Development Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; Vienna, Austria: 2011. [Google Scholar]
- Schwartz GG, et al. Urinary cadmium, impaired fasting glucose, and diabetes in the NHANES III. Diabetes Care. 2003;26:468–470. doi: 10.2337/diacare.26.2.468. [DOI] [PubMed] [Google Scholar]
- Shaikh ZA, et al. Oxidative stress as a mechanism of chronic cadmium-induced hepatotoxicity and renal toxicity and protection by antioxidants. Toxicol Appl Pharmacol. 1999;154:256–263. doi: 10.1006/taap.1998.8586. [DOI] [PubMed] [Google Scholar]
- Shekeeb Shahab M, et al. Evaluation of oxidant and antioxidant status in term neonates: a plausible protective role of bilirubin. Mol Cell Biochem. 2008;317:51–59. doi: 10.1007/s11010-008-9807-4. [DOI] [PubMed] [Google Scholar]
- Singh U, et al. Vitamin E, oxidative stress, and inflammation. Annu Rev Nutr. 2005;25:151–174. doi: 10.1146/annurev.nutr.24.012003.132446. [DOI] [PubMed] [Google Scholar]
- Staessen J, Lauwerys R. Health effects of environmental exposure to cadmium in a population study. J Hum Hypertens. 1993;7:195–199. [PubMed] [Google Scholar]
- Strasak AM, et al. Association of gamma-glutamyltransferase and risk of cancer incidence in men: a prospective study. Cancer Res. 2008;68:3970–3977. doi: 10.1158/0008-5472.CAN-07-6686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tellez-Plaza M, et al. Cadmium exposure and hypertension in the 1999–2004 National Health and Nutrition Examination Survey (NHANES) Environ Health Perspect. 2008;116:51. doi: 10.1289/ehp.10764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venn A, Britton J. Exposure to secondhand smoke and biomarkers of cardiovascular disease risk in never-smoking adults. Circulation. 2007;115:990–995. doi: 10.1161/CIRCULATIONAHA.106.648469. [DOI] [PubMed] [Google Scholar]
- Wellen KE, Hotamisligil GS. Inflammation, stress, and diabetes. J Clin Invest. 2005;115:1111–1119. doi: 10.1172/JCI25102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willett W. Nutritional Epidemiology. Oxford University Press; New York, New York: 1998. [Google Scholar]
- Wu LL, et al. Urinary 8-OHdG: a marker of oxidative stress to DNA and a risk factor for cancer, atherosclerosis and diabetics. Clin Chim Acta. 2004;339:1–9. doi: 10.1016/j.cccn.2003.09.010. [DOI] [PubMed] [Google Scholar]
- Wymenga LF, et al. Routine bone scans in patients with prostate cancer related to serum prostate-specific antigen and alkaline phosphatase. BJU Int. 2001;88:226–230. doi: 10.1046/j.1464-410x.2001.02275.x. [DOI] [PubMed] [Google Scholar]
- Yang YJ, et al. Bisphenol A exposure is associated with oxidative stress and inflammation in postmenopausal women. Environ Res. 2009;109:797–801. doi: 10.1016/j.envres.2009.04.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.