Abstract
Objective
This study evaluated whether social cognitive theory (SCT) variables, as measured by questionnaire and ecological momentary assessment (EMA), predicted exercise in endometrial cancer survivors.
Methods
One hundred post-treatment endometrial cancer survivors received a 6-month home-based exercise intervention. EMAs were conducted using hand-held computers for 10- to 12-day periods every 2 months. Participants rated morning self-efficacy and positive and negative outcome expectations using the computer, recorded exercise information in real time and at night, and wore accelerometers. At the midpoint of each assessment period participants completed SCT questionnaires. Using linear mixed-effects models, we tested whether morning SCT variables predicted minutes of exercise that day (Question 1) and whether exercise minutes at time point Tj could be predicted by questionnaire measures of SCT variables from time point Tj-1 (Question 2).
Results
Morning self-efficacy significantly predicted that day’s exercise minutes (p<.0001). Morning positive outcome expectations was also associated with exercise minutes (p=0.0003), but the relationship was attenuated when self-efficacy was included in the model (p=0.4032). Morning negative outcome expectations was not associated with exercise minutes. Of the questionnaire measures of SCT variables, only exercise self-efficacy predicted exercise at the next time point (p=0.003).
Conclusions
The consistency of the relationship between self-efficacy and exercise minutes over short (same day) and longer (Tj to Tj-1) time periods provides support for a causal relationship. The strength of the relationship between morning self-efficacy and exercise minutes suggest that real-time interventions that target daily variation in self-efficacy may benefit endometrial cancer survivors’ exercise adherence.
Keywords: Social Cognitive Theory, self-efficacy, exercise, cancer survivors, ecological momentary assessment
Exercise benefits the physical and psychological functioning of cancer survivors. Epidemiologic research indicates that leisure-time physical activity is associated with a lower risk of cancer recurrence and increased survival in breast and colon cancer (Holmes, Chen, Feskanich, Kroenke, & Colditz, 2005; Meyerhardt et al., 2006). Additionally, in the general population exercise is associated with a decreased risk of chronic diseases (Physical Activity Guidelines Advisory Committee, 2008), a benefit that is likely to accrue to cancer survivors as well. A recent meta-analysis of 82 randomized trials of exercise interventions for cancer patients and survivors found positive effects both for patients receiving treatment and post-treatment survivors (Speck, Courneya, Masse, Duval, & Schmitz, 2010). Physical activity, fitness levels, and body composition were positively affected in both groups. In addition, exercise had a positive effect on quality of life and emotional well-being, and can be done safely (Speck, et al., 2010). Survivors of endometrial cancer in particular may benefit from exercise. They are often overweight or obese and physically inactive, because these are risk factors for the disease. Although endometrial cancer has a high cure rate, survivors frequently have comorbidities that affect health and quality of life. Physical activity in this population is associated with better quality of life and physical functioning and with less fatigue and pain (Basen-Engquist et al., 2009; Courneya et al., 2005). Even low levels of activity are associated with better physical functioning compared with complete sedentariness (Basen-Engquist, Scruggs, et al., 2009). Given evidence that exercise is safe and beneficial for cancer survivors (Speck, et al., 2010), the American College of Sports Medicine (ACSM) published exercise recommendations for cancer survivors (Schmitz et al., 2010), concluding that, with a few exceptions, cancer patients and survivors should follow the general population exercise recommendations appropriate to their age group.
Exercise adherence in cancer survivors
In light of the demonstrated benefits of exercise for cancer survivors and the ACSM exercise recommendations for this population, the mechanisms of survivors’ exercise adherence must be explored so that more effective interventions can be developed. Several studies have indicated that physical activity declines after a diagnosis of cancer and does not recover to pre-diagnosis levels (Blanchard et al., 2003; Courneya & Friedenreich, 1999).
Theory-based approaches have been helpful for understanding and intervening on health behaviors in the general population. For example, social cognitive theory (Bandura, 1986, 1997) has been used to predict exercise adherence and as a basis for interventions to increase physical activity. Social cognitive theory (SCT) posits that people acquire skills and perform new behaviors by enacting them, being reinforced for performance, and observing others. Behavior is influenced by these direct and observed experiences through the expectations they create, including expectations about the ability to perform the behavior successfully (self-efficacy) and the consequences of the behavior (outcome expectations). In a broad range of populations, including people with chronic disease, self-efficacy has been found to predict exercise behavior (Garcia & King, 1991; Moore, Dolansky, Ruland, Pashkow, & Blackburn, 2003; Plotnikoff, Brez, & Hotz, 2000; Steptoe, Rink, & Kerry, 2000). In research on the relationship between self-efficacy and structured exercise adherence among sedentary, middle-aged or older adults, McAuley concluded that self-efficacy is most influential on exercise adherence at times when the exerciser faces new challenges, such as when beginning a new exercise program (McAuley, 1992; McAuley, Courneya, Rudolph, & Lox, 1994) or when continuing exercise after a structured program ends (McAuley, 1993). Different types of self-efficacy have been assessed. Task self-efficacy is the confidence in one’s ability to physically perform the exercise. Self-regulatory, coping, or barriers self-efficacy is the confidence to overcome barriers to maintain an exercise program. A prospective study of cancer survivors showed that task self-efficacy was associated with exercise intentions but that “scheduling” (barriers) self-efficacy was associated with actual exercise (Rodgers, Hall, Blanchard, McAuley, & Munroe, 2002).
Outcome expectations are expected to influence exercise adherence as well. The link between outcome expectations and exercise has been studied less frequently than that between self-efficacy and exercise. The literature has been inconsistent in demonstrating a relationship between the two independently of self-efficacy, but the association seems to be more consistent among older adults than younger people (Williams, Anderson, & Winett, 2005). Resnick’s research with 201 older adults living in a continuing care retirement community showed that outcome expectations were correlated with exercise behavior independently of self-efficacy (Resnick, 2001).
Several recent studies have investigated self-efficacy and outcome expectations for exercise in cancer survivor populations, and have shown that self-efficacy, either at baseline or changes over time, is associated with exercise behavior at the end of an exercise intervention (Bennett, Lyons, Winters-Stone, Nail, & Scherer, 2007; Jones, Courneya, Fairey, & Mackey, 2005; Mosher et al., 2008; Pinto, Rabin, & Dunsiger, 2009; Vallance, Courneya, Plotnikoff, & Mackey, 2008). Outcome expectations have been tested less frequently among cancer survivors, and the results have been mixed. Pinto (Pinto, et al., 2009) found no significant associations between decisional balance (pros and cons) at baseline and subsequent exercise behavior in her study of home-based exercise for breast cancer survivors. However, Vallance et al. (Vallance, et al., 2008) showed that beliefs about the outcome of exercise (e.g., living longer, reducing the risk of cancer recurrence) mediated the effect of a home-based exercise intervention on exercise intentions. Although literature on theory-based correlates of exercise in cancer survivors is expanding, it is limited to just a few disease sites, such as the breast.
Research on exercise has relied on retrospective questionnaires to report SCT determinants. Such measurements are affected by recall biases that may distort data and study conclusions and miss important day-to-day dynamics in expectancy-behavior relationships (Shiffman, 2000). In contrast, the ecological momentary assessment (EMA) captures data about a respondent’s current state or behavior in real time. It involves real-time assessment of phenomena in a person’s natural environment, allowing the investigation of topics such as variability of cognitions, mood states, or behaviors over time; cyclical patterns such as diurnal or weekly patterns; and covariation of expectations or affect and behavior (Stone, Broderick, Porter, & Kaell, 1997). EMA has rarely been used to study the determinants of exercise and physical activity in adults. One study of physical activity of older adults (Dunton, Atienza, Castro, & King, 2009) used EMA to measure cognitive, social, affective, and contextual variables as well as physical activity. The results showed that self-efficacy and perceived situational control and having a positive social interaction were correlated with minutes of physical activity.
Current Study
The goal of our study was to elucidate the relationships of self-efficacy and outcome expectations to exercise behavior over time in endometrial cancer survivors receiving an exercise intervention using two approaches. First, we evaluated whether the SCT variables of self-efficacy, positive outcome expectations, and negative outcome expectations, measured each morning using EMA, predicted the duration of daily exercise. Second, we analyzed whether exercise self-efficacy, barriers self-efficacy, positive outcome expectations, and negative outcome expectations, measured by questionnaires administered in person 4 times in 6 months, predicted exercise at later time points. We hypothesized that, self-efficacy and outcome expectations would be associated with exercise behavior in both sets of analyses. We further hypothesized that the association between self-efficacy and exercise would be stronger at the beginning of the intervention than at the end. Finally, we explored the joint relationships of self-efficacy and outcome expectations, investigating whether (1) both variables had independent effects on self-efficacy; (2) self-efficacy mediated the relationship between outcome expectations and exercise, comprising a single pathway to exercise behavior; or (3) self-efficacy moderated the relationship between outcome expectations and exercise behavior, such that outcome expectations were related to behavior only when self-efficacy was high.
METHODS
Design and Participants
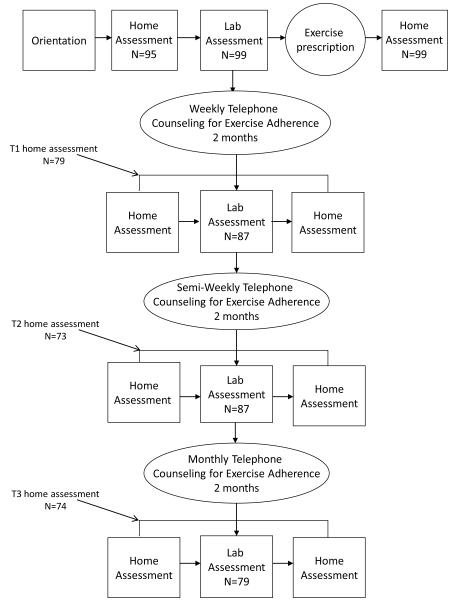
The STH study was a 6-month longitudinal study to assess predictors of exercise behavior in endometrial cancer survivors who received an exercise intervention (see figure 1). Participants completed assessments at baseline, 2 months, 4 months, and 6 months. Participants were 100 women who had been diagnosed with Stage I, II, or IIIa endometrial cancer and were at least 6 months post-treatment with no evidence of disease. Participants were excluded from the study if they met the public health recommendations for physical activity (moderate or greater intensity on at least 5 days per week for 30 min or more, or vigorous intensity activity for 20 min or more on at least 3 days per week) (American College of Sports Medicine, 1998) and had maintained that level of activity for 6 months or longer. Each patient’s physician signed a release form indicating that the survivor was sufficiently healthy to participate in a home-based exercise program and that she had none of the absolute contraindications for exercise testing as defined by the ACSM (American College of Sports Medicine, 2006).
Figure 1.
Design of the Steps to Health (STH) study.
Participants were recruited from the Gynecologic Oncology Center and satellite clinics at The University of Texas MD Anderson Cancer Center (UTMDACC) and at a private gynecologic oncology practice in Houston, TX. At the UTMDACC sites, potentially eligible survivors were identified using medical records and approached at an appointment or contacted by mail and telephone. At the private clinic, a potential study participant was first approached by the health care provider, and if interested the recruitment coordinator would discuss the study with her. The UTMDACC institutional review board approved study procedures. Participants were recruited between January, 2007 and September, 2010.
Procedure
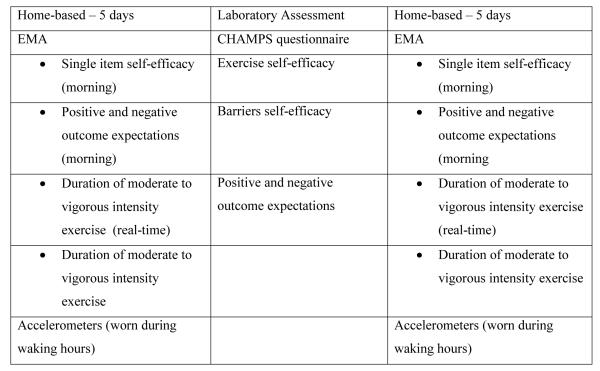
Endometrial cancer survivors who consented to this study completed 7 days of home-based assessment (EMA and assessment of physical activity by accelerometer), then completed a laboratory assessment of cardiorespiratory fitness and exercise-related questionnaires, followed by 5 more days of home-based assessment (see figure 2). This assessment routine was repeated at 2, 4, and 6 months except that the home-based assessment was done for only 5 days before and after the laboratory assessment. Participants received compensation worth $40 for each laboratory assessment completed. The compensation for the EMA in each home assessment period was prorated based on compliance level and ranged from $5-$30.
Figure 2.
Description of measures in each assessment modality.
Home-based assessment
Participants used a handheld computer (Hewlett-Packard iPAQ RX1950) to complete the EMA. Each morning, when the participant awoke, the computer prompted a response to items assessing SCT variables, and in the evening it prompted a response to questions about exercise performed throughout the day. Participants also were instructed to answer questions after each exercise session to record minutes of exercise completed. Random assessments about mood and physical symptoms were conducted 3 times during the day, but that data is not included in this analysis. To capture objective data on physical activity, participants wore a GT1M accelerometer (Actigraph L.L.C., Pensacola, FL), which collected activity count data in 60-second epochs. It was worn on a belt at the waist during waking hours except when participants were in the water (e.g., showering or swimming).
Laboratory assessment
The laboratory assessment consisted of a 2- to 3-hour session held at UTMDACC. Participants completed questionnaires related to quality of life, exercise, and SCT variables and performed implicit cognition tasks related to the SCT model. The participants then completed a submaximal cardiorespiratory fitness test on a cycle ergometer. After the fitness test, the SCT questionnaires and implicit tasks were repeated. In this paper we report only the results of analyses related to the questionnaire measures of SCT variables and exercise.
Measures
Self-efficacy
The hand-held computer was used to record participants’ daily self-efficacy. Each morning the computer prompted them to rate the level of confidence in exercising that day (“How confident are you that you will exercise today for the recommended amount of time?”). The responses ranged from 1 (not at all confident) to 5 (extremely confident). To measure exercise self-efficacy in the laboratory assessment, a questionnaire was developed assessing survivors’ confidence in completing a graded series of exercise tasks (McAuley et al., 1999). Using the item stem “How confident I am that I can…” items started at low levels (e.g., “…walk briskly for 2 minutes without stopping”) and finished at more difficult levels (“…walk briskly for one hour without stopping”). For each item the scale ranged from 1 to 5, with a higher score indicating greater confidence. The total scale had 18 items, and the internal consistency reliability was 0.96 at baseline. The questionnaire was administered before and after the cardiorespiratory fitness test; because the two scores were highly correlated (r=.90-.96, depending on assessment time point), we used the mean of the two scores for exercise self-efficacy.
Barriers self-efficacy measured a participant’s confidence in continuing exercise in the face of common barriers. For example, items used the stem “How confident I am that I can exercise…” with items like “…when I am tired” and “when I am travelling.” We developed this questionnaire using a scale by Marcus et al. (Marcus, Selby, Niaura, & Rossi, 1992) and adding items based on elicitation interviews with breast cancer survivors from a previous study (Basen-Engquist, Baum, Hughes, Scruggs, & Carmack, 2009). The total scale had 14 items and an internal consistency reliability of 0.92 at baseline. For both self-efficacy questionnaires, the score was the mean of the items, yielding a score that ranged from 1 to 5, with a higher score indicating greater self-efficacy.
Outcome expectations
The handheld computer was also used to assess daily outcome expectations, comprised of a of 7 positive items and 3 negative items that were re-worded from the scale described below to reflect expected outcomes of exercising that day (e.g., “I will sleep more soundly tonight if I exercise today” and “Exercising today will be painful”). The internal consistency reliabilities of the positive and negative scales were 0.93 and 0.62, respectively.
Participants completed a questionnaire measuring positive (15 items) and negative (10 items) outcome expectations. Examples of outcome expectation items include “I would sleep more soundly if I exercised regularly” and “Regular exercise would increase my pain.” The scales had internal consistency reliabilities of 0.93 (positive) and 0.83 (negative). This questionnaire was administered before and after the fitness test; because the two scores were highly correlated (r=0.90-0.94 for positive outcome expectations; r=0.87-0.90 for negative outcome expectations), we used the mean of the pre- and post-fitness test scores. For all outcome expectations scales, the score was calculated as the mean of the items, so the scale range was 1 to 5; a higher score indicating greater positive or negative outcome expectations.
Exercise duration
Exercise duration was measured using a combination of methods: EMA questions answered at the time of exercise (real-time exercise minutes), EMA questions answered at the end of the day about exercise completed that day (night-time exercise diary minutes), and minutes of moderate or greater activity performed in bouts of at least 10 minutes as measured with the accelerometer. Because on some days participants were missing certain assessments, we used a combination of the data available from these three methods for our final measure of minutes of exercise. If real-time minutes had not been reported (perhaps because the participant forgot to enter the information), we used the minutes of exercise reported in the night-time diary. If both real-time minutes and night-time minutes were missing, we used the minutes recorded by the accelerometer. Because accelerometers would not register certain exercises (e.g., biking, circuit training), the real-time exercise minutes were considered first. The 3 methods had moderate degrees of intercorrelation for days on which participants had both night-time minutes and accelerometer data (real-time and night-time diary: r=0.57; real-time and accelerometer: r=0.50; night-time diary and accelerometer: r=0.48; p-value <.0001 for each association). Correlations with the combined minutes variables were: real-time minutes, 0.77; night-time diary, 0.62; accelerometer minutes, 0.53 (p<.0001 for all associations). The correlations compare favorably with other studies comparing different types of PA measures (Ainsworth BE, 2000)
Across all participants and assessment periods, there was a total of 3,720 possible days for which data on exercise could be obtained. On 30 to 180 days (depending on the assessment) technical difficulties with the EMA device prevented downloading of data. Real-time exercise minutes were available on 1,453 days (these assessments were only done on days the participant exercised). Night-time exercise diary minutes and accelerometer data were available for 56% and 81% of the EMA days, respectively. Using all three data sources allowed us to calculate exercise minutes for 91% of the potential EMA days.
We also measured current physical activity by using the Community Health Activities Model Program for Seniors (CHAMPS) questionnaire (Stewart et al., 2001), which assesses weekly frequency and duration of physical activities commonly performed by older adults. In previous work the CHAMPS questionnaire had good stability over 6 months (0.58-0.67) and 2-week periods (0.72-0.81 for kcal/week from moderate activities), and was sensitive to change among people receiving an intervention. This questionnaire was administered at each laboratory assessment; the baseline measure was used as a covariate in model 2 for question 1 (see below).
Intervention
At the end of the baseline laboratory assessment, each participant received an exercise recommendation tailored to her fitness level based on ACSM guidelines (American College of Sports Medicine, 2006), provided by a masters-level exercise physiologist. The ultimate goal was for the survivor to work up to moderate-intensity exercise for at least 30 min a day on 5 or more days per week. For the majority of the participants the exercise recommended was moderate-intensity walking, but for participants who had difficulty walking another exercise was recommended.
To encourage participants to adhere to the exercise recommendation we provided telephone counseling, print materials, and a pedometer. Telephone counseling consisted of 20- to 30-minute telephone calls provided weekly in months 1 and 2, twice a month in months 3 and 4, and once a month in months 5 and 6. The calls covered participant progress on exercise goals, exercise barriers, health problems interfering with exercise, and brief teaching of a cognitive or behavioral skill to support the exercise behavior change (e.g., identifying benefits, problem-solving barriers, goal setting, rewards, finding social support, time management). Before each counseling session a newsletter was mailed with content covering the cognitive or behavioral technique that would be taught in the session. The newsletters also included “role model stories,” which consisted of narratives describing other endometrial cancer survivors who had adopted exercise programs. Participants also periodically received items such as pens, refrigerator magnets, and water bottles with the program logo.
Analysis
We used two approaches to examining the hypothesis that self-efficacy and outcome expectations would predict exercise in endometrial cancer survivors. First, we tested whether daily self-efficacy, positive outcome expectations, and negative outcome expectations measured in the morning with the EMA predicted the amount of exercise that was performed that day (Question 1). Second, we tested whether exercise self-efficacy, barriers self-efficacy, positive outcome expectations, and negative outcome expectations measured with the questionnaire at time point Tj-1 predicted exercise minutes measured at time point Tj (Question 2). Both sets of analyses were conducted using linear mixed-effects models, which account for the correlation among repeated measurements within subjects over time (Brown & Prescott, 1999; Verbeke & Molenberghs, 2000). We used the Wald statistic to test for the significance of the coefficient of each independent variable, without adjusting for multiple testing. Selection of the random effects (e.g., intercepts and/or slopes) and the repeated measures correlation structure in the residuals (e.g., autoregressive AR(1)) was made based on the Bayesian information criterion, with a small criterion being preferred. For both questions we also tested for significant interactions with assessment time point to evaluate whether associations changed over time.
Question 1
For this analysis we looked at daily self-efficacy, daily positive outcome expectations, and daily negative outcome expectations measured in the morning using EMA. The purpose of this analysis was to determine whether there was an association between these SCT variables and the outcome variable (exercise duration) and on a within-day basis. We ran 5 models for each SCT variable, adding a potential confounding variable to the model each time. Model 1 included the SCT variable and the covariates of assessment time point, body mass index (BMI), and weekend vs. weekday (binary). Time point and weekend vs weekday were included because they were expected to be related to exercise. BMI and education were included because an analysis comparing participants who had complete data at all four time points with those who did not demonstrated that subjects with higher BMI and less education were less likely to have complete data. To control for baseline differences in physical activity model 2 added the weekly frequency of moderate or greater intensity activity from the CHAMPS questionnaire at baseline. To identify relationships between daily SCT variables and exercise above and beyond the effects more stable individual differences in SCT variables, model 3 added questionnaire measures of the SCT variables (exercise or barriers self-efficacy, or positive or negative outcome expectations) at each time point. We also wanted to determine if there was an effect of daily SCT variables on exercise minutes independent of their usual exercise level and typical level of the SCT variables during a given EMA time period. Thus model 4 added each individual’s mean daily minutes of exercise for the assessment time point and model 5 added the individual’s mean value of the SCT variable from the EMA for the assessment time point. The goal of testing multiple models was to determine whether the SCT variable measured in the morning was a significant predictor of minutes exercised that day, above and beyond baseline level of exercise, questionnaire measures of the SCT variables, and usual level of exercise and SCT variables during the assessment period. By controlling for all these variables we could determine whether the daily variation in SCT variables has important implications for exercise behavior.
Question 2
This analysis would determine whether the SCT variables measured by questionnaire at the laboratory sessions predicted exercise minutes for the following assessment time point throughout the course of the study. The models included the questionnaire measures of the SCT variables as the independent variables, and the exercise duration for the time point following the questionnaire as the outcome variable. Each model controlled for the baseline exercise time (i.e., minutes recorded for the week prior to the first lab assessment), assessment time point, and BMI and education. Baseline exercise minutes and assessment time point were included as covariates because they were expected to be related to exercise minutes at follow-up time points. BMI and education were included as covariates because of the differences between subjects who did and did not have complete data at all time points.
RESULTS
A total of 643 survivors were identified as potentially eligible for the study. Of these, 39 were ineligible upon additional screening and 270 were incompletely screened (i.e., did not respond to letters and phone calls, and did not have appointments during the recruitment period). Of the remaining 334 women, 192 were not interested in the study and 42 were initially interested but did not complete either the consent process or the baseline assessment. Of the 100 who completed baseline assessments, one dropped out immediately after the laboratory baseline assessment and was dropped from all analyses. The number completing home and laboratory assessments at each time point is provided in figure 1. The question 1 analysis included all participants who provided EMA data for at least one time point (n=97) and the question 2 analysis included all participants who had baseline lab assessments plus home assessment data at one additional time point (n=86). Most of the participants were non-Hispanic white, had at least some college education, had been diagnosed with Stage I endometrial cancer, and were treated with surgery only (Table 1). At baseline, the mean age, time since diagnosis, and BMI were 57.0 years, 26.0 months, and 34.2 kg/m2, respectively.
Table 1.
Characteristics of endometrial cancer survivor cohort (N=100)
| Characteristic | N (%) | Mean (SD) | Range | |
|---|---|---|---|---|
| Race | Black/non-Hispanic | 7 (7) | ||
| White/non-Hispanic | 74 (75) | |||
| Asian/non-Hispanic | 5 (5) | |||
| White/Hispanic | 12 (12) | |||
| American Indian/non-Hispanic | 1 (1) | |||
| Education | < High school | 2 (2) | ||
| High school diploma/GED | 13 (13) | |||
| Technical/vocational degree | 8 (8) | |||
| Some college/Two-year degree | 35 (35) | |||
| Four-year degree | 24 (24) | |||
| Advanced degree | 17 (17) | |||
| Disease stage | I | 79 (80%) | ||
| II | 16 (16%) | |||
| IIIa | 4 (4) | |||
| Treatment | Surgery only | 57 (58%) | ||
| Radiotherapy only | 0 (0%) | |||
| Surgery + Radiotherapy | 41 (42) | |||
| Age, years | 57.0 (11.01) | 25-76 | ||
| Time since diagnosis, months | 26.0 (14.3) | 3.7 – 63.8 | ||
| Body mass index, kg/m2 | 34.2 (9.4) | 18.7-69.3 |
Exercise behavior and SCT variables: Descriptive statistics
During the baseline home assessment period before the baseline laboratory assessment (7 days), participants reported a mean (± standard deviation) of 14.5 ± 18.5 minutes of exercise per day over a total of 680 days. Ninety-five participants provided the data. On 46% of these days no exercise was reported, and on 20% of the days at least 30 min of exercise was reported. Eleven percent of participants reported no exercise during their pre-laboratory baseline assessment period. After the baseline lab assessment and providing the exercise recommendation, another home assessment (5 days) was implemented. During this period participants exercised an average of 18.5 ± 19.5 minutes per day over a total of 504 days. At the 2-, 4-, and 6-month assessments the average minutes of exercise were 19.3 ± 20.5, 18.4 ± 20.8, and 17.6 ± 20.7 over 777, 757, and 744 days, respectively.
The SCT variables ranged from 1 to 5. For the EMA measures, the means and standard deviations at baseline were 2.9 ± 0.92 for daily self-efficacy, 3.5 ± 0.77 for daily positive outcome expectations, and 1.9 ± 0.69 for daily negative outcome expectations. For the questionnaire measures, the baseline values were 3.2 ± 0.9 for exercise self-efficacy, 2.5 ± 0.71 for barriers self-efficacy, 3.8 ± 0.7 for positive outcome expectations, and 1.7 ± 0.5 for negative outcome expectations.
Question 1: Effects of Daily Self-Efficacy and Daily Outcome Expectations on Exercise Duration
Out first goal was to determine whether daily variation in SCT variables affected the likelihood of exercise on a given day. For this analysis we used the morning assessment data from the EMA, when daily self-efficacy and daily positive and negative outcome expectations were measured, and tested the association with exercise duration that day. Daily self-efficacy significantly predicted exercise minutes in all 5 models (Table 2); model 5 indicated that each point increase in self-efficacy was associated with an increase of approximately 6 minutes of exercise (estimate = 5.98, standard error of the estimate [SEE]=0.41, F(1, 2254)=215.82, p <.0001). Mean exercise minutes and mean daily self-efficacy for the assessment time point also predicted exercise minutes. Baseline physical activity (CHAMPS), barriers self-efficacy, and exercise self-efficacy measured at the laboratory assessment were not significantly associated with exercise minutes.
Table 2.
Models predicting minutes of exercise from morning assessments of three SCT variables
| Model | Self-efficacy | Positive outcome expectations |
Negative outcome expectations |
|---|---|---|---|
| 1: SCT variables + covariates | 6.31 (0.36)*** | 4.14 (0.76)*** | −0.82 (0.77) |
| 2: Model 1 + Baseline exercise frequency |
6.30 (0.36)*** | 3.78(0.74)*** | −1.12 (0.76) |
| 3: Model 2 + SCT variable measured in laboratory assessment |
6.02 (0.38)*** | 3.92 (0.92)*** | −1.01 (0.85) |
| 4: Model 3 + Mean daily minutes of exercise for assessment time point |
3.58 (0.30)*** | 2.10 (0.72)* | −0.70 (0.68) |
| 5: Model 4 + Mean SCT variable measure for assessment time point |
5.98 (0.41)*** | 3.93(1.09)** | −0.92 (0.92) |
Note: Entries in table are coefficients and standard errors of the estimate for each SCT variable. Models were run separately for each SCT variable. Covariates were assessment time point, BMI, education; and weekend vs. weekday.
p<.01;
p<.001;
p<.0001
Daily positive outcome expectations was a significant predictor of exercise minutes in all 5 models (Table 2), including the final model (estimate = 3.93, SEE= 1.09, F(1, 2263)=13.12, p= 0.0003). In model 5, mean exercise minutes for the time point, mean daily positive outcome expectations, and assessment on a weekday also were significantly associated with the exercise minutes; baseline activity and positive outcome expectations measured during the laboratory assessment were not. Daily negative outcome expectations was not significantly associated with exercise minutes in any of the models tested (Model 5 estimate = −0.92, SEE= 0.92, F(1, 2263)= 1.00, p= 0.3179) (Table 2). In model 5, the only variables significantly associated with exercise minutes were mean exercise minutes for the time point and assessment on a weekday.
Model 5 for daily self-efficacy and daily positive outcome expectations was run adding their interaction with the assessment time point. For daily self-efficacy, the interaction term approached significance (F(4, 2250)=2.03, p=.0879), with the results indicating that daily self-efficacy had a slightly stronger association with exercise minutes at the later assessment points. The interaction term in the daily positive outcome expectations model was not significant (F(4, 2259)=0.14, p=.968.
Because there was a significant association between daily self-efficacy and daily positive outcome expectations (F (1,2313)=399.86, p<0.0001), we also entered these two variables in the same model (model 5). In this analysis, daily self-efficacy continued to be significant (estimate = 6.09, SEE=0.43, F(1, 2251)=203.46; p<0.0001), whereas daily positive outcome expectations was no longer significant (estimate = −0.92, SEE = 1.09, F(1, 2251)=0.71, p=0.3989). To determine if the relationship between daily outcome expectations and exercise minutes was conditional on having high self-efficacy, we also tested a model which included a self-efficacy x positive outcome expectations interaction. The interaction was not significant (estimate=5.38, SEE=4.50, F(1, 2250)=0.22, p=.636.
To evaluate whether the exercise measurement modality affected the results, we conducted the analysis of model 5 with both daily self-efficacy and daily positive outcome expectations by using accelerometer data only to measure exercise duration. The effects were essentially the same. Daily self-efficacy was significantly associated with accelerometer-measured minutes of moderate or greater intensity activity performed in bouts of 10 min or more (estimate = 3.08, SEE=0.40, F(1, 1998)=60.20, p<0.0001). Daily positive outcome expectations was not significant in the model (estimate = −0.18, SEE=1.02, F(1,1998)=0.03, p<0.8603).
We further evaluated the temporality of the relationship between daily self-efficacy or daily positive outcome expectations and exercise minutes. First, we tested whether self-efficacy from the day before predicted exercise minutes and found that it did not (estimate = −0.62, SEE=0.45; F(1, 1822)=1.92, p=0.1656). We also tested whether the same-day self-efficacy was associated with exercise duration when the previous day’s self-efficacy was included in the model; we found that it was (estimate = 6.25, SEE=0.43, F(1, 1821)=173.13, p<0.0001).
Question 2: Prediction of Exercise Minutes by SCT variables Measured at Previous Time Point
We tested whether the SCT theory variables of exercise self-efficacy, barriers self-efficacy, positive outcome expectations, and negative outcome expectations as measured by questionnaires at baseline, 2-month, and 4-month lab sessions were associated with the mean exercise duration per day at subsequent sessions (2, 4, or 6 months), controlling for baseline exercise minutes. The variables were first tested individually in models that included baseline exercise minutes, time point, BMI, education and the SCT variable. Then we tested a model that included baseline exercise minutes, assessment time point, BMI, education and all 4 SCT variables (Table 3). Eighty six participants were included in this analysis. Exercise self-efficacy was the only variable that significantly predicted exercise minutes at the next time point in both the single-SCT variable models (estimate = 2.67, SEE=0.82, F[1, 129]=10.50, p=0.0015) and the 4-SCT variable model (estimate = 2.88, SEE=1.34, F[1, 124]=7.56, p=.0069). Positive outcome expectations neared significance in the single-SCT variable model (estimate = 2.00, SEE=1.18, F[1, 127]=2.89, p=.0914), but was not significant in the 4-SCT model (estimate = 0.78, SEE=1.14, F[1, 127]=0.31, p=.0.581). When the exercise self-efficacy x positive outcome expectations and barriers self-efficacy x positive outcome expectations interactions were added to the model, they were not significant (estimate = 1.04, SEE=1.40, F(1, 122)=0.55, p=0.4589; estimate = −2.58, SEE=1.77, F(1, 122)=2.13, p=0.1472). When SCT variables x time point interactions were added to the full model, the interaction between exercise self-efficacy and time point was significant (F[2, 117]= 5.15, p=0.0072); coefficients indicated that the association between T1 exercise self-efficacy and T2 exercise minutes was stronger than at other time points (estimate =3.43, SEE=2.04, p=0.096). Analyses repeated using minutes of exercise as measured with an accelerometer yielded a similar pattern of outcomes, except that the exercise self-efficacy by time point interaction was not significant.
Table 3.
Association between questionnaire measures of SCT variables and exercise minutes at the following time point: multivariate model.
| Variable | F value | P |
|---|---|---|
| Continuous variables | ||
| Baseline exercise minutes | 23.67 | <.0001 |
| Exercise self-efficacy | 7.56 | 0.0069 |
| Positive out come expectations | 0.31 | 0.581 |
| Negative outcome expectations | 0.04 | 0.8357 |
| Barriers self-efficacy | 1.23 | 0.2705 |
| BMI | 2.12 | 0.1496 |
| Categorical variables | ||
| Time Point | 0.99 | 0.3744 |
| Education | 1.75 | 0.4199 |
| Time point × Exercise self-efficacy | 5.15 | 0.0072 |
| Time Point × Barriers to self-efficacy | 1.94 | 0.1487 |
| Time point × Positive outcome expectations | 0.66 | 0.5171 |
| Time point × Negative outcome expectations | 1.84 | 0.1629 |
SEE = standard error of the estimate.
DISCUSSION
In this 6-month study of the mechanisms of exercise adoption by endometrial cancer survivors, we examined SCT predictors of exercise behavior using 2 approaches: examining within-day prediction of daily exercise behavior by SCT variables measured each morning, and testing whether SCT variables measured by questionnaire at earlier time points predicted exercise at later time points. Results from both approaches indicated that self-efficacy predicted increases in exercise. The within-day analysis also found a relationship between daily positive outcome expectations and behavior, but this relationship appeared to be accounted for by daily self-efficacy. Daily negative outcome expectations was not significantly associated with exercise in either analysis.
Participants performed more exercise on days when their daily self-efficacy and positive outcome expectations were higher. However, in models that included both daily self-efficacy and positive outcome expectations, neither daily positive outcome expectations nor the interaction with daily self-efficacy were significant, indicating that the effect of positive outcome expectations was mediated by self-efficacy, rather than both variables having direct independent effects, or positive outcome expectations having an effect on exercise that was conditional on self-efficacy. In the question 2 analysis (SCT variables from earlier time points predicting exercise behavior at the next time point), there was no significant relationship between either negative or positive outcome expectations and exercise behavior, so we could not evaluate the joint relationship between self-efficacy and outcome expectations.
Our EMA measure of daily self-efficacy was a single item (“How confident are you that you will exercise today?”). This measure likely incorporated both participants’ confidence in their ability to physically do the exercise as well as whether they could implement the exercise given the barriers they expected to encounter during the day. The relationship between daily self-efficacy and exercise persisted even after adjustment for physical activity level at study baseline, self-efficacy measures completed at the laboratory sessions, average level of daily self-efficacy over the 10-day assessment period, and average level of exercise over the same period. This persistence indicates that the daily variability in self-efficacy is an important determinant of exercise behavior, and it suggests that real-time interventions to increase self-efficacy could increase exercise behavior. To evaluate the specificity of the temporal relations we also explored adding self-efficacy from the previous day to the model. We found that this variable was not a significant predictor of the next day’s physical activity; however self-efficacy on the day of exercise remained significant even when the previous day’s measure was included in the model. The temporal relationship between self-efficacy and exercise is similar to that noted in an EMA study of predictors of exercise among older adults, which found that self-efficacy in the previous assessment period predicted moderate to vigorous intensity physical activity (Dunton, et al., 2009).
SCT posits that relationships between behavior and expectancies, such as self-efficacy and outcome expectations, can be bi-directional. Cross-sectional analyses of the relationship between cognitions and behavior do not allow us to distinguish the directionality of the relationship. However, when the goal is to change behavior, the focus is on changing the cognitions as a mechanism for influencing behavior. This approach assumes that the path of causation is stronger from cognitions to behavior than from behavior to cognitions. Because in our study self-efficacy measured each day was associated with minutes of exercise, above and beyond the effect of usual levels of self-efficacy and exercise, a stronger argument can be made for the idea that self-efficacy has a causal relationship to exercise behavior and thus is an appropriate target for interventions to increase exercise behavior. Furthermore, our results showing a significant within-day relationship provide support for intervening in real time to increase exercise by targeting low self-efficacy at the time it is reported. Such “ecological momentary intervention” is now increasingly feasible given emerging smart phone technologies. Our results suggest that an intervention that delivers messages to increase self-efficacy expectations in real time could increase exercise behavior among endometrial cancer survivors. Patrick and colleagues used text messaging interventions for weight management among overweight adults (Patrick et al., 2009). This approach could be tested with cancer survivor populations as well to increase exercise adherence.
In analyses predicting exercise behavior across time points, exercise self-efficacy (the individual’s confidence that she would succeed in doing exercise of varying durations) was associated with increasing exercise minutes. It was significantly associated with minutes of exercise at the next time point in the single-SCT variable model as well as the model that included all four SCT variables. However, barriers self-efficacy (the individual’s expectation that she could continue her exercise even in the face of barriers, such as bad weather, fatigue, and travel), was not significantly associated with exercise minutes. Cross-sectional studies, including some with cancer survivor cohorts, have shown positive associations between both types of self-efficacy and exercise (Anderson, Wojcik, Winett, & Williams, 2006; Rogers, McAuley, Courneya, & Verhulst, 2008). However, longitudinal studies, or studies testing self-efficacy as a mediator of intervention effects, have been more mixed. Several studies using varied populations found that barriers self-efficacy or similar constructs predicted future exercise behavior (Anderson, et al., 2006; Rogers, et al., 2008). For cancer survivors, two studies (Bennett, et al., 2007; Pinto, et al., 2009) looking at predictors of changes in exercise in response to interventions showed that high barriers self-efficacy at baseline was associated with greater increases in exercise. However, intervention studies with community samples (Baruth et al., 2010; Napolitano et al., 2008) found that barriers self-efficacy did not mediate the effects of an intervention on exercise behavior. An intervention study with cancer survivors (C.E. Mosher et al., 2008) arrived at the same conclusion, although in their study change in self-efficacy was associated with increases in exercise. A possible explanation for the lack of an association with barriers self-efficacy despite a positive exercise self-efficacy-exercise association, is that endometrial cancer survivors, who have had a serious health threat and are also likely to have comorbidities (Mols, Coebergh, & van de Poll-Franse, 2007), are less confident in their ability to perform the exercise, and thus self-efficacy for being physically able to exercise is more salient than self-efficacy for managing barriers.
In our study the effect of daily self-efficacy was fairly consistent over time, but in the longitudinal analysis self-efficacy at the 2-month time point was the most influential on later exercise. This result conflicts somewhat with previous findings showing that self-efficacy is more important as exercise is first being adopted (McAuley, 1992; McAuley, et al., 1994). However, we followed participants for only 6 months, which may not be sufficient time to develop an exercise habit. Indeed, research based on the transtheoretical model indicates that individuals rarely enter a maintenance stage until a behavior has been practiced regularly for 6 months (Marcus, et al., 1992). If we had followed participants longer, we might have found that self-efficacy’s importance diminished over time.
Relationships between SCT variables and exercise appeared more robust in the EMA analysis than the time point to time point analyses. This difference could be due in part to EMA’s ability to better capture the dynamic nature of self-efficacy. Research by McAuley et al. (McAuley et al., 2011) indicated that dramatic changes in self-efficacy may occur in the first 3 weeks of an exercise program, suggesting that self-efficacy is a dynamic variable that may have a profound effect on behavior in the short term.
This study indicated that there were no significant associations between outcome expectations (positive or negative) and exercise measured at the next time point. However, in the within-day analysis, daily positive outcome expectations was associated with exercise duration. A survivor’s moment-to-moment appraisal of how she will benefit from exercise may influence decision-making more than a generalized expectation. A review by Williams (Williams, et al., 2005) indicated that expectations about proximal outcomes may be more influential than the distal ones; thus, measuring expectations about the outcomes that may accrue from exercise on that day might carry greater weight than more general expectations.
One limitation of the study is that the sample size for analyzing Question 2 was rather small, in part due to missing data from at least one post-baseline time point in 41.4% of the participants, which reduced the power for finding associations and could have introduced a selection bias. We found that participants who had at least one missing time point differed in BMI and education (p<0.10), and we controlled for these variables in the question 2 analysis. With regard to the EMA results, a third variable could explain the strong relationship between daily self-efficacy and exercise minutes. For example, if a participant had a regular schedule for exercise, she might rate her daily self-efficacy high on the mornings of days that exercise was scheduled and low on days that it was not. As long as she does indeed exercise on the days scheduled, a relationship between daily self-efficacy and exercise might be more due to habit and scheduling than self-efficacy. However, we do not think this is a primary explanation for the self-efficacy/exercise relationship for two reasons. First, all the participants were inactive or had exercise levels below public health recommendations at the start of the study, and the overall increases in activity were not large. Second, if this were the explanation for the daily self-efficacy/exercise relationship, we would expect to see this relationship when the outcome was dichotomized as exercise/no exercise, but not in predicting duration of exercise on days when it was done. However, when we conducted analyses in this way, we found that daily self-efficacy predicted both whether or not exercise was done on that day (estimate=0.92, SE=0.08, p<0.0001), and, using only the days on which exercise was done, the duration of exercise (estimate=2.86, SE=0.52, p<0.0001). These results strengthen our confidence that an individual’s morning self-efficacy plays a causal role in exercise behavior for the day. However, we need to be cautious in making statements about causation, because we did not experimentally manipulate self-efficacy and look at the effect on self-efficacy. Despite this we feel that the observation of these variables in a naturalistic context makes a unique contribution to the literature by giving a sense of how the self-efficacy-behavior relationship operates outside the laboratory.
This study makes a unique contribution to the literature in that it uses multiple methods to measure and evaluate the predictive relationship between SCT variables and exercise. The consistency between the self-efficacy/exercise relationship in both the questionnaire and EMA results provides stronger support for the idea of a causal relationship than cross-sectional studies could. The strength of the results for both self-efficacy and positive outcome expectations in the within-person analysis suggests future directions for exercise interventions in this population. In particular, they highlight the promise of tailored interventions to improve self-efficacy and increase exercise behavior delivered in real time.
Acknowledgements
This research was supported by NIH grants R01CA109919, R25TCA057730, R25ECA056452, and P30 CA016672 (PROSPR Shared Resource).
BIBLIOGRAPHY
- American College of Sports Medicine Position stand. Exercise and physical activity for older adults. Medical and Science in Sports and Exercise. 1998;30(6):992–1008. [PubMed] [Google Scholar]
- American College of Sports Medicine . Guidelines for Exercise Testing and Prescription. 7th ed Lippincott Williams & Wilkins; Philadelphia, PA: 2006b. [Google Scholar]
- Anderson ES, Wojcik JR, Winett RA, Williams DM. Social-cognitive determinants of physical activity: the influence of social support, self-efficacy, outcome expectations, and self-regulation among participants in a church-based health promotion study. Health Psychology. 2006;25(4):510–520. doi: 10.1037/0278-6133.25.4.510. [DOI] [PubMed] [Google Scholar]
- Bandura A. Social Foundations of Thought and Action: A Social-Cognitive Theory. Prentice-Hall; Englewood Cliffs, NJ: 1986. [Google Scholar]
- Bandura A. Self-Efficacy: The Exercise of Control. W. H. Freeman and Company; New York, NY: 1997. [Google Scholar]
- Baranowski T. Validity and reliability of self report measures of physical activity: An information-processing perspective. Research Quarterly for Exercises and Sport. 1988;59(4):314–327. [Google Scholar]
- Baruth M, Wilcox S, Dunn AL, King AC, Marcus BH, Rejeski WJ, et al. Psychosocial mediators of physical activity and fitness changes in the activity counseling trial. Annals of Behavioral Medicine. 2010;39(3):274–289. doi: 10.1007/s12160-010-9178-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basen-Engquist K, Baum G, Hughes DC, Scruggs S, Carmack C. Validity and reliability of a cancer survivors’ exercise barriers self-efficacy scale. Annals of Behavioral Medicine. 2009;37(suppl):s185. [Google Scholar]
- Basen-Engquist K, Scruggs S, Jhingran A, Bodurka DC, Lu K, Ramondetta L, et al. Physical activity and obesity in endometrial cancer survivors: associations with pain, fatigue, and physical functioning. American Journal of Obstetrics and Gynecology. 2009;200(3):288, e281–288. doi: 10.1016/j.ajog.2008.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett JA, Lyons KS, Winters-Stone K, Nail LM, Scherer J. Motivational interviewing to increase physical activity in long-term cancer survivors: a randomized controlled trial. Nurs Res. 2007;56(1):18–27. doi: 10.1097/00006199-200701000-00003. [DOI] [PubMed] [Google Scholar]
- Blanchard C, Denniston MM, Baker F, Ainsworth SR, Courneya KS, Hann DM, et al. Do adults change their lifestyle behaviors after a cancer diagnosis? American Journal of Health Behavior. 2003;27(3):246–256. doi: 10.5993/ajhb.27.3.6. [DOI] [PubMed] [Google Scholar]
- Brown H, Prescott R. Applied Mixed Models in Medicine. John Wiley and Sons, Ltd; West Sussex, UK: 1999. [Google Scholar]
- Courneya KS, Friedenreich CM. Physical exercise and quality of life following cancer diagnosis: a literature review. Annals of Behavioral Medicine. 1999;21(2):171–179. doi: 10.1007/BF02908298. [DOI] [PubMed] [Google Scholar]
- Courneya KS, Karvinen KH, Campbell KL, Pearcey RG, Dundas G, Capstick V, et al. Associations among exercise, body weight, and quality of life in a population-based sample of endometrial cancer survivors. Gynecologic Oncology. 2005;97(2):422–430. doi: 10.1016/j.ygyno.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Dunton GF, Atienza AA, Castro CM, King AC. Using ecological momentary assessment to examine antecedents and correlates of physical activity bouts in adults age 50+ years: a pilot study. Ann Behav Med. 2009;38(3):249–255. doi: 10.1007/s12160-009-9141-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia AW, King AC. Predicting long-term adherence to aerobic exercise: A comparison of two models. Journal of Sport & Exercise Psychology. 1991;13:394–410. [Google Scholar]
- Holmes MD, Chen WY, Feskanich D, Kroenke CH, Colditz GA. Physical activity and survival after breast cancer diagnosis. Journal of the American Medical Association. 2005;293(20):2479–2486. doi: 10.1001/jama.293.20.2479. [DOI] [PubMed] [Google Scholar]
- Jones LW, Courneya KS, Fairey AS, Mackey JR. Does the theory of planned behavior mediate the effects of an oncologist’s recommendation to exercise in newly diagnosed breast cancer survivors? Results from a randomized controlled trial. Health Psychology. 2005;24(2):189–197. doi: 10.1037/0278-6133.24.2.189. [DOI] [PubMed] [Google Scholar]
- Marcus BH, Selby VC, Niaura RS, Rossi JS. Self-efficacy and the stages of exercise behavior change. Research Quarterly for Exercise and Sport. 1992;63(1):60–66. doi: 10.1080/02701367.1992.10607557. [DOI] [PubMed] [Google Scholar]
- McAuley E. The role of efficacy cognitions in the prediction of exercise behavior in middle-aged adults. Journal of Behavior Medicine. 1992;15(1):65–87. doi: 10.1007/BF00848378. [DOI] [PubMed] [Google Scholar]
- McAuley E. Self-efficacy and the maintenance of exercise participation in older adults. Journal of Behavior Medicine. 1993;16(1):103–113. doi: 10.1007/BF00844757. [DOI] [PubMed] [Google Scholar]
- McAuley E, Courneya KS, Rudolph DL, Lox CL. Enhancing exercise adherence in middle-aged males and females. Preventive Medicine. 1994;23:498–506. doi: 10.1006/pmed.1994.1068. [DOI] [PubMed] [Google Scholar]
- McAuley E, Katula J, Mihalko SL, Blissmer B, Duncan TE, Pena M, et al. Mode of physical activity and self-efficacy in older adults: A latent growth curve analysis. Journal of Gerontology: Psychological Sciences. 1999;54B(5):283–292. doi: 10.1093/geronb/54b.5.p283. [DOI] [PubMed] [Google Scholar]
- McAuley E, Mailey EL, Mullen SP, Szabo AN, Wojcicki TR, White SM, et al. Growth trajectories of exercise self-efficacy in older adults: influence of measures and initial status. Health Psychology. 2011;30(1):75–83. doi: 10.1037/a0021567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyerhardt JA, Giovannucci EL, Holmes MD, Chan AT, Chan JA, Colditz GA, et al. Physical activity and survival after colorectal cancer diagnosis. Journal of Clinical Oncology. 2006;24(22):3527–3534. doi: 10.1200/JCO.2006.06.0855. [DOI] [PubMed] [Google Scholar]
- Mols F, Coebergh JW, van de Poll-Franse LV. Health-related quality of life and health care utilisation among older long-term cancer survivors: a population-based study. European Journal of Cancer. 2007;43(15):2211–2221. doi: 10.1016/j.ejca.2007.06.022. [DOI] [PubMed] [Google Scholar]
- Moore SM, Dolansky MA, Ruland CM, Pashkow FJ, Blackburn GG. Predictors of women’s exercise maintenance after cardiac rehabilitation. Journal of Cardiopulmonary Rehabilitation. 2003;23:40–49. doi: 10.1097/00008483-200301000-00008. [DOI] [PubMed] [Google Scholar]
- Mosher CE, Fuemmeler BF, Sloane R, Kraus WE, Lobach DF, Snyder DC, et al. Change in self-efficacy partially mediates the effects of the FRESH START intervention on cancer survivors’ dietary outcomes. Psychooncology. 2008;17(10):1014–1023. doi: 10.1002/pon.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napolitano MA, Papandonatos GD, Lewis BA, Whiteley JA, Williams DM, King AC, et al. Mediators of physical activity behavior change: a multivariate approach. Health Psychology. 2008;27(4):409–418. doi: 10.1037/0278-6133.27.4.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson ME, Rejeski WJ, Blair SN, Duncan PW, Judge JO, King AC, et al. Physical activity and public health in older adults: recommendation from the American College of Sports Medicine and the American Heart Association. Med Sci Sports Exerc. 2007;39(8):1435–1445. doi: 10.1249/mss.0b013e3180616aa2. [DOI] [PubMed] [Google Scholar]
- Patrick K, Raab F, Adams MA, Dillon L, Zabinski M, Rock CL, et al. A text message-based intervention for weight loss: randomized controlled trial. Journal of Medical Internet Research. 2009;11(1):e1. doi: 10.2196/jmir.1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Physical Activity Guidelines Advisory Committee Physical Activity Guidelines Advisory Committee Report, 2008. 2008.
- Pinto BM, Rabin C, Dunsiger S. Home-based exercise among cancer survivors: adherence and its predictors. Psychooncology. 2009;18(4):369–376. doi: 10.1002/pon.1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plotnikoff RC, Brez S, Hotz SB. Exercise behavior in a community sample with diabetes: Understanding the determinants of exercise behavioral change. The Diabetes Educator. 2000;26(3):450–459. doi: 10.1177/014572170002600312. [DOI] [PubMed] [Google Scholar]
- Resnick B. A prediction model of aerobic exercise in older adults living in a continuing-care retirement community. Journal of Aging and Health. 2001;13(2):287–310. doi: 10.1177/089826430101300207. [DOI] [PubMed] [Google Scholar]
- Rodgers WM, Hall CR, Blanchard CM, McAuley E, Munroe KJ. Task and scheduling self-efficacy as predictors of exercise behavior. Psychology and Health. 2002;17(4):405–416. [Google Scholar]
- Rogers LQ, McAuley E, Courneya KS, Verhulst SJ. Correlates of physical activity self-efficacy among breast cancer survivors. American Journal of Health Behavior. 2008;32(6):594–603. doi: 10.5555/ajhb.2008.32.6.594. [DOI] [PubMed] [Google Scholar]
- Schmitz KH, Courneya KS, Matthews C, Demark-Wahnefried W, Galvao DA, Pinto BM, et al. American College of Sports Medicine roundtable on exercise guidelines for cancer survivors. Medicine & Science in Sports & Exercise. 2010;42(7):1409–1426. doi: 10.1249/MSS.0b013e3181e0c112. [DOI] [PubMed] [Google Scholar]
- Shiffman S. Real-time self-report of momentary states in the natural environment: Computerized Ecological Momentary Assessment. In: Stone A, Turkkan JS, Bachrach CA, Jobe JB, Kurtzman HS, Cain VS, editors. The Science of Self-Report: Implications for Research and Practice. Lawrence Erlbaum Associates; Mahwah: 2000. pp. 277–296. [Google Scholar]
- Speck RM, Courneya KS, Masse LC, Duval S, Schmitz KH. An update of controlled physical activity trials in cancer survivors: A systematic review and meta-analysis. Journal of Cancer Survivorship. 2010;4(2):87–100. doi: 10.1007/s11764-009-0110-5. [DOI] [PubMed] [Google Scholar]
- Steptoe A, Rink E, Kerry S. Psychosocial predictors of changes in physical activity in overweight sedentary adults following counseling in primary care. Preventive Medicine. 2000;31:183–194. doi: 10.1006/pmed.2000.0688. [DOI] [PubMed] [Google Scholar]
- Stewart AL, Mills KM, King AC, Haskell WL, Gillis D, Ritter PL. CHAMPS Physical activity questionnaire for older adults: Outcomes for interventions. Medicine & Science in Sports & Exercise. 2001;33(7):1126–1141. doi: 10.1097/00005768-200107000-00010. [DOI] [PubMed] [Google Scholar]
- Stone AA, Broderick JE, Porter LS, Kaell AT. The experience of rheumatoid arthritis pain and fatigue: Examining momentary reports and correlates over one week. Arthritis Care & Research. 1997;10(3):185–193. doi: 10.1002/art.1790100306. [DOI] [PubMed] [Google Scholar]
- Vallance JK, Courneya KS, Plotnikoff RC, Mackey JR. Analyzing theoretical mechanisms of physical activity behavior change in breast cancer survivors: results from the activity promotion (ACTION) trial. Annals of Behavioral Medicine. 2008;35(2):150–158. doi: 10.1007/s12160-008-9019-x. [DOI] [PubMed] [Google Scholar]
- Verbeke G, Molenberghs G. Linear Mixed Models for Longitudinal Data. Springer-Verlag; New York: 2000. [Google Scholar]
- Williams DM, Anderson ES, Winett RA. A review of the outcome expectancy construct in physical activity research. Annals of Behavioral Medicine. 2005;29(1):70–79. doi: 10.1207/s15324796abm2901_10. [DOI] [PubMed] [Google Scholar]