Abstract
Childhood emotional abuse (CEA) is a pervasive problem associated with negative sequelae such as elevated depressive symptoms. Key stress-related genes, such as the 5-HTTLPR polymorphism, interact with childhood abuse to produce elevated depressive symptoms in older adolescent girls, but not in older adolescent boys. To date, studies have not examined this relationship as a function of CEA specifically or among younger adolescents. To extend prior work, we examined the effects of the 5-HTTLPR and CEA on depressive symptoms among 10–12-year-old youth. Based on previous findings, we expected a main effect of CEA on depressive symptoms among all youth, but only expected an interactive effect between the 5-HTTLPR and CEA on depressive symptoms in girls. In the current study, 222 youth (mean age 11.02 years, 44.1% girls, 51.6% Caucasian, 33.0% African American, 2.7% Latino, and 12.7% other) and their parent(s)/guardian(s) completed the Revised Child Anxiety and Depression Scale and the Emotional Abuse subscale of the Childhood Trauma Questionnaire and provided saliva samples for genotyping the 5-HTTLPR. Results indicate that CEA, but not the 5-HTTLPR, was related to elevated depressive symptoms among boys. Among girls, each copy of the s allele of the 5-HTTLPR was related to increased depressive symptoms, but only for those who had experienced CEA. Our results extend prior findings by specifically examining CEA and by focusing on 10–12-year-old youth. These results, although preliminary, suggest that focusing on the interplay between putative genetic markers and a broader range of environmental events, such as CEA, might allow researchers to determine factors differentially influencing the later emergence of sex differences in depressive symptoms.
Keywords: emotional abuse, depressive symptoms, 5-HTTLPR, sex differences, early adolescence
One of eight youth in the United States has been physically, sexually, or emotionally abused (Finkelhor, Ormrod, Turner, & Hamby, 2005), with a significant percentage reporting multiple types of abuse (Felitti et al., 1998). This abuse is a significant public health problem (Centers for Disease Control and Prevention, 2006), and the resulting negative psychological effects following childhood abuse have been documented in adolescents (Kilpatrick et al., 2003) and adults (Arnow, Blasey, Hunkeler, Lee, & Hayward, 2011). Despite strong associations between childhood abuse and negative sequelae such as depression and posttraumatic stress disorder, some youth are resilient and do not develop psychopathology following abuse (Cicchetti, Rogosch, Lynch, & Holt, 1993), which suggests that there are important moderating factors that can help elucidate the postabuse trajectory. A potential moderating factor that may explain how individuals respond to abuse is their underlying genetic makeup.
The genetic polymorphism most frequently implicated as a moderator of the relationship between abuse and depressive symptoms is in the promoter region of the serotonin transporter gene, often referred to as the 5-HTTLPR. The variable number tandem repeat (VNTR) polymorphism at site 5-HTTLPR has short (s) and long (l) allelic variants that influence the function of the resulting serotonin transporter protein; individuals with the s variant have poorer serotonin reuptake and increased levels of synaptic serotonin (Lesch et al., 1995). Numerous gene-by-environment interaction (G × E) studies suggest that adults with this s variant who have experienced trauma/abuse are more likely to develop depression than adults with the l variant (e.g., Caspi et al., 2003; Kaufman et al., 2006), although these implications are debated (e.g., Karg, Burmeister, Shedden, & Sen, 2011; Risch et al., 2009; Sen, Karg, & Burmeister, 2010).
Few studies examining the relationship between the 5-HTTLPR and abuse/trauma on depression have focused on youth. Adolescence is an especially relevant developmental period when rates of depression increase rapidly, particularly among girls (Costello, Mustillo, Erkanli, Keeler, & Angold, 2003). An examination of factors uniquely predicting depressive symptoms among boys versus girls, prior to the emergence of diagnostic differences as a function of sex, may lend clues as to why differential rates later emerge (De Boo & Spiering, 2010). Researchers have shown that preadolescent girls have more risk factors for depression and face more challenges than boys, which increases their later risk for depression (Nolen-Hoeksema & Girgus, 1994). Moreover, depressive symptoms among 8-year-old girls are fairly stable and predict later depressive disorders (Keenan et al., 2008). Finally, there are sex-specific temperamental and coping vulnerabilities for depression among 8–12 year olds that differentially relate to depressive symptoms between boys and girls (De Boo & Spiering, 2010). This suggests it may be necessary to formulate different models, with different risk factors, to predict depression in boys versus girls.
Serotonergic system variability may help explain depression differences that emerge between male and female subjects. Women have lower 5HT2 receptor density (Biver et al., 1996) and lower central nervous system serotonin synthesis (Nishizawa et al., 1997) than men. Nondepressed women carrying two short alleles of the serotonin transporter protein exhibit a proinflammatory resting state analogous to that of individuals with a depression diagnosis (Fredericks et al., 2010). The ss genotype is associated with higher levels of the serotonin metabolite (5-HIAA) in women, whereas in men ss is associated with lower levels of 5-HIAA (Williams et al., 2003), suggesting differential effects of the ss genotype in male and female brains. Moreover, cortisol reactivity, which is particularly elevated among maltreated youth (Harkness, Stewart, & Wynne-Edwards, 2011), is differentially affected by the 5-HTTLPR in women versus men. The ss genotype is associated with larger cortisol responses to challenge tasks among ss females (Jabbi et al., 2007) and with increased cortisol awakening response in ss females (Wüst et al., 2009); these effects do not occur among ss males. This suggests sex-specific effects of the 5-HTTLPR in response to stress among adults.
Interestingly, studies among adolescents have demonstrated that the interaction between the 5-HTTLPR and abuse on depression is only relevant to girls, which mirrors the above biological studies in adults. Among adolescent s carriers who have experienced abuse/trauma, there are elevated levels of depression/depressive symptoms among girls, but not among boys (Åslund et al., 2009; Eley et al., 2004; Hammen, Brennan, Keenan-Miller, Hazel, & Najman, 2010; Sjöberg et al., 2006). Within a sample of 1,990 10–20 year olds, there was a significant interaction between the 5-HTTLPR and environmental risk on depression among girls, but not among boys (Eley et al., 2004). Similarly, among 16–19 year olds, female s allele carriers exposed to traumatic family conflicts had elevated depressive symptoms; this relationship did not hold among boys (Sjöberg et al., 2006). Among 17–18 year olds, there was a main effect of child maltreatment on depression; when the sample was separated by sex, there was a significant interaction between the 5-HTTLPR and childhood maltreatment among girls, but not among boys (Åslund et al., 2009). Continuing this pattern, chronic family stress among 15 year olds predicts elevated depressive symptoms among 20-year-old female s carriers, but not among 20-year-old male s carriers (Hammen et al., 2010). Finally, among 10–14-year-old girls, relational victimization predicted depression, but only among ss homozygotes (Benjet, Thompson, & Gotlib, 2010). Based on these studies, the interaction between the s allele and abuse/trauma on depression appears to only apply to young female participants.
The majority of studies focusing on the relationship between abuse, the 5-HTTLPR, and depression have not examined specific subtypes of abuse. Childhood emotional abuse (CEA), which is defined as verbal assaults or humiliating/demeaning behavior directed toward a child by an adult (Bernstein & Fink, 1998), represents an important avenue of exploration for multiple reasons. First, depressed individuals have experienced significantly higher levels of CEA than nondepressed individuals (McInnis, 2011). Second, CEA is a stronger predictor of depression in adulthood than physical/sexual abuse, and individuals who experienced CEA are two-to-three times more likely to have depression than individuals who have not experienced CEA (Chapman et al., 2004; Gibb, Chelminski, & Zimmerman, 2007). Third, CEA may be especially relevant to depression because the abuser directly provides negative statements that are incorporated into the child’s cognitive self-schema (Antypa & Van der Does, 2010). Fourth, youth experiencing emotional maltreatment/physical neglect have elevated suicidal ideation if they are s allele carriers, but not if they are l homozygotes (Cicchetti, Rogosch, Sturge-Apple, & Toth, 2010), suggesting important genetic effects on this relationship. Currently, little is known about factors interacting with CEA to produce depression; there has not been a single study focusing on the interaction between CEA and the 5-HTTLPR on depression in any age group.
Because the experience of abuse is associated with depression among adolescents regardless of sex (Åslund et al., 2009; Chapman et al., 2004; Gibb et al., 2007), we hypothesized that in our sample of 10–12-year-old youth, the experience of CEA would be associated with elevated depressive symptoms within the full sample. Following from the adolescent literature (e.g., Åslund et al., 2009; Eley et al., 2004; Hammen et al., 2010; Sjöberg et al., 2006), we expected that the interaction between the 5-HTTLPR and CEA would only be associated with elevated depressive symptoms among young adolescent girls. We did not expect the 5-HTTLPR to influence depressive symptoms among young adolescent girls who had not experienced CEA.
Method
Participants
Two hundred twenty-two 10–12-year-old youth (mean age 11.02 years, SD = .815; 44.1% girls; see Table 1) and their families in the Washington, D.C. Metro area were recruited, via media outreach and mailings to area schools, libraries, and Boys and Girls Clubs, to participate in a longitudinal study of risk taking and psychiatric disorders. The study was open to all 5th and 6th grade youth proficient in English; no other exclusion criteria were used. Study procedures and confidentiality requirements were separately described to parent(s)/guardian(s) and youth; informed consent and assent were obtained. Youth and their parent(s)/guardian(s) were administered all measures in separate private rooms. The parent(s)/guardian(s) provided demographics, including age, annual family income (M = $97,809 ± $55,163), education, and race/ethnicity (51.4% European American, 33.0% African American, 2.7% Latino, and 12.7% other) about themselves and their child. For the purpose of genetic analyses (e.g., Cardon & Palmer, 2003), participants were coded as being European American (EA; 51.4%) versus non-European American (non-EA; 48.6%). Families were compensated for study participation. Study procedures were approved by the University of Maryland Institutional Review Board.
Table 1.
Demographic Characteristics
| Characteristic | Girls (n = 98) | Boys (n = 124) | Total (N = 222) |
|---|---|---|---|
| Age (M, SD) | 10.91 (.808) | 11.09 (.815) | 11.02 (.815) |
| Race/ethnicity (n, %) | |||
| European American | 52 (53.1) | 62 (50.0) | 114 (51.4) |
| African American | 32 (32.7) | 41 (33.1) | 73 (32.9) |
| Hispanic/Latino | 1 (1.0) | 5 (4.0) | 6 (2.7) |
| Mixed/other | 13 (13.2) | 15 (12.1) | 28 (12.7) |
| Family income (M, SD) | 88,813 (49,172) | 98,813 (88,715) | 97,809 (55,163) |
| CEA score (M, SD) | 7.47 (3.47) | 7.68 (3.66) | 7.59 (3.53) |
| CEA: yes (n, %) | 10 (10.2) | 15 (12.1) | 25 (11.3) |
| CEA: no (n, %) | 88 (89.8) | 109 (87.8) | 197 (88.7) |
| RCADS depressive symptoms (M, SD) | 5.98 (4.72) | 6.32 (4.03) | 6.17 (4.34) |
Note. Due to some missing data, not all ns add up to 222 (valid percentages presented). CEA = childhood emotional abuse; RCADS = Revised Child Anxiety and Depression Scale. Family income is in dollars.
Measures
Revised Child Anxiety and Depression Scale (RCADS; Chorpita, Yim, Moffitt, Umemoto, & Francis, 2000)
The RCADS is a reliable and valid self-report questionnaire for youth containing six scales corresponding to DSM–IV anxiety and depressive disorders (Chorpita et al., 2000). In this article, we focused on 10 items comprising the depression subscale score (e.g., “I feel sad or empty” or “Nothing is much fun anymore”), which has an adequate Cronbach’s alpha (α = .78). Higher scores on this subscale indicate a greater number of depressive symptoms (Chorpita, Moffitt, & Gray, 2005), which we examined continuously.
Childhood Trauma Questionnaire (CTQ; Fink, Bernstein, Handelsman, & Foote, 1995)
We used the Emotional Abuse scale of the CTQ, a self-report measure, to assess for a lifetime history of childhood emotional abuse; the Physical and Sexual Abuse subscales were not administered. Scores on the Emotional Abuse subscale of the CTQ are stable over time and have discriminant and convergent validity with other measures of trauma (Bernstein et al., 1994). When self-reports are compared with trauma ratings from child welfare records and reports of family members and clinicians, the CTQ has good sensitivity and satisfactory specificity (Bernstein, Ahluvalia, Pogge, & Handelsman, 1997). Internal consistency of the Emotional Abuse subscale in the present sample was good (α= .84). The Emotional Abuse subscale contains five items scored on a 5-point scale 1 (never true) to 5 (very often true) and examines whether participants experienced humiliating or demeaning behavior from adults in their lives, including threats to their well-being or verbal assaults. The average CTQ Emotional Abuse subscale score was 7.59 (SD = 3.53, range = 5–25) in our sample. CEA was defined as a score greater than one standard deviation above the mean.
DNA samples
Saliva was collected and isolated using the Oragene protocol. We examined the functional VNTR polymorphism in the 5′ flanking regulatory region of the gene (SLC6A4) coding for the serotonin transporter protein. This polymorphism (5-HTTLPR) is defined by two common alleles based on the number of repeats: “long” (l) (16 repeats) and “short” (s) (14 repeats). Prior Mspl restriction endonuclease digestion for triallelic classification determined genotype, which allowed classification of l alleles into “lA” and “lG” (lG has lower reuptake efficiency, similar to the s allele) variants. This polymerase chain reaction (PCR) was conducted twice to confirm the accuracy of this categorization. Consistent with previous research the rare lG alleles were classified as s for analyses (Sadeh et al., 2010). PCR and subsequent size fractionation were performed as described elsewhere (Gelernter, Kranzler, & Cubells, 1997). Allele frequencies did not deviate significantly from Hardy-Weinberg equilibrium in the full sample (χ2 = .12, ns) or within the EA (χ2 = .12, ns) and non-EA groups (χ2 = .09, ns). For quality control, 8% of the SLC6A4 genotypes were repeated. The effect of the 5-HTTLPR was examined within an additive genetic model; we coded the alleles as ll = 0, sl = 1, and ss = 2 for statistical analyses.
Statistical Analyses
All data were double-entered, compared, cleaned, and verified using the statistical package SPSS version 18.0 (SPSS Inc., 2009). To test for a gene × environment correlation (testing the extent to which genotype is related to exposure to CEA) a chi-square analysis was conducted. To test for population stratification, a chi-square analysis was conducted to determine whether genotype distribution differed between EAs and non-EAs (Cardon & Palmer, 2003). A chi-square analysis was used to determine whether elevated CEA scores were associated with gender, race/ethnicity, and elevated RCADS depression scores.
Given that we had different hypotheses regarding predictors for depressive symptoms in boys and girls, we conducted separate regression analyses by sex. Step 1 of these linear regressions tested whether CEA and the 5-HTTLPR were significant predictors of RCADS depression, while controlling for demographic covariates (i.e., age and racial/ethnic status). Step 2 of these linear regressions tested these main effects and the interaction of CEA and 5-HTTLPR, on the RCADS depression score, while controlling for the aforementioned demographic covariates. All β coefficients presented are the standardized regression coefficients.
Results
Genotype frequencies did not differ between those who experienced CEA compared with those who did not (χ2 = .33, ns) or between EA versus non-EA participants (χ2 = .06, ns). The frequency of CEA did not differ between EA and non-EA participants (χ2 = .61, ns) or as a function of gender (χ2 = .20, ns). CEA and RCADS depression scores were positively correlated (r = .44, p < .001).
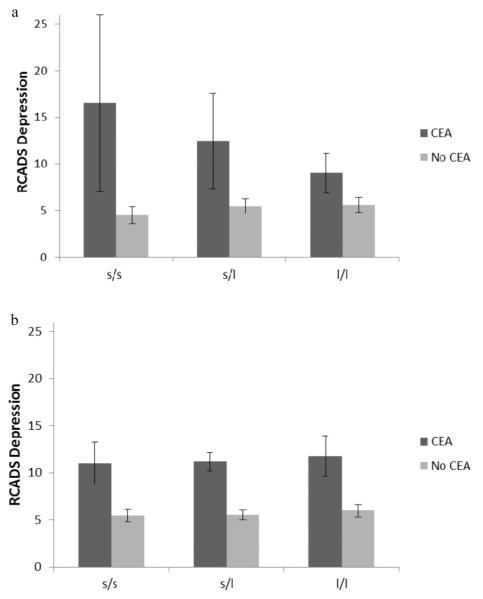
The overall regression models were significant for girls, R2 = .226, F(5, 90) = 5.24, p < .001, and for boys, R2 = .494, F(5, 115) = 7.42, p < .001. For girls, the overall regression model accounted for 22.6% of the variance in RCADS depression scores, whereas for boys, the overall regression model accounted for 24.4% of the variance in RCADS depression scores. In the first step of the model, CEA was a significant predictor of RCADS depression in girls, β= .430, t(90) = 4.52, p < .001, and in boys, β= .244, t(115) = 5.76, p < .001. However, in the final model for girls, the only significant contributor to the RCADS depression score was the interaction between CEA and 5-HTTLPR, β= .312, t(90) = 2.20, p = .03, whereas in the final model for boys, the only significant contributor to the RCADS depression score was CEA, β= .432, t(115) = 3.20, p = .002. For girls, the second step of the model had a significantly better fit than the first step of the model, ΔR2 = .042, ΔF(1, 90) = 4.84, p = .03, whereas for boys, the second step model did not have a significantly better fit than the first step of the model, ΔR2 = .001, ΔF(1, 115) = .117, p = .733. All other predictors were nonsignificant (see Table 2). As shown in Figure 1, an additive effect was found for girls with the s allele of the 5-HTTLPR, in that those who had experienced CEA had a greater likelihood of reporting higher levels of depressive symptoms if they had one or more copies of the s allele (see Table 2). This interaction, as hypothesized, was not found for boys.
Table 2.
Linear Regression Analysis of the Association With RCADS Depressive Symptoms, Separate by Sex
| Girls |
Boys |
|||||
|---|---|---|---|---|---|---|
| β | t | p | β | t | p | |
| Step 1 | ||||||
| Age | −.050 | −.529 | .598 | −.158 | −1.91 | .059 |
| EA (vs. non-EA) | .007 | .078 | .938 | .118 | 1.42 | .157 |
| CEA | .430 | 4.52 | .001 | .469 | 5.76 | .001 |
| 5-HTTLPR (s is risk) | .006 | .065 | .948 | −.025 | −.314 | .754 |
| Step 2 (final model) | ||||||
| Age | −.061 | −.649 | .518 | −.160 | −1.92 | .057 |
| EA (vs. non-EA) | −.009 | −.097 | .923 | .121 | 1.45 | .150 |
| CEA | .203 | 1.46 | .149 | .432 | 3.20 | .002 |
| 5-HTTLPR (s is risk) | −.071 | −.717 | .475 | −.037 | −0.42 | .677 |
| 5-HTTLPR (s is risk) × CEA | .312 | 3.20 | .030 | .048 | 0.34 | .733 |
Note. EA = European American; CEA = childhood emotional abuse; RCADS = Revised Child Anxiety and Depression Scale.
Figure 1.
RCADS depression score as a function of 5-HTTLPR, sex, and CEA. (a) Results for girls; (b) results for boys. Standard errors are represented by the error bars attached to each column.
Discussion
This study aimed to examine the effects of CEA and the 5-HTTLPR on depressive symptoms in a group of 10–12-year-old youth. Among boys, the experience of CEA had a greater impact on depressive symptoms than did the 5-HTTLPR, which is in line with prior studies demonstrating a main effect of abuse/trauma, but no interaction between abuse and the 5-HTTLPR, on depression in boys (Åslund et al., 2009; Eley et al., 2004; Hammen et al., 2010; Sjöberg et al., 2006). Although there was a main effect of CEA on depressive symptoms in the first step of the model for girls, this relationship did not hold when the interaction term was added to the second step of the model. It is unclear whether this was due to lack of power or whether it represents a pattern particularly relevant to this age group. Our findings do suggest that 10–12-year-old girls with the s allele may be particularly sensitive to the effects of CEA, a finding consistent with that observed in 10–14-year-old s carrier females exposed to relational victimization (Benjet et al., 2010) and older adolescents exposed to various types of abuse/stress (Åslund et al., 2009; Eley et al., 2004; Hammen et al., 2010; Sjöberg et al., 2006). Broadly, our study stresses the detrimental effects likely to be experienced by all youth exposed to CEA and highlights a potential pathway of vulnerability that might uniquely contribute to depression differences observed among older adolescent girls and boys. However, replication of the current findings is needed to determine better the significance of the results, and extension of this work is necessary to better understand the implications of these findings, particularly with respect to more severe depression that is typically seen in clinical settings.
There are multiple limitations with this study. First, the CTQ and RCADS are self-report measures, which may be subject to information biases or inaccurate reporting; for example, it is possible that youth with more severe depressive symptoms may have been more likely to self-report abuse experiences than their peers with lower levels of depressive symptoms. However, research demonstrates that CTQ results are in concordance with trauma ratings from child welfare records and reports by family members and clinicians (Bernstein et al., 1997), and RCADS scores are in concordance with interview-based measures of depression (Chorpita et al., 2000), suggesting their suitability. Moreover, children’s self-report measures of CEA are likely to be more informative than information collected from parents or community agencies. Second, ancestral informative markers were not available; thus, we controlled for population stratification based on self-reported racial/ethnic status. Third, the sample size was relatively small for a genetic association study, and we focused on a single polymorphism. Future studies should utilize fine mapping of candidate genes within larger samples. Fourth, we only measured CEA in this sample and did not collect information about physical or sexual abuse, which limits our ability to compare findings across types of abuse. Fifth, we used a convenience sample, which limits the generalizability of our results; future research should attempt to recruit more low-income and Hispanic/Latino families. Sixth, we chose to focus on a continuous measure of depressive symptoms; additional research is needed to determine the extent to which these findings may apply to youth with clinically significant major depressive disorder. Lastly, the present study was cross-sectional, so inferences about the temporal relationships between CEA and depressive symptoms could not be made. Despite these limitations, we believe our study is an important initial step in elucidating this G × E relationship among 10–12-year-old youth. It will be necessary for future work to examine the effects of CEA and the 5-HTTLPR on depressive symptoms across longer periods of development, in larger samples, and with more robust measurement tools.
We believe the present study contributes to the growing body of literature examining the interaction between abuse/trauma and the 5-HTTLPR on depressive symptoms. Although there were a number of limitations, our study also had multiple strengths, including the diversity of the sample, the focus on CEA, and the age range. This is the first study to our knowledge focusing specifically on the interaction between CEA and the 5-HTTLPR on depressive symptoms. Further, we focused on adolescents prior to the emergence of depression differences between the sexes, allowing an examination of particular factors differentially related to elevated depressive symptoms between boys and girls. Thus, our findings not only extend the literature in terms of examining the relationship between these two factors in youth, but also extend the literature by examining CEA more specifically. Further replications and extensions of this work are necessary to better establish causality and to understand the underlying mechanisms of these findings.
There are a number of potential clinical implications of this research. Our findings suggest the importance of assessing for experiences of childhood abuse, including emotional abuse, among youth presenting with depressive symptoms in clinical settings, because depressed individuals with abuse histories may respond less positively to some forms of therapy (Harkness, Bagby, & Kennedy, 2012). They also suggest an important role for CEA in the development of depressive symptoms in youth and suggest that this type of abuse should be targeted by clinicians and others caring for youths. These findings raise the possibility that certain youth (e.g., girls with s allelic variants) who experience trauma early in life might be particularly vulnerable to depression, raising the possibility that targeted intervention strategies might be particularly helpful for these individuals.
The types of interventions that would be of the greatest benefit to youth based on the interaction between their experiences and genotypes are somewhat unclear, and this is a topic of current interest (Beauchaine et al., 2008). Previous research has demonstrated a link between SLC6A4 markers and antidepressant/psychological treatment response (e.g., Bryant et al., 2012; Huezo-Diaz et al., 2009; Keers et al., 2011; Porcelli, Fabbri, & Serretti, 2012; Wilkie et al., 2009). For example, Porcelli and colleagues’ (2012) meta-analysis demonstrated that l allele 5-HTTLPR Caucasian carriers who took selective serotonin reuptake inhibitor (SSRI) antidepressants were more likely to experience remission from depression than their s allele counterparts. Further, s allele carriers are less responsive to the SSRI escitalopram if they have experienced stressful life events; those who have not experienced stressful life events do not respond differentially to the treatment as a function of genotype (Keers et al., 2011). Finally, a relationship between the 5-HTTLPR and cognitive–behavioral therapy response has been shown, where s allele carriers with posttraumatic stress disorder have poorer responses to CBT than their l allele peers (Bryant et al., 2010). As the majority of treatments that have been examined as a function of the 5-HTTLPR have showed poorer outcomes among s allele carriers (e.g., Huezo-Diaz et al., 2009; Porcelli et al., 2012; Wilkie et al., 2009), it is necessary to think of novel techniques that might target some of their underlying vulnerabilities that have been identified. For example, s allele homozygotes have stronger negative attentional biases than long allele carriers (Pergamin-Hight, Bakermans-Kranenburg, van IJzendoorn, & Bar-Haim, 2012), which might suggest that attentional training to reduce these biases could be one of many components of treatment that might benefit girls who experience emotional abuse (Eldar et al., 2012). These patterns of findings should be considered in future research in order to better understand which youth are likely to respond to which interventions. Our findings additionally highlight the importance of the 5-HTTLPR among girls and suggest the need for more aggressive intervention strategies among girls with the s allele, as they may experience worse outcomes. Given that allostatic load and childhood stressors may impact adult mental health problems, this study improves our understanding of factors contributing to depressive symptoms among youth and provides information that can be used as a foundation to identify strategies to modify risk and prevent future illness.
Contributor Information
Anne N. Banducci, Department of Psychology, University of Maryland College Park
Melissa Gomes, Department of Nursing, Virginia Commonwealth University.
Laura MacPherson, Department of Psychology, University of Maryland College Park.
C. W. Lejuez, Department of Psychology, University of Maryland College Park
Marc N. Potenza, Departments of Psychiatry, Neurobiology, and Child Study Center, Yale University
Joel Gelernter, Departments of Psychiatry, Genetics, and Neurobiology, Yale University School of Medicine.
Ananda B. Amstadter, Department of Psychiatry, Virginia Institute of Psychiatric and Behavioral Genetics, Virginia Commonwealth University
References
- Antypa N, Van der Does AW. Serotonin transporter gene, childhood emotional abuse and cognitive vulnerability to depression. Genes, Brain and Behavior. 2010;9:615–620. doi: 10.1111/j.1601-183X.2010.00593.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnow BA, Blasey CM, Hunkeler EM, Lee J, Hayward C. Does gender moderate the relationship between childhood maltreatment and adult depression? Child Maltreatment. 2011;16:175–183. doi: 10.1177/1077559511412067. doi: 10.1177/1077559511412067. [DOI] [PubMed] [Google Scholar]
- Åslund C, Leppert J, Comasco E, Nordquist N, Oreland L, Nilsson KW. Impact of the interaction between the 5HTTLPR polymorphism and maltreatment on adolescent depression. A population-based study. Behavior Genetics. 2009;39:524–531. doi: 10.1007/s10519-009-9285-9. doi:10.1007/s10519-009-9285-9. [DOI] [PubMed] [Google Scholar]
- Beauchaine TP, Neuhaus E, Brenner SL, Gatzke-Kopp L. Ten good reasons to consider biological processes in prevention and intervention research. Development and Psychopathology. 2008;20:745–774. doi: 10.1017/S0954579408000369. doi:10.1017/S0954579408000369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjet C, Thompson RJ, Gotlib IH. 5-HTTLPR moderates the effect of relational peer victimization on depressive symptoms in adolescent girls. Journal of Child Psychology and Psychiatry. 2010;51:173–179. doi: 10.1111/j.1469-7610.2009.02149.x. doi:10.1111/j.1469-7610.2009.02149.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein DP, Ahluvalia T, Pogge D, Handelsman L. Validity of the Childhood Trauma Questionnaire in an adolescent psychiatric population. Journal of The American Academy of Child & Adolescent Psychiatry. 1997;36:340–348. doi: 10.1097/00004583-199703000-00012. doi:10.1097/00004583-199703000-00012. [DOI] [PubMed] [Google Scholar]
- Bernstein DP, Fink L, Handelsman L, Foote J. Initial reliability and validity of a new retrospective measure of child abuse and neglect. The American Journal of Psychiatry. 1994;151:1132–1136. doi: 10.1176/ajp.151.8.1132. [DOI] [PubMed] [Google Scholar]
- Bernstein DP, Fink L. Childhood trauma questionnaire manual. Harcourt, The Psychological Corporation; San Antonio, TX: 1998. [Google Scholar]
- Biver F, Lotstra F, Monclus M, Wikler D, Damhaut P, Mendlewicz J, Goldman S. Sex difference in 5HT receptor in the living human brain. Neuroscience Letters. 1996;204:25–28. doi: 10.1016/0304-3940(96)12307-7. doi:10.1016/0304-3940(96)12307-7. [DOI] [PubMed] [Google Scholar]
- Bryant RA, Felmingham KL, Falconer EM, Pe Benito L, Dobson-Stone C, Pierce KD, Schofield PR. Preliminary evidence of the short allele of the serotonin transporter gene predicting poor response to cognitive behavior therapy in posttraumatic stress disorder. Biological Psychiatry. 2010;67:1217–1219. doi: 10.1016/j.biopsych.2010.03.016. doi:10.1016/j.biopsych.2010.03.016. [DOI] [PubMed] [Google Scholar]
- Cardon LR, Palmer LJ. Population stratification and spurious allelic association. Lancet. 2003;361:598–604. doi: 10.1016/S0140-6736(03)12520-2. doi:10.1016/S0140-6736(03)12520-2. [DOI] [PubMed] [Google Scholar]
- Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, Poulton R. Influence of life stress on depression: Moderation by a polymorphism in the 5 HTT gene. Science. 2003;301:386–389. doi: 10.1126/science.1083968. doi:10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention Child Maltreatment: CDC Activities. 2006 Retrieved from http://www.cdc.gov/ncipc/factsheets/cmactivities.htm.
- Chapman DP, Whitfield CL, Felitti VJ, Dube SR, Edwards VJ, Anda RF. Adverse childhood experiences and the risk of depressive disorders in adulthood. Journal of Affective Disorders. 2004;82:217–225. doi: 10.1016/j.jad.2003.12.013. doi:10.1016/j.jad.2003.12.013. [DOI] [PubMed] [Google Scholar]
- Chorpita BF, Moffitt CE, Gray J. Psychometric properties of the Revised Child Anxiety and Depression Scale in a clinical sample. Behaviour Research and Therapy. 2005;43:309–322. doi: 10.1016/j.brat.2004.02.004. doi:10.1016/j.brat.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Chorpita BF, Yim L, Moffitt C, Umemoto LA, Francis SE. Assessment of symptoms of DSM-IV anxiety and depression in children: A revised child anxiety and depression scale. Behaviour Research and Therapy. 2000;38:835–855. doi: 10.1016/s0005-7967(99)00130-8. doi:10.1016/S0005-7967(99)00130-8. [DOI] [PubMed] [Google Scholar]
- Cicchetti D, Rogosch FA, Lynch M, Holt KD. Resilience in maltreated children: Processes leading to adaptive outcome. Development and Psychopathology. 1993;5:629–647. doi:10.1017/S0954579400006209. [Google Scholar]
- Cicchetti D, Rogosch FA, Sturge-Apple M, Toth SL. Interaction of child maltreatment and 5-HTT polymorphisms: Suicidal ideation among children from low-SES backgrounds. Journal of Pediatric Psychology. 2010;35:536–546. doi: 10.1093/jpepsy/jsp078. doi:10.1093/jpepsy/jsp078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costello EJ, Mustillo S, Erkanli A, Keeler G, Angold A. Prevalence and development of psychiatric disorders in childhood and adolescence. Archives of General Psychiatry. 2003;60:837–844. doi: 10.1001/archpsyc.60.8.837. doi:10.1001/archpsyc.60.8.837. [DOI] [PubMed] [Google Scholar]
- De Boo GM, Spiering M. Pre-adolescent gender differences in associations between temperament, coping, and mood. Clinical Psychology & Psychotherapy. 2010;17:313–320. doi: 10.1002/cpp.664. [DOI] [PubMed] [Google Scholar]
- Eldar S, Apter A, Lotan D, Edgar KP, Naim R, Fox NA, Bar-Haim Y. Attention bias modification treatment for pediatric anxiety disorders: A randomized controlled trial. The American Journal of Psychiatry. 2012;169:213–220. doi: 10.1176/appi.ajp.2011.11060886. doi:10.1176/appi.ajp.2011.11060886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eley TC, Sugden KK, Corsico AA, Gregory AM, Sham PP, McGuffin PP, Craig IW. Gene-environment interaction analysis of serotonin system markers with adolescent depression. Molecular Psychiatry. 2004;9:908–915. doi: 10.1038/sj.mp.4001546. doi:10.1038/sj.mp.4001546. [DOI] [PubMed] [Google Scholar]
- Felitti VJ, Anda RF, Nordenberg D, Williamson DF, Spitz AM, Edwards V, Marks JS. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults: The Adverse Childhood Experiences (ACE) Study. American Journal of Preventive Medicine. 1998;14:245–258. doi: 10.1016/s0749-3797(98)00017-8. doi:10.1016/S0749-3797(98)00017-8. [DOI] [PubMed] [Google Scholar]
- Fink LA, Bernstein D, Handelsman L, Foote J. Initial reliability and validity of the Childhood Trauma Interview: A new multidimensional measure of childhood interpersonal trauma. The American Journal of Psychiatry. 1995;152:1329–1335. doi: 10.1176/ajp.152.9.1329. [DOI] [PubMed] [Google Scholar]
- Finkelhor D, Ormrod R, Turner H, Hamby SL. The victimization of children and youth: A comprehensive, national survey. Child Maltreatment. 2005;10:5–25. doi: 10.1177/1077559504271287. doi:10.1177/1077559504271287. [DOI] [PubMed] [Google Scholar]
- Fredericks CA, Drabant EM, Edge MD, Tillie JM, Hallmayer J, Ramel W, Dhabhar FS. Healthy young women with serotonin transporter SS polymorphism show a pro-inflammatory bias under resting and stress conditions. Brain, Behavior, and Immunity. 2010;24:350–357. doi: 10.1016/j.bbi.2009.10.014. doi:10.1016/j.bbi.2009.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelernter J, Kranzler H, Cubells JF. Serotonin transporter protein (SLC6A4) allele and haplotype frequencies and linkage disequilibria in African- and European-American and Japanese populations and in alcohol-dependent subjects. Human Genetics. 1997;101:243–246. doi: 10.1007/s004390050624. doi: 10.1007/s004390050624. [DOI] [PubMed] [Google Scholar]
- Gibb BE, Chelminski I, Zimmerman M. Childhood emotional, physical, and sexual abuse, and diagnoses of depressive and anxiety disorders in adult psychiatric outpatients. Depression and Anxiety. 2007;24:256–263. doi: 10.1002/da.20238. doi:10.1002/da.20238. [DOI] [PubMed] [Google Scholar]
- Hammen C, Brennan PA, Keenan-Miller D, Hazel NA, Najman JM. Chronic and acute stress, gender, and serotonin transporter gene environment interactions predicting depression symptoms in youth. Journal of Child Psychology and Psychiatry. 2010;51:180–187. doi: 10.1111/j.1469-7610.2009.02177.x. doi:10.1037/j.1469-7610.2009.02177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harkness KL, Bagby R, Kennedy SH. Childhood maltreatment and differential treatment response and recurrence in adult major depressive disorder. Journal of Consulting and Clinical Psychology. 2012;80:342–353. doi: 10.1037/a0027665. doi:10.1037/a0027665. [DOI] [PubMed] [Google Scholar]
- Harkness KL, Stewart JG, Wynne-Edwards KE. Cortisol reactivity to social stress in adolescents: Role of depression severity and child maltreatment. Psychoneuroendocrinology. 2011;36:173–181. doi: 10.1016/j.psyneuen.2010.07.006. doi: 10.1016/j.psyneuen.2010.07.006. [DOI] [PubMed] [Google Scholar]
- Huezo-Diaz P, Uher R, Smith R, Rietschel M, Henigsberg N, Marušič A, McGuffin P. Moderation of antidepressant response by the serotonin transporter gene. The British Journal of Psychiatry. 2009;195:30–38. doi: 10.1192/bjp.bp.108.062521. doi:10.1192/bjp.bp.108.062521. [DOI] [PubMed] [Google Scholar]
- Jabbi M, Korf J, Kema IP, Hartman C, van der Pompe G, Minderaa RB, den Boer JA. Convergent genetic modulation of the endocrine stress response involves polymorphic variations of 5-HTT, COMT and MAOA. Molecular Psychiatry. 2007;12:483–490. doi: 10.1038/sj.mp.4001975. [DOI] [PubMed] [Google Scholar]
- Karg K, Burmeister M, Shedden K, Sen S. The serotonin transporter promoter variant (5-HTTLPR), stress, and depression Meta-analysis revisited evidence of genetic moderation. Archives of General Psychiatry. 2011;68:444–454. doi: 10.1001/archgenpsychiatry.2010.189. doi:10.1001/archgenpsychiatry.2010.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman J, Yang BZ, Douglas-Palumberi H, Grasso D, Lipschitz D, Houshyar S, Gelernter J. Brain-derived neurotrophic factor-5-HTTLPR gene interactions and environmental modifiers of depression in children. Biological Psychiatry. 2006;59:673–680. doi: 10.1016/j.biopsych.2005.10.026. [DOI] [PubMed] [Google Scholar]
- Keenan K, Hipwell A, Feng X, Babinski D, Hinze A, Rischall M, Henneberger A. Subthreshold symptoms of depression in preadolescent girls are stable and predictive of depressive disorders. Journal Of The American Academy of Child & Adolescent Psychiatry. 2008;47:1433–1442. doi: 10.1097/CHI.0b013e3181886eab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keers R, Uher R, Huezo-Diaz P, Smith R, Jaffee S, Reitschel M, Aitchison KJ. Interaction between serotonin transporter gene variants and life events predicts response to antidepressants in the GENDEP project. Pharmacogenomics Journal. 2011;11:138–145. doi: 10.1038/tpj.2010.14. doi: 10.1038/tpj.2010.14. [DOI] [PubMed] [Google Scholar]
- Kilpatrick DG, Ruggiero KJ, Acierno R, Saunders BE, Resnick HS, Best CL. Violence and risk of PTSD, major depression, substance abuse/dependence, and comorbidity: Results from the National Survey of Adolescents. Journal of Consulting and Clinical Psychology. 2003;71:692–700. doi: 10.1037/0022-006x.71.4.692. doi:10.1037/0022-006X.71.4.692. [DOI] [PubMed] [Google Scholar]
- Lesch KP, Gross J, Franzek E, Wolozin BL, Riederer P, Murphy DL. Primary structure of the serotonin transporter in unipolar depression and bipolar disorder. Biological Psychiatry. 1995;37:215–223. doi: 10.1016/0006-3223(94)00147-U. doi:10.1016/0006-3223(94)00147-U. [DOI] [PubMed] [Google Scholar]
- McInnis M. Disorder: Baseline descriptive analysis of individuals with and without bipolar disorders. Symposium presented at the World Congress of Psychiatric Genetics; Washington, D.C.. 2011. [Google Scholar]
- Nishizawa S, Benkelfat C, Young SN, Leyton M, Mzengeza S, de Montigny C, Diksik M. Differences between males and females in rates of serotonin synthesis in human brain. Proceedings of the National Academy of Sciences, USA. 1997;94:5308–5313. doi: 10.1073/pnas.94.10.5308. doi:10.1073/pnas.94.10.5308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolen-Hoeksema S, Girgus JS. The emergence of gender differences in depression during adolescence. Psychological Bulletin. 1994;115:424–443. doi: 10.1037/0033-2909.115.3.424. doi:10.1037/0033-2909.115.3.424. [DOI] [PubMed] [Google Scholar]
- Pergamin-Hight L, Bakermans-Kranenburg MJ, van IJzendoorn MH, Bar-Haim Y. Variations in the promoter region of the serotonin transporter gene and biased attention for emotional information: A meta-analysis. Biological Psychiatry. 2012;71:373–379. doi: 10.1016/j.biopsych.2011.10.030. doi:10.1016/j.biopsych.2011.10.030. [DOI] [PubMed] [Google Scholar]
- Porcelli S, Fabbri C, Serretti A. Meta-analysis of serotonin transporter gene promoter polymorphism (5-HTTLPR) association with antidepressant efficacy. European Neuropsychopharmacology. 2012;22:239–258. doi: 10.1016/j.euroneuro.2011.10.003. doi:10.1016/j.euroneuro.2011.10.003. [DOI] [PubMed] [Google Scholar]
- Risch N, Herrell R, Lehner T, Liang K, Eaves L, Hoh J, Merikangas K. Interaction between the serotonin transporter gene (5-HTTLPR), stressful life events, and risk of depression: A meta-analysis. Journal of the American Medical Association. 2009;301:2462–2471. doi: 10.1001/jama.2009.878. doi:10.1001/jama.2009.878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SPSS Inc. Released 2009. PASW Statistics for Windows. Version 18.0 SPSS Inc; Chicago: [Google Scholar]
- Sadeh N, Javdani S, Jackson JJ, Reynolds EK, Potenza MN, Gelernter J, Verona E. Serotonin transporter gene associations with psychopathic traits in youth vary as a function of socioeconomic resources. Journal of Abnormal Psychology. 2010;119:604–609. doi: 10.1037/a0019709. doi: 10.1037/a0019709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen S, Karg K, Burmeister M. The serotonin transporter promoter variant (5 HTTLPR), stress, and depression meta-analysis revisited: Evidence of genetic moderation. Biological Psychiatry. 2010;67:676. doi: 10.1001/archgenpsychiatry.2010.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjöberg RL, Nilsson KW, Nordquist N, Öhrvik J, Leppert J, Lindström L, Oreland L. Development of depression: Sex and the interaction between environment and a promoter polymorphism of the serotonin transporter gene. International Journal of Neuropsycho-pharmacology. 2006;9:443–449. doi: 10.1017/S1461145705005936. doi:10.1017/S1461145705005936. [DOI] [PubMed] [Google Scholar]
- Uher R, McGuffin PP. The moderation by the serotonin transporter gene of environmental adversity in the etiology of depression: 2009 update. Molecular Psychiatry. 2010;15:18–22. doi: 10.1038/mp.2009.123. doi:10.1038/mp.2009.123. [DOI] [PubMed] [Google Scholar]
- Wilkie MJV, Smith G, Day RK, Matthews K, Smith D, Blackwood D, Wolf CR. Polymorphisms in the SLC6A4 and HTR2A genes influence treatment outcome following antidepressant therapy. Pharmacogenomics Journal. 2009;9:61–70. doi: 10.1038/sj.tpj.6500491. doi:10.1038/sj.tpj.6500491. [DOI] [PubMed] [Google Scholar]
- Williams RB, Marchuk DA, Gadde KM, Barefoot JC, Grichnik K, Helms MJ, Siegler IC. Serotonin-related gene polymorphisms and central nervous system serotonin function. Neuropsychopharmacology. 2003;28:533–541. doi: 10.1038/sj.npp.1300054. doi:10.1038/sj.npp.1300054. [DOI] [PubMed] [Google Scholar]
- Wüst S, Kumsta R, Treutlein J, Frank J, Entringer S, Schulze TG, Rietschel M. Sex-specific association between the 5-HTT gene-linked polymorphic region and basal cortisol secretion. Psycho-neuroendocrinology. 2009;34:972–982. doi: 10.1016/j.psyneuen.2009.01.011. doi:10.1016/j.psyneuen.2009.01.011. [DOI] [PubMed] [Google Scholar]