Abstract
Oxylipins, the oxidation products of unsaturated fatty acids (FA), are potent endogenous mediators being involved in regulation of various biological processes such as inflammation, pain and blood coagulation. Compared to oxylipins derived from arachidonic acid (AA) by cyclooxygenase action, i.e. prostanoides, only limited information is available about the endogenous levels of hydroxy-, epoxy- and dihydroxy-FA of linoleic acid (LA), AA, α-linolenic acid (ALA), eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) in humans. Particularly, it is unknown how metabolic disorders affect endogenous oxylipin levels in humans. Therefore, in the present study we compared the serum concentrations of 44 oxylipins in 20 normolipidemic with 20 hyperlipidemic (total cholesterol > 200 mg/dl; LDL-C > 130 mg/dl; TG > 150 mg/ml) men (age 29–51 y). The serum concentration varied strongly among subjects. For most hydroxy-, epoxy- and dihydroxy-FA the concentration were comparable to those of plasma reported in earlier studies. Despite the significant change in blood lipid levels the hyperlipidemic group showed only minor differences in oxylipin levels. The hyperlipidemic subjects had a slightly higher serum concentration of 8,9-DiHETrE, 5-HEPE, 10,11-DiHDPE, and a lower concentration of 12,13-DiHOME, 12-HETE, 9,10-DiHODE, and 12,13-DiHODE compared to normolipidemic subjects. Overall the hydroxy-, epoxy- and dihydroxy-FA levels were not changed suggesting that mild combined hyperlipidemia has no apparent effect on the concentration of circulating oxylipins. By contrast, serum levels of several hydroxy-, epoxy-, and dihydroxy-FA are dependent on the individual status of the parent FA. Particularly, a strong correlation between the EPA content in the erythrocyte membrane and the serum concentration of EPA derived oxylipins was observed. Given that the synthesis of EPA from other n3-FA in humans is low, this suggests that oxylipin levels can be directly influenced by the diet.
Keywords: Eicosanoids, PUFA, Arachidonic acid, eicosapentaenoic acid, hyperlipidemia, omega-3 fatty acids
INTRODUCTION
Omega-6 fatty acids (n-6 FA) such as linoleic acid (LA, C18:2) or arachidonic acid (AA, C20:4) and n-3 FA such as α-linolenic acid (ALA, C18:3), eicosapentaenoic acid (EPA, C20:5) and docosahexaenoic acid (DHA, C22:6) are essential polyunsaturated FA (PUFA). Humans lack the desaturases to synthesize LA and ALA de novo, while LA and AA as well as ALA, EPA and DHA can be transformed from one into another in a multistage enzymatic chain elongation and desaturation process [1, 2]. However, the conversion rate from ALA to EPA and especially to DHA is low (~ 1%), and thus the status of EPA and DHA relies largely on the dietary intake in man [1, 3, 4]. Numerous studies demonstrate that the intake of EPA and DHA with the diet is associated with various beneficial health effects [5–8].
The oxidation products of PUFA, so called oxylipins, are highly potent mediators [9, 10]. The formation and biology of AA derived oxylipins, i.e. eicosanoids, has been intensively studied during the past 40 years. Comparatively little attention has been paid to the analogous oxidation products of other n-6 PUFA and n-3 PUFA [11, 12].
In mammals, oxylipins are formed via four major routes/pathways (Figure 1): (i) Conversion by cyclooxygenases (COXs) yields instable endoperoxides which are further converted to several highly potent mediators such as prostaglandins, thromboxans and prostacylclin [10]. (ii) Lipoxygenases form regio- and sterio-selective hydroperoxides, which serve as precursors for leukotrienes and hepoxylins and can be reduced to hydroxy-FA (HETE, HODE, HOTRrE, HEPE, HDoHE, Figure 1) [13]. (iii) Metabolism of PUFA by Cytochrom-P-450 monooxygenases (CYPs) leads to hydroxy-FA at ω and ω-1 position (CYP4 family) while family CYP2 dominantly forms epoxy-FA (EpOME, EpETrE, EpODE, EpETE, and EpDoPE) [9, 14, 15]. These highly potent mediators are chemically stable under physiological pH and stable to nucleophilic attack by glutathione. A major route of their degradation is the hydrolyzation to the corresponding dihydroxy-FA (DiHOME, DiHETrE, DiHODE, DiHETE, and DiHDPE, Figure 1) by the soluble epoxide hydrolase (sEH) [16, 17]. (iv) Non-enzymatic autoxidation of PUFA leads to hydroperoxy-FA, hydroxy-FA and PG like prostanoids [18, 19]. Despite a pronounced substrate, regio- and steriospecificity of the oxylipin generating enzymes, the product pattern of the four processes overlaps. For example, 15-HETE can not only be formed by reticulocyte and epidermis 15-LOX but also by COXs, CYPs and autoxidation [13, 14, 18, 20]. Also human LOX enzymes do not only accept AA as substrate. Via an intermediate hydroperoxide, 5-LOX conversion of EPA leads to 5-HEPE [21]. Similarly, platelet type 12-LOX can catalyse the formation of 12-HEPE from EPA [22]. Reticulocyte type 15-LOX conversion of LA leads to 13-HODE while EPA give rise to 15-HEPE [23, 24]. In an analogous manner it can be assumed that 13-HOTrE is a 15-LOX product of ALA.
Figure 1.
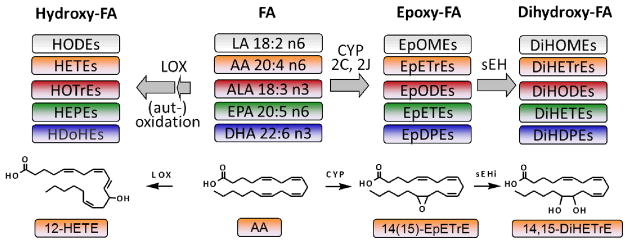
Top: Simplified scheme of the formation of hydroxy-, epoxy- and dihydroxy-fatty acids from their PUFA precursors. Bottom: The structure of several oxylipins are exemplary shown exemplary for the generation of 12-HETE via the lipoxygenase (LOX) pathway and 14(15)-EpETrE and 14,15-DiHETrE by Cytochrom-P450 monooxygenases (CYP) and the soluble epoxide hydrolase (sEH).
Prostaglandins and leukotrienes derived from AA by COX and LOX and their biology are among the most investigated endogenous mediators, and more than half of the currently sold pharmaceutical drugs directly target one of these pathways [25]. The hydroxy-, epoxy- and dihydroxy-FA also show a pronounced biological activity on various regulatory pathways. For example 5-HETE and other hydroxy-FA play a key role in chemotaxis and degranulation of neutrophils [26, 27], being more active than their n-3 PUFA analogs [28]. For 5-HEPE an effect on the glucose-dependent insulin secretion is discussed [29]. 12-HETE mediates the function of many growth factors and thus seems to be a key factor in cancer metastasis [30]. In many studies the blood concentration of hydroxy-FA is used as marker for the activity of a LOX pathway, because they are more stable than the initially formed hydroperoxids and short lived potent mediators as leukotrienes (5-LOX) and hepoxylins (12-LOX). However, the overall biological role of many hydroxy-FA is not fully understood. By contrast, it is well accepted that epoxy-FA play an important role in the regulation of vascular and cardiac function, in particular by acting as vasodilators of human coronary arteries [31, 32]. Moreover, epoxy-FA act anti-inflammatory and analgesic in various animal models, while their corresponding dihydroxy-FA show low or no biological activity [17, 33]. Recently, it was shown that the dihydroxy-FA play an important role in hematopoiesis [34], thus not only the epoxy-FA but the epoxide/diol ratio might be the important factor that governs the regulation of cellular functions.
Up to date, only little information about endogenous levels of hydroxy-, epoxy- and dihydroxy-PUFA in humans is available. Particularly, only few studies comprehensively examined n-3 and n-6 oxylipin profiles including hydroxy-, epoxy-, and dihydroxy-FA in human blood samples [11, 12, 35, 36]. Shearer et al. measured 46 LA, AA, ALA, EPA, and DHA derived oxylipins in plasma after saponification of healthy subjects (n=10) before and after four weeks of n-3 PUFA treatment [12]. Gomolka et al. focused on LOX products of AA, EPA, and DHA by measuring 29 oxylipins, mainly hydroxy-FA, in plasma from healthy subjects (n=5) [35]. Psychogios et al. reported levels of 76 oxylipins in human plasma of which 56 were above the limit of quantification for a large collection of 70 subjects [11]. These studies provide important information on the concentration range of these oxylipins in human blood. However, these studies only examined healthy subjects, which were not further physiologically characterized e.g. by their blood lipid levels.. In order to understand the physiological role of hydroxy-, epoxy- and dihydroxy-FA, knowledge about the endogenous levels in humans and their regulation in response to disease is indispensable. For example, a recent study comprehensively monitoring more than 100 oxylipins could show significant increased plasma 5-HETE and 12-HETE levels as well as their analogous EPA metabolites 5-HEPE and 12-HEPE following heart ischemia during cardiac surgery. Despite the small sample size of only five patients, it could be shown that these LOX metabolites potentially associated with the resolution phase of inflammation were elevated, whereas no changes were observed in the prostaglandin and leukotriene levels [36]. Aside from such severe medical conditions, metabolic disorders may impact the endogenous oxylipin patterns. In the rat model of type-1 diabetes as well as obesity-induced hypertriglyceridemia, significant changes in the oxylipin pattern were observed, which may contribute to the pathophysiological states [37, 38]. Hypertriglyceridemia and Hypercholesterolemia (Hyperlipidemia) are common risk factors for cardiovascular diseases [39] and associated with other metabolic disorders such as the metabolic syndrome [40]. No data are available on if and how hyperlipidemia effect endogenous oxylipin levels in humans. An altered oxylipin pattern could have strong effects on the physiology and thus for example on the development of cardiovascular diseases. In order to investigate the impact of combined hyperlipidemia on oxylipin levels, we compared patterns of 45 free (and non-covalently bound) hydroxy-, epoxy-, and dihydroxy-FA in serum samples of normo- and hyperlipidemic men. Moreover, the data was correlated to the individual status of the five parent PUFA (LA, AA, ALA, EPA, and DHA) to normalize for interindividual varying diets and a potentially changed metabolism of PUFA in the different groups of subjects.
EXPERIMENTAL PROCEDURES
This investigator initiated study was designed and conducted according to the principles of the Good Clinical Practice Guidelines laid down in the Declaration of Helsinki. The study was approved by the Freiburger ethic committee (Freiburg, Germany). All included subjects gave their written informed consent to take part in the study.
Subjects and design
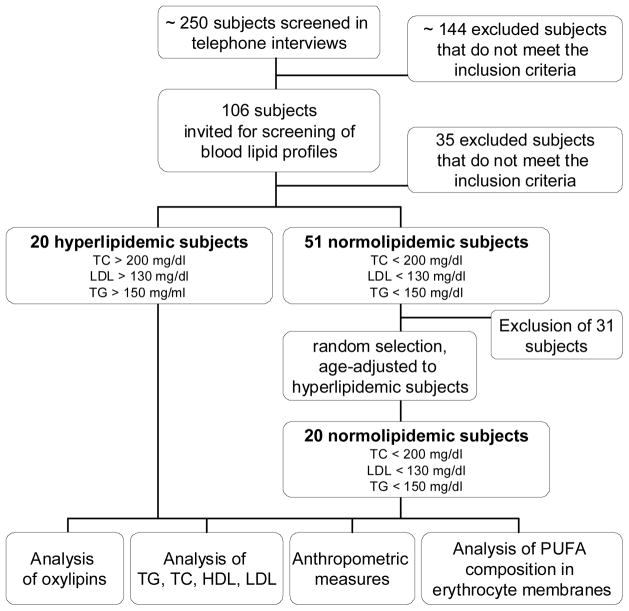
The recruitment of subjects was performed by advertisements in Hannover, Germany. One hundred and six subjects were pre-selected via telephone interviews according to the following exclusion criteria: female; body-mass-index > 35; smoker; intake of any corticosteroids, lipid-lowering or anti-inflammatory drugs; diagnosed chronic, cardiovascular or liver diseases; gastrointestinal disorders; blood coagulation disorders and intake of coagulation-inhibiting drugs; renal failure; periodic intake of laxatives; ingestion of supplements enriched with n-3 PUFA, phytosterols, polyglucosamines, other lipid-binding ingredients. The pre-selected subjects were invited for a screening examination to collect fasting blood and determine serum lipid levels. Among these subjects, twenty men with mild combined hyperlipidemia (threshold value: total cholesterol TC > 200 mg/dl; LDL > 130 mg/dl; TG > 150 mg/ml [41]) and twenty normolipidemic men (TC < 200 mg/dl; LDL < 130 mg/dl; TG < 150 mg/dl) were chosen (Table 1). The recruitment of the study population and selection of the subjects is shown in Figure 2 in more detail. The hyperlipidemic group and the normolipidemic group showed no significant differences in age, height and weight. However, the hyperlipidemic group had a slightly higher mean BMI (p=0.034).
Table 1.
Description of the study population.
| Parameter | Total population (n=40) | Normolipidemic group (n=20) | Hyperlipidemic group (n=20) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| mean | ± | SD | mean | ± | SD | mean | ± | SD | P1 (normo vs. hyper) | |
| Age [years] | 39 | ± | 8 | 38 | ± | 8 | 40 | ± | 9 | 0.337 |
| Body height [cm] | 181 | ± | 7 | 180 | ± | 7 | 181 | ± | 7 | 0.799 |
| Body weight [kg] | 85.2 | ± | 13.7 | 81.1 | ± | 12.8 | 89.3 | ± | 13.6 | 0.057 |
| Body mass index [kg/m2] | 26.1 | ± | 3.49 | 24.9 | ± | 3.40 | 27.3 | ± | 3.26 | 0.034 |
| Total cholesterol [mg/dl] | 223 | ± | 55 | 185 | ± | 17.7 | 261 | ± | 53.2 | <0.001 |
| Triacylglycerol [mg/dl] | 188 | ± | 189 | 100 | ± | 44.6 | 275 | ± | 235 | 0.004 |
| High density lipoprotein [mg/dl] | 51.4 | ± | 10.3 | 56.0 | ± | 10.9 | 47.2 | ± | 8.04 | 0.008 |
| Low density lipoprotein [mg/dl] | 133 | ± | 37.5 | 110 | ± | 19.0 | 160 | ± | 35.7 | <0.001 |
| LDL-C/HDL-C quotient | 2.7 | ± | 0.9 | 2.07 | ± | 0.62 | 3.37 | ± | 0.71 | <0.001 |
| LA [% of total FA] | 15.5 | ± | 2.24 | 15.0 | ± | 2.17 | 16.0 | ± | 2.26 | 0.177 |
| AA [% of total FA] | 14.7 | ± | 2.20 | 15.8 | ± | 1.13 | 13.6 | ± | 2.46 | 0.001 |
| ALA [% of total FA] | 0.32 | ± | 0.23 | 0.25 | ± | 0.09 | 0.40 | ± | 0.29 | 0.046 |
| EPA [% of total FA] | 0.93 | ± | 0.35 | 0.85 | ± | 0.25 | 1.0 | ± | 0.42 | 0.187 |
| DHA [% of total FA] | 4.14 | ± | 1.06 | 4.27 | ± | 0.86 | 4.0 | ± | 1.23 | 0.430 |
All variables were normally distributed.
Significant differences in group means were determined by independent sample t-test.
Figure 2.
Flow chart of recruitment of the human subjects.
Sample collection and analysis of clinical parameters
Blood samples were collected in the morning between 6:00 and 9:30 a.m. after overnight fasting. The samples were obtained by venipuncture of an arm vein into 10 ml BD Vacutainer Blood Collection Tubes (article no.: 367896, Becton Dickinson, Heidelberg, Germany) containing silicate particles as clot activators. After 30 min incubation at room temperature, tubes were centrifuged for 10 min at 2000 × g and serum was transferred into 15 mL falcon tubes (Becton Dickinson) and immediately frozen and stored at −80°C upon extraction and LC-MS analysis. Other sets of blood samples collected simultaneously were sent to external laboratories for the measurement of clinical parameters (Table 1). Serum lipid levels were determined LADR laboratory, Hannover; Germany. Erythrocyte membrane PUFA composition was determined in EDTA stabilized whole blood at Omegametrix, Martinsried, Germany as previously described [42].
Analysis of oxylipins
Analysis of oxylipin levels in serum was carried out by liquid chromatography-electrospray ionization tandem mass spectrometry (LC-ESI-MS/MS) as described [25, 37, 43]. Tthe serum samples (950 μL) were spiked with 20 μL internal standard solution containing 50 nM of 2H11-14,15-DiHETrE,2H4-9-HODE, 2H8-5-HETE, 2H11-11(12)-EpETrE in MeOH. This was followed by addition of antioxidants (10 μL of a 0.2 mg/mL solution of butylated hydroxytoluene and EDTA). In order to extract the free oxylipins in serum, solid phase extraction (SPE) without subsequent protein precipitation was performed. It should be noted that this procedure may also co-extracted oxylipins which are non-covalently bound to proteins. In brief, SPE was carried out as follows: Cartridges (60 mg waters Oasis-HLB, Waters, Milford, MA) were cleaned with one column volume of ethyl acetate (EA) and one of methanol (MeOH). After the serum samples were loaded on preconditioned SPE columns, the columns were washed with two column volumes of 5/95 ACN/water acidified with 0.1% acetic acid. After drying the SPE column applying low vacuum (0.2 bar), the analytes were eluted with 500 μL of MeOH and 1500 μL of EA. The eluted samples were evaporated to dryness and reconstituted in 50 μl of methanol containing 100 nM 1-cyclohexyluriedo-3-dodecanoic acid for the calculation of IS recovery. The resulting extract was filtered using Ultrafree-MC-VV polyvinylidene fluoride filter (pore-size 0.1 μM; Millipore, Bedford, MA), transferred in amber glass vials with insert (Waters) and directly analyzed by LC-ESI-MS/MS. Separation was carried out in 21 minutes on a Agilent 1200 SL LC system (Palo Alto,CA), utilizing a Agilent Zorbax Eclipse Plus C18-reversed phase column (dimensions 2.1 × 150 mm, particle size 1.7 μm) using a gradient of 0.1% acetic acid as solvent A and 80/15/0.1 acetonitrile/methanol/acetic acid as solvent B. The detection was carried out on a 4000 QTRAP instrument (Applied Biosystems, Foster City, CA) operating in negative ion mode. The oxylipins were monitored in so called “scheduled multiple reaction monitoring” mode with a detection window of 90 sec for each compound around the expected retention time and a maximal cycle time of 0.6 sec. Recovery rates of the IS in serum samples were 80±10 % for 2H11-14,15-DiHETrE, for 2H4-9-HODE 70±10%, 70±10% 2H8-5-HETE and 60±10% for 2H11-11(12)-EpETrE and in the same range as previously described [11, 25, 36]. The monitored oxylipins, their transitions and quantification limits are presented the supplementary material (SI, Table S1). Only oxylipins which exceeded the limit of quantification in ≥ 95% of the serum samples were used for data analysis.
Data analysis and statistics
Results for anthropometrical measures, lipid levels as well as erythrocyte membrane PUFA levels are presented as mean ± SD. Serum oxylipin levels are presented as mean ± SE. The influence of the PUFA status on oxylipin levels was investigated by subdividing study participants into two groups with low and high PUFA status. For this purpose, the total study population was divided into tertiles based on PUFA levels in erythrocyte membranes and in the following analysis, groups with low and high PUFA status were compared. Ratios between epoxides/diols as well as between LOX products were calculated on individual level and results are shown as mean ± SD. The sample sets were analyzed for their distribution by the Kolmogorov–Smirnov test. A two tailed t-test for independent samples was used to reveal significant differences between normal distributed groups. In cases of skewed distribution, the Mann–Whitney U test was used. Statistical significance was generally accepted at p≤0.05. Correlation analysis of the normally distributed variables was carried out by the Pearson method. Correlation between the variables was accepted with a correlation coefficient of ≥ 0.5 or ≤ −0.5 and p≤0.05. All statistical analyses were carried out with SPSS software (Version 20, SPSS Inc., Chicago IL, USA).
RESULTS
Among 48 oxylipins measured, 44 hydroxy-, epoxy- and dihydroxy-FA could be detected and quantified in more than 95% of the human serum samples (Table 2, SI Table S1). All oxylipins varied considerably between subjects. The measured concentrations for the analytes ranged over about three orders of magnitude from 50 pM for 11,12-DiHETE to 16 nM for 12-HETE. The concentrations of other hydroxy-FA, ranged from 100 pM to 8 nM. Among these analytes, the metabolites of LA dominated and the concentrations decreased in the order of LA > AA > EPA ≥ ALA. The epoxy-FA were found in a similar concentration range of 200 pM to 6.5 nM. Depending on the parent PUFA, the epoxides were found in the order LA > ALA > AA > DHA > EPA, similar to the dihydroxy-FA levels, which were detected in a concentration range of 50 pM to 11 nM. All LA metabolites were found at a similar concentration of 3 to 8 nM, whereas the levels of hydroxy-, epoxy-, and dihydroxy-FA derived from the other PUFA greatly varied (Table 2). Aside from the dominating metabolite 12-HETE, the epoxy-FA 11(12)-EpETrE and 14(15)-EpETrE and 5-HETE were the major serum AA derived oxylipins with concentrations of about 1.5 nM. By contrast, the dihydroxy-FA 15,16-DiHODE was the major ALA derived metabolite (11 nM), followed by its corresponding epoxide 15(16)-EpHODE (4 nM). Similarly, the epoxy-/dihydroxy-FA metabolites at position n-3 were the major detected oxylipins derived from EPA and DHA. For all three n-3 PUFA, the dihydroxy-FA at position n-3 was found at higher concentration than its corresponding epoxide, whereas the epoxide/diol ratio for all other oxylipins was higher than one (Table 3).
Table 2.
Serum concentration of free oxylipins in total study population and normo- vs. hyperlipidemic groups.
| Parent FA | Oxylipin | Total population (n=40) | Normolipidemic group (n=20) | Hyperlipidemic group (n=20) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| mean [pM] | ± | SE | mean [pM] | ± | SE | mean [pM] | ± | SE | P (normo vs. hyper) | ||||
| n-6 FA | LA | Hydroxy-FA | 9-HODE | 5,900 | ± | 510 | 6,000 | ± | 720 | 5,700 | ± | 750 | 0.7811 |
| 13-HODE | 7,700 | ± | 600 | 8,300 | ± | 820 | 7,100 | ± | 870 | 0.3361 | |||
| Epoxy-FA | 9(10)-EpOME | 5,700 | ± | 660 | 5,500 | ± | 910 | 6,000 | ± | 980 | 0.7421 | ||
| 12(13)-EpOME | 6,500 | ± | 640 | 6,400 | ± | 900 | 6,600 | ± | 930 | 0.8951 | |||
| Dihydroxy-FA | 9,10-DiHOME | 3,500 | ± | 530 | 3,800 | ± | 510 | 3,300 | ± | 940 | 0.6681 | ||
| 12,13-DiHOME | 6,200 | ± | 480 | 7,400 | ± | 700 | 4,900 | ± | 530 | 0.0061 | |||
| AA | Hydroxy-FA | 5-HETE | 1,600 | ± | 130 | 1,700 | ± | 220 | 1,500 | ± | 130 | 0.5571 | |
| 8-HETE | 350 | ± | 13 | 370 | ± | 21 | 330 | ± | 15 | 0.1232 | |||
| 9-HETE | 170 | ± | 8.4 | 180 | ± | 10 | 160 | ± | 13 | 0.3652 | |||
| 11-HETE | 560 | ± | 73 | 690 | ± | 130 | 420 | ± | 50 | 0.0691 | |||
| 12-ETE | 15,500 | ± | 2,900 | 22,100 | ± | 5,200 | 8,900 | ± | 1,900 | 0.025**1 | |||
| 15-HETE | 1,100 | ± | 110 | 1,300 | ± | 190 | 950 | ± | 82 | 0.0881 | |||
| Epoxy-FA | 8(9)-EpETrE | 630 | ± | 62 | 580 | ± | 79 | 680 | ± | 97 | 0.4261 | ||
| 11(12)-EpETrE | 1,400 | ± | 140 | 1,300 | ± | 170 | 1,500 | ± | 230 | 0.4721 | |||
| 14(15)-EpETrE | 1,300 | ± | 130 | 1,200 | ± | 140 | 1,400 | ± | 230 | 0.4031 | |||
| Dihydroxy-FA | 8,9-DiHETrE | 360 | ± | 27 | 270 | ± | 16 | 440 | ± | 45 | 0.0011 | ||
| 11,12-DiHETrE | 560 | ± | 21 | 560 | ± | 33 | 560 | ± | 27 | 0.9391 | |||
| 14,15-DiHETrE | 700 | ± | 22 | 690 | ± | 36 | 720 | ± | 26 | 0.4831 | |||
| n-3 FA | ALA | Hydroxy-FA | 9-HOTrE | 400 | ± | 42 | 440 | ± | 68 | 370 | ± | 50 | 0.4231 |
| 13-HOTrE | 390 | ± | 37 | 440 | ± | 65 | 340 | ± | 37 | 0.1791 | |||
| Epoxy-FA | 9(10)-EpODE | 1,000 | ± | 160 | 1,000 | ± | 230 | 1,100 | ± | 220 | 0.8651 | ||
| 12(13)-EpODE | 530 | ± | 73 | 520 | ± | 110 | 540 | ± | 100 | 0.9131 | |||
| 15(16)-EpODE | 3,700 | ± | 380 | 3,800 | ± | 490 | 3,700 | ± | 590 | 0.9411 | |||
| Dihydroxy-FA | 9,10-DiHODE | 120 | ± | 14 | 160 | ± | 20 | 85 | ± | 15 | 0.0071 | ||
| 12,13-DiHODE | 140 | ± | 14 | 180 | ± | 21 | 100 | ± | 11 | 0.0041 | |||
| 15,16-DiHODE | 11,000 | ± | 950 | 11,000 | ± | 1000 | 9,900 | ± | 1,600 | 0.4561 | |||
| EPA | Hydroxy-FA | 5-HEPE | 330 | ± | 35 | 260 | ± | 31 | 400 | ± | 60 | 0.0421 | |
| 12-HEPE | 460 | ± | 89 | 610 | ± | 160 | 310 | ± | 64 | 0.0931 | |||
| 15-HEPE | 120 | ± | 7.9 | 110 | ± | 9.0 | 130 | ± | 13 | 0.1401 | |||
| Epoxy-FA | 11(12)-EpETE | 170 | ± | 21 | 150 | ± | 27 | 200 | ± | 32 | 0.2661 | ||
| 14(15)-EpETE | 210 | ± | 25 | 180 | ± | 32 | 240 | ± | 38 | 0.2311 | |||
| 17(18)-EpETE | 320 | ± | 27 | 280 | ± | 35 | 370 | ± | 40 | 0.1131 | |||
| Dihydroxy-FA | 11,12-DiHETE | 48 | ± | 3.5 | 42 | ± | 3.3 | 54 | ± | 6.0 | 0.0891 | ||
| 14,15-DiHETE | 83 | ± | 5.6 | 75 | ± | 5.7 | 92 | ± | 9.5 | 0.1311 | |||
| 17,18-DiHETE | 510 | ± | 34 | 470 | ± | 38.1 | 560 | ± | 55 | 0.1841 | |||
| DHA | Epoxy-FA | 10(11)-EpDPE | 700 | ± | 70 | 650 | ± | 97 | 740 | ± | 100 | 0.5151 | |
| 13(14)-EpDPE | 470 | ± | 50 | 440 | ± | 68 | 490 | ± | 75 | 0.5661 | |||
| 16(17)-EpDPE | 480 | ± | 51 | 440 | ± | 66 | 520 | ± | 79 | 0.4131 | |||
| 19(20)-EpDPE | 1,100 | ± | 110 | 930 | ± | 130 | 1,200 | ± | 170 | 0.2921 | |||
| Dihydroxy-FA | 10,11-DiHDPE | 180 | ± | 180 | 140 | ± | 15 | 220 | ± | 29 | 0.0301 | ||
| 13,14-DiHDPE | 200 | ± | 200 | 190 | ± | 16 | 210 | ± | 19 | 0.3991 | |||
| 16,17-DiHDPE | 270 | ± | 270 | 250 | ± | 19 | 300 | ± | 26 | 0.1531 | |||
| 19,20-DiHDPE | 2,100 | ± | 120 | 2,100 | ± | 150 | 2,100 | ± | 180 | 0.9081 | |||
All variables were normally distributed except for 8-HETE, 9-HETE and 12-HETE.
Significant differences in group means were determined by independent sample t-test or
Mann-Whitney U test for skewed distributed variables.
Table 3.
Serum Epoxide/Diol ratios. The serum concentration of epoxy-FA was divided by the concentration of the corresponding dihydroxy-FA on individual level. The mean and SD of the ratio is shown for the total study population and normo- vs. hyperlipidemic groups.
| Parent FA | Epoxide:Diol | Total population (n=40) | Normolipidemic group (n=20) | Hyperlipidemic group (n=20) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| mean | ± | SD | mean | ± | SD | mean | ± | SD | P1 (normo vs. hyper) | ||
| LA | 9(10)-EpOME: 9,10-DiHOME | 2.54 | ± | 0.43 | 2.02 | ± | 0.49 | 3.06 | ± | 0.69 | 0.226 |
| 12(13)-EpOME: 12,13-DiHOME | 1.23 | ± | 0.14 | 0.97 | ± | 0.16 | 1.48 | ± | 0.21 | 0.063 | |
| AA | 8(9)-EpETrE: 8,9-DiHETrE | 1.94 | ± | 0.19 | 2.19 | ± | 0.28 | 1.70 | ± | 0.25 | 0.198 |
| 11(12)-EpETrE: 11,12-DiHETrE | 2.50 | ± | 0.25 | 2.28 | ± | 0.27 | 2.71 | ± | 0.43 | 0.406 | |
| 14(15)-EpETrE: 14,15-DiHETrE | 1.88 | ± | 0.21 | 1.77 | ± | 0.21 | 1.99 | ± | 0.36 | 0.596 | |
| ALA | 9(10)-EpODE: 9,10-DiHODE | 12.2 | ± | 1.98 | 8.05 | ± | 2.08 | 16.3 | ± | 3.15 | 0.035 |
| 12(13)-EpODE: 12,13-DiHODE | 4.30 | ± | 0.55 | 3.14 | ± | 0.51 | 5.46 | ± | 0.92 | 0.035 | |
| 15(16)-EpODE: 15,16-DiHODE | 0.37 | ± | 0.02 | 0.34 | ± | 0.03 | 0.40 | ± | 0.04 | 0.226 | |
| EPA | 11(12)-EpETE: 11,12-DiHETE | 3.89 | ± | 0.44 | 3.57 | ± | 0.53 | 4.22 | ± | 0.72 | 0.475 |
| 14(15)-EpETE: 14,15-DiHETE | 2.69 | ± | 0.32 | 2.38 | ± | 0.35 | 3.0 | ± | 0.54 | 0.342 | |
| 17(18)-EpETE: 17,18-DiHETE | 0.68 | ± | 0.06 | 0.62 | ± | 0.07 | 0.75 | ± | 0.10 | 0.304 | |
| DHA | 10(11)-EpDPE: 10,11-DiHDPE | 4.72 | ± | 0.52 | 5.07 | ± | 0.75 | 4.37 | ± | 0.73 | 0.506 |
| 13(14)-EpDPE: 13,14-DiHDPE | 2.51 | ± | 0.28 | 2.37 | ± | 0.35 | 2.64 | ± | 0.43 | 0.623 | |
| 16(17)-EpDPE: 16,17-DiHDPE | 1.89 | ± | 0.22 | 1.82 | ± | 0.29 | 1.96 | ± | 0.33 | 0.756 | |
| 19(20)-EpDPE: 19,20-DiHDPE | 0.56 | ± | 0.07 | 0.46 | ± | 0.06 | 0.65 | ± | 0.13 | 0.164 | |
All variables were normally distributed.
Significant differences in group means were determined by independent sample t-test.
Comparison of serum oxylipin levels between normo- and hyperlipidemic subjects
The differences in serum oxylipin levels derived from LA, AA, ALA, EPA and DHA between the normo- and hyperlipidemic group were minor (Table 2). Generally, a trend towards lower levels of hydroxy-FA in the hyperlipidemic group was observed, being significant only for 12-HETE (Table 2). On the contrary, the 5-HEPE concentration was significantly elevated compared to the normolipidemic group. For all epoxy-FA a trend to higher levels were found in the hyperlipidemic group, while the levels of the corresponding diols differed dependent on the parent FA. The levels of the LA and ALA derived dihydroxy-FA 12,13-DiHOME, 9,10-DiHODE and 12,13-DiHODE were significantly lower, whereas the concentration of all hydroxy-FA derived from AA, EPA, and DHA were higher in the hyperlipidemic group (significant for 8,9-DiHETrE and 10,11-DiHDPE). When comparing the epoxide/diol ratio on the individual level, no significant differences, expect for the ALA derived oxylipins were observed (Table 3). However, compared to the normolipidemic group, hyperlipidemic men showed a trend to higher epoxide/diol ratios for all monitored oxylipins, except for 10(11)-EpDPE:10,11-DiHDPE and 8(9)-EpETrE:8,9-DiHETrE.
Post-hoc tests for equivalence between the oxylipin pattern of normo- and hyperlipidemic men were carried out by evaluating the 90% confidence intervals (CI) of the mean serum oxylipin level differences of normo- and hyperlipidemic groups from mean levels of the total study population. Only levels of 11,12-DiHETrE and 14,15-DiHETrE were considered as statistically equivalent since the upper and lower CI of the mean differences were within a tolerable variance of ± 20% (SI, Table S2).
Other covariates such as BMI or TG/HDL ratio, a surrogate marker for insulin resistance [44], were checked to possibly influence oxylipin levels. Men with a high BMI (>28) compared to the group with a low BMI (<25) showed no significant differences in the oxylipin pattern. Similarly, no consistent shifts in the oxylipin pattern were found when comparing groups with high and low (> 3.5; <2.2) TG/HDL ratio (SI Figure S2–3).
Correlation of serum oxylipins to their parent PUFA
Levels of free and non-covalently bound oxylipins in serum were compared with the relative PUFA composition in erythrocyte membranes, a robust measure for the PUFA status of an individual [42]. In erythrocyte membranes, the n-6 PUFA LA and AA dominated followed by the n-3 PUFA DHA, EPA and ALA (Table 1), which strongly varied among the subjects (e.g. RSD of EPA 40%). No significant differences in the PUFA composition of erythrocyte membranes were observed for LA, EPA and DHA levels, whereas in hyperlipidemic men AA levels were 7% lower and ALA levels were 38% higher compared to the normolipidemic group (Table 1).
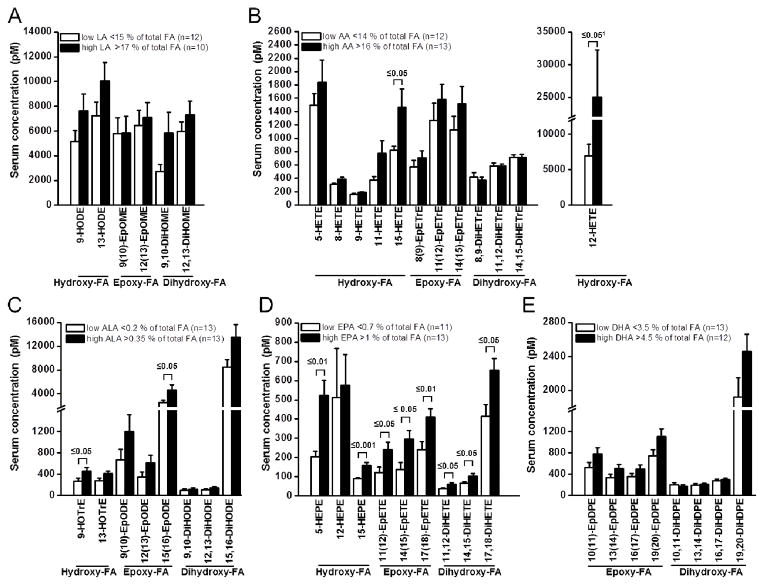
In order to investigate how parent PUFA level affects the formation of their corresponding oxylipins, the total study population was divided into two groups based on a high and low percentage of each PUFA in the erythrocyte membranes. As shown in Figure 3, the serum oxylipin levels were consistently higher with a higher status of their precursor PUFA. However, for the high vs. the low LA, AA, ALA, and DHA status groups, a significant difference was only observed for 12-HETE, 15-HETE and 15(16)-EpODE (Figure 3). By contrast, all EPA derived oxylipins were significantly higher in the high EPA status group compared to the low EPA status group, except for 12-HEPE. For 5-HEPE, 15-HEPE, 11,12-DiHETE and 14,15-DiHETE a significant correlation (r ≥ 0.5) of the EPA status and the oxylipin concentration was found for the total study population (Table 4). Additionally, a positive correlation between ALA levels in erythrocyte membranes and the ALA derived oxylipins 9-HOTrE, 13-HOTrE, 15(16)-EpODE, 9,10-DiHODE, 12,13-DiHODE and 15,16-DiHODE was observed in the hyperlipidemic group (Table 4). No correlation, neither in the total study population, nor in the normolipidemic collective, was found for other parent PUFA (LA, AA, ALA and DHA) and their corresponding oxylipins. Similarly, no significant changes in the AA oxylipin levels between the groups with high and low ALA, EPA and DHA were observed (SI Figure S1).
Figure 3.
Dependence of serum oxylipin levels on parent fatty acid status. Oxylipin levels derived from A) linoleic acid (LA, 18:2 n-6), B) arachidonic acid (AA, 20:4 n-6), C) alpha-linolenic acid (ALA, 18:3 n-3), D) eicosapentaenoic acid (EPA, 20:5 n-3) and E) docosahexaenoic acid (DHA, 22:6 n-3) were compared in tertiles of the whole study population with low and high PUFA percentage in erythrocyte membranes. All results are shown as the mean ± SE. Significant differences were determined by independent sample t-test for normal distributed variables. 1 Mann-Whitney U test was used for skewed distributed variables.
Table 4.
Correlation between serum oxylipin concentration and the percentage of the parent PUFA (LA, AA, ALA, EPA, and DHA) in erythrocyte membranes. Only those oxylipins are presented, where a significant correlation (correlation coefficient ≥ 0.5 and p≤0.05) could be observed. The analysis was carried out in the total study population and independently in the in normo- and hyperlipidemic subgroups.
| Total population (n=40) | EPA | |||
|---|---|---|---|---|
| Hydroxy-FA | 5-HEPE | r | 0.572*** | |
| 15-HEPE | r | 0.524** | ||
| Dihydroxy-FA | 11,12-DiHETE | r | 0.517** | |
| 14,15-DiHETE | r | 0.523** | ||
| Normolipidemic group (n=20) | EPA | |||
| Hydroxy-FA | 5-HEPE | r | 0.650** | |
| Epoxy-FA | 11(12)-EpETE | r | 0.556* | |
| 14(15)-EpETE | r | 0.570* | ||
| 17(18)-EpETE | r | 0.632** | ||
| Hyperlipidemic group (n=20) | ALA | |||
| Hydroxy-FA | 9-HOTrE | r | 0.740*** | |
| 13-HOTrE | r | 0.757*** | ||
| Epoxy-FA | 15(16)-EpODE | r | 0.546* | |
| Dihydroxy-FA | 9,10-DiHODE | r | 0.846*** | |
| 12,13-DiHODE | r | 0.749*** | ||
| 15,16-DiHODE | r | 0.517* | ||
| EP* | ||||
| Hydroxy-FA | 5-HEPE | r | 0.512* | |
| 15-HEPE | r | 0.511* | ||
| Dihydroxy-FA | 14,15-DiHETE | r | 0.525* | |
| 17,18-DiHETE | r | 0.524* |
All variables were normally distributed and correlation analysis was carried out according to Pearson
p<0.05
p<0.005
p<0.001
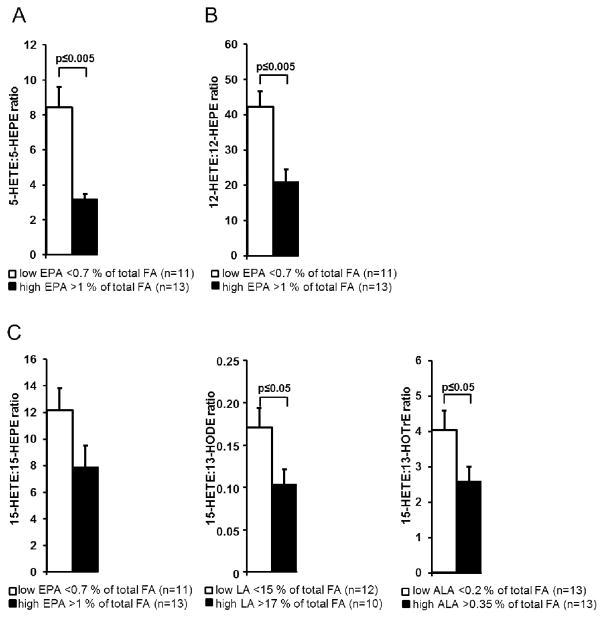
The ratios of AA derived HETE to the corresponding hydroxy-FA of other PUFA were correlated with the status of the precursor PUFA to evaluate changes in the LOX product pattern. For 12-LOX, the ratio of 12-HETE to 12-HEPE was compared between the group with high EPA vs. low EPA levels (Figure 4). For 5-LOX, the ratio of 5-HETE to 5-HEPE was used. Finally for 15-LOX, the ratio of 15-HETE to 13-HODE, 13-HOTrE and 15-HEPE were compared to the status of LA, ALA and EPA, respectively. For all PUFA and all LOX pathways, the ratios were significantly decreased in the high PUFA groups, except for 15-HETE:15-HEPE (Figure 4). The ratio between 5-HETE:5-HEPE and 12-HETE:12-HEPE negatively correlated with the relative erythrocyte membrane EPA content in the total population as well as in the normo- and hyperlipidemic groups (SI Table S3). Additionally, a correlation in the ratio of 15-HETE:13-HOTrE, a potential 15-LOX product of ALA, was observed in the normo- and hyperlipidemic group. Compared to the normolipidemic group, the hyperlipidemic group showed a trend towards lower HETE:hydroxy-PUFA ratios (SI Table S4). With a significant lower ratio of 5-HETE:5-HEPE, 12-HETE:12-HEPE and 15-HETE:15-HEPE, the product ratios of all LOX pathways were changed towards the EPA product.
Figure 4.
Serum hydroxy fatty acid ratios depending on parent fatty acid status. Ratios between 5-lipoxygenase (5-LOX) products (A: 5-HETE:5-HEPE), 12-LOX products (B: 12-HETE:12-HEPE), and 15-LOX products (C: 15-HETE:15-HEPE; 15-HETE:13-HODE; 15-HETE:13-HOTrE) were compared between tertiles of the total study population with low and high PUFA status. All results are shown as the mean ± SE of group measurement. Significant differences in group means were determined by independent sample t-test.
DISCUSSION
While prostanoids and leukotrienes are well characterized, little information is available on the endogenous levels and biological roles of n-6 and n-3 PUFA derived hydroxy-, epoxy-, and dihydroxy-FA in humans. Several studies demonstrate that these oxylipins act as potent modulators of biological processes with opposing effects on inflammation and other biological functions. Thus, knowledge about their endogenous levels in human blood and changes in different physiological and pathophysiological states is of pivotal importance in biology and medicine.
In this study, we present quantitative data on 44 oxylipin serum levels from a cohort of 40 subjects, which had normal and elevated serum triglyceride and cholesterol levels. We found that oxylipin concentrations varied strongly among subjects, consistent with all previous studies monitoring oxylipins in human blood samples [11, 12, 25, 36]. The observed concentration of hydroxy-, epoxy- and dihydroxy-FA in serum were well in line with previously described levels of these oxylipins in plasma [11]. The oxylipin concentration in plasma can be strongly effected by the anticoagulant used [45]. Serum is a standard matrix which is used for the analysis of many biochemical parameters in blood and can be obtained in highly reproducible quality. The deviation between the mean of concentration in 40 human serum samples analyzed in this study compared to 70 plasma samples reported by Psychogios [11] was lower than 50% and within the standard deviations for 17 out of 33 oxylipins (SI Table S5). Giordano et al. described in the plasma of subjects (n=14) almost the same concentration (deviation ≤ 3%) of the three AA derived dihydroxy-FA as found in serum [46], while their reported EpETrE levels in plasma were 2–7 fold lower than the observed levels in serum as well as in plasma described by Psychologios et al.[11]. Comparing the human plasma levels of five oxylipins measured by Gomolka et al, 15-HETE, 12-HEPE were found almost at the same concentration (800 pM and 380 pM), whereas the concentrations of 5-HETE and 5-HEPE were 3–5 fold lower as in our study. The human plasma oxylipin levels, observed in subjects (n=5) shortly before cardiac surgery, reported by Strassburg et al., were overall 2–5 times higher than concentrations obtained in serum [36]. For example, the mean concentration in plasma of 5-HEPE was found to be around 1 nM, compared to our serum analysis (0.3 nM), and the plasma analyses of Gomolka (0.06 nM) and Psychologios (0.2 nM) [11, 35, 36]. The comparison of oxylipin levels to earlier studies demonstrates that the levels of most hydroxy-, epoxy-, and dihydroxy-FA in serum are comparable to those of plasma samples and the deviation between serum and plasma levels is for most analytes not higher than those from two independent plasma analyses. Nevertheless, a dramatic formation of COX-1 metabolites, particularly thromboxanes, has to be taken into account when analyzing serum because of the blood coagulation process involving the platelet activation and degranulation [11]. Similarly, we found markedly higher 12-HETE levels of 16±3 nM in serum compared to 2–4 nM described in plasma [11, 36], probably caused by platelet 12-LOX activated by blood coagulation.
As expected, the serum levels of hydroxy-, epoxy- and dihydroxy-FA were considerably lower, compared to plasma levels after liberation of ester-bound oxylipins. Shearer et al. reported plasma levels of oxylipins after saponification for a group of subjects (n=10) before and after treatment with n-3 PUFA covering the same set of hydroxy-, epoxy- and dihydroxy-FA [12]. A comparison of the plasma levels before n-3 PUFA treatment unveiled that the difference between the serum levels and the sum of free and liberated ester bound oxylipins in plasma, reported by Shearer et al., are strongly dependent on the oxylipin (SI Table 4). For example, the autoxidation product of AA, 9-HETE, is found at about 200 fold higher concentration after saponification indicating that this metabolite is predominantly esterified in plasma, and/or formed during saponification. By contrast, the (free) serum levels of PUFA dihydroxylated at position n-6 and n-3 (12,13-DiHOME, 14,15-DiHETrE, 15,16-DiHODE, 19,20-DiHDPE) were observed in the same range as in plasma after saponification. This indicates that these more polar compounds predominantly circulate as free oxylipins. The corresponding epoxides, as well as their constitution isomers (e.g. 9,10-DiHOME, 8,9-DiHETrE) seems to be predominantly esterified to more than 90% with a 10 fold higher concentration found in plasma after conjugate cleavage (SI Table 4). This is consistent with the observation that the epoxide/diol ratios of the serum levels of n-3-epoxy-FA are < 1, while being on the side of the epoxides for the n-6 and n-9 and all other epoxy-FA (Table 3). The n-3 epoxy-FA are hydrolyzed by sEH at low or medium rate compared to their regioisomers [17]. Thus, the observed differences in the epoxide/diol ratios within the regioisomers of the n-3 FA can not be explained by differences in their degradation and may be caused by differences in their esterification. Oxylipin specific esterification seems to be a key factor in the regulation of the serum concentration of hydroxy-, epoxy- and hydroxy-FA, which warrants further research.
Strong differences in the levels of the different regioisomers of the epoxy-FA and corresponding dihydroxy-FA of each PUFA were observed. Particularly, it is striking that for ALA, EPA, and DHA the n-3 epoxy-FA 15(16)-EpODE, 17(18)-EpETE and 19(20)-EpDPE and their corresponding dihydroxy-FA are present in serum in much higher levels than their n-6 and n-9 analogs. By contrast, the AA is almost equally epoxizdized at the n-6 and n-9 position (Table 2). This observation is consistent with the product specificity of the CYP2J and CYP2C enzymes [9, 47], indicating that these are the CYP families which form the epoxy-FA present in serum.
Correlation of serum oxylipins to their parent PUFA
The overall serum concentration of the different PUFA oxylipins decreased in the order LA > AA = ALA ≥ DHA > EPA and correlates well with the abundance of the PUFA in the human organism (Table 1, 2). Subjects with a high PUFA status, determined as percentage of the PUFA in the erythrocyte membrane, show a trend to higher serum levels of hydroxy-, epoxy-, dihydroxy-FA derived from this PUFA (Figure 3). Hence, it can be concluded as a first approximation that the endogenous formation of these oxylipins is primarily dependent on the availability of the substrate. However, no significant correlation between either LA or AA status and deriving oxylipin serum levels could be observed, except for 15- and 12-HETE (Figure 3). Similarly, high endogenous levels of DHA did not lead to significantly higher levels of its epoxy- and dihydroxy-metabolites (Figure 3). Thus, for these three abundant PUFA, substrate availability seems to be only of moderate importance in the regulation of oxylipin formation.
By contrast, the formation of EPA derived oxylipins seems to be strongly dependent on the availability of the substrate. Subjects with a high EPA status showed significant higher levels of all EPA derived oxylipins except for 12-HEPE, which varied strongly among the subjects (Figure 3). Given that the synthesis rate of EPA from ALA in humans is very low (Brenna 2009 Chambaz 1985), the EPA status largely relies on the diet. Numerous nutritional intervention studies have shown that EPA levels in plasma and erythrocyte membranes increase substantially by supplementation with EPA- and DHA-rich fish oils (Neubronner 2011, Köhler 2010; Vidgren 1997; Cao 2006). Taken together, one can conclude that the EPA oxylipin levels are directly affected by the diet. This hypothesis warrants further investigation particularly because several EPA oxylipins are highly active lipid mediators, e.g. the resolving anti-inflammatory resolvins of the E series [48]. Shearer et al. already demonstrated that treating healthy subjects (n=10) with n-3 PUFA ethyl esters (4 g/day for 4 weeks) significantly increased the plasma levels of several esterified and free EPA derived oxylipins (5-, 12-, and 15-HEPE, 14(15)-, and 17(18)-EpETE; 14,15-, and 17,18-DiHETE) [12]. Moreover, Keenan et al showed that the response of subjects on n-3-PUFA treatment depends on the basal n-3 PUFA status [49]. However, no data about the influence of fish oil supplementation or other dietary interventions on the free oxylipin levels in blood (plasma or serum) have been reported so far.
The beneficial health effects of long chain n-3 PUFA are believed to be partly mediated by a replacement of AA at the active side of LOXs and COXs, thus reducing the eicosanoid formation. In our study, we did not observe any significant differences in the AA oxylipins between subjects with a high and a low status of long chain n-3 PUFA (SI Figure S1). However, when regarding the ratio of LOX products of different PUFA based on the hydroxy-FA as pathway markers, significant changes were observed depending on the PUFA status of the subjects. A significant lower ratio of HETE and the corresponding hydroxy-PUFA product of 5-LOX, 12-LOX and 15-LOX was observed in subjects with a high status of LA, ALA, and EPA (Figure 4, SI, Figure S3). Particularly, the negative correlation of the HETE/HEPE ratio with the EPA status (SI Table S3) shows that the LOX product patterns are significantly influenced by the availability of substrate and thus could be modified with the diet. However, it should be noted that we observed LOX activity solely on the hydroxy-FA, being a product of the initially formed hydroperoxide. The latter can be converted to different oxylipins aside from a reduction to the hydroxy-FA. In order to evaluate the alteration of the LOX product pattern, a larger oxylipin pattern including leukotrienes should be investigated in the future.
Comparison of oxylipin levels between normo- and hyperlipidemic groups
Similar to all previous studies which monitored oxylipins in plasma [11, 12, 36], the serum oxylipin levels, varied considerably among the human subjects. Aside from varying PUFA intake via the diet, differences in lipid metabolism, such as hyperlipidemia, could be an underlying cause for this variation. However, when analyzing the oxylipin levels dependent on the triglyceride and cholesterol status, the same variation of hydroxy-, epoxy- and dihydroxy-FA was observed within the normolipidemic and hyperlipidemic group. Only levels of six oxylipins were significantly different between the groups, slightly exceeding the theoretical number of three incorrectly identified differences (false discovery rate of about 60 variables and an alpha error of 5%). Significant differences were observed for 12-HETE, 5-HEPE and four dihydroxy-FA of different PUFA (Table 2). Since the major portion of 12-HETE is probably formed during serum preparation by platelet 12-LOX, the difference is most likely caused by differences in the blood coagulation between the groups and does not reflect an altered formation in vivo. The major route of dihydroxy-FA formation is the hydrolysis of epoxy-FA by sEH (Figure 1). In order to analyze the altered levels of dihydroxy-FA, levels were therefore compared to those of the corresponding epoxy-FA. For all epoxy-/dihydroxy-FA of the different PUFA, except for 8(9)-EpETrE, a trend to higher epoxide/diol ratio in the hyperlipidemic group was observed (Table 3). A high epoxide/diol correlates with anti-inflammatory, vasodilatory and analgesic effects, caused by inhibition of sEH using sEH inhibitors (sEHi) [50]. However, for most of the epoxide/diol ratios of the different PUFAs the slightly higher epoxide/diol ratios in the hyperlipidemic group were based on higher epoxy-FA levels and thus do not imply an altered sEH activity in hyperlipidemic men (Table 1).
Interestingly, the strongest difference observed in hyperlipidemic men, was the increased ALA status (Table 1). Taking the influence of PUFA status on the oxylipin formation into account, it is no surprise that this was accompanied by changes in the ALA derived oxylipins levels. Interestingly, lower levels of 9(10)-DiHODE and 12,13(DiHODE) were found, leading to a significant increase in the epoxide/diol ratio for these oxylipins in the hyperlipidemic group (Table 1, 2). Moreover, the hyperlipidemic group also showed a significant correlation between ALA status and ALA derived oxylipins (Table 4), whereas no such correlation was found in the normolipidemic group. However, it seems unlikely that the observed changes in oxylipin metabolism are correlated to hyperlipidemia. Rather these changes seem to be caused by the elevated ALA status of the hyperlipidemic subjects, being a confounding factor of the investigated collective of subjects. Similarly, the decreased HETE/HEPE ratios in the hyperlipidemic group are rather correlated with a significant decreased ALA/EPA ratio, than caused by elevated blood lipid levels.
Conclusion
The analysis of serum samples for 44 oxylipins unveiled no apparent differences between normo- and hyperlipidemic subjects. Despite altered blood lipid levels, only very minor or no changes in the serum concentration of hydroxy-, epoxy- and dihydroxy-FA were observed. This indicates that the formation and degradation of this class of lipid metabolites are under tight endogenous control, which is not significantly altered by mild metabolic diseases. However, the interindividual variance in the serum oxylipin concentration was high, which might mask effects of hyperlipidemia on blood oxylipin levels. In the whole collective of subjects, we observed a strong correlation between the EPA content in the erythrocyte membrane and the serum concentration of EPA derived hydroxy-, epoxy-, and dihydroxy-FA. Given that the synthesis of EPA from ALA in the human is low, this suggests, that oxylipin levels can be directly influenced by the diet.
Acknowledgments
This study was supported by a Marie Curie Career Integration Grant to NHS, a Grant of the German Federal Ministry of Education and Research (BMBF) to AH, a Kekulé Ph.D. fellowship of the Fonds der Chemischen Industry to IW and a grants of US National Institutes of Health (NIH), Enviromental Health (NIEHS, P42 ES004699 and R01 ES002710) and Diabetes and Digestive and Kidney Diseases (NIDDK, U24 DK097154) and the West Coast Central Comprehensive Metabolomics Resource Core (WC3MRC) to BDH. BDH is a George and Judy Marcus Senior Fellow of the American Asthma Association.
References
- 1.Burdge GC, Calder PC. Conversion of alpha-linolenic acid to longer-chain polyunsaturated fatty acids in human adults. Reproduction, nutrition, development. 2005;45:581–597. doi: 10.1051/rnd:2005047. [DOI] [PubMed] [Google Scholar]
- 2.Whelan J. The health implications of changing linoleic acid intakes. Prostag Leukotr Ess. 2008;79:165–167. doi: 10.1016/j.plefa.2008.09.013. [DOI] [PubMed] [Google Scholar]
- 3.Brenna JT, Salem N, Jr, Sinclair AJ, Cunnane SC. alpha-Linolenic acid supplementation and conversion to n-3 long-chain polyunsaturated fatty acids in humans. Prostaglandins, leukotrienes and essential fatty acids. 2009;80:85–91. doi: 10.1016/j.plefa.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 4.Plourde M, Cunnane SC. Extremely limited synthesis of long chain polyunsaturates in adults: implications for their dietary essentiality and use as supplements, Applied physiology, nutrition, and metabolism = Physiologie appliquee, nutrition et metabolisme. 2007;32:619–634. doi: 10.1139/H07-034. [DOI] [PubMed] [Google Scholar]
- 5.Cabo J, Alonso R, Mata P. Omega-3 fatty acids and blood pressure. Br J Nutr. 2012;107(Suppl 2):S195–200. doi: 10.1017/S0007114512001584. [DOI] [PubMed] [Google Scholar]
- 6.Delgado-Lista J, Perez-Martinez P, Lopez-Miranda J, Perez-Jimenez F. Long chain omega-3 fatty acids and cardiovascular disease: a systematic review. Br J Nutr. 2012;107(Suppl 2):S201–213. doi: 10.1017/S0007114512001596. [DOI] [PubMed] [Google Scholar]
- 7.Ortega RM, Rodriguez-Rodriguez E, Lopez-Sobaler AM. Effects of omega 3 fatty acids supplementation in behavior and non-neurodegenerative neuropsychiatric disorders. Br J Nutr. 2012;107(Suppl 2):S261–270. doi: 10.1017/S000711451200164X. [DOI] [PubMed] [Google Scholar]
- 8.Rangel-Huerta OD, Aguilera CM, Mesa MD, Gil A. Omega-3 long-chain polyunsaturated fatty acids supplementation on inflammatory biomakers: a systematic review of randomised clinical trials. Br J Nutr. 2012;107(Suppl 2):S159–170. doi: 10.1017/S0007114512001559. [DOI] [PubMed] [Google Scholar]
- 9.Arnold C, Konkel A, Fischer R, Schunck WH. Cytochrome P450-dependent metabolism of omega-6 and omega-3 long-chain polyunsaturated fatty acids. Pharmacol Rep. 2010;62:536–547. doi: 10.1016/s1734-1140(10)70311-x. [DOI] [PubMed] [Google Scholar]
- 10.Buczynski MW, Dumlao DS, Dennis EA. Thematic Review Series: Proteomics. An integrated omics analysis of eicosanoid biology. J Lipid Res. 2009;50:1015–1038. doi: 10.1194/jlr.R900004-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Psychogios N, Hau DD, Peng J, Guo AC, Mandal R, Bouatra S, Sinelnikov I, Krishnamurthy R, Eisner R, Gautam B, Young N, Xia JG, Knox C, Dong E, Huang P, Hollander Z, Pedersen TL, Smith SR, Bamforth F, Greiner R, McManus B, Newman JW, Goodfriend T, Wishart DS. The Human Serum Metabolome. Plos One. 2011;6 doi: 10.1371/journal.pone.0016957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shearer GC, Harris WS, Pedersen TL, Newman JW. Detection of omega-3 oxylipins in human plasma and response to treatment with omega-3 acid ethyl esters. J Lipid Res. 2010;51:2074–2081. doi: 10.1194/jlr.M900193-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brash AR. Lipoxygenases: occurrence, functions, catalysis, and acquisition of substrate. J Biol Chem. 1999;274:23679–23682. doi: 10.1074/jbc.274.34.23679. [DOI] [PubMed] [Google Scholar]
- 14.Bylund J, Kunz T, Valmsen K, Oliw EH. Cytochromes P450 with bisallylic hydroxylation activity on arachidonic and linoleic acids studied with human recombinant enzymes and with human and rat liver microsomes. J Pharmacol Exp Ther. 1998;284:51–60. [PubMed] [Google Scholar]
- 15.Kroetz DL, Zeldin DC. Cytochrome P450 pathways of arachidonic acid metabolism. Current opinion in lipidology. 2002;13:273–283. doi: 10.1097/00041433-200206000-00007. [DOI] [PubMed] [Google Scholar]
- 16.Morisseau C, Hammock BD. Epoxide hydrolases: mechanisms, inhibitor designs, and biological roles. Annual review of pharmacology and toxicology. 2005;45:311–333. doi: 10.1146/annurev.pharmtox.45.120403.095920. [DOI] [PubMed] [Google Scholar]
- 17.Morisseau C, Inceoglu B, Schmelzer K, Tsai HJ, Jinks SL, Hegedus CM, Hammock BD. Naturally occurring monoepoxides of eicosapentaenoic acid and docosahexaenoic acid are bioactive antihyperalgesic lipids. J Lipid Res. 2010;51:3481–3490. doi: 10.1194/jlr.M006007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garscha U, Nilsson T, Oliw EH. Enantiomeric separation and analysis of unsaturated hydroperoxy fatty acids by chiral column chromatography-mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2008;872:90–98. doi: 10.1016/j.jchromb.2008.07.013. [DOI] [PubMed] [Google Scholar]
- 19.Niki E. Lipid peroxidation products as oxidative stress biomarkers. Biofactors. 2008;34:171–180. doi: 10.1002/biof.5520340208. [DOI] [PubMed] [Google Scholar]
- 20.Mulugeta S, Suzuki T, Hernandez NT, Griesser M, Boeglin WE, Schneider C. Identification and absolute configuration of dihydroxy-arachidonic acids formed by oxygenation of 5S-HETE by native and aspirin-acetylated COX-2. J Lipid Res. 2010;51:575–585. doi: 10.1194/jlr.M001719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee TH, Austen KF. Arachidonic acid metabolism by the 5-lipoxygenase pathway, and the effects of alternative dietary fatty acids. Advances in immunology. 1986;39:145–175. doi: 10.1016/s0065-2776(08)60350-8. [DOI] [PubMed] [Google Scholar]
- 22.Yeung J, Holinstat M. 12-lipoxygenase: a potential target for novel anti-platelet therapeutics. Cardiovascular & hematological agents in medicinal chemistry. 2011;9:154–164. doi: 10.2174/187152511797037619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ford-Hutchinson AW, Gresser M, Young RN. 5-Lipoxygenase. Annual review of biochemistry. 1994;63:383–417. doi: 10.1146/annurev.bi.63.070194.002123. [DOI] [PubMed] [Google Scholar]
- 24.Vang K, Ziboh VA. 15-lipoxygenase metabolites of gamma-linolenic acid/eicosapentaenoic acid suppress growth and arachidonic acid metabolism in human prostatic adenocarcinoma cells: possible implications of dietary fatty acids. Prostaglandins, leukotrienes, and essential fatty acids. 2005;72:363–372. doi: 10.1016/j.plefa.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 25.Yang J, Schmelzer K, Georgi K, Hammock BD. Quantitative profiling method for oxylipin metabolome by liquid chromatography electrospray ionization tandem mass spectrometry. Anal Chem. 2009;81:8085–8093. doi: 10.1021/ac901282n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Spector AA, Gordon JA, Moore SA. Hydroxyeicosatetraenoic acids (HETEs) Prog Lipid Res. 1988;27:271–323. doi: 10.1016/0163-7827(88)90009-4. [DOI] [PubMed] [Google Scholar]
- 27.Stenson WF, Parker CW. Prostaglandins, macrophages, and immunity. J Immunol. 1980;125:1–5. [PubMed] [Google Scholar]
- 28.Heidel JR, Taylor SM, Laegreid WW, Silflow RM, Liggitt HD, Leid RW. In vivo chemotaxis of bovine neutrophils induced by 5-lipoxygenase metabolites of arachidonic and eicosapentaenoic acid. The American journal of pathology. 1989;134:671–676. [PMC free article] [PubMed] [Google Scholar]
- 29.Kogure R, Toyama K, Hiyamuta S, Kojima I, Takeda S. 5-Hydroxy-eicosapentaenoic acid is an endogenous GPR119 agonist and enhances glucose-dependent insulin secretion. Biochemical and Biophysical Research Communications. 2011;416:58–63. doi: 10.1016/j.bbrc.2011.10.141. [DOI] [PubMed] [Google Scholar]
- 30.Honn KV, Tang DG, Gao X, Butovich IA, Liu B, Timar J, Hagmann W. 12-lipoxygenases and 12(S)-HETE: role in cancer metastasis. Cancer Metastasis Rev. 1994;13:365–396. doi: 10.1007/BF00666105. [DOI] [PubMed] [Google Scholar]
- 31.Campbell WB, Falck JR. Arachidonic acid metabolites as endothelium-derived hyperpolarizing factors. Hypertension. 2007;49:590–596. doi: 10.1161/01.HYP.0000255173.50317.fc. [DOI] [PubMed] [Google Scholar]
- 32.Fleming I. Vascular cytochrome P450 enzymes: Physiology and pathophysiology. Trends in Cardiovascular Medicine. 2008;18:20–25. doi: 10.1016/j.tcm.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 33.Thomson SJ, Askari A, Bishop-Bailey D. Anti-inflammatory effects of epoxyeicosatrienoic acids. International journal of vascular medicine. 2012;2012:605101. doi: 10.1155/2012/605101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fromel T, Jungblut B, Hu J, Trouvain C, Barbosa-Sicard E, Popp R, Liebner S, Dimmeler S, Hammock BD, Fleming I. Soluble epoxide hydrolase regulates hematopoietic progenitor cell function via generation of fatty acid diols. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:9995–10000. doi: 10.1073/pnas.1206493109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gomolka B, Siegert E, Blossey K, Schunck WH, Rothe M, Weylandt KH. Analysis of omega-3 and omega-6 fatty acid-derived lipid metabolite formation in human and mouse blood samples. Prostaglandins & other lipid mediators. 2011;94:81–87. doi: 10.1016/j.prostaglandins.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 36.Strassburg K, Huijbrechts AM, Kortekaas KA, Lindeman JH, Pedersen TL, Dane A, Berger R, Brenkman A, Hankemeier T, van Duynhoven J, Kalkhoven E, Newman JW, Vreeken RJ. Quantitative profiling of oxylipins through comprehensive LC-MS/MS analysis: application in cardiac surgery. Anal Bioanal Chem. 2012;404:1413–1426. doi: 10.1007/s00216-012-6226-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Inceoglu B, Wagner KM, Yang J, Bettaieb A, Schebb NH, Hwang SH, Morisseau C, Haj FG, Hammock BD. Acute augmentation of epoxygenated fatty acid levels rapidly reduces pain-related behavior in a rat model of type I diabetes. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:11390–11395. doi: 10.1073/pnas.1208708109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shearer GC, Newman JW. Lipoprotein lipase releases esterified oxylipins from very low-density lipoproteins. Prostaglandins, leukotrienes, and essential fatty acids. 2008;79:215–222. doi: 10.1016/j.plefa.2008.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Poss J, Custodis F, Werner C, Weingartner O, Bohm M, Laufs U. Cardiovascular disease and dyslipidemia: beyond LDL. Current pharmaceutical design. 2011;17:861–870. doi: 10.2174/138161211795428858. [DOI] [PubMed] [Google Scholar]
- 40.Gade W, Schmit J, Collins M, Gade J. Beyond obesity: the diagnosis and pathophysiology of metabolic syndrome. Clin Lab Sci. 2010;23:51–61. [PubMed] [Google Scholar]
- 41.Grundy S, Becker D, Clark R, Cooper M, Denke J, Howard D, Hunninghake R, Illingworth R, Luepker P, McBride J, McKenney R, Pasternak N, Stone LV, Horn L. Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) National Cholesterol Education Program National Heart, Lung, and Blood. 2002;2001 NIH Publication No. 01-3670. [Google Scholar]
- 42.Harris WS, Von Schacky C. The Omega-3 Index: a new risk factor for death from coronary heart disease? Preventive medicine. 2004;39:212–220. doi: 10.1016/j.ypmed.2004.02.030. [DOI] [PubMed] [Google Scholar]
- 43.Schebb NH, Inceoglu B, Morisseau C, Ahn KC, Gee SJ, Hammock BD. Investigation of human exposure to triclocarban after showering, and preliminary evaluation of its biological effects. Environmental science & technology. 2011;45:3109–3115. doi: 10.1021/es103650m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McLaughlin T, Abbasi F, Cheal K, Chu J, Lamendola C, Reaven G. Use of metabolic markers to identify overweight individuals who are insulin resistant. Annals of Internal Medicine. 2003;139:802–809. doi: 10.7326/0003-4819-139-10-200311180-00007. [DOI] [PubMed] [Google Scholar]
- 45.Goodfriend TL, Pedersen TL, Grekin RJ, Hammock BD, Ball DL, Vollmer A. Heparin, lipoproteins, and oxygenated fatty acids in blood: a cautionary note. Prostaglandins, leukotrienes, and essential fatty acids. 2007;77:363–366. doi: 10.1016/j.plefa.2007.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Giordano RM, Newman JW, Pedersen TL, Ramos MI, Stebbins CL. Effects of dynamic exercise on plasma arachidonic Acid epoxides and diols in human volunteers. International journal of sport nutrition and exercise metabolism. 2011;21:471–479. doi: 10.1123/ijsnem.21.6.471. [DOI] [PubMed] [Google Scholar]
- 47.Arnold C, Markovic M, Blossey K, Wallukat G, Fischer R, Dechend R, Konkel A, von Schacky C, Luft FC, Muller DN, Rothe M, Schunck WH. Arachidonic acid-metabolizing cytochrome P450 enzymes are targets of {omega}-3 fatty acids. J Biol Chem. 2010;285:32720–32733. doi: 10.1074/jbc.M110.118406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang MJ, Spite M. Resolvins: anti-inflammatory and proresolving mediators derived from omega-3 polyunsaturated fatty acids. Annual review of nutrition. 2012;32:203–227. doi: 10.1146/annurev-nutr-071811-150726. [DOI] [PubMed] [Google Scholar]
- 49.Keenan AH, Pedersen TL, Fillaus K, Larson MK, Shearer GC, Newman JW. Basal omega-3 fatty acid status affects fatty acid and oxylipin responses to high-dose n3-HUFA in healthy volunteers. J Lipid Res. 2012;53:1662–1669. doi: 10.1194/jlr.P025577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Inceoglu B, Wagner K, Schebb NH, Morisseau C, Jinks SL, Ulu A, Hegedus C, Rose T, Brosnan R, Hammock BD. Analgesia mediated by soluble epoxide hydrolase inhibitors is dependent on cAMP. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:5093–5097. doi: 10.1073/pnas.1101073108. [DOI] [PMC free article] [PubMed] [Google Scholar]