Sepsis-induced multi-organ dysfunction syndrome (MODS) still has a high mortality. Improvements await a better understanding of the pathophysiological mechanisms. The angiopoietin (Ang)1/2 and Tie2 (tyrosine kinase with immunoglobulin and epidermal growth factor homology domains 2) ligand/receptor system is an important regulator of endothelial cell responses to severe insults [1]. Plasma Ang2 levels are prognostic in sepsis, but data on Ang/Tie responses in organs in humans are lacking [2-5]. We hypothesized that, in kidneys of patients who died of sepsis with acute kidney injury (AKI), the Ang/Tie signaling system is changed in such a way that microvessels become destabilized.
Patients dying with sepsis-induced MODS were included. In the family conference preceding withdrawal or withholding of therapy, permission was asked for a renal biopsy as a partial autopsy to be performed immediately after death. The family was asked to give signed consent. The unaffected part of kidneys removed for renal cell carcinoma was used as a control (n = 8). mRNA was analyzed as described [3].
Histopathology revealed abnormalities without a recognizable pattern (Table 1). CD31 mRNA, a pan-endothelial marker, did not differ between patients and controls, suggesting equal endothelial content in the biopsies (Figure 1). Neutrophil gelatinase-associated lipocalin, a sensitive marker for renal damage, showed increased mRNA expression compatible with AKI and the histopathological findings. Ang1 mRNA in patients was decreased 10-fold, whereas Ang2 was decreased to a lesser extent. The Ang1/Ang2 ratio was decreased in sepsis. Tie2 mRNA was reduced as was the level of expression of Krüppel-like factor (KLF)2, a shear stress sensor. A correlation was found both in patients and in controls between KLF2 and Tie2 mRNA.
Table 1.
Patient characteristics
| Patient | Age, years | Diagnosis | LOS in ICU, days | RIFLE | RRT needed? | Time to biopsy, minutes a | Number of glomeruli b | Predominant histology c |
|---|---|---|---|---|---|---|---|---|
| 1 |
85 |
Small bowl ischemia |
3 |
R |
No |
32 |
61 |
Apoptosis, flattened epithelium, vacuoles |
| 2 |
62 |
Pneumosepsis |
1 |
I |
No |
25 |
24 |
Apoptosis, flattened epithelium |
| 3 |
55 |
Pneumosepsis |
2 |
I |
Yes |
30 |
40 |
Little apoptosis, flattened epithelium, vacuoles |
| 4 |
62 |
Necrotizing fasciitis |
2 |
F |
No |
150 |
22 |
Focally lymphoid infiltration, evident vacuolization, flattened epithelium |
| 5 |
57 |
Colon perforation |
2 |
F |
Yes |
31 |
15 |
Dilated glomerular capillaries, vacuolization |
| 6 |
83 |
Small bowel ischemia |
1 |
I |
No |
45 |
26 |
Apoptosis, vacuolization |
| 7 |
78 |
Pneumonia |
2 |
F |
Yes |
25 |
19 |
Focally leukocyte infiltration, apoptosis, flattened epithelium |
| 8 |
79 |
Necrotizing fasciitis |
6 |
F |
No |
43 |
47 |
Apoptosis, flattened and lost epithelium, vacuolization |
| 9 |
77 |
Pneumonia |
3 |
F |
No |
50 |
22 |
Apoptosis, flattened epithelium |
| 10 |
53 |
Pneumonia |
2 |
F |
Yes |
35 |
69 |
Apoptosis, flattened epithelium |
| 11 | 83 | Sinusitis, meningitis | 2 | F | Yes | 53 | 27 | Neutrophil infiltration (nodular), vacuolization |
aTime between circulation arrest and renal biopsy; bnumber of glomeruli found in the histology of the biopsy; cpathologic conclusion of the histology of the biopsies (performed by MH and AD). LOS in ICU, length of stay in the intensive care unit; RIFLE, Risk, Injury, Failure, Loss, End-stage kidney injury; RRT, renal replacement therapy.
Figure 1.
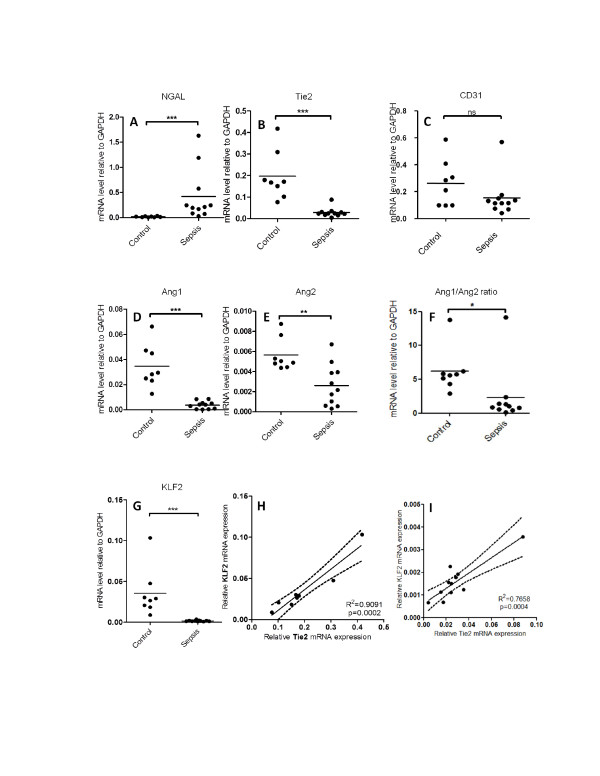
Kidney damage, endothelial Angiopoietin/Tie2 system, and flow responsive gene expression in renal biopsies from sepsis patients compared to healthy kidney part from patients with renal cell carcinoma. Renal neutrophil gelatinase-associated lipocalin (NGAL) (A) (P=0,0004), Tyrosine-protein kinase receptor TIE-2 (Tie2) (B) (P=0,0004), cluster of differentiation 31 (CD31) (C) (NS), Angiopoietin 1 (Ang1) (P=0,0003) (D), Angiopoietin 2 (Ang2) (E) (P=0,005), Angiopoietin 1/Angiopoietin 2 ratio (F) (P=0,0467), Kruppel-like factor 2 (KLF2) (G) (P=0,0003) mRNA gene expression in renal biopsies from sepsis patients (n=11) compared to healthy kidney part from patients with renal cell carcinoma (n=8). Each dot represents the mean of two biopsies per patient. Tie2 gene expression levels in renal biopsies correlates with the mRNA levels of KLF2 in both control biopsies (R2= 0,91; P=0,0002) (H) as well as in kidney biopsies from sepsis patients (R2= 0,70; P<0,0001) (I). Low expression of Tie2 is associated with low levels of KLF2 gene expression. Lines denote best fit, with the dashed lines representing the 95% confidence interval for best fit. ns, Not significant. *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001.
Our hypothesis that the Ang/Tie system is changed in a way that microvessels become destabilized in sepsis-induced AKI is supported by our finding that Ang1, Ang1/Ang2 ratio, and Tie2 mRNA levels were decreased in immediate post-mortem renal biopsies of patients with sepsis. The consequences on protein levels are the subject of further study. We previously reported flow sensitivity of Tie2 expression [5]. Combined with the KLF2 flow sensitivity, the correlation between KLF2 and Tie2 suggests that the decrease in Tie2 in patients dying of sepsis might be due to changes in local blood flow. This mechanism would be additional to, and maybe synergistic with, other mechanisms such as inflammation and apoptosis as described in the literature and seen in the histology in our study.
Studies in organs of critically ill patients immediately after dying, especially examining gene expression status, are scarce. In biopsies taken immediately after circulation arrest, the effects of autolysis are avoided. However, patients dying of sepsis die after a long process with increasing organ damage. Instead of studying an illness-induced change, we might be studying a shutdown process comparable to what happens at a cellular level in apoptosis. We show that immediate post-mortem samples contain high-quality RNA.
Our study is the first to show, on an organ level in humans, that the Ang/Tie2 axis is changed in AKI. The place of immediate post-mortem mRNA in the understanding of MODS should be further evaluated. It might be a new tool complementing human plasma and animal studies.
Abbreviations
AKI: Acute kidney injury; Ang: Angiopoietin; KLF: Krüppel-like factor; MODS: Multi-organ dysfunction syndrome; Tie: Tyrosine kinase with immunoglobulin and epidermal growth factor homology domains.
Competing interests
The authors declare that they have no competing interests.
Contributor Information
Adnan Aslan, Email: a.aslan@umcg.nl.
Rianne M Jongman, Email: r.m.jongman@umcg.nl.
Jill Moser, Email: j.moser@umcg.nl.
Coen A Stegeman, Email: c.a.stegeman@umcg.nl.
Harry van Goor, Email: h.van.goor@umcg.nl.
Arjan Diepstra, Email: a.diepstra@umcg.nl.
Marius C van den Heuvel, Email: m.c.van.den.heuvel@umcg.nl.
Peter Heeringa, Email: p.heeringa@umcg.nl.
Grietje Molema, Email: g.molema01@umcg.nl.
Jan G Zijlstra, Email: j.g.zijlstra@umcg.nl.
Matijs van Meurs, Email: m.van.meurs@umcg.nl.
Acknowledgments
The medical ethical committee considered the study as part of the regular autopsies to confirm the cause of death. The committee allowed direct post-mortem renal biopsies to avoid loss of tissue integrity. The further use of anonymized tissues for research was allowed (University Medical Center Groningen, Medical Ethical Committee, chairman Willem A Kamps, registration number 2011/372). We thank Peter J Zwiers for technical assistance.
References
- van Meurs M, Kumpers P, Ligtenberg JJ, Meertens JH, Molema G, Zijlstra JG. Bench-to-bedside review: Angiopoietin signalling in critical illness - a future target? Crit Care. 2009;13:207. doi: 10.1186/cc7153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumpers P, Lukasz A, David S, Horn R, Hafer C, Faulhaber-Walter R, Fliser D, Haller H, Kielstein JT. Excess circulating angiopoietin-2 is a strong predictor of mortality in critically ill medical patients. Crit Care. 2008;12:R147. doi: 10.1186/cc7130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Meurs M, Wulfert FM, Knol AJ, de Haes A, Houwertjes M, Aarts LP, Molema G. Early organ-specific endothelial activation during hemorrhagic shock and resuscitation. Shock. 2008;29:291–299. doi: 10.1097/SHK.0b013e318145a7c1. [DOI] [PubMed] [Google Scholar]
- van Meurs M, Kurniati NF, Wulfert FM, Asgeirsdottir SA, de Graaf IA, Satchell SC, Mathieson PW, Jongman RM, Kümpers P, Zijlstra JG, Heeringa P, Molema G. Shock-induced stress induces loss of microvascular endothelial Tie2 in the kidney which is not associated with reduced glomerular barrier function. Am J Physiol Renal Physiol. 2009;297:F272–F281. doi: 10.1152/ajprenal.00137.2009. [DOI] [PubMed] [Google Scholar]
- Kurniati NF, Jongman RM, Vom Hagen F, Spokes KC, Moser J, Regan ER, Krenning G, Moonen JR, Harmsen MC, Struys MM, Hammes HP, Zijlstra JG, Aird WC, Heeringa P, Molema G, van Meurs M. The flow dependency of Tie2 expression in endotoxemia. Intensive Care Med. 2013;39:1262–1271. doi: 10.1007/s00134-013-2899-7. [DOI] [PubMed] [Google Scholar]


