Abstract
We investigated the proportion of orthopaedic procedures supported by evidence from randomised controlled trials comparing operative procedures to a non-operative alternative. Orthopaedic procedures conducted in 2009, 2010 and 2011 across three metropolitan teaching hospitals were identified, grouped and ranked according to frequency. Searches of the Cochrane Central Register of Controlled Trials (CENTRAL), the Cochrane Database of Systematic Reviews (CDSR) and the Database of Abstracts of Reviews of Effects (DARE) were performed to identify RCTs evaluating the most commonly performed orthopaedic procedures. Included studies were categorised as “supportive” or “not supportive” of operative treatment. A risk of bias analysis was conducted for included studies using the Cochrane Collaboration's Risk of Bias tool. A total of 9,392 orthopaedic procedures were performed across the index period. 94.6% (8886 procedures) of the total volume, representing the 32 most common operative procedure categories, were used for this analysis. Of the 83 included RCTs, 22.9% (19/83) were classified as supportive of operative intervention. 36.9% (3279/8886) of the total volume of procedures performed were supported by at least one RCT showing surgery to be superior to a non-operative alternative. 19.6% (1743/8886) of the total volume of procedures performed were supported by at least one low risk of bias RCT showing surgery to be superior to a non-operative alternative. The level of RCT support for common orthopaedic procedures compares unfavourably with other fields of medicine.
Introduction
“Evidence based medicine (EBM) is the conscientious, explicit, and judicious use of current best evidence in making decisions about the care of individual patients”[1]. Since this definition by Sackett in 1996, EBM has been adopted and included in most developed medical and health care curricula around the world [2], [3]. The study design that provides the best (least biased) evidence for the efficacy and safety of an intervention is the randomised controlled trial (RCT). The process of randomised allocation equally distributes prognostic factors among study groups, and is therefore the best tool in dealing with confounding [4] Empirical research has shown that healthcare decisions on medical wards are more likely to be based on randomised trial evidence (53%) [5] than those on surgical wards (24% to 26%) [6]–[8].
RCTs assessing operative interventions face particular challenges. Operative procedures are often difficult to standardise [9], [10], are frequently conducted in an emergency setting [11], and patients may find difficulty in accepting either the validity of the non-operative comparison [12] or the risk of potential, and often irreversible, adverse outcomes associated with the operative option [13]. Furthermore, academic surgical units are less common when compared with other specialties [14], and surgeon equipoise, particularly when comparing operative and non-operative treatments, is difficult to obtain [11].
Almost two decades ago, surgical research was ridiculed as a “comic opera” as most peer reviewed publications were found to be case series or professional opinion [15]. While as a proportion of publications surgical RCTs are becoming more common [16]–[18], RCTs comparing operative procedures to (best) non-operative management remain uncommon. These comparisons are important to establish the utility of any operative procedure.
While empirical research has been conducted on the quantity and quality of orthopaedic and surgical randomised trials [7], [19], [20], little is known about whether current clinical orthopaedic practice has an evidence base from randomised trials. An appreciation of what volume and proportion of commonly performed procedures have any evidence from randomised trials (when compared to non-operative interventions) is relevant when deciding whether resources are being used appropriately.
Our aims were: i) to determine the proportion of the total number of commonly performed orthopaedic procedures that are supported by RCT evidence (indicating operative treatment may be superior to non-operative treatment), and ii) to investigate the risk of bias in RCTs that have compared commonly performed orthopaedic procedures to non-operative alternatives. A secondary aim was to establish what proportion of procedure types was supported by RCT evidence in favour of the operative approach over a non-operative approach.
Methods
Identification and Selection of Orthopaedic Procedures
We reviewed the RCT evidence for commonly performed orthopaedic procedures in three major metropolitan teaching hospitals in southwest Sydney, Australia. The hospitals were chosen as they are from a single large health district, but have different roles: one general hospital (mixed minor elective and trauma), one hospital with a large elective orthopaedic caseload, and one with a large trauma orthopaedic workload. Data pertaining to orthopaedic procedures conducted in 2009, 2010 and 2011 were extracted from an electronic clinical database recording details of all procedures undertaken in the operating theatres of these hospitals. All data pertaining to patient records and/or information was de-identified prior to extraction and analysis. Operative procedures - defined as any procedure conducted in an operating theatre by an orthopaedic team that involved penetration of the skin - were identified, grouped under general headings and ranked according to frequency (Appendix S1). Procedure groupings were determined according to anatomical site and/or nature of the procedure performed. For example, “unilateral total arthroplasty of the hip”, “bilateral total arthroplasty of the hip”, “revision of total arthroplasty of the hip” and “revision of partial arthroplasty of the hip” were grouped under the general heading “hip arthoplasty”. In order to obtain a representative sample, the procedures that comprised the top 95% of the total volume of procedures performed (according to frequency) were included in this study.
Identification and Selection of RCTs
A search of the Cochrane Central Register of Controlled Trials (CENTRAL), the Cochrane Database of Systematic Reviews (CDSR) and the Database of Abstracts of Reviews of Effects (DARE) was performed to identify RCTs examining each operative procedure. CENTRAL, CDSR and DARE were chosen as these databases index the vast majority of published (and many unpublished) RCTs [21]. For each procedure, a search strategy was formulated with the help of a medical librarian, incorporating an RCT filter used by the Scottish Intercollegiate Guidelines Network. Retrieved abstracts were independently examined by two reviewers, and included if the study was a full-text RCT comparing the operative procedure to non-operative treatment(s). We defined an RCT as a study in which participants are randomly (that is, by chance) assigned to one of two or more treatment arms of the clinical trial [22]. RCTs comparing different operative modalities, for instance mobile versus fixed bearing implants for total knee arthroplasty, were excluded as the primary aim of the study was to determine the evidence base for operative interventions compared to non-operative alternatives. RCTs were also excluded after full-text assessment if the method of participant allocation was quasi-randomised (e.g. by date of birth or alternation), if the publication related to a follow-up study utilising a cohort from previously published RCTs or if published after December 2010. Excluding recent RCTs increased the likelihood that the study was available at the time of the intervention.
Data Extraction and Categorisation
Included RCTs were independently assessed in their entirety by two reviewers, and the sample size and population, the specific interventions being compared, primary outcome, primary outcome findings, secondary outcomes, findings of secondary outcomes and the authors' conclusions were identified. Given that our objective was to determine whether any RCT evidence may be found for commonly performed orthopaedic procedures, we crudely categorized each RCT as being either 'supportive’ or 'not supportive’ of operative treatment using the following criteria:
For RCTs where a primary outcome was stated, and the findings demonstrated a statistically significant result in favour of operative treatment for the stated primary outcome – regardless of the results of any stated secondary outcome(s) – the study was categorised as being 'supportive of operative treatment'.
For RCTs where a primary outcome was stated, and the findings demonstrated a statistically significant result in favour of the non-operative treatment or where no significant difference was shown for the stated primary outcome, the study was categorised as being 'not supportive of operative treatment'.
For RCTs where a primary outcome was not stated but several outcomes were measured, and where statistically significant results were demonstrated across all measured outcomes in favour of operative treatment, the study was categorized as “supportive of operative treatment”.
For RCTs where a primary outcome was not stated but several outcomes were measured, and where the measured outcomes did not consistently demonstrate statistically significant results in favour of operative treatment, the study was categorised as 'not supportive of operative treatment'".
Discrepancies were resolved by discussion and arbitrated by a third author if necessary.
Risk of Bias Assessment
A risk of bias analysis was conducted using the Cochrane Collaboration's Risk of Bias tool [23]. The tool was applied to extract information for six risk of bias categories: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, attrition bias and selective reporting. For each category, the risk of bias was categorised into high, unclear, or low. An RCT was assessed to be at low risk of bias based on its performance across two domains – allocation concealment and blinding of outcome assessment. These domains were chosen for bias assessment as the significance of good allocation concealment and outcome assessment blinding in minimising bias and, in particular, overestimation of treatment effect is well supported by empirical evidence [24], [25]. While empirical evidence also exists to support the significance of adequate blinding of participants in reducing exaggeration of estimated treatment effects [25] the inherent difficulty of blinding participants in surgical RCTs necessitated the exclusion of this domain in our assessment. Random sequence generation was not included in the final assessment as the exclusion criteria for quasi-randomisation meant that most of the included RCTs performed well within this domain. In contrast to the first four domains, empirical evidence to support the significance of incomplete outcome data on RCT bias is lacking and mainly driven by theoretical considerations [26], [27], and was therefore not included in our assessment.
Data Analysis
Results were analysed and presented as:
The proportion of the total volume of procedures that were assessed by RCTs;
-
The proportion of the total volume of procedures that were:
supported by at least one RCT
supported by at least one low risk of bias RCT
supported by at least one RCT with sample size greater than or equal to the median sample size of included RCTs
Primary analyses were conducted using total procedure volume, as this was a more accurate reflection of surgical volume. Secondary analyses were conducted using procedure type, rather than total procedure volume; this analysis would highlight which procedure types were supported by high-level evidence.
Results
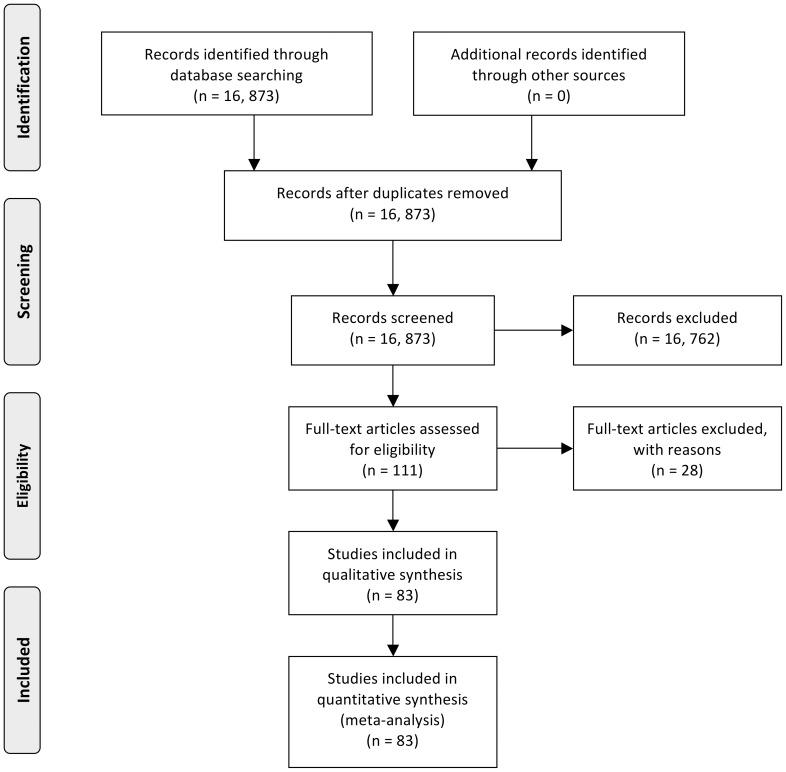
A total volume of 9,392 orthopaedic procedures were performed across the three hospitals in the years 2009, 2010 and 2011 comprising 91 operative procedure categories. 94.6% (8,886 procedures) of the total volume was represented by 32 operative procedure categories. This group was used as the basis for this study. The 10 most commonly performed procedures comprised 80.6% of the 8,886 procedures included in the study (Table 1). 32 different search strategies were executed on CENTRAL, CDSR and DARE databases for dates up to the end of 2010. The search strategies are outlined in Appendix S2. The flowchart of records retrieved from CENTRAL, CDSR and DARE and the screening process to obtain the included articles relevant to each procedure are provided in Table 2. A total of 16,873 abstracts were screened, and of these 83 RCTs were included (Figure 1).
Table 1. 10 most commonly performed orthopaedic surgical procedures in 2009, 2010, 2011 ordered according to frequency.
| Procedure: | Number of procedures performed | |
| 1 | Knee arthroscopy | 1349 |
| 2 | Knee arthroplasty | 1023 |
| 3 | Hip arthroplasty | 917 |
| 4 | Removal/debridement/wound cleaning | 775 |
| 5 | Internal fixation of proximal or shaft fracture of the femur | 766 |
| 6 | Internal fixation of distal radius fracture | 765 |
| 7 | Removal of implants | 697 |
| 8 | Ankle fracture fixation | 435 |
| 9 | Acromioplasty repair of rotator cuff | 237 |
| 10 | Shoulder arthroscopy | 202 |
| Total | 7166 | |
Table 2. Flow of RCT screening, inclusion and surgical procedure support for each search executed on CENTRAL/CDSR/DARE on 32 unique procedures.
| Procedure: | Number of procedures performed | Number of articles retrieved from CENTRAL/CDSR/DARE | Number of RCTs included after title/abstract screening | Number of RCTs included after full text screening | Number of RCTs supporting surgical procedure | Number of RCTs not supporting surgical procedure | Number of low risk of bias RCTs supporting surgical procedure | Number of RCTs with sample size ≥ 72 supporting surgical procedure | |
| 1 | Knee arthroscopy | 1349 | 861 | 12 | 10 | 1 | 9 | 1 | 1 |
| 2 | Knee arthroplasty | 1023 | 1798 | 0 | 0 | - | - | - | - |
| 3 | Hip arthroplasty | 917 | 771 | 0 | 0 | - | - | - | - |
| 4 | Removal/debridement/wound cleaning | 775 | 1967 | 0 | 0 | - | - | - | - |
| 5 | Internal fixation of proximal or shaft fracture of the femur | 766 | 862 | 2 | 2 | 0 | 2 | 0 | 1 |
| 6 | Internal fixation of distal radius fracture | 765 | 683 | 14 | 11 | 2 | 9 | 0 | 1 |
| 7 | Removal of implants | 697 | 859 | 0 | 0 | - | - | - | - |
| 8 | Ankle fracture fixation | 435 | 1028 | 9 | 7 | 2 | 5 | 0 | 1 |
| 9 | Acromioplasty repair of rotator cuff | 237 | 171 | 8 | 5 | 1 | 4 | 1 | 1 |
| 10 | Shoulder arthroscopy | 202 | 224 | 0 | 0 | - | - | - | - |
| 11 | Open reduction of fracture of shaft of tibia with internal fixation | 170 | 337 | 4 | 4 | 0 | 4 | 0 | 2 |
| 12 | Osteotomy | 169 | 560 | 2 | 2 | 1 | 1 | 0 | 0 |
| 13 | Open reduction of joint dislocation (shoulder, acromioclavicular & patella respectively) | 157 | 120, 14, 22 | 9, 3, 6 | 6, 3, 4 | 5, 0, 1 | 1, 3, 3 | 2, 0, 0 | 1, 0, 0 |
| 14 | Knee, repair of cruciate ligament | 157 | 662 | 6 | 3 | 0 | 3 | 0 | 1 |
| 15 | Tibia, plateau of, medial or lateral fracture, open | 100 | 341 | 0 | 0 | - | - | - | - |
| 16 | Repair of achilles tendon rupture | 89 | 281 | 13 | 9 | 0 | 9 | 0 | 2 |
| 17 | Humerus, distal, treatment of fracture by open reduction | 87 | 191 | 3 | 1 | 0 | 1 | 0 | 0 |
| 18 | Olecranon, treatment of fracture by open reduction (intern fix) | 82 | 433 | 0 | 0 | - | - | - | - |
| 19 | Arthroscopy of ankle | 77 | 48 | 0 | 0 | - | - | - | - |
| 20 | Joint arthrodesis | 76 | 742 | 1 | 1 | 0 | 1 | 0 | 0 |
| 21 | Abscess drainage | 69 | 988 | 5 | 5 | 1 | 4 | 0 | 0 |
| 22 | Clavicle, treatment of fracture, open reduction | 61 | 89 | 4 | 3 | 1 | 2 | 0 | 1 |
| 23 | Patella, treatment fracture, by internal fixation open reduction | 53 | 142 | 0 | 0 | - | - | - | - |
| 24 | Humerus, proximal, treatment of fracture, open reduction | 53 | 191 | 3 | 2 | 0 | 2 | 0 | 0 |
| 25 | Amputation | 48 | 678 | 0 | 0 | - | - | - | - |
| 26 | Foot (not talus or calcaneus) fracture fixation | 47 | 285 | 0 | 0 | - | - | - | - |
| 27 | Acetabulum, treatment of fracture by open reduction | 42 | 443 | 0 | 0 | - | - | - | - |
| 28 | Excision of ganglion | 40 | 198 | 0 | 0 | - | - | - | - |
| 29 | Wedge resection of ingrown toenail | 39 | 301 | 0 | 0 | - | - | - | - |
| 30 | Release of carpal tunnel | 37 | 397 | 6 | 5 | 4 | 1 | 0 | 3 |
| 31 | Shoulder Arthroplasty | 37 | 99 | 0 | 0 | - | - | - | - |
| 32 | Fasciotomy | 30 | 87 | 0 | 0 | - | - | - | - |
| Total | 8886 | 16873 | 110 | 83 | 19 | 64 | 3 | 15 | |
Figure 1. Flow diagram of searches executed, abstracts screened, full-texts screened and RCTs included.
Of the 83 RCTs included, 22.9% (19/83) were classified as supportive of operative intervention. The median sample size of the included RCTs was 72. A list of included and excluded studies is found in Appendix S3; characteristics of included studies are summarised in Appendix S4.
Proportion of Total Procedure Volume with RCT Evidence
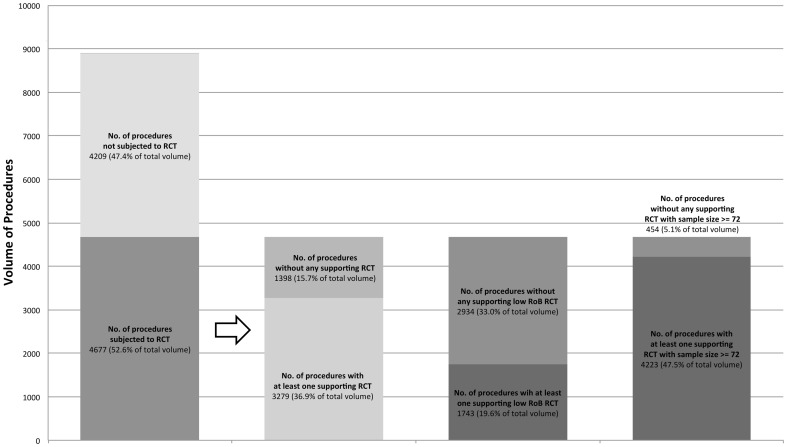
52.6% (4,677/8886) of the total volume of procedures were subjected to an RCT comparing operative to non-operative treatment. 36.9% (3279/8886) of total volume of procedures had at least one RCT supporting operative intervention. When analysed according to risk of bias assessments, 19.6% (1743/8886) of total volume of procedures had at least one low risk of bias RCT supporting operative intervention. When analysed against sample size, 47.5% (4223/8886) of total volume of procedures had at least one RCT with sample size greater than or equal to 72 supporting operative intervention (Figure 2).
Figure 2. Procedure Volume versus Degree of RCT Evidence and Support.
Proportion of Procedure Types with RCT Evidence
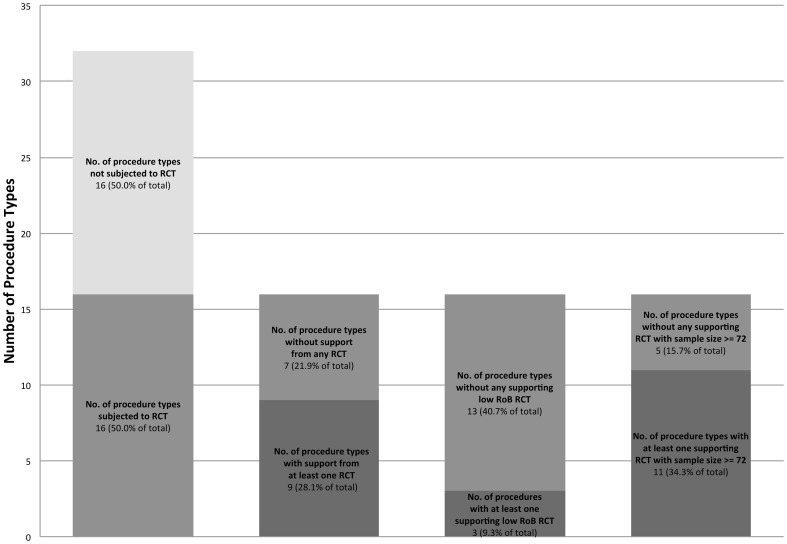
Of the 32 procedure types comprising 94.6% of the total volume of procedures, 16 (50%) had RCTs comparing the procedure to non-operative treatment. 28.1% (13/32) procedure types had at least one RCT supporting operative intervention. When analysed according to risk of bias, 9.3% (3/32) procedure types had at least one low risk of bias RCT supporting the operative intervention. 34.3% (11/32) procedure types had at least one RCT with sample size greater or equal to 72 supporting operative intervention (Figure 3).
Figure 3. Procedure Type versus Degree of RCT Evidence and Support.
Risk of Bias Summary
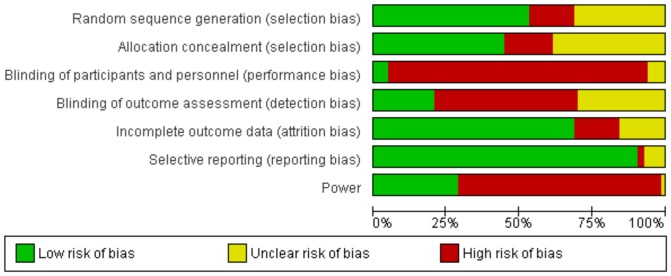
12 of the 83 included RCTs were assessed as having low risk of bias across the two domains: allocation concealment and blinding of outcome assessment. 26 RCTs were assessed as having unclear risk of bias and 45 as having high risk of bias across the two domains. Four of the 12 RCTs assessed as having low risk of bias (Arden 2008, Moosmayer 2010, Wintzell 1999 and Wintzell 2000) were supportive of operative treatment.
A risk of bias summary for each domain is depicted in Figure 4. Overall, studies performed well in dealing with biases related to selective reporting and attrition, but only half of all trials had adequate methods related to randomisation. There was generally a high risk of bias in the domains of blinding, which reflects the difficulty of blinding trials involving operative interventions, particularly when comparing to non-operative treatments. Risk of bias data for each of the 83 included RCTs is included in Appendix S5.
Figure 4. Risk of bias assessment summary for 83 included RCTs using the Cochrane Collaboration's risk of bias tool.
Discussion
We reviewed the available RCT evidence in relation to commonly performed orthopaedic procedures in the years 2009, 2010 and 2011 to establish what proportion of these procedures was supported by RCT evidence. We found that 52.6% of the total volume of orthopaedic procedures performed had been compared to non-operative treatments in at least one RCT, and that 36.9% of the total volume of procedures performed was supported by at least one RCT in favour of the operative treatment. Where RCT evidence was available, the quality of that evidence was largely deemed sub-optimal due to inherent biases in the trial methodology.
While the proportion of RCTs in surgical publications has increased over recent decades, our findings suggest that the proportion of surgical interventions supported by quality RCT-level evidence remains low. In general, this is in keeping with the findings of earlier studies.
Howes et al conducted a prospective study looking at the evidence-base for surgical decisions made on 100 patients admitted to a general surgical ward in a tertiary teaching hospital. The literature concerning the efficacy of each treatment was reviewed and it was found that while 95% of the surgical interventions studied were evidence-based, only 24% were based on RCT-level evidence, defined as at least one RCT in favour of the surgical approach [6]. Similar results were reported in a study by Baraldini et al who analysed the levels of evidence for surgical procedures performed on 49 patients admitted over a 4-week period to a paediatric surgical unit. The study found that while 97% of the procedures performed were evidence based, only 26% were based on RCT-level evidence where there was at least one study in favour of surgery [8]. Findings from a prospective review by Kenny et al of 281 paediatric patients who all received primary surgical interventions again showed a low proportion (11%) of RCT-level evidence for the interventions [17]. These figures are substantially lower than the values of 53% and 57% obtained in general medicine, as reported by Ellis et al and Michaud et al, respectively [5], [28].
For surgical procedures performed commonly, it is not unreasonable to expect decision-making to be based on a high level of scientific evidence. Our findings, however, show that despite 52.6% of the total procedure volume being subjected to at least one RCT, only 19.6% of performed procedures had at least one low risk of bias RCT supporting the operative treatment over non-operative alternatives. Similarly, only a minority of the procedure types were supported by higher quality RCT evidence in favour of the surgical procedure. These comparatively low figures are in part explained by our methodology. In contrast to the three studies highlighted above, our study analysed the results according to the quality of RCT evidence. If the figures obtained in the current study were interpreted using the same definition as that of the previous studies, this would result in 36.9% of total volume of procedures having support from at least one RCT. While this figure may seem comparatively favourable, we consider our assessment to be a more accurate reflection of available evidence.
Despite the methodological differences between studies, the common finding is that there is a paucity of high-level evidence to support a large volume of surgical procedures. This disconnect between evidence and practice may be explained by several factors, including clinician bias. Katz, in his ethnography on the culture of surgeons, contended that “surgeons have been resistant to accepting new scientific findings and applying them to their practice" [29]. The lack of generalisability of randomised controlled trial findings to individual patients is often proposed as a reason for the surgeon's reticence to accept RCT evidence [30]. However well-conducted the trial, the rigidity of RCT design means that the findings can only suggest what was more effective for a specific group of patients with a particular condition, but not whether this evidence is applicable to a particular case or to an individual patient [30]. An RCT is also considered by surgeons to be too simplistic to adequately assess the complex nature of surgical interventions [32]. The various components of a surgical intervention in the pre-operative, intra-operative and post-operative stages all significantly influence the final outcome - the complexity of which cannot be adequately accounted for by an RCT [31]. That the quality of the RCT evidence that is available is generally poor may also serve to explain the lack of acceptance.
The 83 included RCTs had variable levels of bias. The risk of bias summary for each surgical procedure consistently showed poor scores in the performance and outcome detection categories of bias due to difficulties with blinding the surgical intervention. Furthermore, a quarter of studies reported poorly on their randomisation strategy while only a handful performed sample size calculations for power. The only category that was consistently adequate across the included RCTs was follow-up. Of the two included domains, RCTs performed most poorly in blinding of outcome assessment. Just 17 studies specifically attempted to address this potential source of detection bias in their methodology and was the largest avoidable contributor to the overall high risk of bias across the included studies.
When RCTs were analysed against sample size, less than half the total volume of procedures were supported by RCTs with a sample size greater than the median of 72. Though inadequate sample size does not directly influence study bias, underpowered studies are more likely to have a greater rates of type II errors in which studies fail to detect statistically significant treatment effects. An investigation by Lochner et al into the rates of type II errors of randomised trials involving orthopaedic fracture care showed higher than accepted levels of type II errors and, low mean level of study power [33]. The high proportion of underpowered orthopaedic RCTs may be one of the reasons for the low support rate seen in our study.
We acknowledge the limitations of our study. Not subdividing the RCT evidence by indication for the specific procedures in our analysis (for example, the removal of implants for pain as opposed to other indications, or cruciate ligament reconstruction for an isolated tear as opposed to a multi-ligamentous injury) is a potential limitation of this study as the RCT evidence support for a procedure may be restricted to specific indications. Given the primary aim of the study was to assess potential differences between available RCT evidence and current clinical practice, an analysis subdividing each of the included procedure groups for indication would have contributed little value to the primary outcome. As our criteria allowed inclusion of supportive RCTs for any indication, however, it is likely that there was a potential overestimation of RCT support for any procedure group. Our study investigated RCT-level evidence exclusively as this study design provides the least biased evidence for the efficacy and safety of an intervention though its ability to deal with confounding [4]. This does not imply that every operative procedure requires RCT-level evidence to support its efficacy over a non-operative alternative. Large, well-designed prospective cohort studies can adequately minimise bias comparable to that of RCTs [28] and therefore have acceptable levels of validity in cases where an RCT may not be feasible [34], [35].
While improvements have occurred in the quantity and to a lesser extent, the quality of surgical research over the past several decades, this study confirms that, consistent with other surgical specialities, the majority of orthopaedic surgical interventions are not based on RCT evidence. The findings of this study are reflections of the current disconnect between trial evidence and orthopaedic surgical practice and its comparison to other specialities. These findings support the need for better quality RCTs to evaluate the indications for orthopaedic procedures and stakeholder discussions about the lack of support for many procedures currently being performed.
Supporting Information
List of procedures performed ordered according to frequency.
(DOCX)
Syntax of search strategies for each operative procedure category.
(DOCX)
List of included and excluded RCTs after full-text assessment.
(DOCX)
Characteristics of included RCTs.
(DOCX)
Risk of Bias assessment of 83 included RCTs using the Cochrane Collaboration's Risk of Bias tool.
(PDF)
(DOC)
Funding Statement
The authors have no support or funding to report.
References
- 1. Sackett DL, Rosenberg WMC, Gray JAM, Haynes RB, Richardson WS (1996) Evidence based medicine: what it is and what it isn't. BMJ 312: 71–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Crowther H, Lipworth W, Kerridge I (2011) Evidence-based medicine and epistemological imperialism: narrowing the divide between evidence and illness. J Eval Clin Pract 17: 868–872. [DOI] [PubMed] [Google Scholar]
- 3. Ghosh AK (2007) Clinical applications and update on evidence-based medicine. J Assoc Physicians India 55: 787–794. [PubMed] [Google Scholar]
- 4. Schulz KF, Grimes DA (2002) Generation of allocation sequences in randomised trials: chance, not choice. Lancet 359: 515–519. [DOI] [PubMed] [Google Scholar]
- 5. Ellis J, Mulligan I, Rowe J, Sackett DL (1995) Inpatient general medicine is evidence based. A-Team, Nuffield Department of Clinical Medicine. Lancet 346: 407–410. [PubMed] [Google Scholar]
- 6. Howes N, Chagla L, Thorpe M, McCulloch P (1997) Surgical practice is evidence based. Br J Surg 84: 1220–1223. [PubMed] [Google Scholar]
- 7. Solomon MJ, McLeod RS (1993) Clinical studies in surgical journals—have we improved? Dis Colon Rectum 36: 43–48. [DOI] [PubMed] [Google Scholar]
- 8. Baraldini V, Spitz L, Pierro A (1998) Evidence-based operations in paediatric surgery. Pediatr Surg Int 13: 331–335. [DOI] [PubMed] [Google Scholar]
- 9. McArdle CS, Hole D (1991) Impact of variability among surgeons on postoperative morbidity and mortality and ultimate survival. BMJ 302: 1501–1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cook JA, Ramsay CR, Fayers P (2004) Statistical evaluation of learning curve effects in surgical trials. Clin Trials 1: 421–427. [DOI] [PubMed] [Google Scholar]
- 11. McCulloch P, Taylor I, Sasako M, Lovett B, Griffin D (2002) Randomised trials in surgery: problems and possible solutions. BMJ 324: 1448–1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cook JA (2009) The challenges faced in the design, conduct and analysis of surgical randomised controlled trials. Trials 10: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Endarterectomy for asymptomatic carotid artery stenosis. Executive Committee for the Asymptomatic Carotid Atherosclerosis Study. JAMA 273: 1421–1428. [PubMed] [Google Scholar]
- 14. Mann M, Tendulkar A, Birger N, Howard C, Ratcliffe MB (2008) National institutes of health funding for surgical research. Ann Surg 247: 217–221. [DOI] [PubMed] [Google Scholar]
- 15. Horton R (1996) Surgical research or comic opera: questions, but few answers. Lancet 347: 984–985. [DOI] [PubMed] [Google Scholar]
- 16. Ko CY, Sack J, Chang JT, Fink A (2002) Reporting randomized, controlled trials: where quality of reporting may be improved. Dis Colon Rectum 45: 443–447. [DOI] [PubMed] [Google Scholar]
- 17. Kenny SE, Shankar KR, Rintala R, Lamont GL, Lloyd DA (1997) Evidence-based surgery: interventions in a regional paediatric surgical unit. Arch Dis Child 76: 50–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hardin WD Jr, Stylianos S, Lally KP (1999) Evidence-based practice in pediatric surgery. J Pediatr Surg 34: 908–912 discussion 912–903. [DOI] [PubMed] [Google Scholar]
- 19. Kiter E, Karatosun V, Gunal I (2003) Do orthopaedic journals provide high-quality evidence for clinical practice? Arch Orthop Trauma Surg 123: 82–85. [DOI] [PubMed] [Google Scholar]
- 20. Obremskey WT, Pappas N, Attallah-Wasif E, Tornetta P 3rd, Bhandari M (2005) Level of evidence in orthopaedic journals. J Bone Joint Surg Am 87: 2632–2638. [DOI] [PubMed] [Google Scholar]
- 21. Dickersin K, Manheimer E, Wieland S, Robinson KA, Lefebvre C, et al. (2002) Development of the Cochrane Collaboration's CENTRAL Register of controlled clinical trials. Eval Health Prof 25: 38–64. [DOI] [PubMed] [Google Scholar]
- 22.Higgins JPT, Green S (2009) Cochrane definitions and criteria for randomized controlled trials (RCTs) and controlled clinical trials (CCTs) In: Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.2 [updated September 2009] Chapter 6, Section 6.3.a. The Cochrane Collaboration. Available from www.cochrane-handbook.org
- 23. Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, et al. (2011) The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 343: d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pildal J, Hrobjartsson A, Jorgensen KJ, Hilden J, Altman DG, et al. (2007) Impact of allocation concealment on conclusions drawn from meta-analyses of randomized trials. Int J Epidemiol 36: 847–857. [DOI] [PubMed] [Google Scholar]
- 25. Wood L, Egger M, Gluud LL, Schulz KF, Juni P, et al. (2008) Empirical evidence of bias in treatment effect estimates in controlled trials with different interventions and outcomes: meta-epidemiological study. BMJ 336: 601–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Schulz KF, Chalmers I, Hayes RJ, Altman DG (1995) Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 273: 408–412. [DOI] [PubMed] [Google Scholar]
- 27. Kjaergard LL, Villumsen J, Gluud C (2001) Reported methodologic quality and discrepancies between large and small randomized trials in meta-analyses. Ann Intern Med 135: 982–989. [DOI] [PubMed] [Google Scholar]
- 28. Michaud G, McGowan JL, van der Jagt R, Wells G, Tugwell P (1998) Are therapeutic decisions supported by evidence from health care research? Arch Intern Med 158: 1665–1668. [DOI] [PubMed] [Google Scholar]
- 29.Katz P (1999) The Scapel's Edge: The Culture of Surgeons. New Jersey: Allyn and Bacon, The Johns Hopkins University School of Medicine. [Google Scholar]
- 30. Stirrat GM, Farrow SC, Farndon J, Dwyer N (1992) The challenge of evaluating surgical procedures. Ann R Coll Surg Engl 74: 80–84. [PMC free article] [PubMed] [Google Scholar]
- 31. Avery KN, Barham CP, Berrisford R, Blazeby JM, Blencowe NS, et al. (2013) Understanding surgical interventions in RCTs: the need for better methodology. Lancet 381: 27–28. [DOI] [PubMed] [Google Scholar]
- 32. Concato J, Shah N, Horwitz RI (2000) Randomized, controlled trials, observational studies, and the hierarchy of research designs. N Engl J Med 342: 1887–1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lochner HV, Bhandari M, Tornetta P 3rd (2001) Type-II error rates (beta errors) of randomized trials in orthopaedic trauma. J Bone Joint Surg Am 83-A: 1650–1655. [DOI] [PubMed] [Google Scholar]
- 34. Fehlings MG, Vaccaro A, Wilson JR, Singh A, Cadotte DW, et al. (2012) Early versus delayed decompression for traumatic cervical spinal cord injury: results of the Surgical Timing in Acute Spinal Cord Injury Study (STASCIS). PLoS One 7: e32037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hamilton D, Henderson GR, Gaston P, MacDonald D, Howie C, et al. (2012) Comparative outcomes of total hip and knee arthroplasty: a prospective cohort study. Postgrad Med J 88: 627–631. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
List of procedures performed ordered according to frequency.
(DOCX)
Syntax of search strategies for each operative procedure category.
(DOCX)
List of included and excluded RCTs after full-text assessment.
(DOCX)
Characteristics of included RCTs.
(DOCX)
Risk of Bias assessment of 83 included RCTs using the Cochrane Collaboration's Risk of Bias tool.
(PDF)
(DOC)